Abstract
Tumors actively manipulate the immune response through the production of factors that attract immune cells and subsequently alter their ability to recognize and effectively remove the tumor. While this immune evasion mechanism is an important aspect of tumor survival, the factors that serve as primary growth factors for the tumor are less understood. Here, we demonstrated a novel mechanism by which breast cancer cells manipulate tumor-infiltrating myeloid cells to maintain their survival. Tumor-derived interleukin 1α (IL-1α), acting on infiltrating myeloid cells, induced the expression of a critical tumor survival factor, the cytokine thymic stromal lymphopoietin (TSLP). TSLP promoted the survival of the tumor cells through induction of Bcl-2 expression. TSLP signaling was also required for metastasis to the lung. These studies define a novel IL-1α–TSLP-mediated crosstalk between tumor-infiltrating myeloid cells and tumor cells in the control of metastatic breast cancer.
Keywords: TSLP, IL-1α, breast cancer, breast tumor cell survival, tumor metastasis, Bcl-2, neutrophils, Ly6Chi monocytes, tumor-associated macrophages, human classical monocytes
Introduction
Thymic stromal lymphopoietin (TSLP), a key cytokine that initiates and promotes the development of type-2 immunity1, has been implicated in the progression of several human cancers, including breast, pancreatic, gastric, cervical cancer, and B cell lymphoma and myeloma2–7. In breast cancer, TSLP can be produced by both human and murine tumor cells to promote tumor progression by generating type 2-biased inflammation in the tumor microenvironment2,8. However, a recent study showed that TSLP expression by human breast tumors was not universal, suggesting the possibility of alternative sources of TSLP in breast cancer9. The possible source of TSLP in these tumor microenvironments was not fully investigated, and the identity of critical TSLP-responding cells was also not clear. Although TSLP has been suggested to play a role in promoting some human cancers mentioned above, several studies have pointed to an anti-tumor role of TSLP in skin, colon, and early stage breast cancers in murine models, emphasizing the need to understand the impacts of TSLP on various cell types in tumor microenvironments10–13. The TSLP receptor (TSLPR), composed of TSLPR chain and the interleukin 7 receptor α chain (IL-7Rα), is widely expressed on various immune cells, including B cells, mast cells, macrophages, dendritic cells (DCs), basophils, CD4+, CD8+, and regulatory T cells14,15. TSLP signaling in different immune cell types has been reported to affect various immune responses in mice, and to control related human diseases1. Furthermore, TSLPR expression on non-hematopoietic cells has also been seen, including airway and colonic epithelium, with expression regulated by local inflammation13,16,17.
TSLP is expressed primarily by epithelial cells during homeostatic conditions, and expression is induced upon inflammatory stimuli14. TSLP expression in epidermal keratinocytes and lung epithelial and smooth muscle cells results in pathological symptoms in atopic dermatitis and asthma18–20. Hematopoietic cells can also express TSLP as DCs can express TSLP upon Toll- like receptor (TLR) ligand or allergen challenge21,22. TSLP expression is up-regulated in lung epithelial cells when the transcription factor NF-κB is activated by IL-1, tumor necrosis factor (TNF) or TLR ligands23.
IL-1α is a pleiotropic pro-inflammatory cytokine that is broadly associated with many inflammatory responses. Upon binding to its receptor IL-1R1, IL-1α triggers signaling cascades that activate NF-κB and MAPKs. IL-1α can be secreted constitutively or upon cell damage or stimuli by various cell types. IL-1α, produced by tumor cells or by adjacent stromal cells, has been associated with the development of many different human cancers through stimulation of tumor cell growth and induction of vasculogenic factors24. In breast cancer, IL-1α has been reported expressed by human breast tumor cells and expression an active form of IL-1α in a human breast tumor cell line MCF-7 leads to increased tumor growth in vivo in a xenograft breast tumor model25,26.
We employed both orthotopic and autochthonous murine models of metastatic breast cancer to study the role of TSLP in tumor progression. We showed here that TSLP serves as an essential growth and survival factor for breast tumor cells through its ability to induce expression of the anti-apoptotic molecule Bcl-2. Lack of TSLP signaling in breast tumor cells led to profound regression of primary tumor growth and reduced metastasis to lungs due to increased tumor cell death. TSLP expression by myeloid cells, induced by tumor-derived IL-1α, was required for the survival of tumor cells. We also showed TSLP expression in the lung, regardless of the source, was essential for the establishment and growth of metastases. The data provide novel mechanistic insights into the role of TSLP in breast tumor progression and suggest that TSLP blockade as a novel therapeutic strategy for breast cancer.
Results
TSLP signaling in breast tumor cells is required for their growth in vitro and in vivo
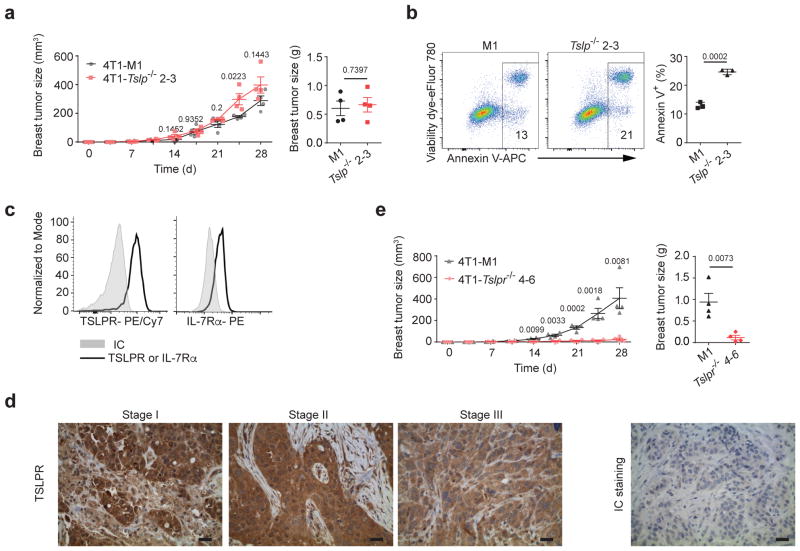
It is still controversial whether TSLP can be directly produced by human breast tumor cells2,9. To directly examine the role of tumor cell-derived TSLP regulating breast tumor progression, we generated TSLP-deficient 4T1 cells (a TSLP-expressing mouse breast tumor cell line that gives rise to a disease resembling human metastatic breast cancer)8,27 (Supplementary Fig. 1a). Interestingly, we found no difference in the primary tumor size in mice transplanted with 4T1-Tslp−/−or control 4T1 (4T1-M1) cells (Fig. 1a), indicating TSLP produced by breast tumor cells is not critical for primary tumor progression in vivo. Although TSLP deficiency did not affect tumor growth in mice, we found decreased survival in vitro, suggesting that a non-tumor source of TSLP regulated tumor survival in vivo (Fig. 1b). To test this hypothesis, we examined TSLP receptor (TSLPR) expression on breast tumor cells and found expression of both TSLPR and IL-7Rα (Fig. 1c). Importantly, human breast tumors from stage I to stage III patients, but not non-tumor breast tissue, also expressed TSLPR (Fig. 1d and Supplementary Fig. 1b). Similar to primary human breast tumor cells, the human breast tumor cell line MDA-MB 468 expressed TSLPR, while a non-tumor human breast epithelial cell line (MCF10A) did not (Supplementary Fig. 1c). To assess the requirement for TSLP signaling for tumor cell progression in vivo, we generated TSLPR-deficient 4T1 (4T1-Tslpr−/−) cells (Supplementary Fig. 1d). Mice transplanted with 4T1-Tslpr−/− cells displayed greatly reduced primary breast tumor size, showing that direct TSLP signaling by the tumor was required to maintain their growth in vivo (Fig. 1e). These data demonstrated a critical role of TSLP signaling in breast tumor cells for their progression in vivo.
