Abstract
Objective
Hypertension is a significant risk factor for atrial fibrillation (AF). The role of pulmonary vein (PV) remodeling in the mechanistic association between hypertension and AF is not definitive. In this study, we aimed to identify changes in the electrophysiology and histology in PVs in two-kidney, one-clip (2K1C) hypertensive rats.
Methods
Fifty male Sprague-Dawley rats were classified into the 2K1C and sham-operated groups. The systolic blood pressure was measured every 2 weeks. The left atrial diameter was measured by transthoracic echocardiography. Left superior PV (LSPV) and left atrial (LA) fibrosis was evaluated by Masson’s trichrome staining. The expression of fibrosis markers [angiotensin II (Ang II), transforming growth factor-β1 (TGF-β1), matrix metalloproteinase-2 (MMP-2), and collagen I (Col I)] and ion channels [Kir2.1, Kir2.3, Cav1.2, and Nav1.5] in LSVP was quantified by western blot. Conventional microelectrodes were used to record the action potential duration at 90% repolarization (APD90) and effective refractory period (ERP) in isolated LA.
Results
At 4 months, the 2K1C hypertensive rats developed LA dilation. Col deposition in LSPV and left atrium and expression of TGF-β1, MMP-2, and Col I in LSPV were significantly increased in 2K1C hypertensive rats. In addition, hypertension reduced the expression of Nav1.5 and Kir2.1, although there were no significant differences in APD90; ERP; and expression of Ang II, Kir2.3, and Cav1.2 between the two groups.
Conclusion
Hypertension may lead to changes in the electrophysiology and histology of rats PVs, which is characterized by significant reduction in the expression of Nav1.5 and Kir2.1 and increase in interstitial fibrosis. These observations may clarify the role of PVs in the mechanistic association between hypertension and AF.
Keywords: atrial fibrillation, fibrosis, hypertension, pulmonary vein, remodeling
Introduction
The relationship between atrial fibrillation (AF) and hypertension has been established in epidemiological as well as histological studies (1-5). Clinical and animal studies have shown that hypertension can lead to histological and electrophysiological remodeling of the atrial tissue, which results in easier maintenance of AF (1, 5). Previous studies have demonstrated that spontaneous ectopic activity in the pulmonary veins (PVs) plays a critical role in the initiation of AF (6-8). However, most previous studies on associations between hypertension and AF have specifically focused on the atria. There is currently a lack of data on the arrhythmogenic role of PVs in the direct link between hypertension and AF.
Histological and electrophysiological remodeling of PV has some links with AF, including PV interstitial fibrosis and altered expression or function of cardiac ion channels (9). Some cardiac diseases modify the operation of ion channels in a way that promotes the occurrence of cardiac rhythm disturbances; the process is termed as “arrhythmogenic remodeling” (10). Therefore, changes in the ion channels may play an important role during the arrhythmogenic process.
We hypothesized that hypertension leads to AF not only through atrial remodeling but also through PV arrhythmogenesis. We investigated the effects of hypertension on histological and electrical remodeling of PV in a rat model. It has been reported that expression of collagen I (Col I), matrix metalloproteinase-2 (MMP-2), and transforming growth factor-β1 (TGF-β1), which imply fibrosis of the tissue, and expression of Nav1.5 and Kir2.1 channel protein are indicators of electrical remodeling. In the present study, interstitial fibrosis, indicated by the expression of Col I, MMP-2, and TGF-β1, and the cardiac ion channels level at PV were evaluated.
Methods
Animal model of hypertension
All experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication NO. 85–23, revised 1996). Male Sprague-Dawley rats (180–200 g, n=50) were maintained in a 12-h light/dark cycle at 22°C with free access to rat chow and water.
Two-kidney, one-clip (2K1C) hypertension was induced in rats, as described previously (11). The rats were randomly assigned to 2K1C (n=30) and sham-operated (n=20) groups, and anesthetized using chloral hydrate (300 mg/kg, intraperitoneally). After a midline laparotomy, a silver clip with an internal diameter of 0.20 mm was placed around the left renal artery close to the aorta. Sham-operated rats underwent the same surgical procedure, except for the placement of the silver clip. Tail systolic blood pressure (SBP) was measured by tail-cuff plethysmography (BP-2000, Visitech Systems, USA) every 2 weeks. The mean of three or four measurements was used as the blood pressure estimate, and the rats were considered to be hypertensive if their SBP exceeded 160 mm Hg after 2 weeks of ligation (11). Approximately 20% of the operated rats failed to achieve this level of SBP.
