Abstract
Multiple limitations for cardiac pharmacologic therapies like intolerance, individual variation in effectiveness, side effects, and high cost still remain, despite the recent progress in diagnosis and health support. Gene therapy is poised to be an attractive alternative in various ways for the future, refractory cardiac diseases being one aspect of it. As a novel therapy to deliver the objective gene to organs of living animals, ultrasound targeted microbubble destruction (UTMD) has therapeutic potential in cardiovascular disorders. UTMD, which binds microbubbles with DNA or RNA carriers into the shell and destroys the located microbubbles with low frequency and high mechanical index ultrasound can release target agents to specific organs. UTMD has the ability to transfect markedly through sonoporation, cavitation and other effects by way of intravenous injection that is minimally invasive and highly specific for gene deliverance. Here, we have summarized the present role of UTMD in pre-clinical studies of cardiac gene therapy which covers myocardial infarction, regeneration, ischaemia/reperfusion injury, hypertension, diabetic cardiomyopathy, adriamycin cardiomyopathy and some discussion for further studies.
Keywords: Ultrasound, microbubble, gene therapy, cardiovascular diseases (CVDs)
Introduction
Greater incidence of cardiovascular diseases (CVDs) globally, is associated with the increase in mortality and morbidity rate every passing year (1). Despite the recent progress in diagnosis and health support, multiple limitations for cardiac pharmacologic therapy still remain, including intolerance, individual variation in effectiveness, side effects and high cost (2). Gene therapy tends to be a promising therapeutic tool and may be beneficial in refractory cardiac disease after raising insight into the molecular mechanisms of CVDs (3,4). However, experimental methods are not yet ready for clinical applications in terms of efficient delivery to the target tissue and sustained expression of transgenes.
Ultrasound targeted microbubble destruction (UTMD) is a novel therapy to deliver the objective gene to organs of living animals. It has been proven to bind non-invasively microbubbles (MBs) with DNA or RNA carriers (assemble adenoviral, plasmid or nanoparticles) into the shell and destroy the located MBs with low frequency and high mechanical index ultrasound, releasing target agents into peculiar organs (5-7). Not only can UTMD improve the transfection efficiency by several orders of magnitude, but also achieve specific target markedly (8). Due to its less invasive method and highly specific gene delivery system, UTMD is considered a promising strategy for gene therapy.
For our study the criteria and keywords of “ultrasound targeted microbubble destruction”, “gene therapy”, “cardiovascular diseases” was used in PubMed. All cited studies have received informed consent from each study participant and protocol approval by the ethics committee and institutional review board. This paper briefly reviews the current applications of UTMD in cardiac gene therapy and suggests avenues for further studies.
Mechanisms of UTMD
UTMD has proven to elevate the gene transfection efficiency by various preclinical studies in vitro and in vivo (9-11), as a potential target specific gene delivery tool. MBs, made of lipids, saccharide, albumin, biocompatible polymers and other materials (12-14), are considered as a promising approach for gene delivery vectors, which expands and contracts through sonoporation, cavitation and other effects.
Sonoporation, based on the specific response of the MBs upon exposure to ultrasound, is the mechanism of transferring gene into cells effectively. When exposed to ultrasound, MBs oscillate and then rupture (Figure 1). Not only can UTMD improve the transfection efficiency by several orders of magnitude, but also achieve specific target markedly. The nuclear membrane in the cell membrane have temporary and reversible holes (about 50 nm in diameter), allowing a molecular (weigh 10–30 KD) to get through (15). Figure 2 shows the process of UTMD gene delivery in tissue (16), which demonstrates the key role in augmentation of transfection efficiency (17).
Figure 1.
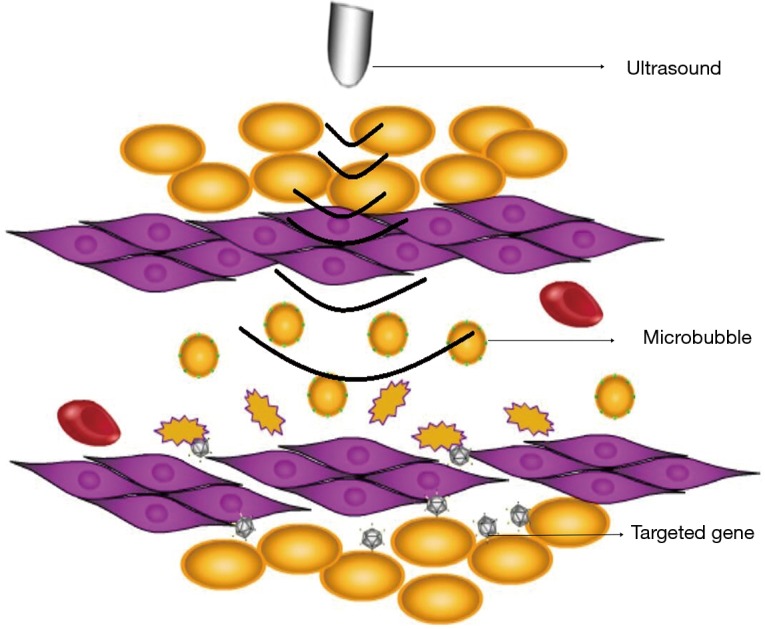
Therapeutic gene is released to the target cell after the microbubbles are destroyed at the site of the target tissue.
Figure 2.
UTMD gene delivery in tissue. The ultrasound application leads to holes in cell membrane and capillary, which facilitates the uptake of therapeutic GFP-mark gene (16). UTMD, ultrasound targeted microbubble destruction.
Cavitation effect is another physical basis of ultrasound targeted microbubble therapy. Cell membrane permeability change is a prerequisite for gene transfection. The cavitation and mechanical effect can enhance the permeability of cell membrane, especially the cavitation effect which can be divided into; steady state and transient cavitation. The latter is a kind of strong biological effect, which can cause cell apoptosis and necrosis at the same time (18-20). It has been reported that ultrasonic cavitation effect can widen the gap between capillary endothelial cells and increase cell membrane permeability, so the microbubble or gene that is released can enter the blood vessel wall and tissue space, so as to increase the effect of targeted gene therapy.
In addition, Meijering et al. (21) proposed the MBs rupture resulted in hydrogen peroxide generation under ultrasound irradiation. At the same time, it caused Ca2+ influx and Ca2+-dependent K channel opening in adjacent cell membrane, thereby causing local membrane potential hyperpolarization. The ultrasound microbubble gets into the cell by the mechanism of endocytosis and pinocytosis. Tran et al. (22) reported, the pressure generated by the MBs rupture induced the formation of a mechanical stimulation, activating specific channels (stretch activated channels) and non-specific ion channels, causing the exogenous molecules (such as MBs containing transfection gene or drugs) to enter cells and play a role.
