Abstract
We put forth our opinion regarding the enhanced plasticity and modulation of mechanical properties of polymeric films obtained through electrospinning process in this article. In majority of the pharmaceutical, biomedical, and packaging applications, it is desirable that polymer based matrices should be soft, flexible, and have a moderate toughness. In order to convert inflexible and brittle polymers, adjuvants in the form of plasticizers are added to improve the flexibility and smoothness of solvent casted polymer films. However, many of these plasticizers are under scrutiny for their toxic effects and environmental hazards. In addition, plasticizers also increase the cost of end products. This has motivated the scientific community to investigate alternate approaches. The changes imparted in membrane casted by electrospinning were tried to be proved by SEM, Mechanical property study, DSC and XRD studies. We have showed dramatic improvement in flexibility of poly(ε-caprolactone) based nanofiber matrix prepared by electrospinning method whereas solvent casting method without any plasticizer produced very brittle, inflexible film of PCL. Modulation capacity of mechanical properties is also recorded. We tried to support our opinion by citing several similar findings available in the open literature. The electrospinning method helps in plasticization and in tuning mechanical properties.
Introduction
Polymers, be it synthetic, semi-synthetic, or natural, have gained wide attention in different technology sectors. They are used in various forms, such as solid films, thin matrices, nanofiber mats or patches, hydrogel films etc.1,2. All these polymer forms are obtained from their original bulk form after appropriate modification of mechanical properties3,4. Further modification is often needed depending on the target application. One such important modification, especially for pharmaceutical, biomedical, and packaging industries, is to increase the flexibility of the polymer films through incorporation of plasticizer. As the plasticity of a polymer film turns out to be one of the most crucial fabrication points, similarly mechanical properties of films should also be considered during different applications. Polymeric films that are widely used as coatings over solid pharmaceutical dosage forms to improve their appearance and mechanical strength, to prevent them from dusting, chipping, and cracking, and to modify release of active ingredients from formulations are taken care of. In their basic form, these film coatings are often hard, brittle, and have lower ductility. Proper adjuvants are added during the manufacturing process to overcome these limitations.
Several investigators have confirmed the utility of polymeric film as a drug delivery carrier to avoid hepatic first pass metabolism and to reduce side effects related with other dosage forms. Systems that involve drug administration through adhesive bases, e.g., transdermal systems, bioadhesive vaginal films, sublingual or buccal films, wound dressing films, are all primarily comprised of polymer films that contain drugs. Further, using films as carrier attains some other goals such as enhancing bioavailability, realizing controlled/sustained and/or targeted drug release, minimizing drug dosage, and elevating patient compliance. Of course all the goals are accomplished only when conformability criterion for these thin films is compiled during formulation, i.e. they are strong and flexible enough for practical use.
Polymeric films have gained attention of the biomedical community as well5. Biomedical device construction is challenging because it is difficult to fabricate devices that are both biocompatible and portable6. Moreover, majority of the conventional biomedical devices lack flexibility which is of serious concern if they are to be worn or implanted to the patient body. The rigidity can be overcome with biopolymer based matrix or thin films. The films are biodegradable and this aspect is an added advantage.
Polymer films, either in membrane or in matrix forms, can contribute hugely in packaging, optical lenses, touch screens, foldable sensors, and each of these domains has their own set of plasticity and mechanical property requirements. In pharmaceutical, biomedical, and packaging industries there is a demand for flexible films. This requirement is fulfilled through incorporation of polymer specific plasticizers such as phtalates, trimellitates, adipates, sebacates, glycols, polyethers and alkyl citrates. In fact for the mentioned target applications plasticizers have become a very important additive in polymer systems, and modification of plasticity with plasticizer forms an integral part during fabrication irrespective of the film fabrication or matrix formation process. Table 1 summarizes how the plasticity of several solvent-cast polymer films is modified with the introduction of plasticizer. The cells (in Table 1) with no entries represent values that are either not calculated or could not be determined due to very brittle nature of film7–20.
Table 1.
