Abstract
Background
Subcentimetre pulmonary nodules can be challenging to locate either during video-assisted thoracoscopic surgery (VATS) or by open techniques. In an era of increasing computed tomography scan availability the number of nodules that are identified that are suspicious for malignancy is rising, and thoracic surgeons require a reliable method to locate these nodules intraoperatively.
Methods
Our aim was to evaluate, for the first time in the UK, resection of pulmonary nodules using radioactive dye labelling. Local research ethics approval was obtained and the study was submitted to the Integrated Research Application System (IRAS). All data were prospectively collected in our dedicated thoracic surgical database and analyzed at the conclusion of the study. This represents a consecutive series of patients, from January 2016 and until April 2017, who underwent this procedure at our institution: James Cook University Hospital, Middlesbrough, United Kingdom. The primary outcome measured was successful resection rate of the target nodules.
Results
Twenty-three patients underwent radiolabeled excision of pulmonary nodules, their average age was 61 years (range, 28–79 years), 13 women and 10 men. The average maximum diameter of the nodule was 8 mm (range, 3–16 mm). All patients underwent successful excision of the target lesion (success rate 100%). One patient (4.3%) sustained pneumothorax following the CT-guided injection of the radio-labelled dye and this required chest drainage prior to general anesthesia.
Conclusions
We conclude that technetium guided pulmonary nodule resection is a very reliable method for localization and resection of subcentimetre nodules which may be otherwise be difficult to identify.
Keywords: Nodule location, pulmonary resections, minimally invasive thoracic surgery, localization, video-assisted thoracoscopic surgery (VATS)
Introduction
Subcentimetre pulmonary nodules can be challenging to locate either by video-assisted thoracoscopic surgery (VATS) or even by open techniques. In an era of increasing CT scan availability, the number of nodules that are identified that are suspicious for malignancy is rising and thoracic surgeons require a reliable method to locate these nodules intraoperatively. This nodule is often too small in order to be biopsied. In cases of suspected primary pulmonary malignancy, to be able to offer diagnosis when the lesion is still very small gives a huge prognostic benefit. Furthermore, the increased demand for adequate tissue for molecular characterization of the disease increases the importance of resection of small pulmonary nodules. VATS is the treatment of choice, but location of small sub pleural and deeper pulmonary nodules is often a problem. Thus, a reliable technique, to localize subcentimetre nodules, is required by surgeons in order to safely remove these nodules for diagnostic and therapeutic reasons.
Background
Several methods have been described for localization of small pulmonary nodules over the last 20 years and they have particular advantages and disadvantages. They include: finger palpation, methylene blue CT-guided injection, intraoperative ultrasound (1), lipiodol marker (2), bronchoscopic barium marker (3), fluoroscopy, hook wire (4), spiral wire and radio-guided localization. Ambrogi et al. (5) reported in 2011 the largest cohort of patients who underwent radio-guided surgery for nodules smaller than 1 cm. They were successful in 208 over 211 cases, using a preoperative CT-guided injection of 99mTechnetium (99mTc)-labelled albumin microspheres. They started their experience in the late nineties (6,7) and they concluded that radio-guided localization was safe, associated with minimal complications and provided high success rate. A literature review by Zaman et al. (8) in 2012 concluded that radio-guided resection was the best choice for location of subcentimetre pulmonary nodules. This technique was also adopted to perform sentinel lymph node biopsy in non-small cell lung cancer (9-11). Gonfiotti et al. (12) compared the outcome of patients underwent radio-guided resections with those who underwent the hook wire technique resection. They concluded that both methods were effective but the radio labelling led to fewer complications. In 2003 Partik et al. (13) proposed the adoption of a dedicated helical tip wire to reduce the risk of dislodgment. Unfortunately, the introducer needle required for the placement was 18 gauges, leading to a high pneumothorax incidence of 31%. At University of Virginia (14) researchers compared the use of 99mTc labelled albumin macroaggregates (MAA) with other radio-tracers in rats. They observed that 99mTc may be the most precise and versatile method and they subsequently offered the procedure to patients reporting successful results. MAA have larger volume than microspheres and so demonstrated a lower local spread.