Gene delivery system in UTMD
Viral vector with high efficiency of transfection and sustained expression is the primary choice of transferring genes to the target cells in UTMD. Pre-clinical studies have proven that UTMD has synergy to combine with viral vectors and offers many benefits (23,24). First of all, transgenic expression was enhanced in the heart tissue associated with adenoviral DNA (25,26). Secondly, after injection of MBs in targeted objects loaded with the expressing luciferase or EGFP, the MBs released the site-specific strength through ultrasound irradiation that improved gene transfection efficiency (27). Also, the microbubble allows intravenous injection as delivery method as it can reduce the degradation rate of viruses by the immune system, simultaneously imposing restriction on the immune response to the viruses thus allowing intravascular administration and repetitive injections (28). Danialou et al. (29) investigated a 3-fold transfection of the gene and a 22-fold increase in level of expression was noted in animals treated using UTMD therapies.
Compared with viral vectors, non-viral carrier systems are potentially safer and more convenient, which can be used not only for gene application but also for direct delivery of exogenous protein. Plasmids, siRNA, miRNA and PiggyBac are common non-viral transposons for UTMD. Table 1 shows the studies in which non-viral vectors worked as the delivery vectors. A highly specific and minimally invasive non-viral gene delivery system is the new direction for future therapeutic procedures.
Table 1. Non-viral carrier systems in UTMD.
| Vector | Study | Target gene |
|---|---|---|
| Plasmids | Fujii et al. 2009 (12) | VEGF, SCF |
| Fujii et al. 2011 (30) | SCF, SDF-1α | |
| Yan et al. 2014 (31) | MMP2 | |
| Deng et al. 2015 (32) | Ang-1 | |
| miRNA | Kopechek et al. 2015 (33) | miR-23a |
| Su et al. 2015 (34) | microRNA-21 | |
| Liu et al. 2015 (35) | microRNA-21 | |
| PiggyBac | Chen et al. 2016 (36) | ANGPTLs |
| Chen et al. 2015 (37) | GLP-1 | |
| Chen et al. 2013 (38) | TB4 | |
| siRNA | Huang et al. 2016 (39) | GRK4 |
UTMD, ultrasound targeted microbubble destruction; Ang-1, angiopoietin-1; MMP2, matrix metalloproteinase 2; VEGF, vascular endothelial growth factor; TB4, thymosin beta 4; GRK4, G protein-coupled receptor kinase type 4.
Applications of UTMD in ischemic heart disease
Myocardial infarction (MI)
Despite the stenting or bypassing of the infarcted artery, ventricular dysfunction may still progress after an extensive MI (40). Novel gene therapies may improve cardiac function by regulating gene expression, promoting tissue regeneration and regional perfusion in the infarcted myocardium (41,42). UTMD, non-invasively and selectively delivers genes to the infarct via microbubble carriers and can help to release plasmid DNA when they are targeted with an ultrasound beam. Intravenously administered lipid MBs have been proven mature in clinical evaluation of myocardial perfusion and pre-clinical cardiac gene delivery (25,43). Recently, previous pre-clinical studies have proven the potential advantages of UTMD, however it warrants pragmatic studies that still needs separate optimized protocols for different diseases. In rats, promising strategies were provided to realize the localized delivery of shRNA against PHD2 (44,45), G-CSF (46), S100A6 (47), MMP2 (31), TATp (48), SCF, SDF-1α (30) and bFGF (49) to protect the heart from acute MI via cationic MBs. Besides, ultrasound microbubble was suggested as an effective vector for VEGF (12), SCF (13) and GDF11 (50) delivery in mice, CD151 (51) and Ang-1 (32) in rabbit, microRNA-21 (34) in swine and HGF (52) in dog. The therapeutic value of UTMD mediated gene transfer into the infarcted heart was enhanced by these pre-clinical treatments (12,30-32,34,44-52).
Regeneration
Approximately 10% of MI patients will die, typically from ventricular arrhythmias, pump failure or myocardial rupture as reported (53). Developing strategies for regeneration of cardiomyocytes and blood vessels in the damaged area of the heart, rather than stenting or bypassing the infarcted artery has been the major goal of therapy for acute MI. Novel strategies, particularly non-invasive approaches that induce stem cell homing to the damaged heart for myocardial regeneration includes embryonic stem cells, induced pluripotent stem cells and bone marrow stem cells, which have been studied in recent years (54-56). Moreover, resident cardiac progenitor cells were discovered in the adult mammalian heart. They are self-renewing, clonogenic and multipotent, and can be differentiated into three major cardiac lineages; cardiac muscle cells, vascular smooth muscle cells and endothelial cells theoretically (57-62), despite being sparse in number. Genes such as SDF-1 (35), SDF-1/CXCR4 (63) and TB4 (38) in rat, MSC (64,65) in dog, MSC (66) in rabbit and BMSC (67) in swine were delivered directly to the heart in order to stimulate resident cardiac progenitor cells. Cardiac progenitor cells proliferate and differentiate into three intact cardiac cell lineages after UTMD therapies. However, whether adult cardiac muscle cells can be formed from these resident progenitor cells in vivo still remains controversial.
Ischemia/reperfusion injury (I/R)
I/R injury is one of the main risks of heart failure, and the regenerative capacity of intrinsic stem cells plays an important role in tissue repair after injury. However, stem cells in ageing individuals have reduced regenerative potential and their tissues lack the capacity to renew. TRAF3IP2 (68), GDF11 (69), Antagomir (70), VEGF-a, IGF-1 and Cav-3 (71) in mice as well as bone marrow cell (BMC) (72), MMP2 (31) and Akt1 (73) in rat undergoing multiple application via UTMD can rejuvenate the aged heart and protect it from I/R injury.
Applications of UTMD in hypertension
Cardiac hypertrophy is induced by hypertension in pre-clinical models, but clinical translation is limited by lack of target cardiac delivery systems. Kopechek et al. (33) founded that UTMD mediated delivery of anti-miR-23a can suppress cardiomyocyte hypertrophy and culture cardiomyocytes in rats, laying the groundwork for future in vivo translational studies, which leads to targeted clinical strategies to therapeutically modulate miRNA activity in the human heart. In addition, Huang et al. (39) discovered the downregulation of renal GRK4 expression via UTMD lowers blood pressure in spontaneously hypertensive rats. UTMD offers a novel strategy for gene therapy in hypertension.