Effect of plasticizer on flexibility of different polymer films.
| Polymer | Plasticizer | without Plasticizer | with Plasticizer | Application | |||||
|---|---|---|---|---|---|---|---|---|---|
| UTS (MPa) |
% Elongation at break |
Young’s Modulus (MPa) |
UTS (MPa) |
% Elongation at break |
Young’s Modulus (MPa) |
||||
| PVA7 | Sorbitol | 14.36 | 61.23 | 75.9 | 5.36 | 110.81 | 22.6 | Vaginal film | |
| Eudragit RS PO8 | Citric Acid | 4.46 | — | 760 | 1.00 | 117.11 | 520 | Controlled drug release film | |
| HPMC9 | TEC | 83.3 | 8.5 | 891.1 | 76.7 | 107.0 | 516.9 | Transdermal drug delivery | |
| Chitosan and Carbopol10 | Glycerol | 4.56 | 44 | — | 4.5 | 65 | — | Transdermal drug delivery | |
| Zein11 | Glycerol | — | — | — | 24 | 0.029 | 1480 | Drug release film | |
| PMVE/MA12 | Glycerol | 18.23 | 1.68 | 24.07 | 4.73 | 14.72 | 1.14 | Bioadhesive patch | |
| PMVE/MA13 | PEG 10000 | — | — | — | 31.02 | 478.43 | 9.32 | Bioadhesive film | |
| PVP-PEGDA-PEG14 | PG | 0.135 | 42.5 | — | 0.09 | 75 | — | Pressure sensitive adhesive | |
| HPC and EC15 | PG | 10.51 | 15.51 | 70.06 | 7.82 | 26.62 | 30.07 | Buccal film | |
| Soluplus16 | TEC | 8.5 | 2.6 | 472.4 | 5.1 | 178.5 | 70.0 | Drug release film | |
| PPG | 13.4 | 25.9 | 326.2 | 6.8 | 119.6 | 121.1 | |||
| Glycerin | 10.5 | 47.3 | 279.1 | 4.7 | 195.5 | 77.5 | |||
| Polymer | Plasticizer | Plasticizer at low level | Plasticizer at high level | Application | |||||
|
UTS
(MPa) |
% Elongation
at break |
Young’s
Modulus (MPa) |
UTS
(MPa) |
% Elongation
at break |
Young’s
Modulus (MPa) |
||||
| HPMC17 | PEG | 5.96 | 106 | 2.1 | 4.96 | 242 | 0.56 | Oral fast dissolving film | |
| Soluplus16 | PEG | 8.8 | 66.4 | 196.0 | 0.37 | 461 | 1.4 | Drug release film | |
| HPC18 | PEG 400 | 7.83 | 51.42 | — | 6.99 | 55.91 | — | Vaginal film | |
| PMVE/MA12 | Glycerol | 4.73 | 14.72 | 1.14 | 2.02 | 36.54 | 0.04 | Bioadhesive patch | |
| Starch19 | Glycerol | 17.5 | 1.3 | 2120 | 1.2 | 5.6 | 26 | Bioadhesive film | |
| Cashew gum and CMC20 | Glycerol | 5.35 | 0.78 | 710 | 1.81 | 59 | 3 | Biotechnology/packaging | |
Ref.7. Garg et al.8. Schilling et al.9. Limpongsa et al.10 Silva et al.11 Singh et al.12 Moss et al.13 Singh et al.14 Dana et al.15 Alanazi et al.16 Lim et al.17 Pandey et al.18 Dobaria et al.19 Rechia et al.20 Britto et al. Abbreviations used - UTS: ultimate tensile strength, PVA: poly vinyl alcohol, HPMC: hyrdoxy propyl methyl cellulose, PMVE/MA: (poly) methylvinyl ether/maleic anhydride, PVP: polyvinyl pyrrolidone, PEGDA: poly(ethylene glycol) diacrylate, PEG: polyethylene glycol, HPC: hydroxypropyl cellulose, EC: ethyl cellulose, PG: propylene glycol, TEC: triethyl citrate, PPG: polypropylene glycol, CMC: carboxy methyl cellulose.
In this paper, for the first time we have investigated and reported potential of electro-spinning method (Fig. 1) over solvent casting method to fabricate polymeric membrane with enhanced flexibility and plasticity. We have systematically evaluated the phenomena by thorough investigation of electrospun and solvent casted membrane. We hypothesize that the mechanical properties and plasticity of electrospun polymer nanofibers will be influenced by the fabrication method. In this study, we employed scanning electron microscopy technology to evaluate the consequence of electrospinning method on morphology of spun fibers as well as on the morphology of solvent casted membrane. The change in mechanical properties and flexibility were also studied. How the fiber alignment itself change the mechanical properties of fiber, were studied. XRD, DSC were employed to support the potentiality of electrospinning process.
Figure 1.
Electrospinning apparatus set-up and nanofiber mesh.