Figure 1. TSLPR is expressed by breast tumor cells and TSLP signaling in breast tumor cells is critical for tumor progression in the primary site.
Primary breast tumor size (left) from D0–D28 and weights of primary tumor (right) at D28 of 4T1-M1 (M1 is a non-target gRNA used as control for CRISPR-Cas9-GFP targeting) vs.4T1-Tslp−/− 2–3 cells in wild-type (WT) mice (a), 4T1-M1 vs. 4T1-Tslpr−/ − 4–6 in WT mice (e). n=4/group. b, Representative flow plots and quantification of total dying (Annexin V+) 4T1-Tslp−/ − 2–3 cells in vitro culture. n=3/group. c, Representative flow histogram plots of TSLPR and IL-7Rα expression on 4T1 tumor cells (solid black line). Isotype control antibody staining in grey (IC). d, Representative images of TSLPR expression on human breast tumors from stage I, II, and III breast cancer patients. Right: Isotype control (IC) antibody staining. Scale bar, 10 μm. n=12 patients. Each symbol in (a, e) represents an individual mouse and in (b) represents individual cell culture. Data are represented as mean ± standard error of the mean (s.e.m.). Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (a, b, e) and (c) are representative of three and two independent experiments, respectively.
Non-tumor derived TSLP from hosts is critical to regulate breast tumor progression in vivo
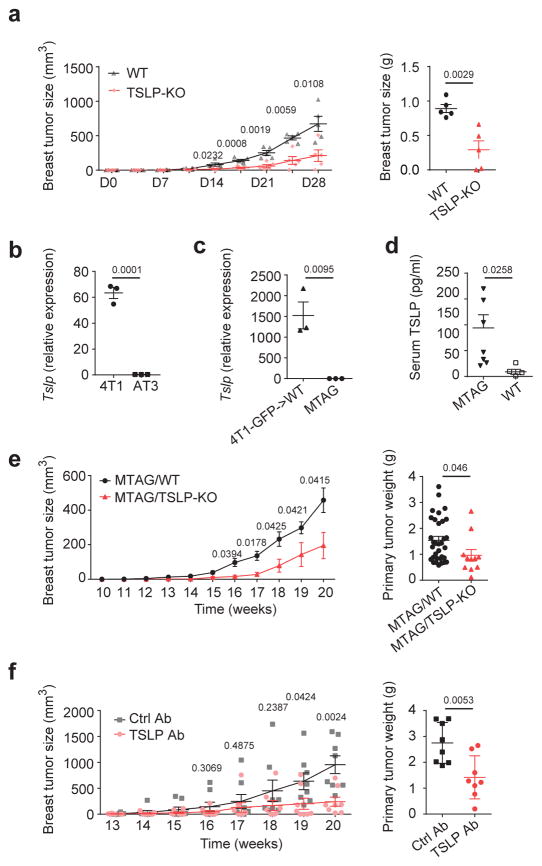
To confirm the need of non-tumor-derived TSLP for tumor cell progression in vivo, we transplanted TSLP-sufficient 4T1 tumor cells to TSLP-deficient mice (TSLP-KO) and found smaller primary breast tumors in these mice, confirming the critical role of non-tumor derived TSLP to maintain primary tumor growth (Fig. 2a). To exclude the possibility that the lack of TSLP in non-tumor cells alters tumor infiltrating T cells for tumor elimination, we examined the frequency of various T cell populations in the tumor. We found no differences in total T cell frequency, proportion of CD4+ and CD8+ T cells, or PD-1 expression in CD4+ or CD8+ T cells in the primary tumors in TSLP-KO tumor-bearing mice, indicating increased tumor cell death is not due to increased T cell recruitment to the tumor site (Supplementary Fig. 2a–c). We next used an autochthonous breast cancer model, MMTV-PyMT mice (referred to here as MTAG)28 to test our hypothesis. Unlike 4T1 cells, AT3 cells (a breast tumor cell line generated from an MTAG primary tumor)29, or tumor cells from MTAG primary tumors, expressed very low amounts of TSLP (Fig. 2b,c and Supplementary Fig. 2d,e for gating strategy for tumor cells in the primary tumors of 4T1-GFP tumor-bearing mice30 and MTAG mouse primary tumors31). However, systemic TSLP concentrations were elevated in MTAG mice, implying a non-tumor source of TSLP was involved in regulating tumor progression in this model (Fig. 2d). In addition, tumor growth in TSLP-deficient MTAG (MTAG/TSLP-KO) mice was reduced as compared to MTAG mice (Fig. 2e). Systemic TSLP blockade with a TSLP antibody, begun when tumors were palpable, significantly reduced primary breast tumor growth in MTAG mice (Fig. 2f). These results demonstrated that TSLP produced by non-tumor cells is critical for tumor progression.
Figure 2. TSLP derived from non-tumor sources is critical for tumor progression in both transplanted and autochthonous murine breast tumor models.
a, Primary breast tumor size (left) from D0-D28 and weights of primary tumor (right) at D28 of 4T1 cells transplanted into WT vs. TSLP-KO mice. n=5 /group. TSLP mRNA expression in AT3 vs. 4T1 (b) and tumor cells sorted from primary tumors of 4T1-GFP tumor-bearing mice at D28 or MTAG mice at week 20 (c). n=3/group. d, TSLP concentrations in serum of MTAG (n=8) or tumor-free WT mice (n=5) at week 20. Measurements of total primary breast tumor growth from week 10–20 (left) and weights of total primary breast tumor mass at week 20 (right) in of MTAG/WT (n=33) vs. MTAG/TSLP-KO (n=11) mice (e) or MTAG mice treated with anti-TSLP or control antibodies weekly from week 14–20 (f) (n=8/group). Each symbol in (a, c, d, e right, f) represents an individual mouse and in (b) represents individual cell culture. Data are represented as mean ± standard error of the mean (s.e.m.). Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (a, d, e, f) represent pooled data; others are representative of two independent experiments.