Applications of UTMD in cardiomyopathies
Diabetic cardiomyopathy (DCM)
DCM, one of the serious chronic complications of diabetes, is the leading cause of morbidity and mortality (74). It results in cardiac functional and structural changes, independent of hypertension, coronary artery disease, or any other known cardiac disease. The structural changes include fibrosis, apoptosis, hypertrophy of myocytes and the functional changes include systolic and diastolic dysfunction (75-80). Previous studies have confirmed the progress of cardiac dysfunction after DCM could be effectively inhibited and even reversed by gene of aFGF (81,82), bFGF (83,84) and FGF-1 (85) in rat combined with the UTMD technique. It provides a promising strategy for DCM-targeted therapy.
Adriamycin cardiomyopathy
Adriamycin cardiomyopathy is an established lethal disease. Approximately 50% of mortality every year is figured out to be capable of maintaining heart function by stimulating adult cardiac progenitor cells to initiate myocardial regeneration when congestive heart failure develops. Lee et al. (86) revealed surviving gene therapy attenuates left ventricular systolic dysfunction in doxorubicin cardiomyopathy by reducing apoptosis and fibrosis, which specifically targeted the underlying biological processes in heart failure. Additionally, Chen et al. employed UTMD to deliver PiggyBac transposon plasmids encoding the intranuclear myocardial gene of ANGPTL8 (36) and GLP-1 (37) to rat hearts with adriamycin cardiomyopathy, which results in stimulating myocardial regeneration respectively.
To summarize, UTMD contributes a tailored approach to improve cardiac diseases. Table 2 showed the encouraging pre-clinical studies, including applications in MI, regeneration, IR, hypertension, DCM and adriamycin cardiomyopathy. However, clinical trials have yet to produce disappointing results, possibly due to incomplete or inaccurate gene delivery (87). Early clinical trials of cell transplantation demonstrated improved perfusion, but limited cell survival may have diminished the benefits of this approach for cardiac restoration (88).
Table 2. Applications of UTMD in cardiac gene delivery.
| Disease | Study | Therapeutic | Target object | Outcome summary |
|---|---|---|---|---|
| Ischemic heart disease | ||||
| MI | Zhang et al. (2017) | PHD2 | Rat | Localized delivery of shRNA against PHD2 protects the heart from acute myocardial infarction through ultrasound-targeted cationic microbubble destruction |
| Yang et al. (2016) | CD151 | Rabbit | Delivery of CD151 by ultrasound microbubbles in rabbit myocardial infarction | |
| Xue et al. (2016) | G-CSF | Rat | UTMD in combination with granulocyte colony-stimulating factor (G-CSF) improves cardiac function in myocardial infarction rats | |
| Li et al. (2016) | PHD2 | Rat | UTMD enhances the PHD2-shRNA to restore myocardial function following ischemic injury in rats via improving the efficacy of therapeutic angiogenesis | |
| Su et al. (2015) | microRNA-21 | Swine | UTMD mediated microRNA-21 transfection regulated PDCD4/NF-κB/TNF-α pathway to prevent coronary microembolization-induced cardiac dysfunction | |
| Mofid et al. (2017) | S100A6 | Rat | UTMD of minicircle-S100A6 attenuates infarct size and improves left ventricular systolic function and perfusion post acute myocardial infarction-reperfusion | |
| Liu et al. (2015) | microRNA-21 | Swine | UTMD enhances gene expression of microRNA-21 in swine heart via intracoronary delivery | |
| Du et al. (2015) | GDF11 | Mice | Repetitive targeted delivery of GDF11 by ultrasound mediated cationic microbubble destruction rejuvenates and protects the aged mouse heart | |
| Deng et al. (2015) | Ang-1 | Rabbit | Improving the efficacy of therapeutic angiogenesis by UTMD mediated Ang-1 gene delivery to the infarcted myocardium | |
| Yan et al. (2014) | MMP-2 | Rat | The use of MMP2 antibody-conjugated cationic microbubble to target the ischemic myocardium, enhances Timp3 gene transfection and improves cardiac function | |
| Zhou et al. (2013) | TATp | Rat | Synergistic effects of UTMD and TAT peptide on gene transfection: an experimental study in vitro and in vivo | |
| Yuan et al. (2012) | HGF | Dog | Intramyocardial injection of HGF and microbubbles in combination with insonation enhances neovascularization and reduces ventricular remodeling and infarct size | |
| Fujii et al. (2011) | SCF, SDF-1α | Rat | Targeted ultrasound delivery of SCF and SDF-1α genes to the infarcted myocardium recruited progenitor cells and increased vascular density | |
| Sheng et al. (2009) | bFGF | Rat | Basic fibroblast growth factor delivered by ultrasound-mediated destruction microbubbles for treatment of acute myocardial infarction | |
| Fujii et al. (2009) | VEGF, SCF | Mice | Noninvasive UTMD successfully delivered VEGF and SCF genes into the infarcted heart, increased vascular density, and improved myocardial perfusion and ventricular function | |
| Regeneration | Li et al. (2015) | SDF-1/CXCR4 | Rat | UTMD improves the migration and homing of Mesenchymal stem cells after myocardial infarction by up-regulating SDF-1/CXCR4: a pilot study |
| Ling et al. (2013) | MSC | Dog | UTMD promotes angiogenesis and heart function by inducing myocardial microenvironment change | |
| Chen et al. (2013) | TB4 | Rat | Stimulation of adult resident cardiac progenitor cells by durable myocardial expression of thymosin beta 4 with ultrasound-targeted microbubble delivery | |
| Zhong et al. (2012) | MSC | Dog | Enhanced homing of mesenchymal stem cells to the ischemic myocardium by UTMD | |
| Xu et al. (2010) | MSC | Rabbit | Myocardium-targeted transplantation of mesenchymal stem cells by diagnostic UTMD improves cardiac function in myocardial infarction of New Zealand rabbits | |
| Li et al. (2009) | BMSC | Swine | UTMD promotes bone marrow mesenchymal stem cell transplantation for myocardial infarction | |
| Ischemia/reperfusion injury | Erikson et al. (2017) | TRAF3IP2 | Mice | Targeting TRAF3IP2 by genetic and interventional approaches inhibits ischemia/reperfusion-induced myocardial injury and adverse remodeling |
| Du et al. (2017) | GDF11 | Mice | Targeted myocardial delivery of GDF11 gene rejuvenates the aged mouse heart and enhances myocardial regeneration after ischemia-reperfusion injury | |
| Kwekkeboom et al. (2016) | Antagomir | Mice | Increased local delivery of antagomir therapeutics to the rodent myocardium using ultrasound and microbubbles | |