Doppler echo study
Transthoracic echocardiography was performed by independent, blinded to physicians. The rats were anesthetized with isoflurane, and transthoracic echocardiography (70 Mhz, VisualSonics company, Canada, Toronto) was conducted at 2, 3, and 4 months postoperatively. Left atrial diameter (LAD), interventricular septal thickness (IVS; d), and left ventricular ejection fraction (EF, %) were measured.
Tissue preparation
At the end of the experimental period, the animals (n=12/group) were anesthetized by intraperitoneal injection of sodium pentobarbital (80 mg/kg). After confirming suppression of the nociceptive reflex, the chest cavity of the rats was opened. The heart and lungs were excised in a block and repeatedly rinsed in ice cold 0.9% saline solution. For each rat, the intact heart was weighed. The left PV (LPV, 5–6 mm long, zone 1 as reported by Bronquard et al.) (12) and the left atrium were then excised from the block. Portions of LPV and LA tissues were immediately fixed in 4% polyformaldehyde for histological studies; the remaining tissues were snap frozen in liquid nitrogen and then stored at −80°C for Western blot analysis.
Histological study
LSPV tissue samples (n=6/group) were fixed in 4% polyformaldehyde for 36 h at room temperature and embedded in paraffin. Deparaffinized tissues were cut into sections (4 µm thick), which were mounted on glass slides and stained with Masson’s trichrome stain (Nanjing Jiancheng Technology Company, China) to evaluate the area occupied by Col fibers. With this technique, cardiomyocytes are stained bright red, whereas Col fibers are stained blue. Images were captured under a microscope, and quantitative analysis of interstitial fibrosis was performed using Image-Pro Plus 6.0 software (USA).
Western blotting
Immunoblotting was performed, as described previously (13). The LSPV tissue (approximately 40 mg) (n=6/group) was homogenized and cleared by ultracentrifugation (14,000×g) at 4°C for 15 min. Homogenates (containing 40 µg of protein) were subjected to 8% SDS-polyacrylamide gel electrophoresis (Beyotime, China) and transferred to PVDF membranes. Membranes were blocked in TBST containing 5% bovine serum albumin at room temperature for 1.5 h before incubation with corresponding primary antibodies: goat polyclonal anti-Col I and rabbit polyclonal anti-MMP-2 (1:200, Santa Cruz, Japan); rabbit monoclonal anti-Ang II (1:2,000, Abcam, UK), rabbit polyclonal anti-TGF-β1 (1:500, Abcam, UK); and rabbit polyclonal anti-Kir2.1, rabbit monoclonal anti-Kir2.3, rabbit monoclonal anti-Cav1.2 and rabbit monoclonal anti-Nav1.5 (1:200, Alomone, Israel). Rabbit monoclonal anti-glyceraldehyde phosphate dehydrogenase (GAPDH; 1:1,000, CST, America) antibody was used as a protein loading control. After overnight incubation with primary antibodies at 4°C, the membranes were washed and incubated with anti-rabbit or anti-goat secondary antibodies (1:5,000, Immunology Consultants Laboratory, USA) for 2 h at room temperature. Membranes were washed, and immunodetection was visualized using an enhanced chemiluminescence method (Thermo, USA) and then developed using X-ray film. Resulting images were analyzed using a densitometer to quantify the protein expression after normalization to GAPDH (Image Lab, BIO-RAD, USA). Mean values of the 2K1C group were compared with the corresponding mean values of the sham-operated group.