TSLP signaling in breast tumor cells regulates tumor cell survival
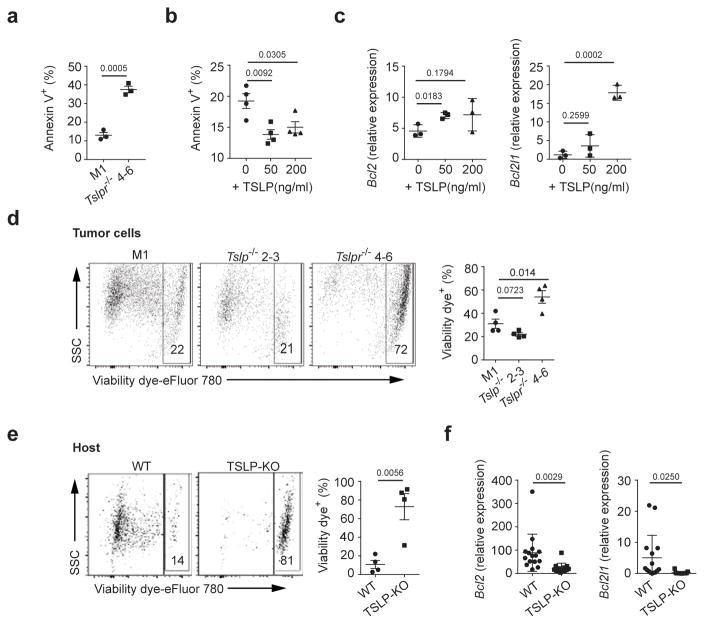
We next investigated how TSLP signaling directly influenced tumor cells. We found in in vitro conditions both 4T1-Tslpr−/− and 4T1-Tslp−/− cells displayed reduced tumor cell viability (Fig. 1b and Fig. 3a); whereas treating parental 4T1 cells with TSLP enhanced their viability through up-regulation of anti-apoptotic molecules Bcl-2 and Bcl-xL (Fig. 3b,c). We observed similar results when treating AT3 cells with TSLP (Supplementary Fig. 3a,b). Next, we explored tumor cell survival in the primary tumor in tumor-bearing mice (gating strategy shown in Supplementary Fig. 3c). Interestingly, we only observed decreased 4T1-Tslpr−/− but not 4T1-Tslp−/− cell survival in the primary tumor when transplanted into wild-type mice, indicating TSLP from non-tumor derived sources maintained tumor cell survival in vivo (Fig. 3d). Importantly, 4T1 cells transplanted into TSLP-KO mice displayed greatly reduced tumor cell survival in the primary tumor with increased cleaved caspase 3 and reduced Bcl-2 and Bcl-xL expression in the tumor cells (Supplementary Fig. 3d and Fig. 3e,f). Furthermore, breast tumor cells from MTAG/TSLP-KO mice displayed decreased Bcl-2 and Bcl-xL expression (Supplementary Fig. 3e). Human breast tumor cell line, MDA-MB-468, showed enhanced cell viability and increased Bcl-2 expression when cultured in the presence of TSLP; whereas non-tumor breast epithelial cell line, MCF10A, was not affected (Supplementary Fig. 3f,g). Although TSLP signaling affects breast tumor survival by regulating anti-apoptotic molecules, it does not affect breast tumor cell proliferation, as deprivation of TSLP in vitro (TSLP- or TSLPR-deficient 4T1 cells) or in vivo (TSLPR-deficient 4T1 cells in wild-type host or TSLP-deficient 4T1 cells or TSLP-deficient host) did not change Ki67 expression in tumor cells (Supplementary Fig. 3h). Taken together, TSLP signaling is important to maintain breast tumor cell survival, likely through induction of anti-apoptotic molecules in vitro and in vivo.
Figure 3. TSLP signaling in breast tumor cells regulates their survival and TSLP producing abilities.
Percentage of dying (Annexin V+) 4T1-Tslpr−/ − cells in vitro (a) (n=3 /group) or 4T1 cells treated with TSLP for 2 days (b) (n=4/group). (c) mRNA expression of Bcl2 and Bcl2l1, examined by qPCR, in 4T1 cells treated with TSLP. Expression values normalized to Gapdh expression. n=3/group. Representative flow plots and quantification of percentage of dying (viability dye+) 4T1-M1 vs. 4T1-Tslpr −/ − 4–6 vs. 4T1-Tslp−/ − 2–3 cells in the primary tumor in WT-tumor bearing mice (d), or dying 4T1 cells in the primary tumors from WT vs. TSLP-KO tumor-bearing mice at D28 (e). n=4/group. f, mRNA relative expression of Bcl2 and Bcl2l1 in sorted 4T1 cells from the primary tumors in (e); measurements as described for panel c. WT, n=15; TSLP-KO, n=13. Each symbol in (d right, e right, f) represents an individual mouse and in (a, b, c) represents individual cell culture. Data are represented as mean ± standard error of the mean (s.e.m.). Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (a–d) and (e) are representative of two and three independent experiments, respectively. Results in (f) represent pooled data.
Myeloid cell-derived TSLP is the critical source to maintain tumor cell survival
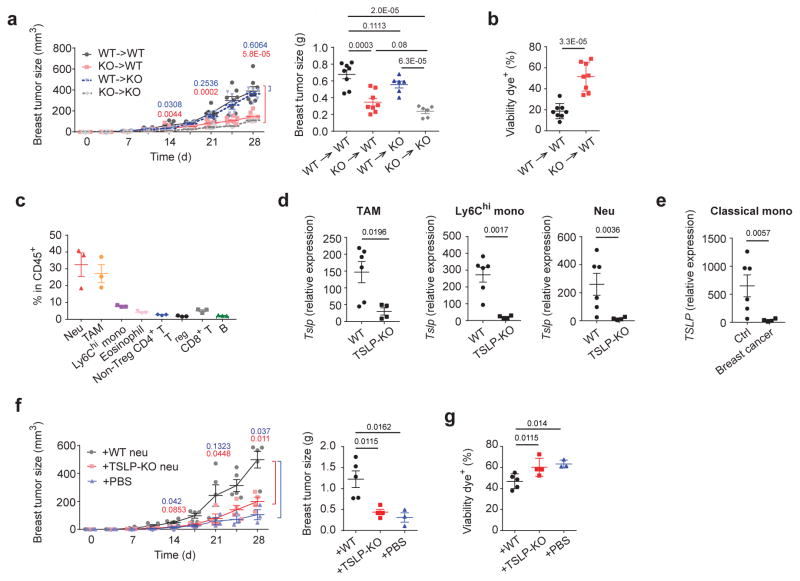
Next, we sought to identify the TSLP-producing cell types during tumor progression. 4T1 cells were transplanted into bone marrow chimeric mice that lacked either hematopoietic cell- or stromal cell-derived TSLP. Surprisingly, we found mice with TSLP deficiency in hematopoietic cells, but not in stromal cells, displayed smaller primary tumors and increased tumor cell death, indicating a role of hematopoietic cell-derived TSLP for tumor cell survival in vivo (Fig. 4a,b). We further found neutrophils, Ly6Chi monocytes, and tumor-associated macrophages (TAMs), the three dominant hematopoietic cell populations in primary tumors, all expressed TSLP (Fig. 4c,d, and Supplementary Fig. 4a for gating strategies in these myeloid cell populations in the primary tumor). As is seen in human breast cancer patients32,33, neutrophils and Ly6Chi monocytes were markedly expanded in tumor bearing m ice and also expressed TSLP. In contrast, these populations in tumor-free mice did not (Supplementary Fig. 4b–e) Similarly, neutrophils derived from MTAG mice displayed increased TSLP expression compared to tumor-free mice (Supplementary Fig. 4f). We further examined TSLP expression in classical monocytes in humans (equivalent to mouse Ly6Chi monocytes)34 and found expression was increased in breast cancer patients compared to healthy subjects (Fig. 4e, Supplementary Fig. 4g,h). To determine whether TSLP from myeloid cells was involved in tumor progression, we transferred neutrophils isolated from wild-type or TSLP-KO tumor-bearing mice into TSLP-KO tumor-bearing mice. We found that neutrophils from wild-type, but not TSLP-KO, tumor-bearing mice were able to restore primary breast tumor size in TSLP-KO hosts (Fig. 4f). The increase in primary tumor volume in TSLP-KO hosts receiving neutrophils from wild-type donors was associated with increased tumor cell survival (Fig. 4g). To confirm decreased tumor cell death was directly due to TSLP produced from transferred neutrophils, sorted wild-type or TSLP-KO neutrophils derived from tumor-bearing mice were co-cultured with 4T1 cells. Wild-type neutrophils, but not TSLP-KO neutrophils, decreased 4T1 cell death, while TSLPR-KO 4T1 cells had no response to either source of neutrophils. Co-culturing Ly6Chi monocytes to 4T1 or TSLPR-KO 4T1 cells showed a similar result (Supplementary Fig. 4i). Taken together, these data demonstrated myeloid cells in tumor setting as critical sources of TSLP for tumor survival in vitro and in vivo.
