Abstract
Objective
Although differentially expressed circRNAs have been proposed to be closely associated with epithelial-mesenchymal transition (EMT), the roles of circRNAs remain unclear in endothelial-to-mesenchymal transition (EndMT), which is a subcategory of EMT. Herein, we characterized the expression and potential function of circRNAs during TGF-β1-induced EndMT in rat coronary artery endothelial cells (CAEC).
Methods
High-throughput RNA sequencing was performed for unbiasedly profiling the expression of circRNAs. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) biological pathway analysis were performed using online forecasting databases. Real-time quantitative polymerase chain reaction (RT-qPCR) was used for confirming the circRNA expression obtained from the sequencing data.
Results
Among the candidated circRNAs, 102 circRNAs were differentially expressed, among which 66 circRNAs and 36 circRNAs were up-regulated and down-regulated, respectively, in TGF-β1-treated rat CAEC. GO analysis findings revealed that numerous differentially expressed circRNAs were closely associated with the biological process. KEGG signaling pathway analysis suggested that the abnormal expression of circRNAs had been implicated in regulating the dynamics endothelial cell junctions. Furthermore, we also found that three EndMT-related circRNAs, chr5:90817794|90827570, chr8:71336875|71337745, and chr6:22033342|22038870, were significantly up-regulated in TGF-β1-treated rat CAEC.
Conclusion
The findings of this study reveal a comprehensive expression and potential functions of differentially expressed circRNAs during TGF-β1-induced EndMT. These findings provide mechanistic insights into the role of circRNAs in EndMT-related cardiovascular diseases (CVDs).
Keywords: circular RNAs, endothelial-to-mesenchymal transition, endothelial cells
Introduction
Endothelial-to-mesenchymal transition (EndMT) refers to a pronounced change in the phenotype of normal endothelial cells that are transdifferentiated into mesenchyma-like cells, which are characterized by induced α-smooth muscle actin (α-SMA) and fibroblast-specific protein-1 (FSP-1) expression and collagen deposition (1). On the contrary, specific markers, platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) and vascular endothelial-cadherin (VE-cadherin), of endothelial cells are down-regulated (2). The course of EndMT can be accelerated by various physiologic and pathologic conditions, such as hyperglycemia (3), hypoxia (4), inflammatory cytokines (5), and a variety of growth factors (6). Recent reports have documented that a high incidence of EndMT drives cardiovascular diseases (CVD), including cardiac neovascularization (7), myocardial fibrosis (8) and atherosclerotic lesions (9). However, molecular mechanisms responsible for the development and progression of EndMT remain to be fully established.
The profibrotic factor transforming growth factor β (TGF-β) plays a crucial role in driving EndMT progression through drosophila mothers against decapentaplegic (Smad)-dependent and independent manner (6). Aortic endothelial cells are stimulated with the TGF-β1 mediated induction of α-SMA expression and concomitant loss in VE-cadherin expression, leading to EndMT (8). In the process of EndMT, TGF-β1 exerts its biological function by regulating messenger RNA (mRNA), microRNA (miRNA), and long non-coding RNA (lncRNA) (10, 11).
Circular RNAs (circRNAs), a known class of special non-coding RNAs, are generally formed by backsplicing and are characterized by high stability, covalently closed continuous loop, without 5’ to 3’ polarity and polyadenylated tail, which give them the distinct ability to counteract RNA exonucleolytic digestion (12). Up till now, circRNAs have been identified as a special kind of endogenous non-coding RNA, which is ubiquitously expressed in various normal and abnormal human tissues (13). CircRNAs have been proposed as significant regulators for understanding growth and development, tissue regeneration, underlying pathological mechanisms, and therapeutic targets for diseases. Emerging evidence shows that circRNAs may play essential roles in the initiation and development of CVDs (14, 15). CircRNA_000203 can act as miRNA sponges in regulating the expression of miR-26b-5p and contributes to the pro-fibrosis effect in cardiac fibroblasts (14). Heart-related circRNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223 (16). Moreover, long non-coding RNA called Antisense Non-coding RNA in the INK4 Locus(ANRIL), one of the INK4/ARF proteins, is reported to play vital roles in proliferation inhibition in cancer and other diseases (17). Burd et al. (18) reported that the expression of circular ANRIL is correlated with the INK/ARF transcription and atherosclerotic vascular disease atherosclerosis risk. However, the relationship between circRNAs and TGF-β1-induced EndMT is rarely reported.
