Abstract
Background
We compared the clinical characteristics and outcomes between the new definition of sepsis-3 septic shock and the definition previously used from 1991 until recently.
Methods
We conducted an observational study using a prospective, multi-center registry of septic shock from October 2015 to February 2017. Registry data were collected by 10 emergency departments (EDs) in tertiary hospitals that are members of the Korean Shock Society. Data on septic shock patients who met the previous septic shock definition were collected. The patients were divided into a sepsis-3 defined septic shock group, made up of those who met the new criteria for refractory hypotension with hyperlactatemia, and a group of those who met only the 1991 definition for septic shock. The primary outcome was 90-day mortality, and secondary outcomes were 28-day mortality and in-hospital mortality.
Results
Of all 1,028 included patients, 574 (55.8%) met the septic shock criteria for sepsis-3, leaving 454 patients who met only the previous definition. Those who met the sepsis-3 criteria demonstrated higher comorbidity than those who met the previous definition (83.1% vs. 75.3%, P<0.01), but there was no difference in infection focus. The sequential organ failure assessment (SOFA) (initial/maximal), the acute physiology, and the chronic health evaluation II scores were significantly higher in for those who met the sepsis-3 criteria [6.5±3.1 vs. 5.0±2.9, 9.3±3.8 vs. 6.6±3.4, and 20.0 (15.0–26.0) vs. 15.0 (10.0–20.3), respectively; P<0.01]. The 90-day mortality was significantly higher in the sepsis-3 group (32.1% vs. 23.3%; P<0.01). In-hospital and 28-day mortality were also higher in the sepsis-3 group (26.8% vs. 17.1% and 25.1% vs. 16.5%, respectively; P<0.01).
Conclusions
The new definition of septic shock successfully selected patients with greater severities and worse outcomes.
Keywords: Sepsis, shock, mortality, prognosis
Introduction
The 1991 American College of Chest Physician and Society of Critical Care Medicine consensus conference developed initial definitions of sepsis to standardize its definition and spectrum (1). The participants focused on the host’s systemic inflammatory response syndrome (SIRS), and sepsis was defined as infection in patients with SIRS (1). Organ dysfunction developing in sepsis was termed severe sepsis, and septic shock was defined as occurring when sepsis-induced hypotension or perfusion abnormality persists despite adequate fluid resuscitation (1). Although these definitions have regularized communication in both the clinical and the research settings, criticisms of the definitions have been reported, such as the limitations of the SIRS criteria and the overly sensitive sepsis definition (2-7).
The Third International Consensus Definitions Task Force recently proposed new criteria of sepsis and septic shock (Sepis-3) (8). Sepsis was defined as evidence of infection plus life-threatening organ dysfunction, clinically characterized by an acute change in the sequential organ failure assessment (SOFA) score ≥2. The definition of septic shock was altered to a subset of sepsis with underlying circulatory and cellular metabolism abnormality (8). Patients with septic shock are identified by a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain mean arterial pressure (MAP) ≥65 mmHg and to have a serum lactate level >2 mmol/L (18 mg/dL) despite adequate volume resuscitation. According to these criteria, hospital mortality is in excess of 40%. Although the use of large databases provides support for the new consensus definitions of sepsis and septic shock (9), there remain concerns over the information used to generate the updated criteria, particularly in the inclusion of serum lactate levels in the definition of septic shock.
Our present study compared clinical characteristics, severities, and outcomes between septic shock defined according to the new sepsis-3 criteria and using the previous criteria.
Methods
Setting and study population
This observational study used a prospective, multi-center registry provided by the Korean Shock Society (KoSS septic shock registry) with data from October 2015 to February 2017 to compare the clinical characteristics and outcomes of patients with septic shock defined by sepsis-3 and the 1991 definition. The KoSS is a collaborative research network that investigates and works to improve the quality of diagnosis and management for sepsis. It was organized in 2013, and KoSS investigators began prospectively collecting data from septic shock patients at the emergency departments (EDs) of 10 teaching hospitals throughout South Korea in October 2015. The institutional review board of each institution approved the study protocol and informed consent was obtained before data collection (Asan Medical Center Institutional Review Board No. 2015-1283) (10).