Electrophysiological studies on LA
Electrophysiological studies on the LA tissue were performed, as previously described (14). Four months postoperatively, the rats (500–600 g; n=4 for the 2K1C group and n=3 for the sham-operated group) were anesthetized with sodium pentobarbital (45 mg/kg, intraperitoneally) and given heparin. left atrium was rapidly removed and the endocardial surface was pinned open in a tissue bath. The atria were superfused with Tyrode’s solution containing KCl, 4.0; NaCl, 131; CaCl2, 1.8; NaHCO3, 24.9; MgSO4, 1.2; KH2PO4, 1.2; and dextrose, 11.1 mmol/L (T=36.5°C±0.5°C, pH 7.4). Bipolar stimulating electrodes were placed on the free wall of left atrium. Conventional microelectrode techniques were used to record action potential duration at 90% repolarization (APD90). Recording sites were located 3–4 mm from the stimulating electrodes. After 20-min equilibration in Tyrode’s solution with pacing from the left atrium at cycle length (CL)=0.4 s, an intermittent burst pacing protocol was applied. This was developed to mimic an intermittently bursting focus in the left atrium. The period of intermittence started at 150 ms, until the atrial effective refractory period (AERP) was recorded and then decreased by 5 ms each time.
Statistical analysis
All data are presented as mean±standard deviation (SD) and analyzed using unpaired Student’s t-test in GraphPad Prism 5.0 (GraphPad Software, USA). The Kolmogorov–Smirnov test was used to identify whether data were normally distributed. P<0.05 was considered to indicate statistical significance.
Results
Systolic blood pressure in 2K1C hypertensive rats
Clipping the left renal artery (2K1C) induced a marked rise in SBP (mm Hg), which was clearly observed, and reached a plateau at 2 weeks postoperatively. Table 1 showed SBP measured using the tail-cuff method at 2 weeks and 1, 2, 3, and 4 months postoperatively.
Table 1.
Comparison of SBP in different time periods between two groups
| 2K1C | Sham | P | |
|---|---|---|---|
| Two weeks | 168.60±21.28 | 121.20±11.12 | <0.001 |
| One month | 170.10±15.11 | 121.20±12.23 | <0.001 |
| Two months | 170.20±24.42 | 125.40±13.59 | <0.001 |
| Three months | 177.70±21.62 | 130.70±9.94 | <0.001 |
| Four months | 176.60±22.20 | 127.30±17.59 | <0.001 |
Data are expressed as mean±SD (n=15). SBP - systolic blood pressure
LA remodeling during the development of 2K1C hypertension
Hypertension-induced morphological alterations in the left atrium of 2K1C hypertensive rats. Increased LAD was observed 3 months postoperatively in the 2K1C groups compared with that in the sham-operated groups (Table 2; p=0.002). However, at 4 months postoperatively, there were no significant differences in heart weight/body weight ratio, (IVS; d) and EF% between the groups (Table 2).
Table 2.
Effects of hypertension on cardiac remodeling at four months after surgery
| 2K1C | Sham | P | |
|---|---|---|---|
| 2-month LAD (mm) | 5.13±0.51 | 4.48±0.36 | 0.099 |
| 3-month LAD (mm) | 5.63±0.29 | 4.32±0.41 | 0.002 |
| 4-month LAD (mm) | 6.01±0.29 | 4.78±0.51 | 0.002 |
| Heart/body weight (%) | 0.33±0.07 | 0.27±0.02 | 0.132 |
| IVS; d (mm) | 1.83±0.09 | 1.76±0.13 | 0.589 |
| EF (%) | 62.83±4.53 | 63.98±8.75 | 0.818 |
| APD90 (ms) | 29.53±9.84 | 43.5±14.24 | 0.399 |
| ERP (mm) | 56±24 | 38±14 | 0.499 |
N=6 for LAD, Heart weight/body weight ratio, (IVS; d) and EF%. N=4 for 2K1C group and N=3 for sham group for APD90, ERP of LA. Data are expressed as mean±SD. APD90 - action potential duration at 90% repolarization; (EF, %) - left ventricular ejection fraction; ERP - effective refractory period; (IVS; d) - interventricular septal thickness; LAD - left atrial diameter
APD90 and ERP were recorded in the excised, perfused LA tissue from the rats in the 2K1C and sham-operated groups. There were no significant differences between 2K1C hypertensive and sham-operated rats in terms of APD90 and ERP (Table 2 and Fig. 1).
Figure 1.