Figure 4. TSLP produced by myeloid cells is an important source for breast tumor progression.
a, Primary breast tumor growth, and D28 primary breast tumor weights in BM chimeric mice. WT->WT and KO->WT, n=8/group; WT->KO and KO->KO, n=6/group. b, Percentage of dying tumor cells in the primary tumor in (a). n=8/group. c, Frequencies of different hematopoietic cell populations within the primary tumor of WT mice at D28 examined by flow cytometric analysis n=3/group. Tslp mRNA expression, examined by qPCR, in sorted neutrophils, Ly6Chi monocytes, or TAMs from the primary tumors (d). WT, n=7; KO, n=4. e, TSLP mRNA relative expression, examined by qPCR, in sorted classical monocytes from breast cancer patients vs. healthy controls. Expression values normalized to Gapdh expression. Ctrl, n=11; breast cancer, n=6. f–g, Neutrophils from WT or TSLP-KO tumor bearing mice at D28 post tumor injection were sorted and then transfer into recipient TSLP-KO tumor-bearing mice at D5 and D15 post tumor injection. Primary tumor progression and tumor weights at D28 of TSLP-KO recipient tumor-bearing mice (f) and percentage of dying tumor cells in the primary tumor at D28 (g) were examined. +WT, n=5; +KO, n=4; +PBS, n= 3. Each symbol represents an individual mouse or human donor. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (a, b, d, e) represent pooled data and rests are representative of three independent experiments.
Breast tumor cell-derived IL-1α regulates TSLP expression in neutrophils
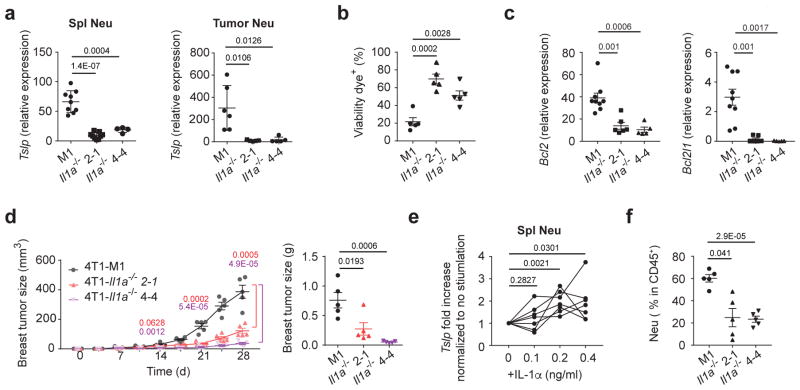
As these myeloid cell populations from naïve mice did not express or expressed low amounts of TSLP, we hypothesized that a tumor-cell derived factor induced TSLP expression. It has been reported that IL-1 induces TSLP expression in epithelial cells23. We then focused on IL-1α as it has been reported to be expressed in human breast tumors25. To explore the role of tumor-derived IL-1α in regulating TSLP expression by myeloid cells in vivo, we generated IL-1α-deficient 4T1 cells (4T1-Il1a−/−) (Supplementary Fig. 5a). We observed not only decreased TSLP expression in neutrophils in tumor-bearing mice transplanted with 4T1-Il1a−/− cells but also increased cell death with reduced Bcl-2 and Bcl-xL expression in 4T1-Il1a−/− cells in the primary tumor even though IL-1α deficiency did not alter 4T1 cell death in vitro (Fig. 5a–c and Supplementary Fig. 5b). Primary tumor size in these mice also showed smaller compared to mice transplanted with control 4T1 cells (Fig. 5d). To test if IL-1α directly affected TSLP expression in neutrophils, neutrophils sorted from D21 tumor-bearing mice were stimulated with recombinant IL-1α. We found IL-α stimulation increased TSLP expression in neutrophils in a dose-dependent manner (Fig. 5e). Consistent with a report showing IL-1α recruited neutrophils to lungs during viral infection35, we observed a decrease in neutrophil recruitment to primary tumors in mice transplanted with 4T1-Il1a−/− cells (Fig. 5f). These results demonstrated IL-1α produced by tumor cells, can induce TSLP production by myeloid cells that infiltrate the tumor and maintain tumor cell survival and lead to primary tumor progression.
Figure 5. 4T1 derived IL-1α induces TSLP expression in neutrophils.
TSLP mRNA expression in sorted splenic neutrophils (left) (M1, n=9; Il1a−/ − 2-1, n=9, Il1a−/ − 4-4, n=4) and tumor-infiltrating neutrophils (right) (M1, n=6; Il1a−/ − 2-1, n=5, Il1a−/ − 4-4, n=5) (a), percentage of dying tumor cells in the D28 primary tumors (n=5/group) (b), Bcl2 and Bcl2l1 mRNA expression in sorted tumor cells (M1, n=9; Il1a−/ − 2-1, n=6, Il1a−/ − 4-4, n=5) (c) in the primary tumor at D28, and primary tumor progression and tumor weights (n=5/group) at D28 (d) in WT mice transplanted with 4T1-M1, 4T1-Il1α −/ − 2-1, and 4T1- Il1α −/ − 4-4 for 28 days. Expression values normalized to Gapdh expression. e, Fold increase of TSLP mRNA expression, examined by qPCR, in splenic neutrophils treated with IL-1α from D21 post 4T1 transplanted into WT mice. n=7/group. f, Percentage of neutrophils in the primary tumors (D28) of WT mice transplanted with IL-1α-KO 4T1 cells. n=5/group. Each symbol in (a, b, c, d, f) or line in (e) represents an individual mouse. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (a, c, e) represent pooled data and rests are representative of three independent experiments
TSLP is critical for maintaining metastasis survival in the lung
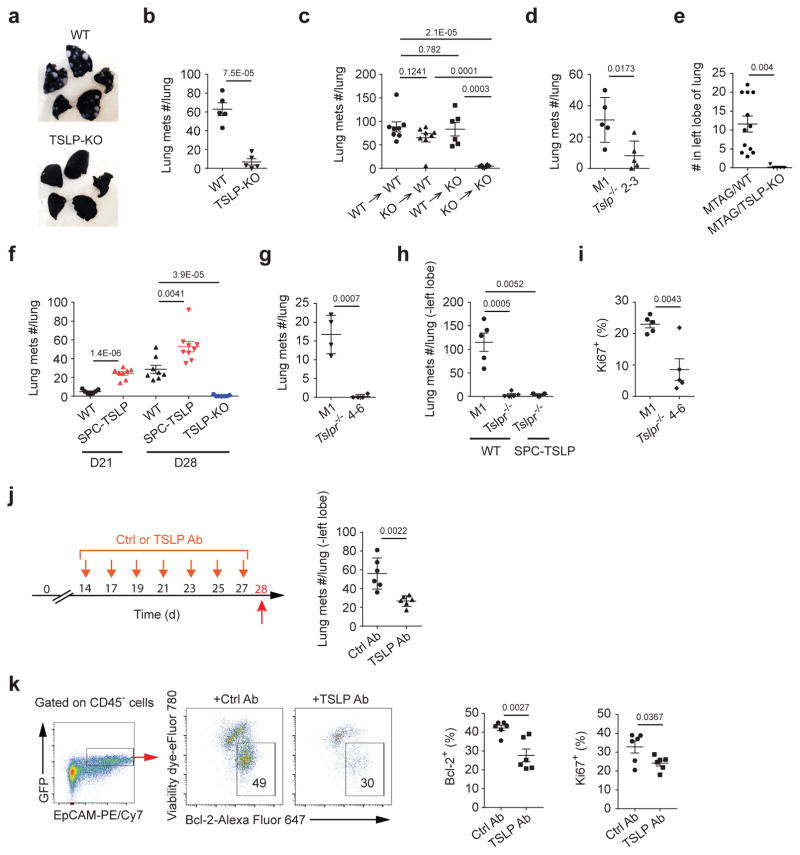
Having shown that TSLP derived from myeloid cells served as a survival factor for tumor cells at the primary tumor site, we then investigated if it also played a role in lung metastasis, which is the leading cause of cancer associated mortality in breast cancer36,37. Indeed, 4T1 transplanted TSLP-KO mice not only showed reduced primary tumor size but also fewer lung metastases (Fig. 6a,b). To exclude the possibility that the reduction in lung metastases was due to a smaller primary tumor, we injected 4T1 cells into TSLP-KO mice intravenously (i.v.) and found these mice still had fewer lung metastases (Supplementary Fig. 6a). We found no difference in the number of lung metastases in the bone marrow chimeric mice with TSLP deficiency in stromal or hematopoietic cells, suggesting that lack of either TSLP source can be compensated by another one (Fig. 6c). Although 4T1-Tslp−/− cells transplanted to wild-type mice displayed similar primary tumor size as control 4T1 cells, they developed fewer lung metastases (Fig. 6d). We further confirmed these results in MTAG mice and found lung metastases were also reduced in MTAG/TSLP-KO mice (Fig. 6e). Taken together, these results suggested TSLP present in lungs, regardless its source, was able to promote metastasis. Breast cancer patients with a history of asthma experienced increased lung metastases, implying that increased TSLP production associated with asthma can increase metastasis38,39. To test this, we transplanted 4T1 cells into a mouse strain that has increased amounts of