Abstract
Occurrence of multiple primary lung cancers (MPLC) in individuals undergoing low-dose computed tomography (LDCT) screening has not been thoroughly addressed. We investigated MPLC in subjects recruited in the ITALUNG randomized clinical trial. Cases of cytologically/histologically proven MPLC detected at screening LDCT or follow-up CT were selected and pathologically re-evaluated according to the WHO 2015 classification. Overall 16 MPLC were diagnosed at screening LDCT (n=14, all present at baseline) or follow-up CT (n=2) in six subjects (4 in one subject, 3 in two and 2 in three subjects), representing 0.43% of the 1,406 screenees and 15.8% of the 38 subjects with at least one screen-detected primary lung cancer. MPLC included 9 adenocarcinomas in three subjects and a combination of 7 different tumour histotypes in three subjects. MPLC, mostly adenocarcinomas, are not uncommon in smokers and ex-smokers with at least one LDCT screen detected primary lung cancer.
Keywords: Multiple primary lung cancer, lung cancer, low-dose CT screening, adenocarcinoma
Introduction
Smoking makes pulmonary tissue diffusely prone to cancer development (“field cancerization” theory) (1,2). Accordingly, smokers and former smokers can develop multiple lung cancers in their lives. Available data on multiple primary lung cancers (MPLC) derive mainly from surgical series (3). However, low-dose computed tomography (LDCT) screening can provide an alternative source of information about MPLC. To the best of our knowledge, so far this has not been thoroughly addressed. We reviewed the occurrence and type of MPLC in subjects undergoing LDCT screening in the ITALUNG randomized clinical trial (4).
Methods
ITALUNG is a randomized clinical trial carried out in Italy evaluating efficacy of LDCT screening in reducing lung cancer mortality as compared to “usual care” (5). The study was conducted in compliance with the Helsinki Declaration (http://www.wma.net/en/30publications/10policies/b3/index.html) and the study protocol was approved by the Local Ethic Committees of the participating centers (Firenze, approval number 29–30, 30 September 2003; Pisa, number 23, 27 October 2003; Pistoia, number 00028543, 13 May 2004). Each subject provided an informed written consent to participate to the study.
The ITALUNG study design and protocol were previously reported (6,7). Briefly, 3,206 smokers or former smokers identified by general practitioners and invited by mail were randomized to receive four annual LDCT (n=1,613) or usual care (n=1,593). Management protocol for positive LDCT examinations included follow-up LDCT, 2-[18F]fluoro-2-deoxy-D glucose positron emission tomography, and CT-guided fine-needle aspiration biopsy (FNAB).
Follow-up data at 8.5 years in ITALUNG indicated that LDCT screening could reduce lung cancer and overall mortality (4). In the actively screened arm, 1,406 smokers or former smokers (910 men with mean age of 61.1 years and 496 women with mean age of 60.6 years) underwent annual screening LDCT at baseline and in the next 3 years.
In ITALUNG the subjects with screen-detected primary lung cancer entered follow-up with contrast-enhanced full-dose head, chest and abdomen CT that was performed every 6 months for the first 2 years and annually for 3 years thereafter. All subjects with screen-detected primary lung cancer had completed the 5 years of follow-up CT at the time of writing.
For identification of MPLC in ITALUNG we applied a three step procedure (see below) and the criteria proposed by Shen et al. (8) that define 3 types of lesions: (I) those that share the same histology but are distributed in different pulmonary lobes, in absence of N2, N3 or systemic metastases; (II) those that show different histological or molecular-genetic characteristics and arise separately from foci of carcinoma in situ; (III) those that share the same histology but are separated by at least 4 years interval and without systemic metastases between the detection of multiple tumors.
The three step procedure included the following:
Step 1: records of all screened subjects with diagnosis of lung cancer based on the results of FNAB or surgical pathology during LDCT screening or full-dose CT follow-up were reviewed searching for cases of multiple primary or secondary lung cancer.