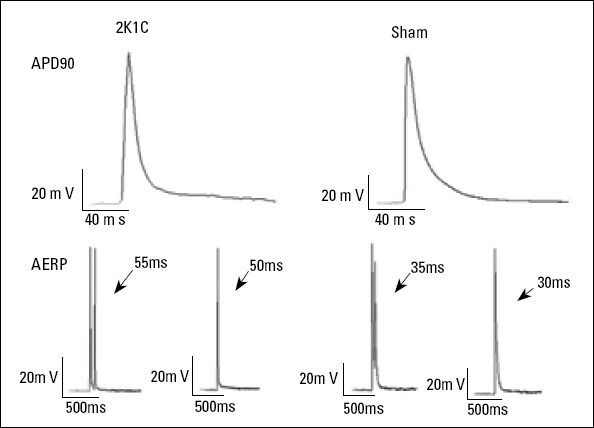
Representative action potential duration at 90% repolarization (APD90) and effective refractory period (ERP) of the left atrium in the 2K1C and sham-operated groups
APD90, action potential duration at 90% repolarization; ERP, effective refractory period
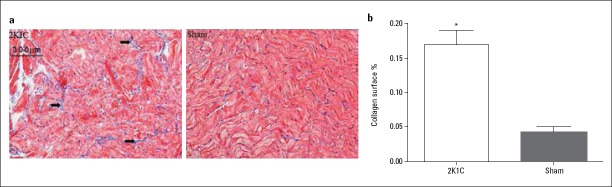
LA fibrosis was examined by Masson’s trichrome staining of tissue sections. At 4 months, the degree of LA fibrosis was markedly greater in the 2K1C group than in the sham-operated group (Fig. 2a). The mean percentage of fibrosis in sections from the 2K1C group was also greater than that observed in the sham-operated group (Fig. 2b; 16.96%±3.57% vs. 4.32%±1.98%; p=0.012).
Figure 2.
Atrial fibrosis in 2K1C hypertensive and sham-operated rats (a) Representative examples of Masson’s trichrome-stained left atrial sections from 2K1C hypertensive and sham-operated rats (original magnification 200× arrows indicate areas of fibrosis). (b) Percentage fibrosis measured as the area that was stained blue as a percentage of the total area using Image-Pro Plus 6.0
Data are expressed as mean±SD. *p=0.012 vs. sham-operated group (n=6)
Histological and electrophysiological remodeling in LSPV
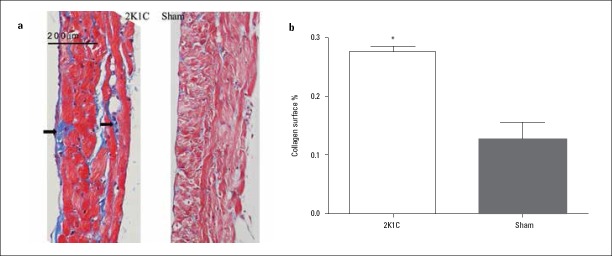
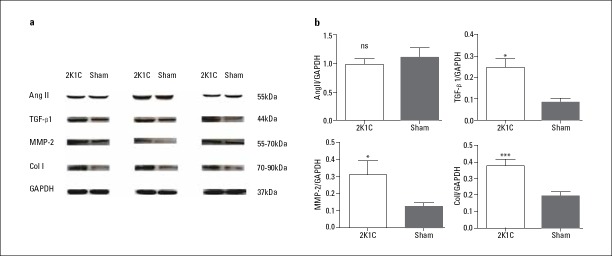
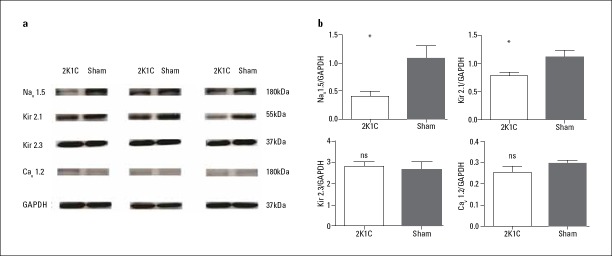
Increased interstitial fibrosis in LSPV was detected by Masson’s staining in 2K1C hypertensive rats compared with that in sham-operated rats (Fig. 3; 27.55%±2.20% vs. 12.71%±9.09%; p=0.016). Expression of Col I (0.37±0.11 vs. 0.19±0.06; p<0.001), TGF-β1 (0.25±0.08 vs. 0.09±0.03; p=0.029), and MMP-2 (0.31±0.17 vs. 0.12±0.03; p=0.029) were increased, while expression of Ang II was unchanged in 2K1C hypertensive rats compared with that in sham-operated rats (p=0.574) (Table 3; Fig. 4). Changes in ion channel protein were detected as decreased expression of Nav1.5 (0.40±0.20 vs. 1.08±0.52; p=0.032) and Kir2.1 (0.78±0.15 vs. 1.11±0.27; p=0.041), although no major changes in the expression of Cav1.2 and Kir2.3 were observed (p=0.4, p=0.691) (Table 3; Fig. 5).