| Chen et al. (2016) | BMC | Rat | UTMD enhances delayed BMSC delivery and attenuates post-infarction cardiac remodeling by inducing engraftment signals | |
| Yan et al. (2014) | MMP2 | Rat | The use of MMP2 antibody-conjugated cationic microbubble to target the ischemic myocardium, enhances Timp3 gene transfection and improves cardiac function | |
| Dorner et al. (2013) | VEGF-a, IGF-1 and Cav-3 | Mice | Ultrasound-mediated stimulation of microbubbles after acute myocardial infarction and reperfusion ameliorates left-ventricular remodeling in mice via improvement of border zone vascularization | |
| Li et al. (2012) | Akt1 | 293FT cells/cardiomyocytes | The effect of Akt1 gene on rat cardiomyocytes by ultrasound/microbubbles destruction | |
| Hypertension | Huang et al. (2016) | GRK4 | Rat | Downregulation of renal G protein-coupled receptor kinase type 4 expression via UTMD lowers blood pressure in spontaneously hypertensive rats |
| Kopechek et al. (2015) | miR-23a | Rat | Targeted delivery of an anti-mir to cardiomyocytes using ultrasound and microbubbles suppresses hypertrophy | |
| Cardiomyopathy | ||||
| DCM | Zhao et al. (2016) | aFGF | Rat | Prevent diabetic cardiomyopathy in diabetic rats by combined therapy of aFGF-loaded nanoparticles and UTMD technique |
| Zhao et al. (2016) | bFGF | Rat | Using basic fibroblast growth factor nanoliposome combined with ultrasound-introduced technology to early intervene the diabetic cardiomyopathy | |
| Zhang et al. (2016) | aFGF | Rat | Advanced interfere treatment of diabetic cardiomyopathy rats by aFGF-loaded heparin-modified microbubbles and UTMD technique | |
| Zhao et al. (2014) | bFGF | Rat | Functional and pathological improvements of the hearts in diabetes model by the combined therapy of bFGF-loaded nanoparticles with UTMD | |
| Tian et al. (2013) | FGF-1 | Rat | Targeted delivery of fibroblast growth factor-1 by UTMD reduces myocyte apoptosis and myocardial fibrosis and improves ventricular systolic and diastolic function in rats having DCM | |
| DIC | Chen et al. (2016) | ANGPTLs | Rat | ANGPTL8 reverses established adriamycin cardiomyopathy by stimulating adult cardiac progenitor cells |
| Chen et al. (2015) | GLP-1 | Rat | Myocardial regeneration in adriamycin cardiomyopathy by nuclear expression of GLP1 using UTMD | |
UTMD, ultrasound targeted microbubble destruction; PHD2, prolyl hydroxylase-2; CD151, cluster of differentiation 151; G-CSF, granulocyte colony-stimulating factor; GDF11, growth differentiation factor 11; Ang-1, angiopoietin-1; MMP2, matrix metalloproteinase 2; TATp, TAT peptide; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; SCF, stem cell factor; MSC, mesenchymal stem cell; TB4, thymosin beta 4; BMSC, bone marrow mesenchymal stem cell; TRAF3IP2, TRAF3 interacting protein 2; FGF-1, fibroblast growth factor-1; GRK4, G protein-coupled receptor kinase type 4; MI, myocardial infarction; DCM, dilated cardiomyopathy; DIC, disseminated intravascular coagulation; BMC, bone marrow cell.
Discussion
UTMD is definitely a promising strategy to improve efficiency of cardiac gene delivery because of the low toxicity, low immunogenicity of vectors, minimum invasiveness, with the great potential for multiple application, and organs which can be targeted with its high specificity proven by increasing evidences. Pre-clinical studies have demonstrated the combination of UTMD with viral or non-viral vectors in gene delivery. UTMD not only enhances the efficiency of the viral vector, but also avoids its immunogenicity. Therefore, a novel and feasible way to support gene therapy trial for individuals with CVDs has come into effect in the past few years.
Nevertheless, future work remains to be done for the technological improvement of UTMD before clinical application (53,54). First, microbubble preparation technology needs to be optimized to efficiently carry gene payloads while maintaining acoustic activity and prolonging circulation time to prevent clearance by the mononuclear cell as well as improving targeting techniques to enhance tissue binding force in areas of high sheer stress. The illustration of optimal ultrasound parameters for each microbubble and its intended application also works (89). Second, the techniques of microbubble surface modification tend to not be mature, such as the technology to connect drugs, gene or ligand to the microbubble. The number of genes or drugs which the microbubble carries are limited to the micro vesicle transport in the blood vessels because of blood fluidity and impact resistance, which leads to short contact time between the MBs and the receptor. Also, there is shortage of formation for targeted microbubble receptor which is often below the treatment of threshold (90). Moreover, to some degree, the parameters of ultrasound influences the transfection rate of MBs. Recent studies point out the use of low frequency probe can produce a wider and more uniform sound field and that strong cavitation is the key to thrombolysis. However, the optimal parameters have not been formed yet (7). In addition, when MBs exist in the capillaries, ultrasonic irradiation can cause microvascular leakage, intracardiac hemolysis capillary rupture, bleeding, formation, inflammatory cell infiltration, myocardial cell damage and other adverse reactions (7,89,90). Therefore, more technological revolution should be taken to enhance the biological application of UTMD.
When it comes to the biological efficacy and safety of UTMD, the injured endothelial cells in part of the vessel wall, limited by toxicity as well as lack of immunogenicity and the potential for repetitive and targeted applications should be taken into account. UTMD is primarily an intravascular method of gene delivery, with the vascular endothelium being the primary target (91), while several studies were not mainly for cardiomyocyte transfection. In addition, effective application of UTMD in larger animal models is one of the major obstacles hampering UTMD application (92) and most cited studies demonstrate feasibility in rodents. Here, Table 3 shows the specific gene delivery in target subjects, which significantly reveals the target animal models, cell types and disease states treated by UTMD. It may help to select the appropriate subjects based on the therapeutic agent. Taken together, mounting experiments with large animal models and specific target cell types as well as accurate disease states are warranted to facilitate the translation into human applications.