Results
Surface Morphology
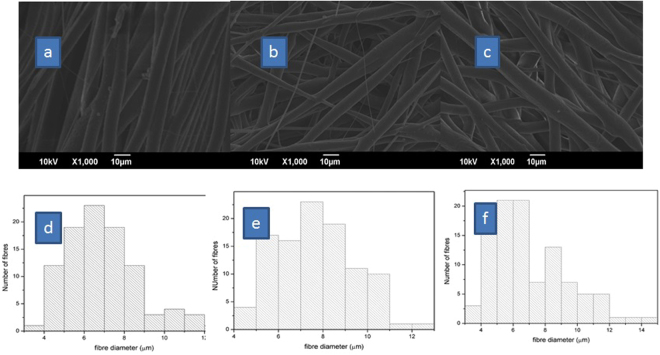
Electrospinning is extensively used to develop fiber of high tensile strength, which is desirable in applications that need lightweight but durable materials. The ability to align and control fiber diameter makes this technique highly attractive. Here, we prepared three electrospun membranes with different kinds of alignment using a rotating mandrel. For simplicity, we designated membrane with uni-directionally aligned fibers as aligned, those randomly aligned as random, and those with aligned fibers overlaid on a randomly aligned as a bilayer. These differences tuned the morphology and size of the fiber because the aligned consisted of uniformly sized fibers that mainly ranged from 5–8 µm. In contrast, the random featured a mixed array of fibers in the range of 5–11 µm and the bilayer exhibited fiber diameter around 4–7 µm (Fig. 2). The SEM micrographs revealed the surface morphologies of the electrospun and the solvent-casted membranes (Figs 2 and 3). Evidently, electrospinning afforded membranes with aligned or non-aligned nano-sized fibers in contrast to that obtained from solvent-casting, which have an irregular surface morphology characterized by the absence of fibers and presence of crystals due to the slow evaporation process. Presumably, the nodules in the solvent-cast membranes are the crystallized domains.
Figure 2.
SEM images of electrospun membrane.
Figure 3.

SEM images of solvent casted membrane.
Mechanical characterization
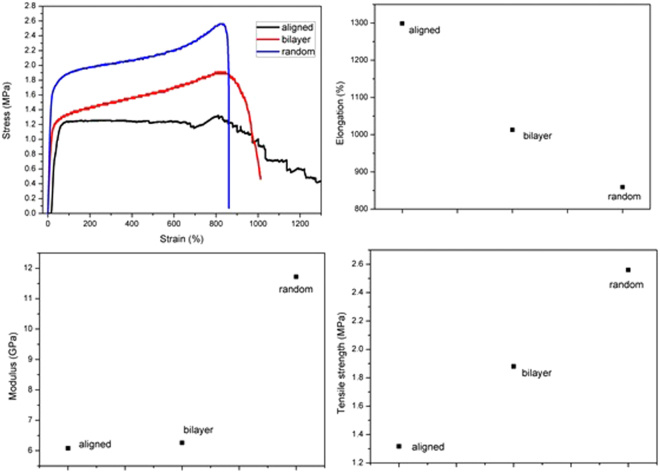
Our investigations revealed that the electrospun PCL membranes excellent elongation rates with tensile strengths of 1.3, 2.6, and 1.9 MPa for aligned, random and bi-layered, respectively. The SEM micrographs of the random membrane reveal fusion among the fibers, a phenomenon that likely increases Young’s modulus and decreases elasticity. Fusion is lacking in the aligned and bilayer membranes, and should results in better orientation and stretching of fibers during mechanical stress. Indeed, the aligned and bilayer gave better elongation than the random (Fig. 4)21–23. We were unable to characterize the mechanical property of the solvent-cast membrane due to their brittleness.
Figure 4.
Mechanical properties of electrospun membrane.
DSC study
We employed DSC to probe the thermal-induced transitions in the electrospun and solvent-cast membranes. The solvent casting process involves slow solvent evaporation process, leading to the formation of crystals, which are not highly oriented. The thermal process during the DSC experiment partially realigned the crystalline domains as evidenced by the presence of a crystallization temperature at 100 °C (Fig. 5). In electrospinning process, the rapid solvent evaporation precludes crystal formation, and indeed, the DSC data reveal no crystallization peak.Regardless of the differences in the crystallization behaviors of these membranes, their melting temperatures were similar (~170 °C), but their enthalpy of melting (Hm) differed with the electrospun exhibiting higher enthalpy as evidenced by the higher endothermic peak. Perhaps higher heat energy is required to melt the highly oriented fiber in the electrospun membrane in contrast to the partially oriented crystalline domains in the solvent-cast membrane24.
Figure 5.