Objectives of our study
The aim of our study was to assess the safety and efficacy of 99mTc injection of subcentimetre pulmonary nodules, and subsequent radioactivity aided intraoperative localization and resection of these nodules. The surgery was accomplished by minimally invasive thoracoscopic surgery techniques. The primary outcome measured was successful resection of target nodules. Secondary outcomes measured were operative complications, length of hospital stay and conversion to open surgery rate. This was the first such use of 99mTc labelling in the UK and this study was subject to a research certificate for the administration of radioactive medicinal products issued by Administration of Radioactive Substances Advisory Committee (ARSAC) after a risk assessment was performed by the Medical Physics Department of our institution.
Methods
The research project was submitted to the Integrated Research Application System (IRAS, project ID number 152329), was assessed and accepted by the Lancaster Research Ethics Committee in June 2015 after consultation with the ARSAC. Written prospective consent was required for all patients entering this study but any form of randomization for patients was required. The selection was only based on the perceived benefit to the patient and not based on any desire to include the patient in a study. Inclusion criteria was for patients with nodule diameter less than 15 mm, which required resection for diagnostic or therapeutic intent, and in whom the surgeon expected difficulties with intraoperative location. Principal exclusion criteria were patients less than 16 years of age, ability to remove mass without radiolabelling, anatomic location of nodule that made it technically difficult to CT-guided radiolabelling, patient not willing to undergo the procedure, inability to consent to the operation and pregnancy. Each eligible case was discussed at the multidisciplinary lung cancer meeting (MDT meeting), which was attended by the radiologist, oncologist, respiratory physician, thoracic surgeon and affiliated specialist nurses. The patient’s medical history and radiology were reviewed collectively. The candidate was then invited to attend the outpatient department where this procedure and the alternatives were discussed with the thoracic surgeon. Also, an informative leaflet was provided at the end of the consultation. The consent was obtained by the consultant prior to surgery, usually 1 or 2 weeks afterwards. In cooperation with the interventional radiology department, the patient was then offered this novel procedure.
The patient underwent CT-guided injection of 0.1 mL of 99mTc-labelled human albumin MAA (Pulmocis, Bio International, IBA group) in 0.2 and 1 mL of Methylene blue near the lesion. The biopsy package was used on a Siemens Definition AS CT scanner. With aseptic technique, Lidocaine is injected under the skin and in the subcutaneous tissue. A 90-mm, 22-gauge needle was usually used. If the lesion was deeper a 150-mm 22-gauge Chiba needle was necessary. The radioactive dye was followed by 1 mL of Prove Blue (Methylene Blue). The radioactivity injected was within 5 and 7 MBq in the form of human albumin MAA labelled with 99mTc.
The agreed, accepted distance between the target lesion and the site of the injection was maximum of 1 centimetre away. This allowed labelling of nodules that were close to important vascular structures (Figure 1), to the visceral mediastinum or to the diaphragm. Also, lesions that were too small for routine CT scan-guided biopsy could be marked with the radioactive solution. After the radiolabelling, the patient was accompanied to the operating room. This always occurred on the same day and ideally within 3 hours from the labelling (the half life for gamma emission of the 99mTc is 6 hours). With the aid of a Geiger counter probe, the same used for sentinel lymph node location by the breast surgeons (Europrobe 3, Capintec, Inc.,), we used the radioactivity signal to locate the lesion (Figure 2). We first identified the area of highest signal prior to making any incisions in order to guide the port placement. We then used it intraoperatively to guide us to the lesion. This was performed through a 3-port anterior or posterior approach or, most usually, with anterior approach uniportal surgery. The probe was 12 mm in diameter and so fits through the larger port. It gives a numeric and an audio frequency feedback which directs the location of the lesion.
Figure 1.