Figure 3.
Left superior pulmonary vein (LSPV) fibrosis in 2K1C hypertensive and sham-operated rats (a) Representative examples of Masson’s trichrome-stained LSPV sections from 2K1C hypertensive and sham-operated rats (original magnification 200×, arrows indicate areas of fibrosis). (b) Percentage fibrosis measured as the area that was stained blue as a percentage of the total area using Image-Pro Plus 6.0
Data are expressed as mean±SD. *p=0.016 vs. sham-operated group (n=6)
Table 3.
Comparison of protein expression between two groups
| 2K1C | Sham | P | |
|---|---|---|---|
| ColI | 0.37±0.11 | 0.19±0.06 | <0.001 |
| TGF-β1 | 0.25±0.08 | 0.09±0.03 | 0.029 |
| MMP-2 | 0.31±0.17 | 0.12±0.03 | 0.029 |
| AngII | 0.99±0.27 | 1.11±0.47 | 0.574 |
| Nav1.5 | 0.40±0.20 | 1.08±0.52 | 0.032 |
| Kir2.1 | 0.78±0.15 | 1.11±0.27 | 0.041 |
| Cav1.2 | 0.25±0.05 | 0.30±0.02 | 0.4 |
| Kir2.3 | 2.78±0.47 | 2.65±0.81 | 0.691 |
N=6 for each group. AngII - Angiotensin II; Cav1.2 - L-type Ca2+-subunit Cav1.2; ColI – collagenI; Kir - Inward rectifyimg potassium channel; MMP-2 - matrix metalloproteinase-2; Nav1.5 - Voltage-gated sodium channel subunit Nav1.5; TGF-β1 - transforming growth factor-β1
Figure 4.
Protein levels of the fibrotic markers Ang II, TGF-β1, MMP-2, and Col I, analyzed by Western blot and normalized to GAPDH
Data are expressed as mean±SD. *p<0.05 vs. sham-operated group. ***p<0.01 vs. sham-operated group (n=6)
Ang II, angiotensin II; Col I, collagen I; GAPDH, glyceraldehyde phosphate dehydrogenase; MMP-2, matrix metalloproteinase-2; ns, not significant; TGF-β1, transforming growth factor-β1
Figure 5.
Protein levels of the ion channel components Nav1.5, Kir2.1, Kir2.3, and Cav1.2, analyzed by western blot and normalized to GAPDH
Data are expressed as mean±SD. *p<0.05 vs. sham-operated group (n=6)
Cav1.2, L-type Ca2+-subunit Cav1.2; GAPDH, glyceraldehyde phosphate dehydrogenase; Kir, inward rectifyimg potassium channel; Nav1.5, voltage-gated sodium channel subunit Nav1.5; ns, not significant
Discussion
In this study, we demonstrated structural and electrophysiological remodeling in PVs in a rat model of hypertension. This shows a newly discovered mechanistic association between hypertension and AF, beyond atrial remodeling, which has been established as an arrhythmogenic substrate for AF (15).
Mechanism of PV arrhythmogenicity
It has been nearly 20 years since Haissaguerre found that ectopic activity originated from PVs accounts for initiation of paroxysmal AF (6). Since then, numerous studies have focused on electro-anatomic features of PVs. Triggered automaticity has been implicated as possible mechanisms of PV firing (16-18). In addition, PV fibrosis may maintain a possible re-entry in the development of AF through impaired anisotropic conductions (9, 19).