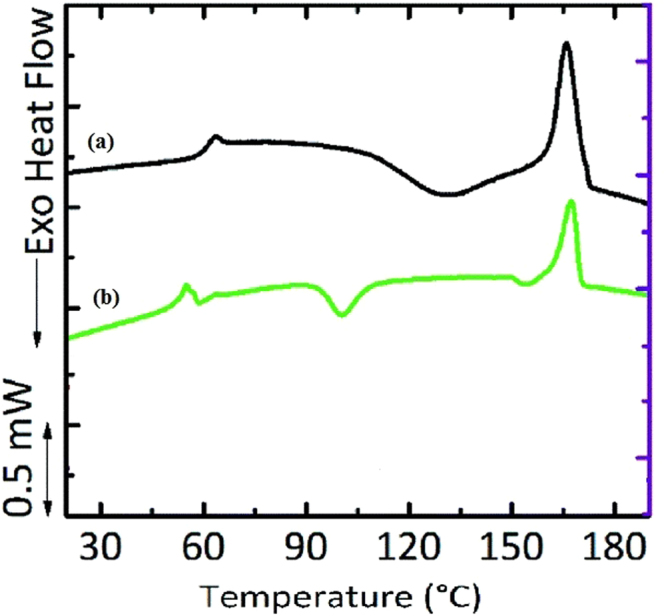
Representative DSC of (a) electrospun membrane (b) solvent casted membrane.
XRD study
Powdered XRD experiments provide information on the degree of crystallinity in polymers. We conducted XRD experiments to gain insights into the degree of crystallinity in the membranes (Fig. 6). Apparently, the absence of crystalline reflections of PCL in the electrospun membrane suggests lack of crystalline domains. This contrasts the XRD diffractogram of the solvent-cast membrane, where sharp peaks were evident due to the presence of crystalline domains25.
Figure 6.
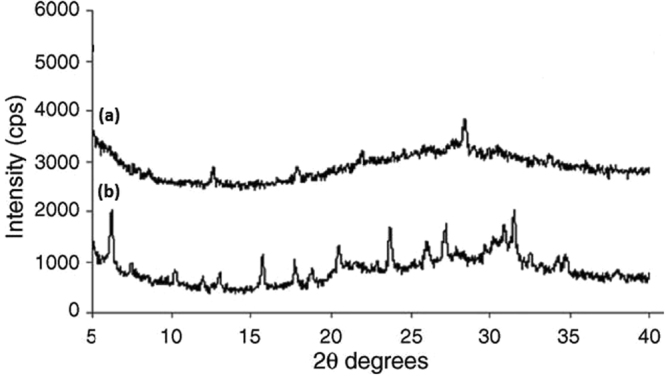
XRD study of (a) electrospun membrane (b) solvent casted membrane.
Discussions
In this present study, our goal includes understanding the fundamental difference in the mechanical behavior of differently oriented electrospun membranes, as well as the differences in surface morphology and thermal behavior of membranes fabricated via electrospinning and the popular solvent-cast. This knowledge is essential from the processing and practical application perspectives and provides parameters to tune mechanical, thermal and surface properties of membranes. To understand the mechanical properties, we characterized the ultimate tensile strength (UTS), Young’s modulus, and elongation at break (%EB). The orientation of the fiber within the electrospun membrane profoundly tunes UTS, %EB, and Young modulus. A random alignment yielded a membrane with higher UTS and Young modulus compared to orderly aligned fibers. The previous studies indicate that the mechanical properties of electrospun fibers depend on the direction of fiber alignment within the membrane. For instance, electrospun random membranes collected on static collectors have lower tensile strength than their aligned counterpart. In contrast, we observed that, with a rotating mandrel as the collector, the random membrane has higher tensile strength than the aligned. Also, in principle, electrospinning improves plasticity, forming flexible films. To the best of our knowledge, several researchers have enhanced plasticity using electrospinning, but a detailed mechanistic explanation is lacking. By comparing our amorphous electrospun to semi-crystalline solvent-cast membranes, we establish a link between the enhanced plasticity of electrospun with amorphousness. Our findings are very optimistic because it provides a viable alternative to overcome the common inflexibility problems of polymeric films prepared by solvent casting method without using plasticizers. Typically, plasticizers penetrate polymer networks reducing cohesive intermolecular forces among polymer chain, a phenomenon that reduces the tensile strength and increasing strain elongation and ultimately improves flexibility26–28.