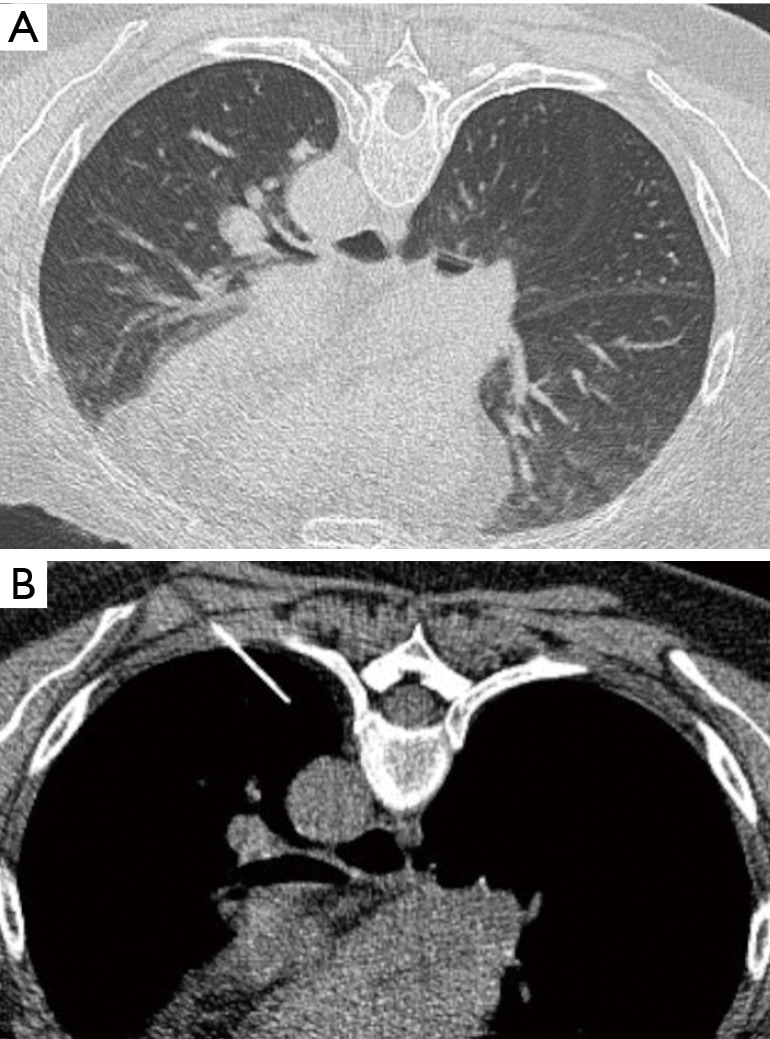
Patient 7. (A) CT scan of a 60-year-old lady with previous history of breast cancer and presented with a small nodule in the apical segment of the left lower lobe, very close to the aorta; (B) labelling of the lesion by the interventional radiologist.
Figure 2.
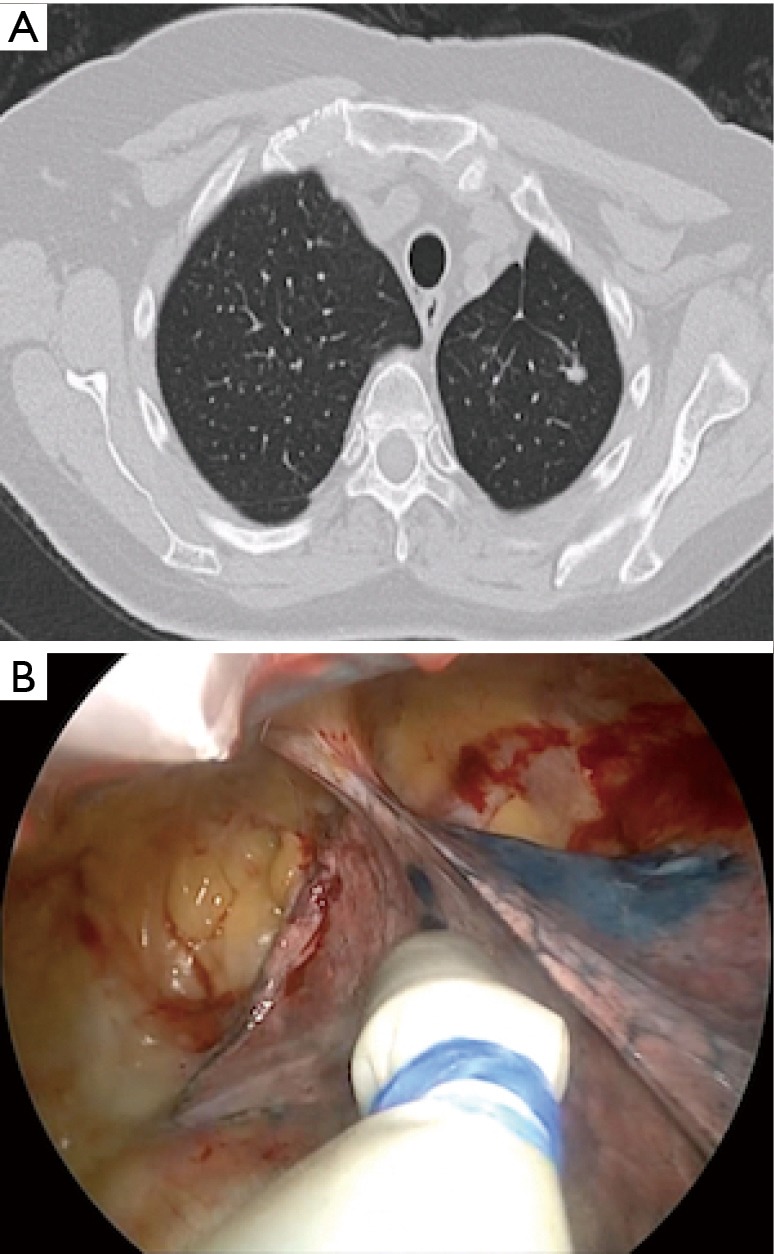
Patient 10. (A) CT scan of a lady with previous history of left upper lobectomy for lung cancer and presented 3 years afterward with an 8-mm pulmonary nodule in the remaining lobe; (B) the Geiger counter probe examines the lung surface, where also methylene blue is visible, during left uniportal VATS radioactivity guided wedge resection. VATS, video-assisted thoracoscopic surgery.