Step 2: one experienced lung pathologist (C.E.C) reviewed and classified all the surgical or fine needle aspiration specimens of the selected cases according to the 2015 WHO criteria taking into account morphology and molecular/genetic features (9,10). The ITALUNG pathology protocol referred to the EU-US shared pathology protocol for CT-screening trials (EU-US Spiral CT Collaborative Group) and is detailed in supplementary material. Pathologic or clinical staging of MPLC was performed according to the 7th Edition of the American Joint Committee on Cancer Staging Manual (11).
Step 3: two senior chest radiologists (M.M. and F.F.) reviewed all the LDCT and follow-up full-dose CT examinations of the subjects with pathologically confirmed MPLC. They established the first LDCT or follow-up CT showing focal abnormalities that were ultimately diagnosed as lung cancer and described them (12).
Results
Eight subjects were diagnosed with multiple lung cancers during LDCT screening or full-dose CT follow-up. Two had multiple secondary lesions (from renal cancer and colorectal cancer) detected at LDCT and diagnosed at FNAB. Six subjects were ultimately diagnosed with MPLC (overall =16:2 in three subjects, 3 in two subjects and 4 in one subject) (Table 1 and Figures 1,2). They represented 15.8% (6/38) of all subjects with at least one screen-detected primary lung cancer.
Table 1. Subjects with multiple primary lung cancers in ITALUNG trial.
| Case/lesion/sex/age/pack-year | Year of CT appearance screening round | Initial CT features: site/size*/shape/margins/density | Time to diagnosis | Histology or cytology | Molecular findings# | Stage | Therapy/outcome |
|---|---|---|---|---|---|---|---|
| I/1/male/70/<30 | 2006 baseline | RUL/32 mm/roundish/spiculated/solid | Immediate | Adenocarcinoma (80% acinar, 15% papillary, 5% solid) | KRAS G12C | pT2N0M0 | Surgery |
| I/2 | 2006 baseline | RUL/18 mm/round/ill-defined, non-solid | Immediate | Adenocarcinoma (90% lepidic, 10% acinar) | EGFR exon19 deletion | pT2N0M0 | Surgery |
| I/3 | 2006 baseline | RLL/6 mm/oval/regular/solid | Immediate | Adenocarcinoma (60% acinar, 30% papillary, 10% micropapillary) | EGFR exon19 deletion | pT2N0M0 | Surgery |
| I/4 | 2006 baseline | LUL, 25 mm/roundish/ill-defined/part-solid | Immediate | Adenocarcinoma (FNAB) | / | T1bN0M0 | Radiation therapy; 2016 alive |
| II/1/male/61/>30 | 2004 baseline | RUL/22 mm/round/regular/solid | Immediate | Adenocarcinoma (70% acinar, 30% solid) | / | pT1bN0M0 | Surgery |
| II/2 | 2004 baseline | LUL/6 mm/oval/regular/solid | 3 months | Small cell carcinoma | / | pT1aN2M0 | Surgery, chemotherapy; dead 9 months later |
| III/1/male/70/<30 | 2005 baseline | LUL/10 mm/complex/irregular/solid | 24 months | Adenocarcinoma (90% papillary, 10% acinar) | / | pT1bN0M0 | Surgery |
| III/2 | 2005 baseline | RLL/18 mm/round lobulated/part-solid (associated with cystic airspace) | 27 months | Adenocarcinoma (60% acinar, 40% papillary) | EGFR exon19deletion | pT1aN0M0 | Surgery; dead 4 days later |
| IV/1/male/66/>30 | 2006 baseline | LLL/10 mm/round/ill-defined/non-solid | 12 months | Adenocarcinoma (50% lepidic, 30% solid, 20% acinar) | / | pT1bNoM0 | Surgery |
| IV/2 | 2006 baseline | Lingula/9 mm/round/regular/solid | 12 months | Carcinoid | / | pT1aN0M0 | Surgery |
| IV/3 | 2012 follow-up | RUL/4 mm/round/regular/solid | 24 months | Adenocarcinoma (FNAB) | KRAS G13D | cT1aN2M1b | Chemotherapy; dead 6 months later |
| V/1/female/79/>30 | 2005 baseline | RUL/14 mm/irregular/spiculated/solid | 3 months | Pleomorphic carcinoma | / | pT1aN0M0 | Surgery |
| V/2 | 2010 follow-up | LLL/25 mm/regular/excavated | 2 months | Squamous cell carcinoma | / | cT1bN0M1b | Chemotherapy; dead 3 months later |
| VI/1/male/70/>30 | 2005 baseline | RUL/9 mm/oval/regular/solid | 24 months | Adenocarcinoma (70% solid, 20% lepidic, 10% acinar) | KRAS G12C | pT1aN0M0 | Surgery |
| VI/2 | 2005 baseline | RLL/12 mm/round/ill-defined/non-solid | 24 months | Adenocarcinoma (70% lepidic, 15% papillary, 15% acinar) | / | pT1aN0M0 | Surgery |
| VI/3 | 2005 baseline | LLL/10 mm/oval/ill-defined/part-solid | 96 months | Adenocarcinoma (70% lepidic, 30% acinar) | / | pT1aN0M0 | Surgery; 2016 alive |
*, mean diameter; #, only positive molecular markers are reported.