Adult (≥18 years old) septic shock patients, defined according to the 1991 septic shock definition, which included suspected or confirmed infection and evidence of refractory hypotension or hypoperfusion, were enrolled in the registry (11-13). Refractory hypotension was defined as persistent hypotension: systolic blood pressure (SBP) <90 mmHg, MAP <70 mmHg, or SBP decrease >40 mmHg after adequate intravenous fluid challenge (20–30 mL/kg or at least 1 L or more of crystalloid solution administered over 30 min) or as the need for vasopressors after fluid resuscitation (14,15). Hypoperfusion was defined as a serum lactate concentration of 4 mmol/L or greater (16). Patients who signed a “do not attempt resuscitation” order, did not meet the inclusion criteria within 6 h after ED arrival, were transferred from other hospitals without meeting the inclusion criteria upon ED arrival, or were directly transferred from ED to other hospitals, were not enrolled in in the KoSS septic shock registry.
The case report form, standard definitions of 200 variables, including clinical characteristics, therapeutic interventions, and outcomes of patients with septic shock and an investigator manual were developed based on a literature review and the consensus of the study investigators. Data was collected via a standardized registry form and was entered into a web-based electronic database registry. Outliers or incorrect values were primarily filtered by this data-entry system. Each site’s principal investigator had a designated local research coordinator, who was responsible for ensuring the accuracy of data entry and verifying records. In each ED, a quality management committee (QMC), which consisted of emergency physicians, local research coordinators, and investigators were organized to monitor and regularly review data quality. The QMCs gave feedback to the research coordinators and investigators of the results of their QM processes through the query function in the system or directly by phone to clarify data.
Data collection
Demographic and clinical data, including age, sex, previous medical history, initial vital signs, severity, laboratory values on admission, and interventions were retrieved from the septic shock registry. Among septic shock patients in the KoSS registry, patients who had refractory hypotension with hyperlactatemia (≥2 mmol/L) were defined as having sepsis-3 septic shock (8). The patients were divided into a sepsis-3-defined septic-shock group and a group that only met the previous 1991 definition of septic shock.
The patient’s severity was assessed using a disease severity score. The maximum SOFA and acute physiology and chronic health evaluation (APACHE) II scores were evaluated using the worst parameters within 24 h of ED arrival. The outcome variables included in-hospital, 28-, and 90-day mortalities and length of hospital stay.
Statistical analysis
Continuous variables are expressed as means ± standard deviations (SD) or medians with interquartile ranges (IQR) if the assumption of a normal distribution was violated. Categorical variables were expressed as numbers and percentages. Baseline characteristics and laboratory examinations were analyzed for the sepsis-3 defined septic shock group and the group that only met previous 1991 defined septic shock. The Student’s t-test was used to compare the means of normally distributed continuous variables, whereas the Mann-Whitney U-test was used to compare non-continuous variables. The chi-square or Fisher’s exact test was used to compare categorical variables.
All tests in this study were two-sided, and a p values <0.01 were considered statistically significant. All statistical analyses were performed using SPSS for Windows version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
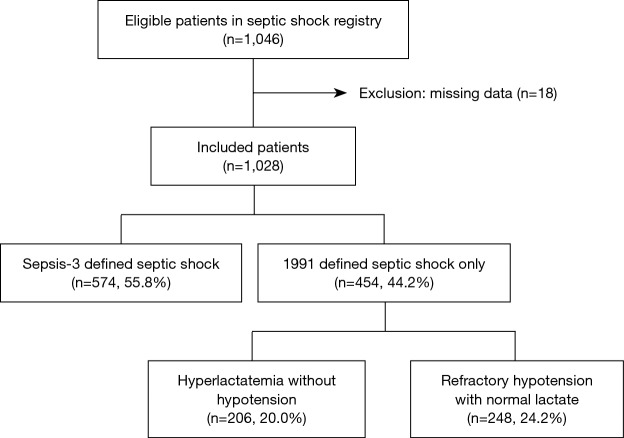
Of the 1,046 eligible patients in KoSS septic shock registry, we excluded 18 patients who had missing data. The included 1,028 patients were divided into 574 (55.8%) who met the sepsis-3 criteria for septic shock, namely, refractory hypotension with hyperlactatemia, leaving 454 patients who met only the 1991 definition of septic shock, of whom 206 (20.0%) had hyperlactatemia without hypotension and 248 (24.2%) had refractory hypotension with normal lactate (Figure 1).