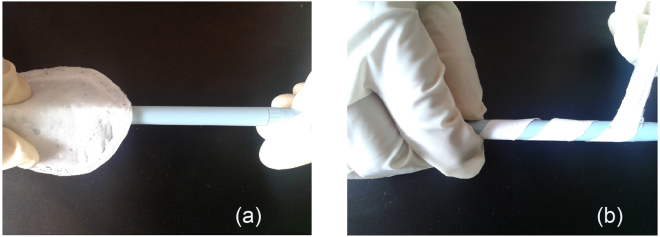
Table 2 enlists some literature where it is notified that mechanical properties of the polymer films had been modulated or modified by many folds solely via electrospinning process29–34. Several process parameters tune the mechanical properties of the polymer films obtained via electrospinning process29–34. Very soft, smooth, and flexible but moderately strong thin polymer films are obtainable by electrospinning process35,36. To achieve this, investigators optimizes electrospinning parameters such as voltage, the distance between nozzle and collector, polymer concentration37,38. In our electrospinning experiment, we applied an electric voltage over polymer solution to draw nano-microfibers39,40 and deposit them as a non-woven matrix. Apparently, this non-woven matrix comprises of randomly aligned polymer chains entangled with each other with distinct voids or porosity (Fig. 3). The porosity between the polymer strands reduces cohesive force between polymer molecules. Also, these voids allow more space for the polymer chains to relax. Therefore, the polymer matrix will be easily strained under particular stress condition due to low cohesion and when the stress is removed polymer chains efficiently relax due to the presence of voids. In this study, the electrospun membranes were flexible in contrast to the membrane were cast from the same solvent blend and same polymer concentration but without plasticizer. We were unable to characterize the mechanical property of the solvent-cast membrane due to their brittleness. Indeed, Fig. 7 shows inflexibility of solvent-cast film and flexibility of electrospun matrix.
Table 2.
Selected polymeric films obtained through electrospinning method.
| Polymer | Findings | Application |
|---|---|---|
| CA29 | Electrospun patches exhibit improved flexibility over the solvent cast films. | Transdermal patch |
| GE-GEM-GM30 | Electrospun matrix showed viscoelastic behavior similar to blood vessels. | Small diameter vascular graft |
| PCL31 | Electrospinning method produced strong but soft and flexible matrix like buttefly wing or human skin. | Tissue engineering |
| PU32 | Large surface area and high porosity confirmed flexibility of the electrospun matrix in comparison to casted film. | Drug delivery |
| PVA33 | Films made through solvent casting are brittle unless a high amount of glycerol is added. Through electrospinning method, strong and flexible films may be obtained even at very low concentration of glycerol. | Drug delivery |
| Zein34 | Plasticizers used in solvent casting method affect barrier property of zein film negatively. Electrospinning produces flexible films without affecting barrier properties. | Food packaging |
Figure 7.
(a) Inflexible solvent-cast film and (b) flexible electrospun matrix of 12% w/v PCL solution.
Conclusions
The mechanical properties of polymer films determine their processing properties and applications. Electrospinning enhances the plasticity of polymer films and membranes, improving flexibility without compromising durability. Here, we establish a link between the enhanced plasticity of an electrospun membrane to amorphousness. Indeed, membranes cast from the same solvent and featuring semi-crystalline domains were brittle and inflexible in contrast to amorphous electrospun membranes. Also, the orientation of the fibers within the membrane tuned the mechanical properties.
Materials and Methods
Materials
poly(ε-caprolactone) (Mw 80,000) was obtained from Sigma Aldrich, USA while analytical grade chloroform was obtained from SRL, Mumbai.
Sample preparation
To formulate the casting and electrospinning solution, we dissolved poly(ε-caprolactone) in chloroform at a concentration of 12% w/v and ensured homogeneity by agitation. The procedure is as follow: an appropriate amount of the polymer was added to the required amount of solvent and stirred for 2 hrs. Then, the resulting mixture was sonicated for 15 minutes and again stirred for another 12 hrs. This solution was used to fabricate the nanofibrous polymer matrix as well as the solvent-cast polymer films. Before electrospinning and casting, the solution was sonicated for 15 minutes and was allowed to sit for 30 minutes to de-aerate.
Electrospinning method
The electrospinning process involves the use of a high DC voltage of several kV (10–20 kV) to induce electrical charges on a jet of polymer solution which dries leaving a polymer nanofiber, eventually forming a nanofibrous mat. The experimental setup includes an electrode connected to the spinneret or metal needle of the syringe that holds the spinning solution, another electrode connected to an 11 × 7 cm aluminum sheet collector, and a syringe pump that drives the polymer solution through the syringe (Fig. 1). To carried out the electrospining, we transferred the polymer solution to a 10-mL syringe fitted with a 0.7 mm gauge blunt needle tip, and electrospun (Holmark, Model- HO- NEV-02) at varying voltages (10 kV to 20 kV) while maintaining the flow rate at 1 mL/hr. The electrospinning was carried out using a rotating mandrel at different speeds (300 to 1000 rpm). The process yielded three kinds of membranes, aligned, random, and bilayer (aligned fibers deposited on random mats), which were used for further studies.