Structural and electrical remodeling of the atrium and PV in 2K1C hypertensive rats
In our study, we attempted to elucidate changes in fibrosis and expression of ion channel proteins within PV in 2K1C hypertensive rats. Experimental 2K1C hypertension led to marked histological and electrophysiological remodeling of LSPV; the former refers to changes in fibrosis associated with increased expression of Col I, TGF-β1, and MMP-2, whereas the latter is involved in downregulation of the expression of Nav1.5 and Kir2.1 channel proteins. To date, these hypertension-related changes in PVs remain unclear.
The precise mechanism leading to the onset and persistence of AF has not been completely elucidated. Structural remodeling, particularly atrial fibrosis, is regarded as the major mechanism accounting for AF persistence (20). In addition to fibrosis, atrial dilatation has also been associated with heterogeneity of conduction (21). Previous studies have also indicated that structural remodeling is the main contributor to the persistence of AF and might be present before the development of AF caused by associated diseases (22). Our histological study confirmed the presence of a higher degree of fibrosis in the left atrium of 2K1C hypertensive rats compared with that observed in sham-operated rats. Furthermore, transthoracic echocardiography showed LA dilatation in 2K1C hypertensive rats. These structural changes in the atria may contribute to increased susceptibility to AF in patients with hypertension.
LA dilatation and increased fibrosis were observed in 2K1C hypertensive rats. These characteristics are considered the hallmark of structural remodeling in AF and the substrate for AF perpetuation (15). However, there were no significant differences in APD90 and AERP between the groups. Previous studies have demonstrated numerous changes in APD and ERP in the atria of hypertensive animals, such as shortened APD and ERP (1, 23) and prolonged (24, 25) or unchanged (26, 27) APD and ERP. This variability could be due to species differences, blood pressure level, or even measurement techniques. Inconsistency in APD and ERP also illustrated diverse mechanisms of AF among different disease models.
As important profibrotic factors, the expression of Ang II, TGF-β1, and MMP-2 were investigated to elucidate the mechanisms by which fibrosis develops within PV. However, the difference in the expression of the Ang II protein between the two groups did not reach the level of statistical significance in our study. This result may be due to two factors. First, angiotensin I-converting enzyme activity increased in 2K1C hypertensive rats, but decreased to normal levels after 10 weeks. This finding suggests that the renin-angiotensin-aldosterone system activation plays an important role in the initial phase of 2K1C hypertension, but not in the late phase (28). Second, 2K1C results in a sustained increase in blood pressure because of increased renin activity, which in turn, increases circulating Ang II levels. In the early stages, hypertension is renin-angiotensin dependent because of the absence of salt and water retention as the second, unclipped kidney is intact. However, after approximately 6 weeks, the increased Ang II releases aldosterone leading to a gradual retention of salt and water, which in return decreases renin production (29). The critical role of TGF-β1 in the development of AF has been confirmed in transgenic mice overexpressing TGF-β1, which led to selective atrial fibrosis, enhanced AF susceptibility, and increased conduction heterogeneity, although atrial action potential duration and ventricular structure and function were normal (30). Researchers have hypothesized that TGF-β1 levels in PVs may be related to the arrhythmogenicity of these vessels (31); however, expression of TGF-β1 in PVs has not been investigated widely. Also, accumulating evidence obtained in animal studies has implicated imbalanced MMP activity, particularly of MMP-2, in hypertension-induced vascular remodeling (32, 33). In the present study, we observed a significant increase in the expression of Col I protein and increased fibrosis in LSPV in 2K1C hypertensive rats. These effects may be, at least in part, caused by TGF-β1 and MMP-2.
In our study, we observed decreased expression of the ion channel proteins Nav1.5 and Kir2.1 in LSPV in 2K1C hypertensive rats compared with that in sham-operated rats. It is reported that reduction in the expression of INa not only contributes to conduction retardation, which favors re-entry (34), but also promotes early afterdepolarization (35). The 2K1C hypertensive rats in the present study exhibited reduced expression of the Kir2.1 protein compared with that in sham-operated rats, while no significant differences in the expression of Kir2.3 protein were observed. Kir2.1 and Kir2.3 encode the principal cardiac inward-rectifier Ik1 subunit. Previous studies have shown that reduction in Ik1 causes increased membrane resistance, leading to a larger voltage deflection for a given quantity of depolarizing membrane current; this may be an important mechanism underlying the promotion of afterdepolarization-induced arrhythmias (26, 36). Decreased expression of the protein may implicate a decrease in the relevant channel function. However, the direct functional link between expression of the protein and ion channels needs to be further studied.