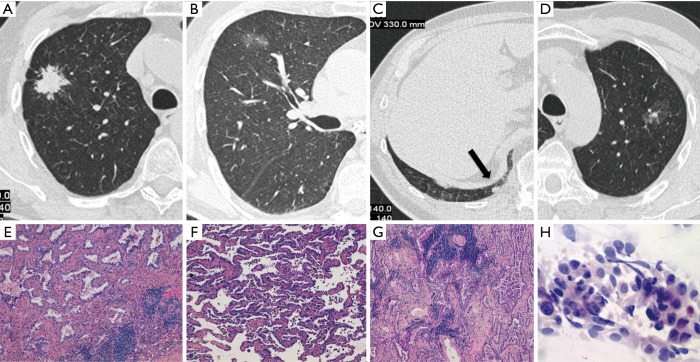
Figure 1.
Case I (Table 1). Four primary adenocarcinomas in a 70-year-old smoker, which were all detected at baseline LDCT screening round. They appeared as a spiculated lung nodule in the right upper lobe (RUL) (A), a ground glass opacity in the same lobe (B), a small solid nodule in the right lower lobe (RLL) (arrow in C) and a ground glass opacity with a small solid component in the left upper lobe (LUL) (D). Haematoxylin and eosin histologic staining (original magnification ×200) demonstrate an invasive adenocarcinoma, acinar predominant (E) in the RUL lesion corresponding to (A), an invasive adenocarcinoma, lepidic predominant (F) in the RUL lesion corresponding to (B) and an invasive adenocarcinoma, acinar predominant (G) in the RLL lesion corresponding to (C). Papanicolaou stain (original magnification ×40) of fine needle aspiration biopsy shows papillary pattern of uniform malignant cells with irregular nuclei consistent with adenocarcinoma (H) in the LUL lesion corresponding to (D).
Figure 2.
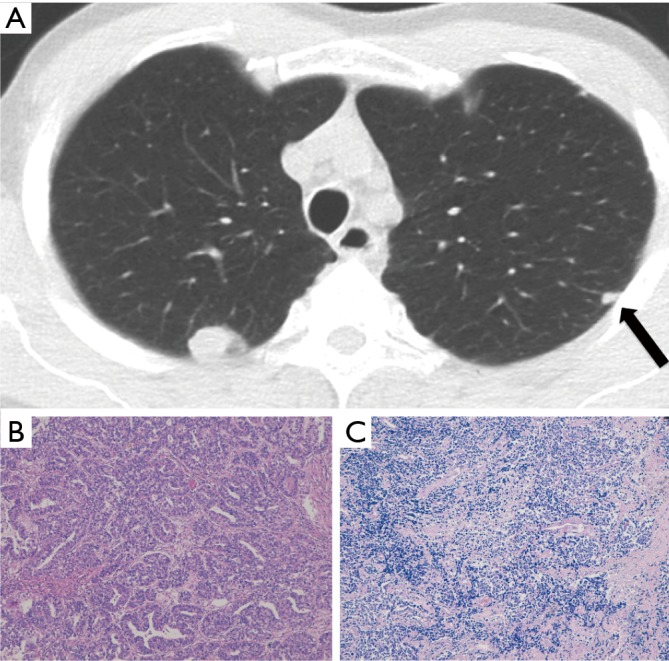
Case II (Table 1). Adenocarcinoma and small cell lung cancer in a 61-year-old smoker both detected at baseline LDCT screening round. They appeared as a rounded lung nodule in the RUL and a subpleural small solid nodule (arrow) in the LUL (A). Haematoxylin and eosin histologic staining demonstrate invasive adenocarcinoma, acinar predominant (original magnification ×200) (B) in the RUL lesion and small cell lung carcinoma (original magnification ×100) (C) in the LUL lesion.