Table 3. Specific gene delivery in target subjects.
| Animal model | Target tissue/cell type | Disease state | Therapeutic agent | Study |
|---|---|---|---|---|
| Rat | Heart/cardiomyocyte | Myocardial infarction | S100A6 | Mofid et al. (2017) |
| TATp | Zhou et al. (2013) | |||
| Ischemia-reperfusion injury | Akt1 | Li et al. (2012) | ||
| MMP2 | Yan et al. (2014) | |||
| Hypertension | miR-23a | Kopechek et al. (2015) | ||
| DIC | GLP-1 | Chen et al. (2015) | ||
| Heart/vascular endothelial | Myocardial infarction/DCM | bFGF | Zhao et al. (2016); Zhao et al. (2014); Sheng et al. (2009) |
|
| Myocardial infarction | SCF, SDF-1α | Fujii et al. (2011) | ||
| G-CSF | Xue et al. (2016) | |||
| Regeneration | SDF-1/CXCR4 | Li et al. (2015). | ||
| DCM | FGF-1 | Tian et al. (2013) | ||
| aFGF | Zhao et al. (2016) | |||
| Heart/vascular smooth muscle cells | Hypertension | GRK4 | Huang et al. (2016) | |
| Heart/cardiac stem cells | Myocardial infarction | PHD2 | Zhang et al. (2017); Li et al. (2016) | |
| DIC | ANGPTLs | Chen et al. (2016) | ||
| Heart/cardiac stem cells | Regeneration | TB4 | Chen et al. (2013) | |
| Ischemia-reperfusion injury | BMC | Chen et al. (2016) | ||
| Mice | Heart/cardiomyocyte | Ischemia-reperfusion injury | TRAF3IP2 | Erikson et al. (2017) |
| Heart/cardiac stem cells | Ischemia-reperfusion injury | Antagomir | Kwekkeboom et al. (2016) | |
| Myocardial infarction/ischemia-reperfusion injury | GDF11 | Du et al. (2015); Du et al. (2017) | ||
| Heart/vascular endothelial | Ischemia-reperfusion injury | VEGF-a, IGF-1 and Cav-3 | Dorner et al. (2013) | |
| Myocardial infarction | VEGF, SCF | Fujii et al. (2009) | ||
| Swine | Heart/cardiomyocyte | Myocardial infarction | microRNA-21 | Su et al. (2015); Liu et al. (2015) |
| Heart/cardiac stem cells | Regeneration | BMSC | Li et al. (2009) | |
| Rabbit | Heart/cardiomyocyte | Myocardial infarction | CD151 | Yang et al. (2016) |
| Heart/cardiac stem cells | Regeneration | MSC | Xu et al. (2010) | |
| Heart/vascular endothelial | Myocardial infarction | Ang-1 | Deng et al. (2015) | |
| Dog | Heart/cardiomyocyte | Myocardial infarction | HGF | Yuan et al. (2012) |
| Heart/cardiac stem cells | Regeneration | MSC | Ling et al. (2013); Zhong et al. (2012) |
UTMD, ultrasound targeted microbubble destruction; PHD2, prolyl hydroxylase-2; CD151, cluster of differentiation 151; G-CSF, granulocyte colony-stimulating factor; GDF11, growth differentiation factor 11; Ang-1, angiopoietin-1; MMP2, matrix metalloproteinase 2; TATp, TAT peptide; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; MSC, mesenchymal stem cell; TB4, thymosin beta 4; BMSC, bone marrow mesenchymal stem cell; TRAF3IP2, TRAF3 interacting protein 2; FGF-1, fibroblast growth factor-1; GRK4, G protein-coupled receptor kinase type 4; DCM, dilated cardiomyopathy; DIC, disseminated intravascular coagulation; BMC, bone marrow cell.
Taken together, the fascinating pre-clinical UTMD studies discussed here represent only a fraction among a wide variety of applications in cardiac gene therapy. UTMD has a strong potential to be used as an adjuvant therapy for candidates with cardiac disorders in the future.
Acknowledgements
We thank for the kind permission to reproduce the figures from other publications, in particular Figure 2 BioMed Central Ltd. (Chen et al., Cardiovasc Ultrasound 2013;11:11. Figure 7D-I).
Funding: This study was supported by Jiangsu Provincial Key Discipline of Medicine (ZDXKA2016003), by the Priority Academic Program Development of Jiangsu higher Education Institutions (PAPD), by Natural Science Foundation of Jiangsu Province (grant No. BK20161057) and National Natural Science Foundation of China (grant No. 81301616, 81601516 and 81271589).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare
References
- 1.Gaziano TA. Economic burden and the cost-effectiveness of treatment of cardiovascular diseases in Africa. Heart 2008;94:140-4. 10.1136/hrt.2007.128785 [DOI] [PubMed] [Google Scholar]
- 2.Fishbein I, Chorny M, Levy RJ. Site-specific gene therapy for cardiovascular disease. Curr Opin Drug Discov Devel 2010;13:203-13. [PMC free article] [PubMed] [Google Scholar]
- 3.Won YW, Lee M, Kim HA, et al. Hypoxia-inducible plasmid expressing both miSHP-1 and HO-1 for the treatment of ischemic disease. J Control Release 2013;165:22-8. 10.1016/j.jconrel.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo X, Huang L. Recent advances in nonviral vectors for gene delivery. Acc Chem Res 2012;45:971-9. 10.1021/ar200151m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen ZY, Liang K, Qiu RX, et al. Ultrasound- and liposome microbubblemediated targeted gene transfer to cardiomyocytes in vivo accompanied by polyethylenimine. J Ultrasound Med 2011;30:1247-58. 10.7863/jum.2011.30.9.1247 [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Xie M, Wang X, et al. Efficient gene delivery to myocardium with ultrasound targeted microbubble destruction and polyethylenimine. J Huazhong Univ Sci Technolog Med Sci 2008;28:613-7. 10.1007/s11596-008-0528-4 [DOI] [PubMed] [Google Scholar]
- 7.Chen ZY, Liang K, Sheng XJ, et al. Optimization and apoptosis induction by RNAi with UTMD technology in vitro. Oncol Lett 2012;3:1030-6. 10.3892/ol.2012.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logeart D, Hatem SN, Heimburger M, et al. How to optimize in vivo gene transfer to cardiac myocytes: mechanical or pharmacological procedures? Hum Gene Ther 2001;12:1601-10. 10.1089/10430340152528101 [DOI] [PubMed] [Google Scholar]
- 9.Phillips LC, Klibanov AL, Wamhoff BR, et al. Targeted gene transfection from microbubbles into vascular smooth muscle cells using focused ultrasound-mediated delivery. Ultrasound Med Biol 2010;36:1470-80. 10.1016/j.ultrasmedbio.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhou J, Zhang Y, et al. Delivery of TFPI-2 using SonoVue and adenovirus results in the suppression of thrombosis and arterial re-stenosis. Exp Biol Med (Maywood) 2010;235:1072-81. 10.1258/ebm.2010.010046 [DOI] [PubMed] [Google Scholar]