Here, we utilized high-throughput sequencing and experimental validation in vitro to uncover the differentially expressed circRNAs in TGF-β1-treated rat coronary artery endothelial cells (CAECs). Next, we performed GO and KEGG biological pathway analysis for predicting potential functions of differentially expressed circRNAs during TGF-β1-induced EndMT. Furthermore, EndMT-related circRNAs were validated using RT-qPCR.
Methods
Isolation and culture of rat CAECs
Ten-week-old male Wistar rats (body weight, 280±20 g; n=10) were obtained from Sun Yay-sen University Experimental Animal Center (Guangzhou, China) and were allowed to acclimate to the environment for 1 week. Rat CAECs were isolated as described (19). Isolated cells were resuspended in Dulbecco’s modified Eagle’s medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 20% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2 atmosphere at 37°C.
Immunofluorescence staining
Rat CAECs were fixed in 4% paraformaldehyde and permeabilized with 0.3% Trition-X100. After blocking with 10% BSA for 1 h, normal endothelial cells and TGF-β1-treated endothelial cells were stained for CD31 (Abcam, Cambridge, UK) and FSP-1 (Abcam, Cambridge, UK). And then endothelial cells were incubated with AlexaFluor-conjugated secondary antibodies (Invitrogen Technology, USA) at room temperature in the dark for 1 h. Cells were visualized under a scanning confocal microscope (LSM 510 META, Carl Zeiss, Germany; TCS SP5, Leica, Germany).
High-throughput RNA sequencing of circRNA
Rat CAECs were incubated in 10 ng/ml TGF-β1 (TGF-β1 group) (Sigma, St. Louis, MO, USA) or vehicle solution (Control group), and cells were harvested. Total RNA was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer’s protocol. The concentration and purity of total RNA were measured using NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). After removing ribosomal RNA and building a library, a high-throughput RNA sequencing was performed. According to the method by Memczak et al. (20), the clean reads were aligned to the reference genome using Bowtie2 (http://bowtie-bio.sourceforge.net/bowtie2/manual.shtml). For unmapped reads, the junctions were picked out using back-splice algorithm. Lastly, circRNAs were verified with the software developed by Shanghai OE Biotech (Shanghai, China) which was considered as the reference sequence for further analysis. The expression level of circRNAs was measured using “Mapped backsplicing junction reads per million mapped reads” (RPM).
Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) biological pathway analysis
Database for Annotation, Visualization and Integrated Discovery was used for analyzing the potential functions of differentially expressed circRNA-host genes. KEGG pathway analysis was performed for determining the involvement of linear transcripts in different biological pathways, as described (21). KOBAS software was used for testing the statistical enrichment of the differentially expressed circRNA-host genes in the KEGG pathways (http://www.genome.jp/kegg/) (22).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
For confirming the sequencing results, RT-qPCR detection was performed for evaluating the expression levels of circRNAs using a SYBR Green PCR kit (GeneCopoeia, Inc. Rockville, MD, USA) with ABI7300 System (Applied Biosystems, Foster City, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was utilized for normalizing circRNA expression. The relative expression levels of circRNAs were calculated using the 2-ΔΔCt method (23).
Validation of circRNA in Rat CAECs
PCR was performed using these convergent and divergent primers, with cDNA or gDNA as templates. The PCR procedure was performed as described (22). PCR products were separated using agarose gel electrophoresis and visualized with UV-photography (GeneGenius; Syngene; UK).
Statistical analysis
Data are presented mean ± standard deviation for each group. All statistical analyses were performed using PRISM version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Student’s t-test was used for analyzing two-group differences. P<0.001 was considered to indicate a statistically significant difference.