lung-specific TSLP (SPC-TSLP mice)40. We found 4T1 transplanted in these mice showed increased lung metastases at both D21 and D28 post tumor injection, with no difference in primary tumor size (Fig. 6f and Supplementary Fig. 6b). We also found that mice given 4T1-Tslpr−/− cells, either transplanted in the 4th mammary gland or injected i.v. showed reduced lung metastases (Fig. 6g,h). SPC-TSLP mice injected i.v. with 4T1-Tslpr−/− cells displayed a similar reduction of lung metastases as wild-type mice (Fig. 6h). Furthermore, we observed a decrease in proliferating (Ki67-positive) 4T1-Tslpr−/− cells in lungs of transplanted mice (Fig. 6i). Treating 4T1-transplanted mice with a neutralizing TSLP antibody specifically in lungs (via intranasal administration) after lung metastasis had begun (D14 post-transplant) reduced lung metastases while having no effect on the growth of the primary tumor (Fig. 6j and Supplementary Fig. 6c). TSLP blockade in lungs not only reduced tumor cell survival by reducing Bcl-2 expression, but also reduced their proliferation abilities (Fig. 6k). Taken together, these data demonstrated TSLP derived from either tumor cells or non-tumor cells enhances tumor cell survival and proliferation in lungs and local TSLP blockage efficiently reduced tumor metastases.
Figure 6. TSLP is also critical for breast tumor metastasis in lungs.
a, Representative images of India ink-injected lungs from WT- vs. TSLP-KO-tumor-bearing mice at D28 post 4T1 or 4T1-GFP transplantation. Counts of tumor metastases in lungs at D21 or D28 in mice injected with 4T1 cells (b, c, d, f, g, j). n=5/groups (b); WT->WT and KO->WT, n=8/group; WT->KO and KO->KO, n=6/group (c); n=5/group (d); D21 WT, n=7, D21 SPC-TSLP, n=8, D28 WT, n=8, D28 SPC-TSLP, n=9, D29 TSLP-KO, n=7 (f), n=4 /group (g). Counts of tumor metastasis in lungs (h) (4T1-M1 in WT mice, n=5; 4T1-Tslpr−/ − in WT mice, n=5, 4T1-Tslpr−/ − in SPC-TSLP mice, n=3) and percentage of Ki67+ tumor cells in lungs (i) in mice at D19 received 4T1 via i.v.injection (n=5 /group). e, Counts of tumor metastasis in lungs from MTAG/TSLP-KO vs. MTAG mice at W20, examine by serial sections of the left lobe. MTAG, n=12; MTAG/TSLP-KO, n=5. j, The schematic summary and counts of tumor metastases in lungs at D28 of lung TSLP blockade in 4T1-tumor bearing mice. n=6/group. k, Representative flow plots of Bcl-2 expressions on live (viability dye−) 4T1 cells and quantification of frequencies of live (viability dye−/Bcl-2+) and Ki67 expression in live 4T1 cells in lungs at D28 in mice in (j). n=6/group. Each symbol represents an individual mouse. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (c, e, f) represent pooled data. Results in (a, b, d, g) and (h, i, j, k) are representative of three and two independent experiments, respectively.
Discussion
A role for TSLP in tumor development was first suggested by studies in human breast and pancreatic cancer, where TSLP was found to skew the tumor microenvironment toward a type 2 T helper (TH2) inflammation that favors tumor protection2,3,41. The current study revealed an unexpected, but critical, role for TSLP as an essential survival factor for breast tumor cells. We further demonstrated that tumor-derived IL-1α was important to induce TSLP expression from tumor-infiltrating myeloid cells. Importantly, treating tumor-bearing mice with a neutralizing TSLP antibody after tumor growth had initiated inhibited subsequent tumor growth. In addition, lung-specific blockade of TSLP following the initiation of metastasis reduced the number of tumor metastases, demonstrating a role for TSLP throughout the life of the tumor and providing a potential therapeutic target for all stages of breast cancer.
TSLP signaling has been shown to increase cell survival through induction of Bcl-2 or increased proliferation of responder cells, likely through phosphorylation of signal transducer and activator of transcription 5 (STAT5)6,42–44. Our findings support this idea as we found TSLP-induced expression of Bcl-2 in the breast tumor cells. As TSLP also has been reported to be up-regulated in other human cancers3–5,7 it is worth exploring if this pathway is also operative in other cancer types. The functional TSLPR complex was expressed on breast tumor cells, but not normal breast tissue, suggesting TSPR expression is induced during neoplastic transformation. How TSLPR and IL-7Rα expression is regulated during this process remains unknown. A similar finding was reported in colon cancer, where the tumor, but not normal tissues expressed functional TSLPR13.
Consistent with its role in the survival of the primary tumor, we also showed that TSLP is critical for the lung metastasis. However, in the lung TSLP from any source is sufficient to promote metastasis. Taken together with the finding that TSLPR-deficient 4T1 cells failed to grow in the lung following i.v. administration, these data support our model for TSLP as an obligate survival factor for the tumor cells. However, a recent study using the MTAG model suggested that skin-derived TSLP, induced prior to breast tumor initiation, might have an inhibitory effect on early breast tumor development12. One possibility for TSLP inducing different outcomes in the same tumor model is that increased TSLP from skin, prior to tumor induction, promotes local inflammation that leads to tumor elimination. As previous studies have found that elevated TSLP concentrations in the skin can inhibit local tumor growth10,11,45, it may be acting differently in the skin as compared to other sites. These data suggest that elevated expression of TSLP, especially in the skin, prior to tumor initiation can inhibit tumor progression through activation of local T cells, while TSLP induced following the establishment of a tumor is protective and promotes tumor growth. These data suggest that the role of TSLP in tumorigenesis can be complex and is determined by the specific TSLP-responding cell type(s) involved and their importance in regulating tumor development.