The 16 MPLC included 9 morphologically and molecularly heterogeneous adenocarcinomas in three subjects (Figure 1) and combination of different tumor histotypes (2 adenocarcinomas plus 1 carcinoid, 1 adenocarcinoma plus 1 small cell carcinoma, 1 pleomorphic carcinoma plus 1 squamous cell carcinoma) in three subjects (Figure 2). Adenocarcinomas accounted for 75% (12/16) of MPLC.
Fourteen of 16 MPLC were observed during LDCT screening in four subjects and 2 during full-dose CT follow-up in two subjects. All the former were already present at baseline LDCT examination (Figures 1,2) and may be considered as synchronous lesions. The latter appeared during follow-up CT 5 and 6 years after surgical removal of the first lesions and may be considered metachronous lesions. Overall, MPLC appeared in the LDCT or follow-up CT in which they were retrospectively identified for the first time as solid (n=9), partially solid (n=3), non-solid (n=3) or excavated (n=1) lesions with regular (n=7), ill-defined (n=5), spiculated (n=2), irregular or lobulated (n=2) margins. Their initial size ranged between 4 and 32 mm (mean diameter 14.4±8.1 mm).
Of the three subjects with 2 lesions, one died of surgical complication and two of advanced disease. Of the three subjects with more than 2 lesions, two are alive (10 and 11 years after treatment of multifocal adenocarcinomas) and one died of advanced disease.
Discussion
Data about occurrence of MPLC in observational or randomized LDCT screening studies are fragmentary and summarized in Table 2. At least one subject with MPLC was reported in 11/33 studies (5,13-18,24-35), with a mean cumulative frequency of 0.18% (136 subjects) in 71,901 screenees and of 11.6% in 1,170 subjects with at least one screen-detected lung cancer (19-23,36-44). The MPLC were considered synchronous in 123/136 (90%) subjects and metachronous in 13 (10%). Unfortunately, in many cases the time of lesions appearance and histological diagnoses were not available. Moreover uncertainties and intervening modifications of the staging system of lung cancer and unavailability of morphologic and genetic/molecular features hinder recollection of information about MPLC in previous reports of LDCT screening (and surgical series). In particular, multiple pulmonary nodules may have been considered stage III or IV tumors (18), and this may have led to under-reporting of cases of MPLC, especially in case of multifocal adenocarcinoma.
Table 2. Multiple primary lung cancers (MPLC) in low-dose CT (LDCT) screening studies.