Figure 1.
Diagram of included patients.
The sepsis-3 criteria group demonstrated higher comorbidity with hypertension (46.5% vs. 37.9%; P=0.005), diabetes (35.2% vs. 21.6%; P<0.001), and chronic liver disease (14.6% vs. 7.9%; P=0.001) than the 1991 septic shock group. The vital signs of the sepsis-3 shock group were more severe than 1991 septic shock group. They were more hypotensive in both the systolic (87.3±21.3 vs. 90.9±23.5; P=0.011) and diastolic (52.7±15.1 vs. 55.5±15.9; P=0.004) measures, had tachycardia (109.2±24.0 vs. 101.5±23.1; P<0.001), and experienced mental change (26.5% vs. 21.1%; P=0.047) (Table 1).
Table 1. Baseline and clinical characteristics of the study population.
| Characteristics | Total (n=1,028) | 1991 septic shock (n=454) | Sepsis-3 shock (n=574) | P value |
|---|---|---|---|---|
| Age, years | 68.6±13.4 | 68.2±13.5 | 68.9±13.3 | 0.394 |
| Male | 585 (56.9) | 258 (56.8) | 327 (57.0) | 0.964 |
| Past medical history | ||||
| Hypertension | 439 (42.7) | 172 (37.9) | 267 (46.5) | 0.005 |
| Stroke | 142 (13.8) | 61 (13.4) | 90 (15.7) | 0.313 |
| Diabetes | 300 (29.2) | 98 (21.6) | 202 (35.2) | <0.001 |
| Coronary artery disease | 151 (14.7) | 67 (14.8) | 75 (13.1) | 0.435 |
| Chronic pulmonary disease | 82 (8.0) | 48 (10.6) | 34 (5.9) | 0.006 |
| Metastatic cancer | 223 (21.7) | 109 (24.0) | 114 (19.9) | 0.109 |
| Chronic renal disease | 85 (8.3) | 30 (6.6) | 55 (9.6) | 0.086 |
| Chronic liver disease | 120 (11.7) | 36 (7.9) | 84 (14.6) | 0.001 |
| Vital signs at shock recognition | ||||
| SBP, mmHg | 88.9±22.4 | 90.9±23.5 | 87.3±21.3 | 0.011 |
| Diastolic blood pressure, mmHg | 53.9±15.5 | 55.5±15.9 | 52.7±15.1 | 0.004 |
| Pulse rate, beats/min | 105.8±23.9 | 101.5±23.1 | 109.2±24.0 | <0.001 |
| Respiratory rate, breaths/min | 22.1±5.7 | 21.7±5.7 | 22.3±5.6 | 0.086 |
| Body temperature, °C | 37.5±1.2 | 37.5±1.1 | 37.5±1.2 | 0.740 |
| Altered mentality | 248 (24.1) | 96 (21.1) | 152 (26.5) | 0.047 |
| Infection focus | ||||
| Lung | 330 (32.1) | 145 (31.9) | 185 (32.2) | 0.921 |
| Urinary tract | 250 (24.3) | 104 (22.9) | 146 (25.4) | 0.348 |
| Hepatobiliary & pancreas | 208 (20.2) | 80 (17.6) | 128 (22.3) | 0.064 |
| Gastrointestinal | 176 (17.1) | 78 (17.2) | 98 (17.1) | 0.964 |
| Unknown focus | 72 (7.0) | 38 (8.4) | 34 (5.9) | 0.127 |
| Duration of first antibiotics use, minutes | 70 [15–138] | 70 [0–133] | 70 [26–142] | 0.046 |
| Steroid use | 209 (20.3) | 58 (12.8) | 151 (26.3) | <0.001 |
Values are expressed as means ± SD, medians [IQRs], or numbers (%). SBP, systolic blood pressure; SD, standard deviation; IQR, interquartile ranges.
The most common foci of infection were the lung (32.1%) and urinary tract (24.3%), and the hepatobiliary–pancreatic area (20.2%) and gastrointestinal tract (17.1%) followed. However, the distribution was not significant for either group. In both groups, empirical antibiotics were administrated 70 minutes from recognition of shock; however, the IQR of the sepsis-3 shock group was more delayed than was that of the 1991 septic shock group (P=0.046) (Table 1).