Myeloid cell production of TSLP was surprising as previous work has shown that TSLP is predominantly expressed by epithelial cells20. It is of note that only neutrophils from tumor-bearing mice, but not tumor-free mice, expressed TSLP, while Ly6Chi monocytes from naïve animals expressed low amounts of TSLP, which was significantly increased in the tumor-bearing mice. In addition, classical monocytes derived from human breast cancer patients showed increased TSLP producing capacity, suggesting that signals from the tumor promote the expression of TSLP by myeloid-lineage cells. Indeed, these myeloid cells in tumor settings have been reported to be functionally distinct from those in tumor free condition46. Up-regulation of TSLP expression might be one of the new features that favor tumor progression. It will be interesting to understand whether TSLP produced by myeloid cells is a universal feature of other tumor models or human cancers. We identified tumor-derived IL-1α as one important factor capable of inducing TSLP expression in neutrophils. Interestingly, we observed a less robust increase in TSLP expression from naïve neutrophils (neutrophils sorted from tumor free mice) stimulated with IL-1α in vitro (data not shown). Although these data imply other stimuli act coordinately with IL-1α to enhance TSLP expression, our data demonstrate that IL-1α is essential for neutrophil-derived TSLP. It will be interesting to explore if IL-1α induction of TSLP expression in neutrophils is universal or specific to breast cancer as IL-1α can be released by many other cancer types24.
A previous study using the 4T1 model also suggested a role for TSLP in supporting tumor growth via direct signaling by CD4+ T cells8. While our results support a role for TSLP in 4T1-mediated tumor progression, our data demonstrated that TSLP exerted its pro-tumor properties by acting on tumor cells. Interestingly, we observed that TSLPR deficiency in all T cells did not affect tumor growth; in fact, primary tumor growth was somewhat greater in these mice (data not shown), in agreement with the notion that TSLP signaling in T cells may inhibit tumors10–12. Finally, the rescue of tumor growth by transfer of TSLP-sufficient, but not TSLP-deleted, neutrophils demonstrates that TSLP from non-tumor cells is the primary driver in overall tumor progression.
In summary, our data demonstrate that breast tumors produce IL-1α, which then acts to induce subsequent TSLP production from tumor-infiltrating myeloid cells, with TSLP then acting as a critical survival factor for the tumor. These data suggest TSLP or IL-1α blockade may be an effective therapeutic intervention in metastatic breast cancer to not only block the growth of the primary tumor but also inhibit metastasis to the lung.
METHODS
Mice and cell lines
All 4T1 experiments were performed in 8- to10-week-old female BALB/c mice. CD45.2+ WT mice were purchased from Charles River Laboratories. TSLP-KO47, and SPC-TSLP40 mice were all backcrossed to BALB/c background for more than 10 generations. MTAG in C57BL/6 background mice were provided by S. Abrams (Roswell Park Cancer Institute). MTAG/TSLP-KO mice were generated from crossing MTAG mice to TSLP-KO mice in C57BL/6 background mice. All experiments were performed as approved by Benaroya Research Institute animal facility IACUC and in compliance with all relevant animal use guidelines and ethical regulations. All mice were bred and housed in specific-pathogen free conditions in Benaroya Research Institute animal facility.
The cell lines 4T1 (ATCC® CRL-2539™), MDA-MB-468 (ATCC® HTB-132™), and MCF 10A (ATCC® CRL-10317™) were all purchased from ATCC and cultured according to ATCC guidelines. AT3 cell line was generous provided by S. Abrams (Roswell Park Cancer Institute).
Murine breast tumor models
Orthotopic: female BALB/c mice were injected with 104 4T1 mammary carcinoma cells at the 4th mammary gland at D0. Some experiments used 4T1 cells expressing eGFP following lentivirus infection or transduced with lentivirus containing CRISPR-Cas9-GFP that targeted to genes of interest. Mouse breast tumor size was measured in 3 dimensions (length × width × height) by digital caliper (Mitutoyo). To evaluate breast tumor metastasis to lungs, mouse lungs were injected with 15 % Indian ink via trachea after sacrifice and then fixed with Fekete’s solution. Lungs that were not seeded with lung metastases were black after India ink infusion but tumor metastases part displayed in white. Number of lung metastases was counted as white nodules on the lung surface.
Lung homing model of metastasis: 4T1 cells (5 × 104) were injected i.v. via tail vein at D0. Tumor burdens in lungs were evaluated at D19 using the India ink method and lung metastases were counted as described above.
Autochthonous breast tumor model: Breast tumor growth of MTAG mice were monitored and measured from 10- to 20-weeks old. Because MTAG mice could develop up to 10 discrete mammary gland tumors, total tumor volume in female MTAG mice was determined as the sum of each distinct tumor volume within an individual mouse. Each breast tumor was measured in 3 dimensions (length × width × height) by digital caliper (Mitutoyo) weekly to 16-week old and then twice a week afterwards. To evaluate tumor metastasis to lungs, the left lobe of mouse lungs was fixed, embedded in paraffin, and serial sectioned. Lung sections were picked every 200 μm and lung metastases in each section were counted under microscope. Total lung metastases were the sum from all picked sections.
All tumor-bearing mice were monitored as approved by the Benaroya Research Institute Institutional Animal Care and Use Committee.
BM chimeras
Recipient mice were lethally irradiated with twice 450 Rads within the same day, followed by 5 ×106 BM cells via i.v. injection. Mice were then used 8 weeks after transplant.
TSLP blockade in mice
To deplete TSLP in mouse lungs in 4T1 model, 25 μg of TSLP antibody (M702)48 or rat IgG2a (BioXCell) in 40 μl of 1× PBS (Sigma-Aldrich) was intranasally delivered into mice at D14, D17, D19, D21, D23, D25, and D27 post 4T1 tumor cell transplant.
To deplete TSLP systemically in MTAG mice, 500 μg of TSLP antibody (M702) or rat IgG2a in 200 μl of 1× PBS was intraperitoneally delivered into MTAG mice weekly, starting from W14 to W20.
In vitro co-culture of 4T1 cells with neutrophils or Ly6Chi monocytes
Sorted neutrophils or Ly6Chi monocytes from spleens of tumor-bearing mice were co-cultured with 4T1 cells at the same number as 4T1 cells were seeded a day before, which makes the cell number ratio of 4T1 to neutrophils or Ly6Chi monocytes about 2 at the time of starting co-culturing. 4T1 cell viability and Ly6Chi monocyte differentiation were evaluated 2 days post-co-culturing.
CRISPR/Cas9 gene targeting
Lentivirus vector for CRISPR-Cas9-GFP targeting was generous provided by D.B. Stetson (University of Washington)49. In brief, 4T1 cells were transduced with lentivirus containing CRISPR-Cas9-GFP-TSLP or CRISPR-Cas9-GFP-TSLPR or CRISPR-Cas9-GFP-IL-1α to deplete TSLP or TSLPR or IL-1α, respectively. CRISPR-Cas9-GFP-M1 (M1 is a non-targeting gRNA) was used as control. Cells were then selected in puromycin for 7 days. Several GFP+ clones were selected to test mRNA expression of target genes. mRNA expression of other genes, including Mmp9, Gmcsf, Ccl2, Ccl5, Tnf, Cxcl1, Ccl17, were also tested to confirm selected clones have similar expression as parental 4T1 cells. Protein expression encoded by the selected target genes was determined by flow cytometric analysis or ELISA. Primer sequences for these genes are in the supplementary experimental procedures.
gRNA sequences:
non-targeting M1 gRNA: 5′-GCGAGGTATTCGGCTCCGCG-3′.
Tslpr 4-6 gRNA: 5′-GGAGACGGTGGAGGTCACGT-3′.