Solvent casting method
The polymer solution was transferred to a Petri dish, covered with an inverted funnel, and allowed to sit overnight to dry. After drying, the sample was collected and stored in desiccators until further use.
SEM study
Electrospun mat or the solvent-casted film sectioned into specific length and width were coated with a platinum layer using a JEOL JFC 1600 Auto fine coater. The images were obtained at 20 kV and then analyzed by software Digimizer® in order to calculate the fiber diameter.
Mechanical characterization
Following ASTM D 882 standard, we characterized the tensile strength of the electrospun nanofibers using Universal Tensile Machine (UTM) INSTRON 1408. Tensile testing was carried out using 500 N load cells at a speed of 1 mm/min onto the specimen. The samples were prepared with a width of 5 mm, a gauge length of 20 mm, and a thickness of 0.2 mm. We performed these experiments in triplicate.
Differential scanning calorimetry
Thermal analysis was performed by DSC (Pyris Diamond TG/DTA; Perkins Elmer Instruments, Mumbai, India). Samples (about 5–10 mg) were heated from 25 to 200 °C at a heating rate 10 °C/min under a steady stream of inert nitrogen gas (flow rate 150 ml/min).
Powdered XRD spectroscopy
The X-ray measurements of nanofibers and solvent-casted membrane were carried out using a Bruker D8 ADVANCE X-ray diffractometer with a scanning rate of 2° per min using Cu–Kα radiation. The XRD data were collected in the 2θ range of 5–60°.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The research has been sponsored by the University Grants Commission under the UGC D.S. Kothari postdoctoral fellowship scheme.
Author Contributions
K.G. designed the experiments and implemented majority of it. A.C. and C.A. contributed ideas and help with manuscript write-up. P.G. and S.S. performed some experiments. S.R., S.T. and I.P. contributed ideas.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kajal Ghosal, Email: kajal.ghosal@gmail.com.
Ivo Provaznik, Email: provaznik@feec.vutbr.cz.
References
- 1.Ahmed A. S., Mandal U. K.,Taher M, Susanti D., Jaffri, J. M. PVA-PEG physically cross-linked hydrogel film as a wound dressing: Experimental design and optimization. Pharm. Dev. Technol (just-accepted) 1–25 (2017). [DOI] [PubMed]
- 2.Walicová V., Gajdziok J., Pavloková S., Vetchý D. Design and evaluation of mucoadhesive oral films containing sodium hyaluronate using multivariate data analysis. Pharm. Dev. Technol 1–8 (2016). [DOI] [PubMed]
- 3.Ghosal K, Manakhov A, Zajíčková L, Thomas S. Structural and surface compatibility study of modified electrospun poly (ε-caprolactone)(PCL) composites for skin tissue engineering. AAPS Pharm Sci Tech. 2017;18(1):72–81. doi: 10.1208/s12249-016-0500-8. [DOI] [PubMed] [Google Scholar]
- 4.Mazza E, Ehret AE. Mechanical biocompatibility of highly deformable biomedical materials. J MechBehav Biomed Mater. 2015;48:100–24. doi: 10.1016/j.jmbbm.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Dhivya S, Saravanan S, Sastry TP, Selvamurugan N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. Journal of nanobiotechnology. 2015;13(1):40. doi: 10.1186/s12951-015-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosal K, Latha MS, Thomas S. Poly (ester amides)(PEAs)–Scaffold for tissue engineering applications. Eur. Polym. J. 2014;60:58–68. [Google Scholar]
- 7.Du L, Xu H, Zhang Y, Zou F. Electrospinning of polycaprolatonenanofibers with DMF additive: The effect of solution proprieties on jet perturbation and fiber morphologies. Fiber Polym. 2016;17(5):751–9. doi: 10.1007/s12221-016-6045-3. [DOI] [Google Scholar]
- 8.Schilling SU, Shah NH, Malick AW, Infeld MH, McGinity JW. Citric acid as a solid‐state plasticizer for Eudragit RS PO. J. Pharm. Pharmacol. 2007;59(11):1493–500. doi: 10.1211/jpp.59.11.0005. [DOI] [PubMed] [Google Scholar]