| Study | No. of screenees | No. of LDCT rounds | Subjects with screen-detected primary lung cancer (%) | No. of adenocarcinomas | Subjects with MPLC | Subjects with MPLC/subjects with screen-detected primary lung cancer (%) |
|---|---|---|---|---|---|---|
| Nawa et al. 2002 (13) | 7,956 | 1 | 40 (0.5) | 39 | 1 (synchronous) | 2.5 |
| Diederich et al. 2002 (14) | 817 | 4 | 11 (1.3) | 5 | 1 (synchronous) | 9.0 |
| Flieder et al. 2006 (15) | 2,968 | 11 | 77 (2.6) | 81 | 16 (synchronous) | 20.8 |
| Carter et al. 2007 (16) | 10,056 | 11 | 250 (2.5) | 177 | 31 (synchronous), 5 (metachronous) | 14.4 |
| Lindell et al. 2007 (17) | 1,520 | 5 | 59 (3.9) | 34 | 1 (synchronous), 1 (metachronous) | 3.4 |
| Pelosi et al. 2008 (18) | 5,202 | 3 | 89 (1.7) | 72 | 10 (synchronous) | 11.2 |
| Vazquez et al. 2009 (19) | 27,456 | 12 | 338 (1.2) | 279 | 49 (synchronous) | 14.5 |
| van Klaveren et al. 2009 (20) | 7,557 | 3 | 124 (1.6) | NA | 5 (synchronous) | 4.0 |
| Infante et al. 2009 (21) | 1,276 | 4 | 60 (4.7) | 27 | 2 (synchronous), 1 (metachronous) | 5.0 |
| Saghir et al. 2012 (22) | 4,104 | 5 | 69 (1.7) | 48 | 6 (synchronous) | 8.7 |
| Sanchez-Salcedo et al. 2015 (23) | 2,989 | 14 | 53 (1.8%) | 33 | 1 (synchronous), 6 (metachronous) | 13.2 |
In the active arm of ITALUNG, after the 4 years of active screening and 5 years of follow-up we observed few subjects (n=6; 0.43% of the screenees) harboring or developing MPLC. However they represented 15.8% of all those subjects with at least one screen-detected primary lung cancer. These percentages are in line with those reported in LDCT screening studies (Table 2).
In our series, adenocarcinoma was the most frequent histotype. This is in line with the type of primary lung cancers that are discovered by LDCT (Table 1) and in a previous study (18). Despite the small numbers of MPLC cases in our study that indicates need of further studies, two scenarios may be drawn. The first deals with morphological and molecular/genetically heterogeneous multifocal adenocarcinomas. The second deals with combination of adenocarcinoma with others histological types including small cell lung cancer, carcinoid and squamous cell cancer. Only one case of double squamous lung cancer was reported (23). As in previous reports (19,22), most (14/16 lesions in five subjects) of the screen-detected MPLC in ITALUNG were present at baseline and were diagnosed after further LDCT screening rounds, because of increased size or density (12,17,27).
Remarkably, our two patients with synchronous multifocal adenocarcinoma with 4 and 3 lesions, respectively, were alive many years after lesion treatment. This is consistent with the view that multifocal adenocarcinoma can behave as indolent lesion that should not be confused with aggressive primary lung cancers with intrapulmonary metastases (18). Awareness of this possibility may have significant impact on management of multiple lesions detected at LDCT screening that is currently recommended in the US (39) and is under evaluation in Europe (4).
In ITALUNG after a median follow-up time of 8.5 years, we observed 2 metachronous cancers during full-dose CT follow-up, which were both fatal. Obviously, longer surveillance is expected to increase the yield of metachronous MPLC. In fact one LDCT study reported 6 cases of metachronous MPLC in 2,989 screenees followed for 14 years (23).
Prevalence of MPLC in 18 surgical series outside screening (mean range, 1.1–8.6%) (3) was lower than the frequency in LDCT screening studies reporting at least one such a case. This is not surprising since subjects undergoing LDCT are asymptomatic and prone to show developing cancers in their earlier stages.
Occurrence of MPLC in a single subject per se suggests the possibility of genetic predisposition. However search of genetic features associated with lung cancer (45) was beyond the scope of the present report.
Admittedly, since our study is based on cytopathologically or histopathologically proven primary lung cancer, it is possible that we underestimated MPLC in the cohort of subjects undergoing LDCT screening. In particular, lesions presenting as pure ground glass opacity that can be associated with minimally invasive adenocarcinoma (9) may be missed.
In conclusion, MPLC, mostly adenocarcinomas, are not uncommon in smokers and former smokers with at least one LDCT screen detected primary lung cancer. The potential distinction of two subtypes of MPLC, represented by multifocal adenocarcinomas and by combination of adenocarcinoma with others histological types, having different prognoses and treatment implications warrants further study.