Results
Rat CAEC isolation, morphological identification, and EndMT establishment
After the rat CAEC was isolated, cellular morphology was identified using phase contrast microscope. The results demonstrated that CAEC had typical endothelial cobblestone morphology (Fig. 1a, 1e). We also found that the CD31+ cells stained positively (green color) (Fig. 1b). Upon cultivation in TGF-β1 (10 ng/ml) condition for 7 days, rat CAEC acquired the filamentous antennal morphology, associated with the decreased CD31 expression and the increased FSP1 expression, typical of EndMT (Fig. 1c, 1d, 1f). These results indicate that TGF-β1-based treatment can be used for establishing the EndMT cell model.
Figure 1.
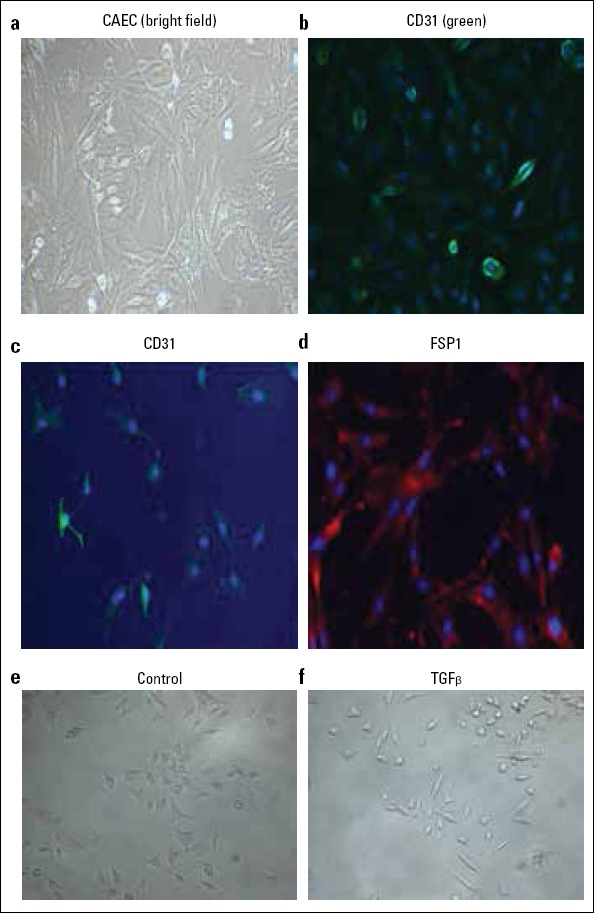
TGF-β1-induced EndMT in rat CAEC. The morphology of rat CAEC was shown as bright field image (a). Immunofluorescence staining of CD31 (green) in rat CAEC (b). Immunofluorescence staining of CD31 (c) and FSP1 (d) in TGF-β1-treated rat CAEC. E&F: typical morphology comparison for EndMT in rat CAEC between Control and TGF-β1 group
Endothelial circRNA expression profile in TGF-β1-induced EndMT
For investigating the differentially expressed circRNAs in TGF-β1-induced EndMT, Rat CAEC was exposed to TGF-β1 (10 ng/mL) condition for 7 days, and then the circRNA expression profile was screened using high-throughput circRNA sequencing. Differentially expressed circRNAs were selected using FDR ≤ 0.0001 and log2 (fold change) ≥1 or ≤-1. The results demonstrated that 102 circRNAs were differentially expressed between the normal control and TGF-β1-treated groups, among which 66 circRNAs and 36 circRNAs were up-regulated and down-regulated, respectively (Fig. 2a). For further analysis of the potential functions of differentially expressed circRNAs and their host genes, GO annotations and KEGG pathways analysis were performed. GO analysis was classified into the following three categories: cellular component (CC), biological process (BP) and molecular function (MF). Notably, numerous differentially expressed circRNAs were closely associated with BP, among which the most enriched and meaningful BP terms were metabolic process, cellular process, CC organization, developmental process, pigmentation, response to stimulus, localization, and biological regulation. As for CC, cell, organelle, and cell part were the most enriched terms. Furthermore, catalytic activity and binding were the most enriched MF terms (Fig. 2b). KEGG signaling pathway analysis was performed using their host genes and showed that 20 pathways were filtered according to Risk Factor (<0.05) and associated with differentially expressed circRNAs and their host genes. The top seven pathways were as follows: the regulation of actin cytoskeleton, proteoglycans in cancer, protein processing in endoplasmic reticulum, focal adhesion, endocytosis, cAMP signaling pathway, and bacterial invasion of epithelial cells (Fig. 2c).