Study limitations
The study was performed at 4 months after 2K1C surgery. However, more obvious changes in the structure and electrophysiology may have been observed if the observation time was extended. Moreover, electrophysiological LPV data, such as APD, ERP, and ion current, was not calculated.
Conclusion
Hypertension is associated with histological and electrophysiological PV remodeling characterized by fibrosis and some changes in the expression of the ion channel protein These observations provide new mechanistic association between hypertension and AF beyond the previously well-established role of atrial remodeling.
Footnotes
Funding: This work was supported by the Natural Science Foundation for Young Investigators of Jiangsu Province (BK2012487).
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – W.Z.J.; Design – W.Z.J.; Supervision – W.Z.J.; Materials – P.P. X., L.J.L.; Data collection &/or processing – R.D.Q., J.J.S.; Analysis &/or interpretation – P.P.X., R.D.Q.; Literature search – M.L.C.; Writing – P.P.X., L.J.L.; Critical review – W.Z.J.
References
- 1.Lau DH, Shipp NJ, Kelly DJ, Thanigaimani S, Neo M, Kuklik P, et al. Atrial arrhythmia in ageing spontaneously hypertensive rats: unraveling the substrate in hypertension and ageing. PLoS One. 2013;8:e72416. doi: 10.1371/journal.pone.0072416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, et al. Atrial fibrillation at baseline and during follow-up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) J Am Coll Cardiol. 2009;54:2023–31. doi: 10.1016/j.jacc.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Neilan TG, Mongeon FP, Shah RV, Coelho-Filho O, Abbasi SA, Dodson JA, et al. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging. 2014;7:1–11. doi: 10.1016/j.jcmg.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239–51. doi: 10.1016/j.jacc.2015.03.557. [DOI] [PubMed] [Google Scholar]
- 5.Choisy SC, Kim SJ, Hancox JC, Jones SA, James AF. Effects of candesartan, an angiotensin II receptor type I blocker, on atrial remodeling in spontaneously hypertensive rats. Physiol Rep. 2015;3:e12274. doi: 10.14814/phy2.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 7.Jaïs P, Hocini M, Macle L, Choi KJ, Deisenhofer I, Weerasooriya R, et al. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation. 2002;106:2479–85. doi: 10.1161/01.cir.0000036744.39782.9f. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 9.Corradi D, Callegari S, Benussi S, Nascimbene S, Pastori P, Calvi S, et al. Regional left atrial interstitial remodeling in patients with chronic atrial fibrillation undergoing mitral-valve surgery. Virchows Arch. 2004;445:498–505. doi: 10.1007/s00428-004-1040-2. [DOI] [PubMed] [Google Scholar]
- 10.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–56. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 11.Rizzi E, Ceron CS, Guimaraes DA, Prado CM, Rossi MA, Gerlach RF, et al. Temporal changes in cardiac matrix metalloproteinase activity, oxidative stress, and TGF-beta in renovascular hypertension-induced cardiac hypertrophy. Exp Mol Pathol. 2013;94:1–9. doi: 10.1016/j.yexmp.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Bronquard C, Maupoil V, Arbeille B, Fetissof F, Findlay I, Cosnay P, et al. Contractile and relaxant properties of rat-isolated pulmonary veins related to localization and histology. Fundam Clin Pharmacol. 2007;21:55–65. doi: 10.1111/j.1472-8206.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 13.Goette A, Staack T, Röcken C, Arndt M, Geller JC, Huth C, et al. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–77. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 14.Ozgen N, Dun W, Sosunov EA, Anyukhovsky EP, Hirose M, Duffy HS, et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res. 2007;75:758–69. doi: 10.1016/j.cardiores.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac Fibrosis in Patients with Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol. 2015;66:943–59. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 16.Chou CC, Nihei M, Zhou S, Tan A, Kawase A, Macias ES, et al. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–97. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 17.Arentz T, Haegeli L, Sanders P, Weber R, Neumann FJ, Kalusche D, et al. High-density mapping of spontaneous pulmonary vein activity initiating atrial fibrillation in humans. J Cardiovasc Electrophysiol. 2007;18:31–8. doi: 10.1111/j.1540-8167.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 18.Patterson E, Jackman WM, Beckman KJ, Lazzara R, Lockwood D, Scherlag BJ, et al. Spontaneous pulmonary vein firing in man: relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro. J Cardiovasc Electrophysiol. 2007;18:1067–75. doi: 10.1111/j.1540-8167.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 19.Hassink RJ, Aretz HT, Ruskin J, Keane D. Morphology of atrial myocardium in human pulmonary veins: a postmortem analysis in patients with and without atrial fibrillation. J Am Coll Cardiol. 2003;42:1108–14. doi: 10.1016/s0735-1097(03)00918-5. [DOI] [PubMed] [Google Scholar]
- 20.Gal P, Marrouche NF. Magnetic resonance imaging of atrial fibrosis: redefining atrial fibrillation to a syndrome. Eur Heart J. 2017;38:14–19. doi: 10.1093/eurheartj/ehv514. [DOI] [PubMed] [Google Scholar]
- 21.Chang SL, Chen YC, Chen YJ, Wangcharoen W, Lee SH, Lin CI, et al. Mechanoelectrical feedback regulates the arrhythmogenic activity of pulmonary veins. Heart. 2007;93:82–8. doi: 10.1136/hrt.2006.089359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89:754–65. doi: 10.1093/cvr/cvq357. [DOI] [PubMed] [Google Scholar]
- 23.Lader JM, Vasquez C, Bao L, Maass K, Qu J, Kefalogianni E, et al. Remodeling of atrial ATP-sensitive K(+) channels in a model of salt-induced elevated blood pressure. Am J Physiol Heart Circ Physiol. 2011;301:H964–74. doi: 10.1152/ajpheart.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Brooks AG, Worthington M, et al. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. 2010;7:1282–90. doi: 10.1016/j.hrthm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Worthington M, Rajendram A, et al. Short-term hypertension is associated with the development of atrial fibrillation substrate: a study in an ovine hypertensive model. Heart Rhythm. 2010;7:396–404. doi: 10.1016/j.hrthm.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 26.Choisy SC, Arberry LA, Hancox JC, James AF. Increased susceptibility to atrial tachyarrhythmia in spontaneously hypertensive rat hearts. Hypertension. 2007;49:498–505. doi: 10.1161/01.HYP.0000257123.95372.ab. [DOI] [PubMed] [Google Scholar]
- 27.Kistler PM, Sanders P, Dodic M, Spence SJ, Samuel CS, Zhao C, et al. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur Heart J. 2006;27:3045–56. doi: 10.1093/eurheartj/ehl360. [DOI] [PubMed] [Google Scholar]
- 28.Ceron CS, Rizzi E, Guimaraes DA, Martins-Oliveira A, Cau SB, Ramos J, et al. Time course involvement of matrix metalloproteinases in the vascular alterations of renovascular hypertension. Matrix Biol. 2012;31:261–70. doi: 10.1016/j.matbio.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Badyal DK, Lata H, Dadhich AP. Animal Models of Hypertension and Effect of Drugs. Indian J Pharmacol. 2003;35:349–62. [Google Scholar]
- 30.Verheule S, Sato T, Everett T, 4th, Engle SK, Otten D, Rubart-von der Lohe M, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–65. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro MM, Tanus-Santos JE, Gerlach RF. Matrix metalloproteinases: targets for doxycycline to prevent the vascular alterations of hypertension. Pharmacol Res. 2011;64:567–72. doi: 10.1016/j.phrs.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, et al. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2011;57:123–30. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 34.Melnyk P, Ehrlich JR, Pourrier M, Villeneuve L, Cha TJ, Nattel S. Comparison of ion channel distribution and expression in cardiomyocytes of canine pulmonary veins versus left atrium. Cardiovasc Res. 2005;65:104–16. doi: 10.1016/j.cardiores.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D, Zhang L, Kneller J, Nattel S. Potential ionic mechanism for repolarization differences between canine right and left atrium. Circ Res. 2001;88:1168–75. doi: 10.1161/hh1101.091266. [DOI] [PubMed] [Google Scholar]