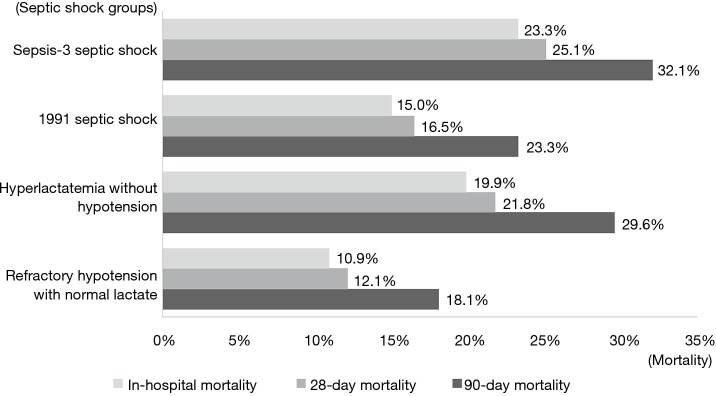
All severity scores were also significantly higher in the sepsis-3 shock group than in the 1991 septic shock group (Table 2). In-hospital mortality, 28- and 90-day mortality were higher in the sepsis-3 shock group as well [23.3% vs. 15.0%, OR: 1.73 (95% CI: 1.25–2.39); 25.1% vs. 16.5%, OR: 1.67 (95% CI: 1.22–2.28); and 32.1% vs. 23.3%, OR: 1.40 (95% CI: 1.04–1.88), respectively]. Within the 1991 defined septic shock group, Hyperlactatemia without hypotension had higher in-hospital, 28-day, and 90-day mortality than refractory hypotension with normal lactate group [19.9% vs.10.9%, OR: 2.03 (95% CI: 1.20–3.44); 21.8% vs. 12.1%, OR: 2.18 (95% CI: 1.31–3.63); 29.6% vs. 18.1% OR: 1.96 (95% CI: 1.23–3.12), respectively] (Figure 2). However, the length of stay did not differ significantly (Table 2).
Table 2. Comparison of the severities and outcomes between 1991 and sepsis-3 defined septic shock.
| Characteristics | 1991 septic shock (n=454) | Sepsis-3 shock (n=574) | P value |
|---|---|---|---|
| Laboratory findings | |||
| White blood cell count, 103/μL | 11.1 [6.4–17.9] | 10.4 [5.1–17.9] | 0.077 |
| Hemoglobin, g/dL | 11.1 [9.3–12.7] | 11.2 [9.5–12.8] | 0.562 |
| Blood urea nitrogen, mg/dL | 23.8 [16.3–38.7] | 29.8 [21.0–44.4] | <0.001 |
| Creatinine, mg/dL | 1.2 [0.8–1.8] | 1.6 [1.1–2.5] | <0.001 |
| Aspartate transaminase, IU/L | 35.0 [23.0–71.3] | 46.0 [27.0–117.0] | <0.001 |
| Alanine transaminase, IU/L | 24.0 [13.0–45.3] | 30.0 [16.0–73.0] | <0.001 |
| Initial lactate, mmol/L | 1.8 [1.2–4.3] | 4.3 [2.8–6.1] | <0.001 |
| Severity score | |||
| Initial SOFA score | 5.0±2.9 | 6.5±3.1 | <0.001 |
| Maximum SOFA score | 6.6±3.4 | 9.3±3.8 | <0.001 |
| APACHE-II score | 15.0 [10.0–20.3] | 20.0 [15.0–26.0] | <0.001 |
| Interventions | |||
| Mechanical ventilator | 100 (22.0) | 201 (35.0) | <0.001 |
| Duration of mechanical ventilation, days | 5.0 [2.0–12.0] | 5.0 [2.0–10.0] | 0.596 |
| Renal replacement therapy | 35 (7.7) | 130 (22.6) | <0.001 |
| Mortality | |||
| In-hospital mortality | 68 (15.0) | 134 (23.3) | 0.001 |
| 28-day mortality | 75 (16.5) | 144 (25.1) | 0.003 |
| 90-day mortality | 106 (23.3) | 184 (32.1) | 0.002 |
| Length of stay, days | |||
| Hospital stay | 12.0 [7.0–20.0] | 13.0 [7.0–24.0] | 0.088 |
| ICU stay | 4.0 [2.0–8.0] | 5.0 [3.0–8.0] | 0.322 |
Values are expressed as means ± SDs, medians [IQRs], or numbers (%). SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; SD, standard deviation; IQR, interquartile ranges.