Figure 2.
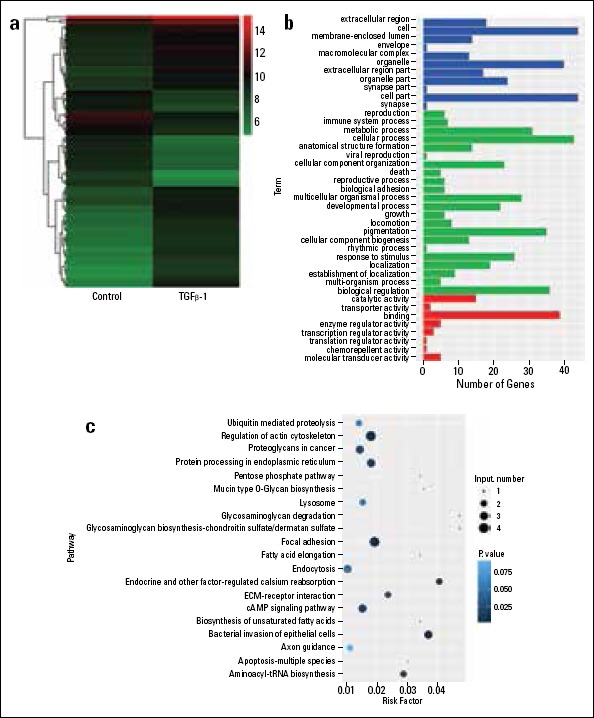
Endothelial circRNA expression profile in TGF-β1-induced EndMT. Hierarchical cluster analysis and heat map showed the differently expressed circRNAs in rat CAEC with or without TGF-β1 treatment (a). GO annotations of differently expressed circRNA-host genes in rat CAEC with the threshold of P<0.05 were listed (b). KEGG pathway analysis was performed to determine the correlation between differentially expressed circRNA and pathways during TGF-β1-induced EndMT (c)
Verification of key circRNA expression during TGF-β1-induced EndMT
Four circRNAs (chr5:90817794|90827570, chr8:71336875|71337745, chr6:22033342|22038870, chr11:34060062|34073206) and their host genes associated with the progression of EndMT were selected and experimentally tested using RT-qPCR. The expression levels of chr5:90817794|90827570, chr8:71336875|71337745, and chr6:22033342|22038870 were dramatically increased in TGF-β1-treated rat CAEC compared with normal control group (p < 0.001) (Fig. 3a); these results showed the same trend of high-throughput sequencing. However, the expression tendency of chr11:34060062|34073206 showed an inconsistent result between RT-qPCR and high-throughput sequencing. In addition, both convergent and divergent primers were designed for detecting the circular or linear form of these three circRNAs (chr5:90817794|90827570, chr8:71336875|71337745, and chr6:22033342|22038870). The results demonstrated that the circular form was only amplified using the divergent primers and cDNA as template; however, the amplified bands showed no signs of genomic DNA (gDNA) with divergent primers. Both cDNA and gDNA could amplify linear RNA using convergent primers by RT-qPCR assay (Fig. 3b). These findings confirmed that a head-to-tail splicing existed in these circRNAs.
Figure 3.