- 11.Tinkov S, Coester C, Serba S, et al. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Control Release 2010;148:368-72. 10.1016/j.jconrel.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 12.Fujii H, Sun Z, Li SH, et al. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovasc Imaging 2009;2:869-79. 10.1016/j.jcmg.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 13.Stride E. Physical principles of microbubbles for ultrasound imaging and therapy. Cerebrovasc Dis 2009;27:1-13. 10.1159/000203122 [DOI] [PubMed] [Google Scholar]
- 14.Lentacker I, Wang N, Vandenbroucke RE, et al. Ultrasound exposure of lipoplex loaded microbubbles facilitates direct cytoplasmic entry of the lipoplexes. Mol Pharm 2009;6:457-67. 10.1021/mp800154s [DOI] [PubMed] [Google Scholar]
- 15.Geers B, Dewitte H, De Smedt SC, et al. Crucial factors and emerging concepts in ultrasound-triggered drug delivery. J Control Release 2012;164:248-55. 10.1016/j.jconrel.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 16.Chen Z Y, Lin Y, Yang F, et al. Gene therapy for cardiovascular disease mediated by ultrasound and microbubbles. Cardiovasc Ultrasound 2013;11:11. 10.1186/1476-7120-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Kong F, Li X, et al. Gene therapy following subretinal AAV5 vector delivery is not affected by a previous intravitreal AAV5 vector administration in the partner eye. Mol Vis 2009;15:267-75. [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y, Lin L, Cheng M, et al. Effect of acoustic parameters on the cavitation behavior of SonoVue microbubbles induced by pulsed ultrasound. Ultrason Sonochem 2017;35:176-84. 10.1016/j.ultsonch.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Wang J, Pacella JJ, et al. Dynamic Behavior of Microbubbles during Long Ultrasound Tone-Burst Excitation: Mechanistic Insights into Ultrasound-Microbubble Mediated Therapeutics Using High-Speed Imaging and Cavitation Detection. Ultrasound Med Biol 2016;42:528-38. 10.1016/j.ultrasmedbio.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazan-Peregrino M, Arvanitis CD, Rifai B, et al. Ultrasound-induced cavitation enhances the delivery and therapeutic efficacy of an oncolytic virus in an in vitro model. J Control Release 2012;157:235-42. 10.1016/j.jconrel.2011.09.086 [DOI] [PubMed] [Google Scholar]
- 21.Meijering BD, Juffermans LJ, van Wamel A, et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res 2009;104:679-87. 10.1161/CIRCRESAHA.108.183806 [DOI] [PubMed] [Google Scholar]
- 22.Tran TA, Roger S, Le Guennec JY, et al. Effect of ultrasound-activated microbubbles on the cell electrophysiological properties. Ultrasound Med Biol 2007;33:158-63. 10.1016/j.ultrasmedbio.2006.07.029 [DOI] [PubMed] [Google Scholar]
- 23.Müller OJ, Schinkel S, Kleinschmidt JA, et al. Augmentation of AAV-mediated cardiac gene transfer after systemic administration in adult rats. Gene Ther 2008;15:1558-65. 10.1038/gt.2008.111 [DOI] [PubMed] [Google Scholar]
- 24.Beeri R. New efficient catheter-based system for myocardial gene delivery. Circulation 2002;106:1756-9. 10.1161/01.CIR.0000035240.92015.E4 [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Shohet RV, Bekeredjian R, et al. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J Am Coll Cardiol 2003;42:301-8. 10.1016/S0735-1097(03)00627-2 [DOI] [PubMed] [Google Scholar]
- 26.Naka T, Sakoda T, Doi T, et al. Ultrasound enhances retrovirus--mediated gene transfer. Prep Biochem Biotechnol 2007;37:87-99. 10.1080/10826060701199007 [DOI] [PubMed] [Google Scholar]
- 27.Lee YH, Peng CA. Enhanced retroviral gene delivery in ultrasonic standing wave fields. Gene Ther 2005;12:625-33. 10.1038/sj.gt.3302444 [DOI] [PubMed] [Google Scholar]
- 28.Howard CM, Forsberg F, Minimo C, et al. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J Cell Physiol 2006;209:413-21. 10.1002/jcp.20736 [DOI] [PubMed] [Google Scholar]
- 29.Danialou G, Comtois Dudley RW, Nalbantoglu J, et al. Ultrasound increases plasmid-mediated gene transfer to dystrophic muscles without collateral damage. Mol Ther 2002;6:687-93. 10.1016/S1525-0016(02)90723-9 [DOI] [PubMed] [Google Scholar]
- 30.Fujii H, Li SH, Wu J, et al. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur Heart J 2011;32:2075-84. 10.1093/eurheartj/ehq475 [DOI] [PubMed] [Google Scholar]
- 31.Yan P, Chen KJ, Wu J, et al. The use of MMP2 antibody-conjugated cationic microbubble to target the ischemic myocardium, enhance Timp3 gene transfection and improve cardiac function. Biomaterials 2014;35:1063-73. 10.1016/j.biomaterials.2013.10.043 [DOI] [PubMed] [Google Scholar]
- 32.Deng Q, Hu B, Cao S, et al. Improving the efficacy of therapeutic angiogenesis by UTMD-mediated Ang-1 gene delivery to the infarcted myocardium. Int J Mol Med 2015;36:335-44. 10.3892/ijmm.2015.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopechek JA, Carsonar AR, McTiernan CF, et al. Targeted delivery of an anti-mir to cardiomyocytes using ultrasound and microbubbles suppresses hypertrophy. Circulation 2015;132:A14662. [Google Scholar]
- 34.Su Q, Li L, Liu Y, et al. Ultrasound-targeted microbubble destruction-mediated microRNA-21 transfection regulated PDCD4/NF-κB/TNF-α pathway to prevent coronary microembolization-induced cardiac dysfunction. Gene Ther 2015;22:1000-6. 10.1038/gt.2015.59 [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Li L, Su Q, et al. Ultrasound-targeted microbubble destruction enhances gene expression of microRNA-21 in swine heart via intracoronary delivery. Echocardiography 2015;32:1407-16. 10.1111/echo.12876 [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Chen J, Meng XL, et al. ANGPTL8 reverses established adriamycin cardiomyopathy by stimulating adult cardiac progenitor cells. Oncotarget 2016;7:80391-403. 10.18632/oncotarget.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, Chen J, Huang P, et al. Myocardial regeneration in adriamycin cardiomyopathy by nuclear expression of GLP1 using ultrasound targeted microbubble destruction. Biochem Biophys Res Commun 2015;458:823-9. 10.1016/j.bbrc.2015.02.038 [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Shimoda M, Chen J, et al. Stimulation of adult resident cardiac progenitor cells by durable myocardial expression of thymosin beta 4 with ultrasound-targeted microbubble delivery. Gene Ther 2013;20:225-33. 10.1038/gt.2012.89 [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Li X, Zheng S, et al. Downregulation of Renal G Protein-Coupled Receptor Kinase Type 4 Expression via Ultrasound-Targeted Microbubble Destruction Lowers Blood Pressure in Spontaneously Hypertensive Rats. J Am Heart Assoc 2016;5. pii: e004028. 10.1161/JAHA.116.004028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St John Sutton M, Pfeffer MA, Moye L, et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation 1997;96:3294-9. 10.1161/01.CIR.96.10.3294 [DOI] [PubMed] [Google Scholar]