Figure 2.
In-hospital 28- and 90-day mortalities of each septic shock groups.
Discussion
In this study, we compared clinical manifestations between sepsis-3 defined septic shock patients within 1991 defined septic shock registry and leaving septic shock patients who met only 1991 definition in the registry. We found in our current investigation that 55.8% of patients with septic shock as defined in 1991 met the new sepsis-3 defined septic shock criteria. Their 90-day mortality was 32.1%, which was significantly higher than that of the group only meeting the 1991 definition of septic shock (23.3%). It means refractory hypotension with hyperlactatemia was more severe than either refractory hypotension or hyperlactatemia alone. Unlike the previous 1991 criteria septic shock, sepsis-3 shock emphasizes cellular metabolic abnormalities (8). This is because the pathophysiologic aspect of shock is circulatory failure that results in inadequate cellular oxygen utilization (17). In tissue with hypoxia, whether global or localized, lactate is overproduced and underutilized as a result of impaired mitochondrial oxidation (18). For this reason, hyperlactatemia is an essential part of the septic-3 definition of shock (8). Anaerobic metabolism, as well as β2 receptor stimulation by endogenous and exogenous catecholamines, overproduces serum lactate (19-21). Therefore, in septic shock, the degree of hyperlactatemia reflects the severity of disease, and the guidelines for surviving sepsis recommend serum lactate measurement within 3 h of recognition of shock (16,22,23).
A previous study reported that risk-adjusted hospital mortality was significantly higher (P<0.001 compared to the reference group) in patients with fluid-resistant hypotension requiring vasopressors and hyperlactatemia (42.3% and 49.7% at thresholds for serum lactate level of >2 or >4 mmol/L, respectively) compared to either hyperlactatemia alone (25.7% and 29.9% mortality for those with serum lactate level of >2 and >4 mmol/L, respectively) or with fluid-resistant hypotension requiring vasopressors but with a lactate level of 2 mmol/L or less (30.1%) (8). These results were reproduced by two unrelated large electronic health record datasets [University of Pittsburgh Medical Center (12 hospitals; 2010–2012; n=5,984) and Kaiser Permanente Northern California (20 hospitals; 2009–2013; n=54,135)]. The combination of hypotension, vasopressor use, and lactate level greater than 2 mmol/L identified patients with mortality rates of 54% and 35%, higher than mortality in patients with hypotension alone (25.2% and 18.8%) or in patients with lactate levels greater than 2 mmol/L alone (20.0% and 8.0%) (8). Another study compared lactate levels to determine the association of in-hospital mortality; the researchers showed that hypotension with hyperlactatemia of lactate >2 mmol/L had a significant higher mortality than hypotension only [OR: 1.16 (95% CI: 1.05–1.27) in lactate 2–3 mmol/L; OR: 1.21 (95% CI: 1.09–1.35) in lactate 3–4 mmol/L, and OR: 2.10 (95% CI: 1.93–2.27) in lactate >4 mmol/L, respectively] (24). Moreover the mortality of each group was higher than that of only hyperlactatemia, with >4 mmol/L (30.6% in lactate 2–3 mmol/L, 31.6% in lactate 3–4 mmol/L, and 44.5% in lactate >4 mmol/L, with hypotension vs. 29.0% in only hyperlactatemia >4 mmol/L) (24).
Since the revision of the definition of sepsis, there have been some studies comparing groups according to the two definitions. One study, which performed a secondary analysis of a multicenter randomized control trial, presented higher in-hospital mortality in their sepsis-3 shock group than their 1991 septic shock group (28.5% vs. 14.4%, P<0.001) (25). Another cohort study, collected at a single center, reported a similar in-hospital mortality rate (sepsis-3 shock, 22.9% vs. previous consensus septic shock, 21.7%) (26). Sterling et al. included patients who met 1991 defined septic shock, and categorized them as meeting sepsis-3 criteria of septic shock and those who met only the old criteria for septic shock. However they excluded patients who had an elevated serum lactate without SBP less than 90 mmHg. Moreover as all patients in their parent studies did not have a MAP documented, only SBP less than 90 mmHg was used for determining hypotension in the analysis (25). On the other hand, Henning et al. combined three different cohorts and each cohort had heterogeneous inclusion criteria. Cohort 1 patients had to obtain blood culture, cohort 3 had to receive antibiotics and meanwhile cohort 2 included by only infection related diagnosis. Since they wanted to know a diagnostic value of sepsis-3 definition among suspected infection patients, they did not purify the population, which differ from our study (26).