- 9.Limpongsa E, Umprayn K. Preparation and evaluation of diltiazem hydrochloride diffusion-controlled transdermal delivery system. AAPS. Pharm. Sci. Tech. 2008;9(2):464–70. doi: 10.1208/s12249-008-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva CL, Pereira JC, Ramalho A, Pais AA, Sousa JJ. Films based on chitosan polyelectrolyte complexes for skin drug delivery: development and characterization. Journal of Membrane Science. 2008;320(1):268–79. doi: 10.1016/j.memsci.2008.04.011. [DOI] [Google Scholar]
- 11.Singh N, Georget DM, Belton PS, Barker SA. Physical properties of zein films containing salicylic acid and acetyl salicylic acid. J Cereal Sci. 2010;52(2):282–7. doi: 10.1016/j.jcs.2010.06.008. [DOI] [Google Scholar]
- 12.Moss GP, Gullick DR, Woolfson AD, McCafferty DF. Mechanical characterization and drug permeation properties of tetracaine-loaded bioadhesive films for percutaneous local anesthesia. Drug DevInd Pharm. 2006;32(2):163–74. doi: 10.1080/03639040500466049. [DOI] [PubMed] [Google Scholar]
- 13.Singh TR, McCarron PA, Woolfson AD, Donnelly RF. Physicochemical characterization of poly (ethylene glycol) plasticized poly (methyl vinyl ether‐co‐maleic acid) films. J Appl Polymer Sci. 2009;112(5):2792–9. doi: 10.1002/app.29523. [DOI] [Google Scholar]
- 14.Dana SF, Nguyen DV, Kochhar JS, Liu XY, Kang L. UV-curable pressure sensitive adhesive films: effects of biocompatible plasticizers on mechanical and adhesion properties. Soft Matter. 2013;9(27):6270–81. doi: 10.1039/c3sm50879j. [DOI] [Google Scholar]
- 15.Alanazi FK, Rahman AA, Mahrous GM, Alsarra IA. Formulation and physicochemical characterisation of buccoadhesive films containing ketorolac. J. Drug. Deliv Sci. Tec. 2007;17(3):183–92. doi: 10.1016/S1773-2247(07)50034-1. [DOI] [Google Scholar]
- 16.Lim H, Hoag SW. Plasticizer effects on physical–mechanical properties of solvent cast Soluplus® films. Aaps Pharm sci tech. 2013;14(3):903–10. doi: 10.1208/s12249-013-9971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosal K, Thomas S, Kalarikkal N, Gnanamani A. Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent: preliminary physico-chemical studies for skin substitute. J Polym Res. 2014;1(21(5)):410. doi: 10.1007/s10965-014-0410-y. [DOI] [Google Scholar]
- 18.Dobaria NB, Badhan AC, Mashru RC. A novel itraconazolebioadhesive film for vaginal delivery: design, optimization, and physicodynamic characterization. AapsPharmscitech. 2009;10(3):951. doi: 10.1208/s12249-009-9288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rechia LM, Morona JB, Zepon KM, Soldi V, Kanis LA. Mechanical properties and total hydroxycinnamic derivative release of starch/glycerol/Melissa officinalis extract films. Braz. J. Pharm. Sci. 2010;46(3):491–7. doi: 10.1590/S1984-82502010000300012. [DOI] [Google Scholar]
- 20.deBritto D, de Rizzo JS, Assis OB. Effect of Carboxymethylcellulose and Plasticizer Concentration on Wetting and Mechanical Properties of Cashew Tree Gum–Based Films. Int J Polym Anal Ch. 2012;17(4):302–11. doi: 10.1080/1023666X.2012.668449. [DOI] [Google Scholar]
- 21.Wei X, Xia Z, Wong S-C, Baji A. “Modelling of mechanical properties of electrospun nanofibre network”. International Journal of Experimental and Computational Biomechanics. 2009;1(1):45–57. doi: 10.1504/IJECB.2009.022858. [DOI] [Google Scholar]
- 22.Baji A, Mai Y-W, Wong S-C, Abtahi M, Chen P. “Electrospinning of polymer nanofibers: effects on oriented morphology, structures and tensile properties”. Composites Science and Technology. 2010;70(5):703–718. doi: 10.1016/j.compscitech.2010.01.010. [DOI] [Google Scholar]
- 23.Zhang M, Atkinson KR, Baughman RH. “Multifunctional carbon nanotube yarns by downsizing an ancient technology”. Science. 2004;306(5700):1358–1361. doi: 10.1126/science.1104276. [DOI] [PubMed] [Google Scholar]
- 24.Ramdhanie LI, et al. Thermal and mechanical characterization of electrospun blends of poly (lactic acid) and poly (glycolic acid) Polymer journal. 2006;38(11):1137–1145. doi: 10.1295/polymj.PJ2006062. [DOI] [Google Scholar]