- 41.Jayasankar V, Woo YJ, Bish LT, et al. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation 2003;108:II230-6. 10.1161/01.cir.0000087444.53354.66 [DOI] [PubMed] [Google Scholar]
- 42.Yau TM, Fung K, Weisel RD, et al. Enhanced myocardial angiogenesis by gene transfer with transplanted cells. Circulation 2001;104:I218-22. 10.1161/hc37t1.094896 [DOI] [PubMed] [Google Scholar]
- 43.Bekeredjian R, Grayburn PA, Shohet RV. Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J Am Coll Cardiol 2005;45:329-35. 10.1016/j.jacc.2004.08.067 [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Sun Z, Ren P, et al. Localized delivery of shRNA against PHD2 protects the heart from acute myocardial infarction through ultrasound-targeted cationic microbubble destruction. Theranostics 2017;7:51-66. 10.7150/thno.16074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Sun Z, Xie M. GW27-e1160 Ultrasound-targeted Microbubble Destruction Enhances the PHD2-shRNA to restore Myocardial Function following Ischemic Injury in Rats via Improving the Efficacy of Therapeutic Angiogenesis. J Am Coll Cardiol 2016;68:C68 10.1016/j.jacc.2016.07.249 [DOI] [Google Scholar]
- 46.Xue J, Yuan JJ, Deng T, et al. Ultrasound-targeted microbubble destruction (UTMD) in combination with granulocyte colony-stimulating factor (G-CSF) improves cardiac function in myocardial infarction rats. Int J Clin Exp Med 2016;9:1748-55. [Google Scholar]
- 47.Mofid A, Newman NS, Lee PJ, et al. Cardiac Overexpression of S100A6 Attenuates Cardiomyocyte Apoptosis and Reduces Infarct Size After Myocardial Ischemia-Reperfusion. J Am Heart Assoc 2017;6:e004738. 10.1161/JAHA.116.004738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z, Zhang P, Ren J, et al. Synergistic effects of ultrasound-targeted microbubble destruction and TAT peptide on gene transfection: an experimental study in vitro and in vivo. J Control Release 2013;170:437-44. 10.1016/j.jconrel.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 49.Sheng WW, Jing H, Zhou DY, et al. Basic fibroblast growth factor delivered by ultrasound-mediated destruction microbubbles for treatment of acute myocardial infarction. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13:1613-6. [Google Scholar]
- 50.Du GK, Wu J, Shao Z, et al. Repetitive targeted delivery of GDF11 by ultrasound mediated cationic microbubble destruction rejuvenates and protects the aged mouse heart. Can J Cardiol 2015;31:S146-7. 10.1016/j.cjca.2015.07.317 [DOI] [Google Scholar]
- 51.Yang SL, Tang KQ, Tao JJ, et al. Delivery of CD151 by Ultrasound Microbubbles in Rabbit Myocardial Infarction. Cardiology 2016;135:221-7. 10.1159/000446639 [DOI] [PubMed] [Google Scholar]
- 52.Yuan QY, Huang J, Chu BC, et al. A targeted high-efficiency angiogenesis strategy as therapy for myocardial infarction. Life Sci 2012;90:695-702. 10.1016/j.lfs.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 53.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive Summary: Heart Disease and Stroke Statistics—2012 Update:A Report From the American Heart Association. Circulation 2012;125:188-97. 10.1161/CIR.0b013e3182456d46 [DOI] [PubMed] [Google Scholar]
- 54.van Laake LW, Passier R, Doevendans PA, et al. Human embryonic stemcell-derived cardiomyocytes and cardiac repair in rodents. Circ Res 2008;102:1008-10. 10.1161/CIRCRESAHA.108.175505 [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Fernandez A, Nelson TJ, Yamada S, et al. iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circ Res 2009;105:648-56. 10.1161/CIRCRESAHA.109.203109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, trans- lational findings, and therapeutic implications for cardiac disease. Circ Res 2011;109:923-40. 10.1161/CIRCRESAHA.111.243147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378:1847-57. 10.1016/S0140-6736(11)61590-0 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Smart N, Riley PR. The epicardium as a candidate for heart regeneration. Future Cardiol 2012;8:53-69. 10.2217/fca.11.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 2011;9:527-40. 10.1016/j.stem.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smart N, Bollini S, Dubé KN, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011;474:640-4. 10.1038/nature10188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bollini S, Smart N, Riley PR. Resident cardiac progenitor cells: at the heart of regeneration. J Mol Cell Cardiol 2011;50:296-303. 10.1016/j.yjmcc.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 62.Zaruba MM, Soonpaa M, Reuter S, et al. Cardiomyogenic potential of C-kit(þ)-expressing cells derived from neonatal and adult mouse hearts. Circulation 2010;121:1992-2000. 10.1161/CIRCULATIONAHA.109.909093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Wu S, Liu Z, et al. Ultrasound-Targeted Microbubble Destruction Improves the Migration and Homing of Mesenchymal Stem Cells after Myocardial Infarction by Upregulating SDF-1/CXCR4: A Pilot Study. Stem Cells Int 2015;2015:691310. 10.1155/2015/691310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ling ZY, Shu SY, Zhong SG, et al. Ultrasound targeted microbubble destruction promotes angiogenesis and heart function by inducing myocardial microenvironment change. Ultrasound Med Biol 2013;39:2001-10. 10.1016/j.ultrasmedbio.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 65.Zhong S, Shu S, Wang Z, et al. Enhanced homing of mesenchymal stem cells to the ischemic myocardium by ultrasound-targeted microbubble destruction. Ultrasonics 2012;52:281-6. 10.1016/j.ultras.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 66.Xu YL, Gao YH, Liu Z, et al. Myocardium-targeted transplantation of mesenchymal stem cells by diagnostic ultrasound-mediated microbubble destruction improves cardiac function in myocardial infarction of New Zealand rabbits. Int J Cardiol 2010;138:182-95. 10.1016/j.ijcard.2009.03.071 [DOI] [PubMed] [Google Scholar]