Tslp 2-3 gRNA: 5′-TCCGGGCAAATGTTTTGTCG-3′.
Il1a 2-1 gRNA: 5′-CCTCAACCAAACTATATATC-3′.
Il1a 4-4 gRNA: 5′-AAATCACTCTGGTAGGTGTA-3′.
Human samples
All human samples were collected after approval by Benaroya Research Institute IRB and with written informed consent acquired through VM-BRITE program. Frozen PBMC samples, fresh breast tumors, adjacent non-tumor breast tissue, and unidentified information from breast cancer patients were provided by VM-BRITE program. Healthy control frozen PBMC samples and unidentified donor information were provided by Benaroya Research Institute Healthy Control Registry and Biorepository. Breast cancer patient PBMC samples were age and sex matched with healthy controls.
Enzyme-linked immunosorbent assay
TSLP protein abundance was measured by Mouse or human TSLP DuoSet kit (R&D Systems).
Immunohistochemistry staining
Tissues were collected and fixed in 10% formalin and then were dehydrated and embedded in paraffin for sectioning. Sections (5 μm) were then deparaffinized and rehydrated for immune-histochemical staining with the primary or isotype control antibodies, followed by DAB substrate for visualization. All sections were counterstained with hematoxylin for nuclei staining. Information on all antibody reagents used is provided in Supplementary Note 1. Images were taken by Leica DME brightfield microscope and processed by Leica Las EZ imaging software. To quantify cleaved caspase 3 staining in mouse tumors, 5 different fields were randomly picked from each mouse tumor and staining intensity was quantified by Fiji software (Fiji.sc).
Preparation of single-cell suspensions and flow cytometry
Mouse breast tumors or lungs were chopped with scissors and then digested with 50 μg/ ml of Liberase TM (Roche) and 20 units/ml of DNase I (Sigma-Aldrich) on shaker at 1500 rpm at 37 °C for 30 min. Cells were then lightly pressed through 100-μm cell strainers (Falcon) and erythrocytes were lysed by 1× BD Pharm Lyse (BD Biosciences). Cells were washed, counted, and stained for flow cytometric analysis. For intranuclear staining, cells were fixed and permeabilized by using Transcription Factor Staining Buffer Set (eBioscience). Dead cells were excluded by Live/Dead fixable dead cell stain (eBioscience).
Acquisition of samples was performed using a FACS LSR II or FACS Canto (BD Biosciences) instrument, and data were analyzed with Flowjo software (version X). Cell sorting was performed in FACS Aria II or FACS Aria Fusion (BD Biosciences). Information on all antibody reagents used for flow cytometry is provided in Supplementary Note 1.
Primer sequences for quantitative real-time PCR in mouse cells were as follow
Bcl2 forward, 5′-CATGTGTGTGGAGAGCGTCAA-3′
Bcl2 reverse, 5′-GTCTTCAGAGACAGCCAGGA-3′
Bclxl forward, 5′-CAGAGCTTTGAGCAGGTAGAG-3′
Bclxl reverse, 5′-GAAGAGTGAGCCCAGCAGAA-3′
Tslp forward, 5′-TACTCTCAATCCTATCCCTGGCTG-3′
Tslp reverse, 5′-TGTGAGGTTTGATTCAGGCAGATG-3′
Il1a forward, 5′-TCCAGATCATGGGTTATGGACTG-3′
Il1a reverse, 5′-AGTATCAGCAACGTCAAGCAA-3′
Tslpr forward, 5′-TGACGTCACGGGGTGATGTC-3′
Tslpr reverse, 5′-GAGGATGCACCCGGAAGTGA-3′
Gapdh forward, 5′-TGCACCACCAACTGCTTAG-3′
Gapdh reverse, 5′-GGATGCAGGGATGATGTTC-3′
Mmp9 forward, 5′-CAGACGTGGGTCGATTCCA-3′
Mmp9 reverse, 5′-CATCTCTCGCGGCAAGTCTT-3′
Ccl2 forward, 5′-GCATCCACGTGTTGGCTCA-3′
Ccl2 reverse, 5′-CTCCAGCCTACTCATTGGGATCA-3′
Ccl5 forward, 5′-AGATCTCTGCAGCTGCCCTCA-3′
Ccl5 reverse, 5′-GGAGCACTTGCTGCTGGTGTAG-3′
Cx3cl1 forward, 5′-AGACTCCAGCCACACTCC-3′
Cx3cl1 reverse, 5′-TGACAGCGCAGCTCATTG-3′
Ccl17 forward, 5′-CACCAATGTAGGCCGAGAGT-3′
Ccl17 reverse, 5′-GTTGAAACCATGGACAGCAGC-3′
Gmcsf forward, 5′-GGCCTTGGAAGCATGTAGAGG-3′
Gmcsf reverse, 5′-GGAGAACTCGTTAGAGACGACTT-3′
Tnf forward, 5′-CTGTAGCCCACGTCGTAGC-3′
Tnf reverse, 5′-TTGAGATCCATGCCGTTG-3′
Primer sequences for quantitative real-time PCR in human cells were as follow
Human GAPDH forward, 5′-GGATTTGGTCGTATTGGG-3′
Human GAPDH reverse, 5′-GGAAGATGGTGATGGGATT-3′
Human TSLP forward, 5′-TCTCCTCTTCTTCATTGCCTG-3′
Human TSLP reverse, 5′-TCGCCATGAAAACTAAGGCT-3′
Human TSLPR forward, 5′-CTGATGCCACGAAAATCTCA-3′
Human TSLPR reverse, 5′-TTCTCCATCAGGAATGGGAC-3′
Human BCL2 forward, 5′-GATTTTATTTCGCCGGCTC-3′
Human BCL2 reverse, 5′-TGATGTGAGTCTGGGCTGAG-3′
Human IL7R forward, 5′-AGGCACTTTACCTCCACGAG-3′
Human IL7R reverse, 5′-AATGGATCGCAGCACTCACT -3′
Quantitative real-time PCR
Total RNA was extracted by Nucleo Spin RNA kit (Macherey-Nagel). First-strand cDNA was synthesized using PrimeScript Reverse Transcriptase (Takara). To amplify cDNA, 2× SYBR Premix Ex Taq II (Takara) was used, and then real-time quantitative PCR was carried out by using the ABI 7900HT instrument an analyzed by SDS 2.4 software. PCR cycling conditions were 95 °C for 30 s for polymerase activation, then amplification for 40 cycles: 95 °C for 5 s, 60 °C for 30 s. Relative expression levels were calculated using Gapdh as endogenous control. All primer sequences used are listed in the Supplemental experimental procedures.
Statistical analysis
All data are presented as mean values ± standard error of the mean (s.e.m.). The statistical significance of differences in mean values was analyzed with the unpaired, two-tailed t test with 95 % confidence intervals. P values less than 0.05 were considered significant. All statistical analyses were performed by using Prism 7 (GraphPad) and Excel 2013 (Microsoft).
Life Sciences Reporting Summary
Further information on experimental design and reagents is available in the Life Sciences Reporting Summary.
Data availability
All data that support the findings of this study are available from the corresponding author upon request.
Supplementary Material
Acknowledgments
We thank G. Kaber for generating 4T1-GFP cell lines. We thank D.B. Stetson and E. Gray (University of Washington) for providing CRISPR vectors and their protocol. We thank J. B. Tan, F. Roan, A. Sheih, S. de Jesus Carrion, and S. Ong for the aid in randomizing samples for blinded experiments. We thank S. Abrams (Roswell Park Cancer Institute) for providing MTAG mice in C57BL/6 background and the AT3 cell line. We thank P. Y. Johnson and M. Beauchamp for TSLPR and TSLP staining in human breast tumors and in mouse breast tumors, respectively. We thank all staff in Virginia Mason BRITE program and BRI clinical core for sample recruitment and preparation. We thank D. J. Campbell, J. Hamerman, and T. N. Wight for discussions, and R. Penn for administrative support. Supported by the US National Institute of Health (R01-CA182783 to S.F.Z.).