ITALUNG pathology protocol
Tumors were sampled as fresh tissue immediately after surgery. The entire tumors, fragments of distant non-neoplastic parenchyma, all detectable lymph nodes, and mediastinal lymph nodes were sampled. Specimens were fixed in buffered formalin for 16–24 hours and processed for standard histological examination. Multiple adenocarcinoma cases underwent molecular analysis for the identification of ALK gene translocation and of the known driver mutations in the following genes: EGFR, KRAS, NRAS, BRAF, PIKCA, ERBB2, DDR2, MAP2K1 and RET. A representative paraffin-embedded block containing about 80% viable tumor was selected for each case. Immunohistochemical staining procedures were conducted on 4 µm-thick sections of paraffin-embedded tissue using a Ventana Benchmark Ultra automated immunostainer (Ventana Medical System, Tucson, AZ, USA) with the anti-ALK monoclonal antibody (clone D5F3, ready to use, Ventana Medical System). The OptiView DAB Detection kit was used as revelation system, adding the OptiView Amplification kit (Ventana Medical System) for ALK detection. Three consecutive 10 µm-thick sections were performed from the same blocks for molecular analysis. These were conducted by a mass spectrometry-based multiplex assay (MassArray technology, Sequenom, San Diego, CA) using the “Myriapod Lung Status” kit (Diatech Pharmacogenetics, AN, IT) capable of identifying the major mutations of the following genes: EGFR (exons 18, 19, 20, 21), KRAS (codons 12, 13, 61), NRAS (codons 12, 61), BRAF (codons 466, 469, 594, 597, 600), PIKCA (codons 542, 545, 1043, 1047), ERBB2 (exon 20), DDR2 (codons 239, 638, 768), MAP2K1 (codons 56, 57, 67), RET (codon 918).
Acknowledgements
Funding: The study was supported by the local government of Tuscany (Decree N. 1014 of 02.25.2004) and by a Research Grant of the Italian Ministry of Education University and Research (PRIN 2003) to Professor Mario Mascalchi. The funding source had no role in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Ethical Statement: The study was conducted in compliance with the Helsinki Declaration (http://www.wma.net/en/30publications/10policies/b3/index.html) and the study protocol was approved by the Local Ethic Committees of the participating centers (Firenze, approval number 29–30, 30 September 2003; Pisa, number 23, 27 October 2003; Pistoia, number 00028543, 13 May 2004). Each subject provided an informed written consent to participate to the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Slaughter DP, Southwich HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium: clinical implications of multi-centric origin. Cancer 1953;6:963-8. [DOI] [PubMed] [Google Scholar]
- 2.Kadara H, Wistuba II. Field cancerization in non-small cell lung cancer: implications in disease pathogenesis. Proc Am Thorac Soc 2012;9:38-42. 10.1513/pats.201201-004MS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voltolini L, Rapicetta C, Luzzi L., et al. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node negative subgroup. Eur J Cardiothorac Surg 2010;37:1198-204. 10.1016/j.ejcts.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 4.Paci E, Puliti D, Lopes Pegna A, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017;72:825-31. 10.1136/thoraxjnl-2016-209825 [DOI] [PubMed] [Google Scholar]
- 5.Picozzi G, Paci E, Lopes-Pegna A, et al. Screening of lung cancer with low-dose spiral CT: results of a three-year pilot study and design of the randomized controlled trial “Italung-CT”. Radiol Med 2005;109:17-26. [PubMed] [Google Scholar]
- 6.Lopes Pegna A, Picozzi G, Mascalchi M, et al. ITALUNG Study Research Group Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34-40. 10.1016/j.lungcan.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Lopes Pegna A, Picozzi G, Falaschi F, et al. ITALUNG Study Research Group Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol 2013;8:866-75. 10.1097/JTO.0b013e31828f68d6 [DOI] [PubMed] [Google Scholar]