All data were prospectively collected in our dedicated thoracic surgical database and analyzed at the conclusion of the study. This represents a consecutive series of patients, from January 2016 and until April 2017, who underwent this procedure at our institution: James Cook University Hospital, Middlesbrough, United Kingdom. This was a pilot study and thus the sample size was obtained as a convenience sample of patients that could be accrued over 1 year.
Results
Over 15 months, 23 patients underwent radiolabelled excision. The average age was 61 years (range, 28–79 years). There were 13 females and 10 males. The average maximum diameter of the nodule was 8 mm (range, 3–16 mm) and the location of pathology is shown in Table 1. Eleven nodules were left sided and 12 were right sided (6 left lower lobe, 5 left upper lobe, 4 right upper lobe, 8 right lower lobe). In 13 patients (56.5%) the nodule was 8 mm of diameter or less.
Table 1. Patients’ demographic details and surgical details.
| Patient | Age (years) | Side | Location | Diameter (CT) (mm) | History of cancer | Incisions | Procedure | Methylene blue | 99mTc | Chest drain | Final histology | Comments | OT (minutes) | LOS (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | Left | LLL | 16 | Colon ca. | 3-port P | Wedge resection | Visible | Detected | Apical | Colon met | – | 55 | 2 |
| 2 | 70 | Right | RUL | 10 | Laryngeal ca. | 3-port A | Wedge resection | Visible | Detected | Apical | Primary adeno. | – | 50 | 2 |
| 3 | 47 | Right | RLL | 4 | Colon ca. | 3-port A | Two wedge resections + lymphadenectomy | Spread | Detected | Apical | Colon met | – | 110 | 1 |
| 4 | 28 | Left | LUL | 13 | Teratoma | 3-port A | Lingulectomy | Not seen | Detected | Apical | Teratoma | – | 110 | 3 |
| 5 | 66 | Left | LUL | 5 | None | 3-port P | Wedge resection + resection of node | Visible | Detected | Apical | Benign (+ malignant node) | Converted because of bleeding from PA invaded by malignant lymph node | 178 | 3 |
| 6 | 61 | Left | LLL | 11 | IPMN | 3-port A | Wedge resection + lymphadenectomy | Not seen | Detected | Apical | Hamartoma | – | 120 | 6 |
| 7 | 60 | Left | LLL | NR | Breast + small bowel | 3-port P | Wedge resection | Visible | Detected | Apical | Breast adeno. | – | 57 | 2 |
| 8 | 73 | Left | LLL | 15 | None | U-VATS | Wedge resection | Visible | Detected | Apical | Primary adeno. | – | 45 | 2 |
| 9 | 53 | Right | RLL | 5 | None | 3-port A | Wedge resection | Not seen | Detected | Apical | Melanoma | – | 35 | 2 |
| 10 | 75 | Left | LLL | 6 | None | U-VATS | Wedge resection | Visible | Detected | Apical | SCC | Re-do surgery, previous left upper lobectomy | 50 | 6 |
| 11 | 49 | Right | RUL | 7 | Colon ca. | U-VATS | Wedge resection | Spread | Detected | Apical | Colon met | Previous chemotherapy, significant adhesions | 70 | 5 |
| 12 | 65 | Right | RLL | 3 | Colon ca. | U-VATS | Wedge resection | Visible | Detected | Apical | Colon met | – | 39 | 0 |
| 13 | 57 | Left | LLL | 5 | None | U-VATS | Wedge resection | Spread | Detected | Apical | Lymphoma | – | 55 | 7 |
| 14 | 68 | Right | RUL | 7 | None | U-VATS | Three wedge resections | Not seen | Detected | Apical | Benign | Also lung biopsies for ILD | 54 | 1 |