In our present study ignoring participants who met the criteria for sepsis-3 shock, leaving those who met the 1991 definition septic shock only, sepsis-induced hypoperfusion without hypotension (lactate >4 mmo/L and MAP ≥70 mmHg, n=206) and refractory hypotension without hyperlactatemia (MAP <70 mmHg and lactate <2 mmol/L, n=248) were examined. Compared to the sepsis-3 shock group, such patients had less severe organ failure scores and mortality (Table 2). The in-hospital mortality of the sepsis-3 group vs. the 1991 group was 26.8% vs. 17.6%, P<0.001. In contrast to previous studies, we analyzed 28-day mortality as well as 90-day mortality, which were significantly higher in the sepsis-3 shock group (25.1% vs. 16.5%, P=0.003; 32.1% vs. 23.3%, P=0.002).
In a previous study, the median SOFA score was higher in the sepsis-3 group than the 1991 only group (9.0 vs. 5.0, P<0.001) (25), and this was similar in our study (9.3 vs. 6.6, P<0.001). In addition, when we analyzed the APACHE-II score, it also higher in the sepsis-3 group (20.0 vs. 15.0, P<0.001), which means the sepsis-3 definition of septic shock successfully selects patients with greater severity. However, the remaining patients in the 1991 group still had high severity scores (6.6 SOFA and 15.0 APACHE-II). Each score predicts 15–20% and 24% of mortality (27,28), and their overall 28- and 90-day mortality rates were 16.5% and 23.3%, respectively. Moreover, the length of stay in ICU and total hospital stay of the groups did not differ significantly (5.0 vs. 4.0, P=0.322; 13.0 vs. 12.0, P=0.088). In a previous multicenter study, although ICU day was longer in the sepsis-3 shock group (3.2 vs. 2.5, P=0.006), the median total hospital stay was 8.0 days for both groups (P=0.466) (25).
Most recent published two studies which compare sepsis-3 septic shock and previous septic shock definition, reported that there were higher ICU mortality (38.9% vs. 34.0%; 46.7% vs. 25.6%) and in hospital mortality (47% vs. 43%; 55.5% vs.35.1%) in sepsis-3 septic shock, too. Moreover, APACHE-II scores were also higher in sepsis-3 septic shock group (27±8 vs. 26±8; 22.0±7.1 vs. 19.2±6.8) (29,30). However they could not comparing analyze to assess the significance of the different outcomes between two groups, because their two groups had overlapping patients from a single source population.
Limitations
This study had several limitations of note. First, it was an observational study, and the groups were not blinded. Although patients were treated protocol-driven septic shock management, this could have affected our results. Second, because it was a multicenter study, the enrollment periods and case volumes varied according to hospital. Third, because it was a prospective registry study of septic shock, we could not collect all infected patients as well as some patients were excluded from this study for their refusal to give informed consent. Fourth, as the KoSS registry has been collected by international surviving sepsis guideline (16), hypotension defined MAP <70 mmHg but, sepsis-3 defined septic shock is MBP ≤65 mmHg (8). It may overestimate the number of patients included in the sepsis-3 septic shock. And finally we focused on early septic shock patients in the ED, not the ICU, which might have led to selection bias.
Conclusions
In this KoSS septic shock registry, which included 10 EDs, the prevalence of septic shock according to the sepsis-3 criteria was 55.8%. The group meeting the sepsis-3 criteria had higher mortality and severity than the remaining group, meeting only the 1991 definition of septic shock. However, only the 1991 group still had high mortality and severity. They also needed similar lengths of stay in the ICU and hospital management. The new definition of septic shock successfully selected patients with higher severities and worse outcomes. However, the previous definition of septic shock still helped to screen critical patients earlier. Therefore, our results could inform the choice of septic shock criteria for identifying patients who may die (sepsis-3) or who will need early screening (old definition).
Acknowledgements
None.