- 8.Shen KR, Meyers BF, Larner JM, et al. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:290-305. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Brambilla E, Burke AP, et al. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC Press, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors. Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 12.Mascalchi M, Picozzi G, Falchini M, et al. Initial LDCT appearance of screen-detected lung cancers in the ITALUNG trial. Eur J Radiol 2014;83:2080-6. 10.1016/j.ejrad.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 13.Nawa T, Nakagawa T, Kusano S, et al. Lung cancer screening using low-dose spiral CT. Results of baseline and 1-year follow-up studies. Chest 2002;122:15-20. 10.1378/chest.122.1.15 [DOI] [PubMed] [Google Scholar]
- 14.Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222:773-81. 10.1148/radiol.2223010490 [DOI] [PubMed] [Google Scholar]
- 15.Flieder DB, Vazquez M, Carter D, et al. Pathologic findings of lung tumors diagnosed on baseline CT screening. Am J Surg Pathol 2006;30:606-13. 10.1097/01.pas.0000202040.51967.d0 [DOI] [PubMed] [Google Scholar]
- 16.Carter D, Vazquez M, Flieder D, et al. Comparison of pathologic findings of baseline and annual repeat cancers diagnosed on CT screening. Lung Cancer 2007;56:193-9. 10.1016/j.lungcan.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 17.Lindell RM, Hartman TE, Swensen SJ, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology 2007;242:555-62. 10.1148/radiol.2422052090 [DOI] [PubMed] [Google Scholar]
- 18.Pelosi G, Sonzogni A, Veronesi G, et al. Pathologic and molecular features of screening low-dose computed tomography (LDCT)-detected lung cancer: A baseline and 2-year repeat study. Lung Cancer 2008;62:202-14. 10.1016/j.lungcan.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Vazquez M, Carter D, Brambilla E, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009;64:148-54. 10.1016/j.lungcan.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Klaveren RJ, Ouderk M, Prokop M, et al. Management of lung nodules detected by volume CT screening. N Engl J Med 2009;361:2221-9. 10.1056/NEJMoa0906085 [DOI] [PubMed] [Google Scholar]
- 21.Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography.Three-year results from the DANTE trial. Am J Respir Crit Care Med 2009;180:445-53. 10.1164/rccm.200901-0076OC [DOI] [PubMed] [Google Scholar]
- 22.Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301. 10.1136/thoraxjnl-2011-200736 [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Salcedo P, Berto J, de-Torres JP, et al. Lung cancer screening: fourteen year experience of the Pamplona early detection program (P-IELCAP). Arch Bronconeumol 2015;51:169-76. [DOI] [PubMed] [Google Scholar]
- 24.Sone S, Li F, Yang ZG, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer 2001;84:25-32. 10.1054/bjoc.2000.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20:911-20. 10.1200/JCO.2002.20.4.911 [DOI] [PubMed] [Google Scholar]
- 26.Garg K, Keith RL, Byers T, et al. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology 2002;225:506-10. 10.1148/radiol.2252011851 [DOI] [PubMed] [Google Scholar]
- 27.Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362:593-7. 10.1016/S0140-6736(03)14188-8 [DOI] [PubMed] [Google Scholar]
- 28.Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomised feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47:9-15. 10.1016/j.lungcan.2004.06.007 [DOI] [PubMed] [Google Scholar]
- 29.Chong S, Lee KS, Jin M, et al. Lung cancer screening with low-dose helical CT in Korea: experiences at the Samsung Medical Center. J Korean Med Sci 2005;20:402-8. 10.3346/jkms.2005.20.3.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephenson SM, Mech KF, Sardi A. Lung cancer screening with low-dose spiral computed tomography. Am Surg 2005;71:1015-7. [PubMed] [Google Scholar]
- 31.Bastarrika G, García-Velloso MJ, Lozano MD, et al. Early lung cancer detection using spiral computed tomography and positron emission tomography. Am J Respir Crit Care Med 2005;171:1378-83. 10.1164/rccm.200411-1479OC [DOI] [PubMed] [Google Scholar]
- 32.Novello S, Fava C, Borasio P, et al. Three-year findings of an early lung cancer detection feasibility study with low-dose spiral computed tomography in heavy smokers. Ann Oncol 2005;16:1662-6. 10.1093/annonc/mdi314 [DOI] [PubMed] [Google Scholar]