| 15 | 58 | Right | RML | 9 | None | U-VATS | Wedge resection | Visible | Detected | Apical | Colon met | – | 38 | 1 |
| 16 | 58 | Left | LUL | 10 | None | U-VATS | Wedge resections bilaterally, 3 totals | Visible | Detected | Bilateral Apical | Colon met | Radiolabelled on the left side | 115 | 3 |
| 17 | 57 | Right | RLL | 4 | Laryngeal SCC | U-VATS | Wedge resection | Visible | Detected | Apical | Benign | – | 35 | 3 |
| 18 | 68 | Right | RLL | 8 | Ovarian cancer | U-VATS | Two wedge resections | Visible | Detected | Apical | Chondroid hamartomas | – | 45 | 2 |
| 19 | 48 | Right | RLL | 5 | Colon ca. | U-VATS | Wedge + middle lobectomy | Visible | Detected | Apical | Colon met | – | 115 | 1 |
| 20 | 76 | Right | RLL | 9 | Lung adeno. | U-VATS | Wedge resection + 11 lymph node samples | Visible | Detected | Apical | Pseudotumour | Re-do surgery, previous right upper lobectomy | 65 | 2 |
| 21 | 79 | Left | LUL | 7 | None | U-VATS | Wedge resection | Visible | Detected | Apical | Adenosquamous | – | 45 | 2 |
| 22 | 53 | Right | RUL | 6 | Tonsillar ca. | U-VATS | Wedge resection | Not seen | Detected | Apical | SCC | Had pneumothorax | 38 | 2 |
| 23 | 67 | Left | LUL | 12 | None | U-VATS | Two wedge resections | Spread | Detected | Apical | Primary adeno. | – | 55 | 1 |
LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RLL, right lower lobe; RML, right middle lobe; adeno., adenocarcinoma; Ca., carcinoma; U-VATS, uniportal video-assisted thoracoscopic surgery; 3-port P, posterior tri-portal approach; 3-port A, anterior tri-portal approach; OT, operative time; LOS, length of stay; ILD, interstitial lung disease; SCC, primary squamous cell carcinoma; PA, pulmonary artery; IPMN, Intraductal papillary mucinous neoplasm.
Thirteen patients (56.5%) had previous history of cancer (see Table 1) and only two patients had pre-operative histology of the target nodule. These lesions were often too small to be biopsied, previous biopsy was unsuccessful or there were other contraindications, for example the nodule was too close to vascular structures. The procedure was usually performed when a CT-guided biopsy was technically not possible as the radiologist only needs to get within 1 cm of the lesion, and can use a smaller needle. The radiologist reported the exact relation between the dye and the nodule in all the cases.
The mean ASA of this group was 2.3 (range, 1–4) while the average performance status was 0.6 (range, 0–2). The average dyspnea score was 1.6 (range, 1–5) and five individuals were affected by chronic obstructive pulmonary disease. Fifteen patients (65.2%) were ex-smokers, two (8.7%) were current smoker and six patients (26.1%) were non-smokers. In the smokers group the average pack year history was 39.1 (range, 5.0–80.0).
Fifteen patients (65.2%) underwent a uniportal approach (U-VATS) and eight patients (34.8%) received a triportal VATS as shown in Figure 3. Of these 8, 3 (13.0%) were treated with posterior and 5 (21.7%) with anterior approach triportal VATS. In the uniportal group, an Alexis (Applied Medical) soft tissue retractor was adopted. Importantly, the Geiger counter was also used over the chest wall with reliable feedback. The signal often directed the strategy of the port placement according to the location of the nodules.
Figure 3.