- 67.Li JY, Tong JY, Feng Y, et al. Ultrasound-mediated microbubble promotes bone marrow mesenchymal stem cell transplantation for myocardial infarction. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13:4421-5. [Google Scholar]
- 68.Erikson JM, Valente AJ, Mummidi S, et al. Targeting TRAF3IP2 by genetic and interventional approaches inhibits ischemia/reperfusion-induced myocardial injury and adverse remodeling. J Biol Chem 2017;292:2345-58. 10.1074/jbc.M116.764522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du GQ, Shao ZB, Wu J, et al. Targeted myocardial delivery of GDF11 gene rejuvenates the aged mouse heart and enhances myocardial regeneration after ischemia-reperfusion injury. Basic Res Cardiol 2017;112:7. 10.1007/s00395-016-0593-y [DOI] [PubMed] [Google Scholar]
- 70.Kwekkeboom RF, Sluijter JP, van Middelaar BJ, et al. Increased local delivery of antagomir therapeutics to the rodent myocardium using ultrasound and microbubbles. J Control Release 2016;222:18-31. 10.1016/j.jconrel.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 71.Dörner J, Struck R, Zimmer S, et al. Ultrasound-mediated stimulation of microbubbles after acute myocardial infarction and reperfusion ameliorates left-ventricular remodelling in mice via improvement of borderzone vascularization. PLoS One 2013;8:e56841. 10.1371/journal.pone.0056841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y, Zhang C, Shen S, et al. Ultrasound-targeted microbubble destruction enhances delayed BMC delivery and attenuates post-infarction cardiac remodelling by inducing engraftment signals. Clin Sci (Lond) 2016;130:2105-20. 10.1042/CS20160085 [DOI] [PubMed] [Google Scholar]
- 73.Li D, Jiang X, Xu T, et al. The effect of Akt1 gene on rats cardiomyocytes by ultrasound/microbubbles destruction. World Congress of Cardiology Scientific Sessions 2012;125:E920-E920. [Google Scholar]
- 74.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 2012;59:635-43. 10.1016/j.jacc.2011.08.080 [DOI] [PubMed] [Google Scholar]
- 75.Fowlkes V, Clark J, Fix C, et al. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci 2013;92:669-76. 10.1016/j.lfs.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mishra PK, Chavali V, Metreveli N, et al. Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Can J Physiol Pharmacol 2012;90:353-60. 10.1139/y11-131 [DOI] [PubMed] [Google Scholar]
- 77.Mishra PK, Givvimani S, Metreveli N, et al. Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy. Biochem Biophys Res Commun 2010;401:175-81. 10.1016/j.bbrc.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zou MH, Xie Z. Regulation of interplay between autophagy and apoptosis in the diabetic heart: new role of AMPK. Autophagy 2013;9:624-5. 10.4161/auto.23577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papa G, Degano C, Iurato MP, et al. Macrovascular complication phenotypes in type 2 diabetic patients. Cardiovasc Diabetol 2013;12:20. 10.1186/1475-2840-12-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Zhang Y, Li Z, et al. Left ventricular radial systolic dysfunction in diabetic patients assessed by myocardial acceleration derived from velocity vector imaging. J Ultrasound Med 2012;31:1179-86. 10.7863/jum.2012.31.8.1179 [DOI] [PubMed] [Google Scholar]
- 81.Zhao YZ, Zhang M, Wong HL, et al. Prevent diabetic cardiomyopathy in diabetic rats by combined therapy of aFGF-loaded nanoparticles and ultrasound-targeted microbubble destruction technique. J Control Release 2016; 223:11-21. 10.1016/j.jconrel.2015.12.030 [DOI] [PubMed] [Google Scholar]
- 82.Zhang M, Yu WZ, Shen XT, et al. Advanced Interfere Treatment of Diabetic Cardiomyopathy Rats by aFGF-Loaded Heparin-Modified Microbubbles and UTMD Technique. Cardiovasc Drugs Ther 2016;30:247-61. 10.1007/s10557-016-6639-4 [DOI] [PubMed] [Google Scholar]
- 83.Zhao YZ, Tian XQ, Zhang M, et al. Functional and pathological improvements of the hearts in diabetes model by the combined therapy of bFGF-loaded nanoparticles with ultrasound-targeted microbubble destruction. J Control Release 2014;186:22-31 10.1016/j.jconrel.2014.04.054 [DOI] [PubMed] [Google Scholar]
- 84.Zhao YZ, Zhang M, Tian XQ, et al. Using basic fibroblast growth factor nanoliposome combined with ultrasound-introduced technology to early intervene the diabetic cardiomyopathy. Int J Nanomedicine 2016;11:675-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian X, Ru A, Zhao Y, et al. Targeted delivery of fibroblast growth factor-1 by ultrasound-targeted microbubble destruction reduces myocyte apoptosis and myocardial fibrosis and improves ventricular systolic and diastolic function in diabetic cardiomyopathy in rats. Circulation 2013;128:A14341. [Google Scholar]
- 86.Lee PJ, Rudenko D, Kuliszewski MA, et al. Survivin gene therapy attenuates left ventricular systolic dysfunction in doxorubicin cardiomyopathy by reducing apoptosis and fibrosis. Cardiovasc Res 2014;101:423-33. 10.1093/cvr/cvu001 [DOI] [PubMed] [Google Scholar]
- 87.Fortuin FD, Vale P, Losordo DW, et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol 2003;92:436-9. 10.1016/S0002-9149(03)00661-1 [DOI] [PubMed] [Google Scholar]
- 88.Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation 2003;107:1359-65. 10.1161/01.CIR.0000061911.47710.8A [DOI] [PubMed] [Google Scholar]
- 89.Ren J, Zhang P, Tian J, et al. A targeted ultrasound contrast agent carrying gene and cell-penetrating peptide: preparation and gene transfection in vitro. Colloids Surf B Biointerfaces 2014;121:362-70. 10.1016/j.colsurfb.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 90.Shen ZP, Brayman AA, Chen L, et al. Ultrasound with microbubbles enhances gene expression of plasmid DNA in the liver via intraportal delivery. Gene Ther 2008;15:1147-55. 10.1038/gt.2008.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang HB, Yang L, Wu J, et al. Reduced Ischemic Injury After Stroke in Mice by Angiogenic Gene Delivery Via Ultrasound-Targeted Microbubble Destruction. J Neuropathol Exp Neurol 2014;73:548-58. 10.1097/NEN.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 92.Miao CH, Noble ML, Song S, et al. Effective ultrasound-targeted microbubble destruction (UTMD)-mediated gene transfer into the livers of small and large animals. Proc Mtgs Acoust 2013;19:075056 10.1121/1.4801418 [DOI] [Google Scholar]