Footnotes
Author Contributions E.L.K. designed and performed experiments. E.L.K. and S.F.Z. wrote and edited the manuscript.
Competing Financial Interests Statement The authors declare no competing financial interests.
Supplementary information is linked to the online version of the paper at www.nature.com/ni.
References
- 1.He R, Geha RS. Thymic stromal lymphopoietin. Ann N Y Acad Sci. 2010;1183:13–24. doi: 10.1111/j.1749-6632.2009.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedroza-Gonzalez A, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. Journal of Experimental Medicine. 2011;208:479–490. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Monte L, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. Journal of Experimental Medicine. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barooei R, Mahmoudian RA, Abbaszadegan MR, Mansouri A, Gholamin M. Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Med Oncol. 2015;32:217. doi: 10.1007/s12032-015-0653-4. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima S, et al. Induction of thymic stromal lymphopoietin in mesenchymal stem cells by interaction with myeloma cells. Leuk Lymphoma. 2014;55:2605–2613. doi: 10.3109/10428194.2014.881478. [DOI] [PubMed] [Google Scholar]
- 6.Vetter T, et al. Blockade of thymic stromal lymphopoietin (TSLP) receptor inhibits TSLP-driven proliferation and signalling in lymphoblasts from a subset of B-precursor ALL patients. Leuk Res. 2016;40:38–43. doi: 10.1016/j.leukres.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Xie F, et al. Cervical Carcinoma Cells Stimulate the Angiogenesis through TSLP Promoting Growth and Activation of Vascular Endothelial Cells. Am J Reprod Immunol. 2013;70:69–79. doi: 10.1111/aji.12104. [DOI] [PubMed] [Google Scholar]
- 8.Olkhanud PB, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. The Journal of Immunology. 2011;186:5656–5662. doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghirelli C, et al. No evidence for TSLP pathway activity in human breast cancer. 2016:1–10. doi: 10.1080/2162402X.2016.1178438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demehri S, et al. Elevated Epidermal Thymic Stromal Lymphopoietin Levels Establish an Antitumor Environment in the Skin. Cancer cell. 2012;22:494–505. doi: 10.1016/j.ccr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Piazza M, et al. Loss of Cutaneous TSLP-Dependent Immune Responses Skews the Balance of Inflammation from Tumor Protective to Tumor Promoting. Cancer cell. 2012;22:479–493. doi: 10.1016/j.ccr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Demehri S, et al. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J Clin Invest. 2016;126:1458–1470. doi: 10.1172/JCI83724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue W, et al. Thymic stromal lymphopoietin (TSLP) inhibits human colon tumor growth by promoting apoptosis of tumor cells. Oncotarget. 2016;7:16840–16854. doi: 10.18632/oncotarget.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roan F, et al. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J Leukoc Biol. 2012;91:877–886. doi: 10.1189/jlb.1211622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwagi M, et al. Direct control of regulatory T cells by keratinocytes. Nat Immunol. 2017;18:334–343. doi: 10.1038/ni.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miazgowicz MM, Elliott MS, Debley JS, Ziegler SF. Respiratory syncytial virus induces functional thymic stromal lymphopoietin receptor in airway epithelial cells. J Inflamm Res. 2013;6:53–61. doi: 10.2147/JIR.S42381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao B, et al. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy. 2015;70:1169–1180. doi: 10.1111/all.12667. [DOI] [PubMed] [Google Scholar]
- 18.Reche PA, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, et al. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L375–82. doi: 10.1152/ajplung.00045.2007. [DOI] [PubMed] [Google Scholar]
- 20.Soumelis V, et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat Immunol. 2002:1–8. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 21.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic Stromal Lymphopoietin Is Produced by Dendritic Cells. J Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5:184–193. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 23.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Paolo NC, Shayakhmetov DM. Interleukin 1α and the inflammatory process. Nat Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtzman SH, et al. Cytokines in human breast cancer: IL-1alpha and IL-1beta expression. Oncol Rep. 1999;6:65–70. doi: 10.3892/or.6.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, et al. Interleukin-1. The American Journal of Pathology. 2010;163:2531–2541. doi: 10.1016/s0002-9440(10)63608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller BE, Roi LD, Howard LM, Miller FR. Quantitative selectivity of contact-mediated intercellular communication in a metastatic mouse mammary tumor line. Cancer Research. 1983;43:4102–4107. [PubMed] [Google Scholar]
- 28.Guy CT, et al. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart TJ, Abrams SI. Altered immune function during long-term host-tumor interactions can be modulated to retard autochthonous neoplastic growth. J Immunol. 2007;179:2851–2859. doi: 10.4049/jimmunol.179.5.2851. [DOI] [PubMed] [Google Scholar]
- 30.Shi G, et al. Isolation of rare tumor cells from blood cells with buoyant immuno-microbubbles. PloS one. 2013;8:e58017. doi: 10.1371/journal.pone.0058017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cremers N, et al. CD24 Is Not Required for Tumor Initiation and Growth in Murine Breast and Prostate Cancer Models. PloS one. 2016;11:e0151468. doi: 10.1371/journal.pone.0151468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng AL, et al. CD16+ monocytes in breast cancer patients: expanded by monocyte chemoattractant protein-1 and may be useful for early diagnosis. Clinical & Experimental Immunology. 2011;164:57–65. doi: 10.1111/j.1365-2249.2011.04321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Research. 2017;19:2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 35.Barry KC, Fontana MF, Portman JL, Dugan AS, Vance RE. IL-1 Signaling Initiates the Inflammatory Response to Virulent Legionella pneumophila In Vivo. J Immunol. 2013;190:6329–6339. doi: 10.4049/jimmunol.1300100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Kang Y. Emerging therapeutic targets in metastatic progression: A focus on breast cancer. Pharmacol Ther. 2016;161:79–96. doi: 10.1016/j.pharmthera.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scully OJ, et al. Breast cancer metastasis. Cancer Genomics Proteomics. 2012;9:311–320. [PubMed] [Google Scholar]
- 38.Taranova AG, et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Research. 2008;68:8582–8589. doi: 10.1158/0008-5472.CAN-08-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West EE, Kashyap M, Leonard WJ. TSLP: a key regulator of asthma pathogenesis. Drug Discovery Today: Disease Mechanisms. 2012;9:e83–e88. doi: 10.1016/j.ddmec.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 41.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 2008;181:7699–7705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheeren FA, et al. Thymic stromal lymphopoietin induces early human B-cell proliferation and differentiation. Eur J Immunol. 2010;40:955–965. doi: 10.1002/eji.200939419. [DOI] [PubMed] [Google Scholar]
- 44.Xie F, et al. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364:106–117. doi: 10.1016/j.canlet.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 45.Cipolat S, Hoste E, Natsuga K, Quist SR, Watt FM. Epidermal barrier defects link atopic dermatitis with altered skin cancer susceptibility. Elife. 2014;3:e01888. doi: 10.7554/eLife.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han H, et al. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012;5:342–351. doi: 10.1038/mi.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larson RP, et al. Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. The Journal of Immunology. 2010;184:2974–2984. doi: 10.4049/jimmunol.0803478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA sensing pathway. Science. 2015 doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are available from the corresponding author upon request.