Radio-guided pulmonary nodules resection: preliminary British experience (15). The surgical video demonstrates three cases from the cohort. The first two patients were treated with uniportal technique, and the last one with a 3-port posterior approach VATS. VATS, video-assisted thoracoscopic surgery. Available online: http://asvidett.amegroups.com/article/view/22886
The average operative time was 69 minutes (range, 35–178 minutes) and the blood loss was universally minimal. Of note, in nine patients (39.1%) the resection of the marked nodule was associated with other procedures as shown in Table 1. Namely, the associated procedures were: other wedges, lymphadenectomy, lymph node sampling, in one case (4.3%) the resection of a malignant lymph node which was invading the pulmonary artery, a maneuver that required conversions for bleeding, 2 wedge resections on the contralateral side in one patient (4.3%), and 1 middle lobectomy. No conversion to open surgery was required in order to locate the marked nodule. In the case mentioned above, the conversion to posterolateral thoracotomy took place after the resection of the marked nodule was already successfully accomplished. All the nodules except for one lingulectomy were resected by mean of a non-anatomical resection. In all the patients one apical chest drain was placed (two drains in the bilateral case). The mean numbers of days with chest drain in situ was 1 (range, 0–5) and the final histologic diagnosis are shown in Tables 1,2. Successful resection of target nodules was achieved in all cases (100%, all the nodules labelled with radioactive dye and methylene blue were resected with microscopically clear margins, R0). Of note, two procedures (8.7%) were re-do cases, as the patients underwent previous lobectomy for primary malignancy (see patient number 10 and number 20 in Table1).
Table 2. Disease location and histology of the group studied.
| Variables | LUL | LLL | RUL | RLL |
|---|---|---|---|---|
| Patients number | 5 | 6 | 4 | 8 |
| Final histology | Teratoma, benign, colonic adeno., two primary adeno. | Colonic adeno., breast adeno., hamartoma, primary adeno., SCC, lymphoma | Primary adeno., SCC, colonic adeno., benign | Four colonic adeno., melanoma, benign, hamartoma, pseudotumour |
LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RLL, right lower lobe; SCC, primary squamous cell carcinoma; adeno., adenocarcinoma.
None of the patient died as a result of surgery. The mean length of stay was 2.5 days (range, 0.0–7.0 days). One individual (4.3%) sustained pneumothorax during injection of the radio-tracer and was treated with chest tube insertion prior to general anesthesia and mechanical ventilation. On the basis of our intraoperative findings, Methylene blue alone seems very unreliable as it tends to spread in the lung parenchyma, and sometimes in the pleural cavity. In fact, in 9 of the 23 patients (39.1%) the methylene blue was not useful in order to identify the nodule (Table 1). In four cases it spread widely while in five patients this was too deep in order to be seen on the lung surface. On the other hand, the radioactivity of the 99mTc was always detected by the probe and it guided successfully the resection. This finding was previously noted by other authors (14). Of note, 5 out of 23 procedures (21.7%) were performed by the resident under consultant supervision.
Conclusions
Radioactivity aided resections of pulmonary nodules is a feasible procedure in order to locate and resect subcentimetre lesions without direct palpation of the pulmonary parenchyma. This procedure saved the patients from a more invasive approach (thoracotomy or mini-thoracotomy), or the alternative of repeat CT scanning and delays to potential treatment. It provides the benefit of minimally invasive surgery with modest additional risks.
The radiolabelling was successfully adopted for patients in whom CT-guided biopsy was not feasible or already unsuccessful. The needle for injection is smaller than a biopsy needle making the procedure safer even in patients affected by emphysema. Also, it does not need to puncture the lesion, as it only needs to be placed within 1 cm from the lesion.
The radiologist always reported the site of injection in relation to the nodule and the distance from the nodule. The date of surgery was always agreed in advance as the patients underwent CT-guided injection in the morning and surgery in the afternoon. All the above make cooperation between teams of vital importance in order to provide the service efficiently.
We suggest that this procedure is cost effective. The gamma probe and Geiger counter were present already in the hospital as they are routinely used in breast surgery. This will be the case in every hospital that offers sentinel lymph node surgery. The additional cost of CT scan injection and radio tracer kit are largely compensated by the reduced hospital stay, that these patients experienced because of the VATS approach. We can speculate that the length of stay would have been an average of 2 days longer if these patients sustained a posterolateral thoracotomy in order to locate the nodules by hand palpation.
We hope that this study, which demonstrates good safety and efficacy, will allow other centres in the UK to adopt this technique. Based on the outcome of this study a comparative or a randomised study could be set up to evaluate this technique further and before it can be recommended for routine use in patients presenting with small lung nodules. Finally, our findings are in keeping with the conclusions of larger studies available in the international literature (16-18).