- 33.MacRedmond R, McVey G, Lee M, et al. Screening for lung cancer using low dose CT scanning: results of 2 year follow up. Thorax 2006;61:54-6. 10.1136/thx.2004.037580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanchon T, Bréchot JM, Grenier PA, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58:50-8. 10.1016/j.lungcan.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 35.Wilson DO, Weissfeld J, Fuhrman C, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178:956-61. 10.1164/rccm.200802-336OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menezes RJ, Roberts HC, Paul NS, et al. Lung cancer screening using low-dose computed tomography in at-risk individuals: The Toronto experience. Lung Cancer 2010;67:177-83. 10.1016/j.lungcan.2009.03.030 [DOI] [PubMed] [Google Scholar]
- 37.Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. 10.1097/CEJ.0b013e328351e1b6 [DOI] [PubMed] [Google Scholar]
- 38.Veronesi G, Maisonneuve P, Rampinelli C, et al. Computed tomography screening for lung cancer: results of ten years of annual screening and validation of COSMOS prediction model. Lung Cancer 2013;82:426-30. 10.1016/j.lungcan.2013.08.026 [DOI] [PubMed] [Google Scholar]
- 39.National Lung Screening Trial Research Team , Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. 10.1056/NEJMoa1209120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow-up after randomization. J Thorac Oncol 2015;10:890-6. 10.1097/JTO.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 41.Rzyman W, Dziedzic R, Jelitto-Górska M, et al. Results of an open-access lung cancer screening program with low-dose computed tomography: the Gdańsk experience. Pol Arch Med Wewn 2015;125:232-9. [DOI] [PubMed] [Google Scholar]
- 42.Yi CA, Lee KS, Shin MH, et al. Low-dose CT screening in an Asian population with diverse risk for lung cancer: A retrospective cohort study. Eur Radiol 2015;25:2335-45. 10.1007/s00330-015-3620-8 [DOI] [PubMed] [Google Scholar]
- 43.Field JK, Duffy SW, Baldwin DR, et al. UK lung cancer RCT pilot screening trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. 10.1136/thoraxjnl-2015-207140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sverzellati N, Silva M, Calareso G, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol 2016;26:3821-9. 10.1007/s00330-016-4228-3 [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Kheradmand F, Davis CF, et al. Focused analysis of exome sequencing data for rare germline mutations in familial and sporadic lung cancer. J Thorac Oncol 2016;11:52-61. 10.1016/j.jtho.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tumors were sampled as fresh tissue immediately after surgery. The entire tumors, fragments of distant non-neoplastic parenchyma, all detectable lymph nodes, and mediastinal lymph nodes were sampled. Specimens were fixed in buffered formalin for 16–24 hours and processed for standard histological examination. Multiple adenocarcinoma cases underwent molecular analysis for the identification of ALK gene translocation and of the known driver mutations in the following genes: EGFR, KRAS, NRAS, BRAF, PIKCA, ERBB2, DDR2, MAP2K1 and RET. A representative paraffin-embedded block containing about 80% viable tumor was selected for each case. Immunohistochemical staining procedures were conducted on 4 µm-thick sections of paraffin-embedded tissue using a Ventana Benchmark Ultra automated immunostainer (Ventana Medical System, Tucson, AZ, USA) with the anti-ALK monoclonal antibody (clone D5F3, ready to use, Ventana Medical System). The OptiView DAB Detection kit was used as revelation system, adding the OptiView Amplification kit (Ventana Medical System) for ALK detection. Three consecutive 10 µm-thick sections were performed from the same blocks for molecular analysis. These were conducted by a mass spectrometry-based multiplex assay (MassArray technology, Sequenom, San Diego, CA) using the “Myriapod Lung Status” kit (Diatech Pharmacogenetics, AN, IT) capable of identifying the major mutations of the following genes: EGFR (exons 18, 19, 20, 21), KRAS (codons 12, 13, 61), NRAS (codons 12, 61), BRAF (codons 466, 469, 594, 597, 600), PIKCA (codons 542, 545, 1043, 1047), ERBB2 (exon 20), DDR2 (codons 239, 638, 768), MAP2K1 (codons 56, 57, 67), RET (codon 918).