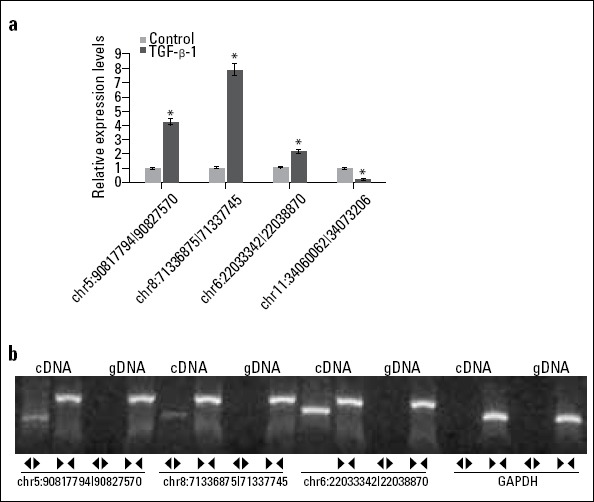
Verification of key circRNA expression during TGF-β1-induced EndMT. EndMT-related circRNAs were validated using RT-qPCR in rat CAEC with or without TGF-β1 treatment (A). The convergent and divergent primers were designed for detecting the circular or linear form of EndMT-related circRNAs (B). *P<0.001 vs. Control
Discussion
In the present study, to the best of our knowledge, we reported for the first time that circRNAs were involved in TGF-β1-induced EndMT in rat CAEC, and 66 of 102 differentially expressed circRNAs were markedly up-regulated and 36 of 102 were down-regulated in TGF-β1-treated rat CAEC. In addition, 102 differentially expressed circRNAs originated from 120 host genes. We also found that 13 host genes could produce more than two circRNAs during TGF-β1-induced EndMT.
For elaborating the regulatory roles of circRNAs during TGF-β1-induced EndMT, GO analysis was performed to annotate the biological functions of host linear transcripts in the progression of EndMT. The most enriched terms were cell, organelle, cell part, cellular process, and binding, which share similarity with circRNAs functions (21, 24). CircRNA was reported to bind to specific miRNAs, which can inhibit the expression of miRNAs and deregulate the target genes of miRNAs (20, 25). For example, circCDR1as harbors more than 70 conserved binding sites for sponging miR-7 (20). Similarly, sex-determining region Y (SRY)-based circRNA possesses 18 putative binding sites for miR-138 (25). These circRNAs can bind to miRNAs regulation of functional gene expression.
KEGG signaling pathway analysis suggested that regulation of actin cytoskeleton and focal adhesion were the top two pathways that were closely connected with circRNAs abnormal expression during TGF-β1-induced EndMT. The endothelial actin cytoskeletal is involved in cell migration, proliferation, barrier properties of the endothelium, and vascular remodeling, which attributes to its contractile machinery and is mediated by the Rho GTPases (26, 27). Reorganization of the actin cytoskeleton is also associated with EMT and EndMT, and the expression of numerous actin regulatory genes is up-regulated (28-30). Cell–cell adhesion complexes anchored to the actin cytoskeleton are critical to the establishment of homotypic cell–cell junctions (31). The expression of endothelial cell–cell adhesion proteins such as E-cadherin is down-regulated, but mesenchymal markers such as N-cadherin, vimentin, and fibronectin are up-regulated in the process of EndMT (32). Both actin cytoskeleton and focal adhesion have been implicated in regulating the dynamics endothelial cell junctions. These findings suggest that circRNA may regulate actin cytoskeleton and focal adhesion alteration of cell–cell junctions during TGF-β1-induced EndMT.
The roles of circRNAs in the endothelial cells have recently been rapidly elaborated, suggesting that circRNAs mainly exhibits miRNA decoys to regulate the circRNA-miRNA-mRNA interaction network (33-35). For example, circRNA_0010729 regulates vascular endothelial cell proliferation and apoptosis by inhibiting miR-186 and induction of HIF-1α (36). CircRNA-HIPK3 can inhibit miR-30a-3p activity, which leads to the up-regulation of VEGFC, FZD4, and WNT2 expression (35). A recent report demonstrates that the production of numerous abundant circRNAs is regulated in response to TGF-β-induced EMT in human mammary epithelial cells (37). In this study, circRNAs were regulated as a response to TGF-β in rat CAEC, and endothelial cell–cell adhesion was closely associated with differentially expressed circRNAs, suggesting that they carried out functions associated with the mesenchymal transition. Moreover, TGF-β1 is increased by circRNA_010567 and promotes myocardial fibrosis in diabetic mice (15). This may create a strong positive feedback loop between TGF-β1 and circRNAs in some pathologic conditions.