- 25.Lee KH, Kim HY, Khil MS, Ra YM, Lee DR. Characterization of nano-structured poly (ε-caprolactone) nonwoven mats via electrospinning. Polymer. 2003;44(4):1287–1294. doi: 10.1016/S0032-3861(02)00820-0. [DOI] [Google Scholar]
- 26.Zhu Y, Shah NH, Malick AW, Infeld MH, McGinity JW. Solid-state plasticization of an acrylic polymer with chlorpheniramine maleate and triethyl citrate. Int. J. Pharm. 2002;241(2):301–10. doi: 10.1016/S0378-5173(02)00244-2. [DOI] [PubMed] [Google Scholar]
- 27.Rabek CL, Stelle RV, Dziubla TD, Puleo DA. The effect of plasticizers on the erosion and mechanical properties of polymeric films. J. Biomater. Appl. 2014;28(5):779–89. doi: 10.1177/0885328213480979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honary S, Orafai H. The effect of different plasticizer molecular weights and concentrations on mechanical and thermomechanical properties of free films. Drug DevInd Pharm. 2002;28(6):711–5. doi: 10.1081/DDC-120003863. [DOI] [PubMed] [Google Scholar]
- 29.Taepaiboon P, Rungsardthong U, Supaphol P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007;67(2):387–97. doi: 10.1016/j.ejpb.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Thomas V, Zhang X, Catledge SA, Vohra YK. Functionally graded electrospun scaffolds with tunable mechanical properties for vascular tissue regeneration. Biomed. Mater. 2007;2(4):224. doi: 10.1088/1748-6041/2/4/004. [DOI] [PubMed] [Google Scholar]
- 31.Bölgen N., Menceloğlu Y. Z., Acatay K., Vargel I., Pişkin E. In vitro and in vivo degradation of non-woven materials made of poly (ε-caprolactone) nanofibers prepared by electrospinning under different conditions. J. Biomater. Sci. Polym. Ed. 16(12), 1537–55 (2005). [DOI] [PubMed]
- 32.Saha, K., Butola, B. S., Joshi, M. Drug release behavior of polyurethane/clay nanocomposite: Film vs. nanofibrous web. J Appl Polymer Sci. 131(19) (2014 Oct 5).
- 33.Tyagi C, et al. Electrospun nanofiber matrix with a mucoadhesive backing film for oramucosal drug delivery. Int. J. Mat. MechManufact. 2014;2:81–5. [Google Scholar]
- 34.Fabra MJ, Lopez-Rubio A, Lagaron JM. High barrier polyhydroxyalcanoate food packaging film by means of nanostructured electrospun interlayers of zein. Food Hydrocoll. 2013;32(1):106–14. doi: 10.1016/j.foodhyd.2012.12.007. [DOI] [Google Scholar]
- 35.Yin C, Jatoi AW, Bang H, Gopiraman M, Kim IS. Fabrication of silk fibroin based three dimensional scaffolds for tissue engineering. Fiber Polym. 2016;17(8):1140–5. doi: 10.1007/s12221-016-5852-x. [DOI] [Google Scholar]
- 36.Li Y, Thouas GA, Chen Q. Novel elastomeric fibrous networks produced from poly (xylitol sebacate) 2:5 by core/shell electrospinning: Fabrication and mechanical properties. J MechBehav. Biomed Mater. 2014;40:210–21. doi: 10.1016/j.jmbbm.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Rizvi MS, Pal A. Statistical model for the mechanical behavior of the tissue engineering non-woven fibrous matrices under large deformation. J. Mech Behav Biomed Mater. 2014;37:235–50. doi: 10.1016/j.jmbbm.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Ranjbar-Mohammadi M, Kargozar S, Bahrami SH, Joghataei MT. Fabrication of curcumin-loaded gum tragacanth/poly (vinyl alcohol) nanofibers with optimized electrospinning parameters. J. Ind. Text. 2017;46(5):1170–1192. doi: 10.1177/1528083715613631. [DOI] [Google Scholar]
- 39.Tonglairoum P, Ngawhirunpat T, Rojanarata T, Opanasopit P. Lysozyme-immobilized electrospun PAMA/PVA and PSSA-MA/PVA ion-exchange nanofiber for wound healing. Pharm. Dev. Technol. 2015;20(8):976–983. doi: 10.3109/10837450.2014.954726. [DOI] [PubMed] [Google Scholar]
- 40.Ngawhirunpat T, et al. Development of meloxicam-loaded electrospun polyvinyl alcohol mats as a transdermal therapeutic agent. Pharm. Dev. Technol. 2009;14(1):73–82. doi: 10.1080/10837450802409420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.