Ethical Statement: The institutional review board of each institution approved the study protocol and informed consent was obtained before data collection (Asan Medical Center Institutional Review Board No. 2015-1283).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. 10.1378/chest.101.6.1644 [DOI] [PubMed] [Google Scholar]
- 2.Ryoo SM, Kim WY, Huh JW, et al. Prognostic value of B-type natriuretic peptide with the sequential organ failure assessment score in septic shock. Am J Med Sci 2015;349:287-91. 10.1097/MAJ.0000000000000422 [DOI] [PubMed] [Google Scholar]
- 3.Sohn CH, Ryoo SM, Seo DW, et al. Outcome of delayed resuscitation bundle achievement in emergency department patients with septic shock. Intern Emerg Med 2014;9:671-6. 10.1007/s11739-014-1092-5 [DOI] [PubMed] [Google Scholar]
- 4.Gille-Johnson P, Hansson KE, Gardlund B. Severe sepsis and systemic inflammatory response syndrome in emergency department patients with suspected severe infection. Scand J Infect Dis 2013;45:186-93. 10.3109/00365548.2012.720025 [DOI] [PubMed] [Google Scholar]
- 5.Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015;372:1629-38. 10.1056/NEJMoa1415236 [DOI] [PubMed] [Google Scholar]
- 6.Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010;303:739-46. 10.1001/jama.2010.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent JL, Opal SM, Marshall JC, et al. Sepsis definitions: time for change. Lancet 2013;381:774-5. 10.1016/S0140-6736(12)61815-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801-10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham E. New Definitions for Sepsis and Septic Shock: Continuing Evolution but With Much Still to Be Done. JAMA 2016;315:757-9. 10.1001/jama.2016.0290 [DOI] [PubMed] [Google Scholar]
- 10.Shin TG, Hwang SY, Kang GH, et al. Korean Shock Society septic shock registry: a preliminary report. Clin Exp Emerg Med 2017;4:146-53. 10.15441/ceem.17.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. 10.1056/NEJMoa1500896 [DOI] [PubMed] [Google Scholar]
- 12.Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-93. 10.1056/NEJMoa1401602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. 10.1056/NEJMoa1404380 [DOI] [PubMed] [Google Scholar]
- 14.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6. 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530-8. 10.1007/s00134-003-1662-x [DOI] [PubMed] [Google Scholar]
- 16.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013;369:1726-34. 10.1056/NEJMra1208943 [DOI] [PubMed] [Google Scholar]
- 18.Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 2014;371:2309-19. 10.1056/NEJMra1309483 [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care 2014;18:503. 10.1186/s13054-014-0503-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy B, Desebbe O, Montemont C, et al. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock 2008;30:417-21. 10.1097/SHK.0b013e318167378f [DOI] [PubMed] [Google Scholar]
- 21.Levy B. Lactate and shock state: the metabolic view. Curr Opin Crit Care 2006;12:315-21. 10.1097/01.ccx.0000235208.77450.15 [DOI] [PubMed] [Google Scholar]
- 22.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486-552. 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 23.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304-77. 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 24.Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med 2015;43:567-73. 10.1097/CCM.0000000000000742 [DOI] [PubMed] [Google Scholar]
- 25.Sterling SA, Puskarich MA, Glass AF, et al. The Impact of the Sepsis-3 Septic Shock Definition on Previously Defined Septic Shock Patients. Crit Care Med 2017;45:1436-42. 10.1097/CCM.0000000000002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henning DJ, Puskarich MA, Self WH, et al. An emergency department validation of the SEP-3 sepsis and septic shock definitions and comparison with 1992 consensus definitions. Ann Emerg Med 2017;70:544-52.e5. 10.1016/j.annemergmed.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998;26:1793-800. 10.1097/00003246-199811000-00016 [DOI] [PubMed] [Google Scholar]
- 28.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 29.Driessen RGH, van de Poll MCG, Mol MF, et al. The influence of a change in septic shock definitions on intensive care epidemiology and outcome: comparison of sepsis-2 and sepsis-3 definitions. Infect Dis (Lond) 2017:1-7. 10.1080/23744235.2017.1383630 [DOI] [PubMed] [Google Scholar]
- 30.Shankar-Hari M, Harrison DA, Rubenfeld GD, et al. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth 2017;119:626-36. 10.1093/bja/aex234 [DOI] [PubMed] [Google Scholar]