Acknowledgements
The authors thank the James Cook University Hospital, which facilitated the study and where all the patients underwent surgery under the care of the senior author of this manuscript. We also thank all the medical staff involved in the care of the patients described. This study was the subject of a poster at the British Thoracic Oncology Group meeting, Dublin 2018.
Ethical Statement: The research project was submitted to the Integrated Research Application System (IRAS, project ID number 152329), was assessed and accepted by the Lancaster Research Ethics Committee in June 2015 after consultation with the ARSAC. Written prospective consent was required for all patients entering this study but any form of randomization for patients was required.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Santambrogio R, Montorsi M, Bianchi P, et al. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg 1999;68:218-22. 10.1016/S0003-4975(99)00459-2 [DOI] [PubMed] [Google Scholar]
- 2.Nomori H, Horio H, Naruke T, et al. Fluoroscopy-assisted thoracoscopic resection of lung nodules marked with lipiodol. Ann Thorac Surg 2002;74:170-3. 10.1016/S0003-4975(02)03615-9 [DOI] [PubMed] [Google Scholar]
- 3.Okumura T, Kondo H, Suzuki K, et al. Fluoroscopy-assisted thoracoscopic surgery after computed tomography-guided bronchoscopic barium marking. Ann Thorac Surg 2001;71:439-42. 10.1016/S0003-4975(00)02378-X [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. 10.1007/s00464-010-1502-3 [DOI] [PubMed] [Google Scholar]
- 5.Ambrogi MC, Melfi F, Zirafa C, et al. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc 2012;26:914-9. 10.1007/s00464-011-1967-8 [DOI] [PubMed] [Google Scholar]
- 6.Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. 10.1016/S1010-7940(00)00411-5 [DOI] [PubMed] [Google Scholar]
- 7.Boni G, Bellina CR, Grosso M, et al. Gamma probe-guided thoracoscopic surgery of small pulmonary nodules. Tumori 2000;86:364-6. [DOI] [PubMed] [Google Scholar]
- 8.Zaman M, Bilal H, Woo CY, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. 10.1093/icvts/ivs068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melfi FM, Chella A, Menconi GF, et al. Intraoperative radioguided sentinel lymph node biopsy in non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:214-20. 10.1016/S1010-7940(02)00763-7 [DOI] [PubMed] [Google Scholar]
- 10.Boni G, Manca G, Melfi FM, et al. Radioguided Biopsy of the Sentinel Lymph Node in Patients with Non-Small Cell Lung Cancer. In: Mariani G, Giuliano AE, Strauss HW. editors. Radioguided Surgery. New York, NY: Springer, 2008:166-71. [Google Scholar]
- 11.Melfi FM, Davini F, Boni G, et al. Sentinel lymph node in lung cancer surgery. Thorac Surg Clin 2012;22:205-14. 10.1016/j.thorsurg.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 12.Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. 10.1016/j.ejcts.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 13.Partik BL, Leung AN, Müller MR, et al. Using a dedicated lung-marker system for localization of pulmonary nodules before thoracoscopic surgery. AJR Am J Roentgenol 2003;180:805-9. 10.2214/ajr.180.3.1800805 [DOI] [PubMed] [Google Scholar]
- 14.Daniel TM, Altes TA, Rehm PK, et al. A novel technique for localization and excisional biopsy of small or ill-defined pulmonary lesions. Ann Thorac Surg 2004;77:1756-62; discussion 1762. [DOI] [PubMed]
- 15.Nardini M, Bilancia R, Papoulidis P, et al. Radio-guided pulmonary nodules resection: preliminary British experience. Asvide 2018;5:088. Available online: http://asvidett.amegroups.com/article/view/22886 [DOI] [PMC free article] [PubMed]
- 16.Grogan EL, Jones DR, Kozower BD, et al. Identification of small lung nodules: technique of radiotracer-guided thoracoscopic biopsy. Ann Thorac Surg 2008;85:S772-7. 10.1016/j.athoracsur.2007.10.105 [DOI] [PubMed] [Google Scholar]
- 17.Bellomi M, Veronesi G, Trifirò G, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg 2010;90:1759-64. 10.1016/j.athoracsur.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 18.Stiles BM, Altes TA, Jones DR, et al. Clinical experience with radiotracer-guided thoracoscopic biopsy of small, indeterminate lung nodules. Ann Thorac Surg 2006;82:1191-6; discussion 1196-7. 10.1016/j.athoracsur.2006.04.059 [DOI] [PubMed] [Google Scholar]