Study limitations
The present study is very preliminary. In vitro and in vivo experiments are needed to investigate the mechanism of the three End-β-1-induced circRNAs in myocardial fibrosis.
Conclusion
In summary, our data indicated that the abundant and aberrantly expressed circRNAs were explored during TGF-β1-induced EndMT using high-throughput sequencing technology. Our data provide rational and mechanistic insights into the role of circRNAs during TGF-β1-induced EndMT. Moreover, we also found that three EndMT-related circRNAs, chr5:90817794|90827570, chr8:71336875|71337745, and chr6:22033342|22038870 were significantly up-regulated in TGF-β1-treated rat CAEC. Examining the roles of individual circRNAs in EndMT-induced myocardial fibrosis will be the subject of future investigations.
Footnotes
Funding: This study was supported by Southern Medical University Clinical Research Project (LC2016ZD002) and the President’s Fund of Nanfang Hospital (2016B015).
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – X.H., J.P.; Design – X.H., J.P.; Supervision – J.P.; Fundings – J.P.; Materials – Y.C., J.X.; Data collection &/or processing – Z.H.; Analysis &/or interpretation – Z.H.; Literature search – L.H., D.X.; Writing – X.H., Y.C.; Critical review – J.P.
References
- 1.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012;125:1795–808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SH, Hong ZY, Nam JK, Lee HJ, Jang J, Yoo RJ, et al. A Hypoxia-Induced Vascular Endothelial-to-Mesenchymal Transition in Development of Radiation-Induced Pulmonary Fibrosis. Clin Cancer Res. 2015;21:3716–26. doi: 10.1158/1078-0432.CCR-14-3193. [DOI] [PubMed] [Google Scholar]
- 3.Feng B, Cao Y, Chen S, Chu X, Chu Y, Chakrabarti S. miR-200b Mediates Endothelial-to-Mesenchymal Transition in Diabetic Cardiomyopathy. Diabetes. 2016;65:768–79. doi: 10.2337/db15-1033. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, Zeisberg EM. Snail Is a Direct Target of Hypoxia-inducible Factor 1α(HIF1α) in Hypoxia-induced Endothelial to Mesenchymal Transition of Human Coronary Endothelial Cells. J Biol Chem. 2015;290:16653–64. doi: 10.1074/jbc.M115.636944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie L, Lyros O, Medda R, Jovanovic N, Schmidt JL, Otterson MF, et al. Endothelial-mesenchymal transition in normal human esophageal endothelial cells cocultured with esophageal adenocarcinoma cells: role of IL-1βand TGF-β2. Am J Physiol Cell Physiol. 2014;307:C859–77. doi: 10.1152/ajpcell.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, et al. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012;2:1684–96. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–90. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spillmann F, Miteva K, Pieske B, Tschöpe C, Van Linthout S. High-density lipoproteins reduce endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. 2015;35:1774–7. doi: 10.1161/ATVBAHA.115.305887. [DOI] [PubMed] [Google Scholar]
- 9.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–28. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang Y, Zhang Y, Tang Y, Li Q. MALAT1 Modulates TGF-β1-Induced Endothelial-to-Mesenchymal Transition through Downregulation of miR-145. Cell Physiol Biochem. 2017;42:357–72. doi: 10.1159/000477479. [DOI] [PubMed] [Google Scholar]
- 11.Hong JP, Li XM, Li MX, Zheng FL. VEGF suppresses epithelial-mesenchymal transition by inhibiting the expression of Smad3 and miR192, a Smad3-dependent microRNA. Int J Mol Med. 2013;31:1436–42. doi: 10.3892/ijmm.2013.1337. [DOI] [PubMed] [Google Scholar]
- 12.Hentze MW, Preiss T. Circular RNAs: splicing's enigma variations. EMBO J. 2013;32:923–5. doi: 10.1038/emboj.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Tang CM, Zhang M, Huang L, Hu ZQ, Zhu JN, Xiao Z, et al. CircRNA_000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci Rep. 2017;7:40342. doi: 10.1038/srep40342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem Biophys Res Commun. 2017;487:769–75. doi: 10.1016/j.bbrc.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37:2602–11. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 17.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–75. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida M, Carley WW, Gerritsen ME, Ellingsen O, Kelly RA, Smith TW. Isolation and characterization of human and rat cardiac microvascular endothelial cells. Am J Physiol. 1993;264:H639–52. doi: 10.1152/ajpheart.1993.264.2.H639. [DOI] [PubMed] [Google Scholar]
- 20.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Guo J, Chen Y, Chang C, Xu C. Comprehensive CircRNA expression profile and selection of key CircRNAs during priming phase of rat liver regeneration. BMC Genomics. 2017;18:80. doi: 10.1186/s12864-016-3476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao W, Cheng Y, Zhang C, You Q, Shen X, Guo W, et al. Genome-wide identification and characterization of circular RNAs by high throughput sequencing in soybean. Sci Rep. 2017;7:5636. doi: 10.1038/s41598-017-05922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, et al. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–30. doi: 10.1016/0092-8674(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 26.Weber M, Kim S, Patterson N, Rooney K, Searles CD. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am J Physiol Heart Circ Physiol. 2014;306:H1192–203. doi: 10.1152/ajpheart.00521.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidemann A, Breyer J, Rehm M, Eckardt KU, Daniel C, Cicha I, et al. HIF-1alpha activation results in actin cytoskeleton reorganization and modulation of Rac-1 signaling in endothelial cells. Cell Commun Signal. 2013;11:80. doi: 10.1186/1478-811X-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankar J, Nabi IR. Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. PloS One. 2015;10:e0119954. doi: 10.1371/journal.pone.0119954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris HT, Machesky LM. Actin cytoskeletal control during epithelial to mesenchymal transition: focus on the pancreas and intestinal tract. Br J Cancer. 2015;112:613–20. doi: 10.1038/bjc.2014.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clouthier DL, Harris CN, Harris RA, Martin CE, Puri MC, Jones N. Requisite role for Nck adaptors in cardiovascular development, endothelial-to-mesenchymal transition, and directed cell migration. Mol Cell Biol. 2015;35:1573–87. doi: 10.1128/MCB.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Zhang Y, Zhong B, Luo CF. SMAD7 prevents heterotopic ossification in a rat Achilles tendon injury model via regulation of endothelial-mesenchymal transition. FEBS J. 2016;283:1275–85. doi: 10.1111/febs.13667. [DOI] [PubMed] [Google Scholar]
- 33.Boeckel JN, Jaé N, Heumüller AW, Chen W, Boon RA, Stellos K, et al. Identification and Characterization of Hypoxia-Regulated Endothelial Circular RNA. Circ Res. 2015;117:884–90. doi: 10.1161/CIRCRESAHA.115.306319. [DOI] [PubMed] [Google Scholar]
- 34.Yao Z, Luo J, Hu K, Lin J, Huang H, Wang Q, et al. ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol. 2017;11:422–37. doi: 10.1002/1878-0261.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, et al. Circular Non-Coding RNA HIPK3 Mediates Retinal Vascular Dysfunction in Diabetes Mellitus. Circulation. 2017;136:1629–42. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 36.Dang RY, Liu FL, Li Y. Circular RNA hsa_circ_0010729 regulates vascular endothelial cell proliferation and apoptosis by targeting the miR-186/HIF-1alpha axis. Biochem Biophys Res Commun. 2017;490:104–10. doi: 10.1016/j.bbrc.2017.05.164. [DOI] [PubMed] [Google Scholar]
- 37.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–34. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]


