Abstract
Objective
Cisplatin (CDDP) has been known to be an effective antineoplastic drug; however, it has a cardiotoxic effect. Curcumin (CMN) and beta-carotene (BC) have been suggested to protect biological systems against CDDP-induced damage. The current study was conducted to evaluate the possible protective roles of CMN and BC on CDDP-induced cardiotoxicity in rat cardiac tissues.
Methods
A total of 49 adult female
Wistar albino rats were equally divided into seven groups as follows
control (no medication), sesame oil (1 mg/kg), CDDP (single dose injection two times as once a week, 5 mg/kg/week), BC (100 mg/kg), CDDP+BC (pretreated BC for 30 min before CDDP injection), CMN (200 mg/kg), and CDDP+CMN (pretreated CMN for 30 min before CDDP injection). These treatments were applied intraperitoneally for CDDP and with gavage for CMN and BC. The oxidative/antioxidant indicators, inflammatory cytokines, and histopathological alterations were examined.
Results
These alterations included a marked increase in malondialdehyde (MDA) level, significant decrease in catalase (CAT) and superoxide dismutase (SOD) activities, and significant elevation of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, interleukin (IL)-6 in the CDDP group compared with the other groups. Histopathologically, CDDP-induced severe myocardial degenerative changes were observed. However, the CDDP-induced disturbances in the above-mentioned parameters significantly improved by treatment with BC and particularly CMN.
Conclusion
This study indicated that CDDP treatment markedly caused cardiotoxicity; however, treatment with CMN or BC ameliorated this cardiotoxicity in rats. Furthermore, these findings revealed that treatment with CMN has a higher cardioprotective effect than that with BC against CDDP-induced cardiotoxicity in rat cardiac tissues.
Keywords: cisplatin, cardiotoxicity, curcumin, beta-carotene, rat heart tissue
Introduction
Chemotherapy, particularly using cisplatin [cis-diamminedichloroplatinum (II); CCDP], declines the normal homeostasis of the body (1). CCDP is a platinum-based effective chemotherapeutic drug that widely used in oncological applications. Owing to its potential activity on carcinoma cells, CCDP has been used to treat various types of malignancies such as bladder, cervical, ovarian, testicular, breast, lung, and head and neck cancer; however, it has frequent adverse effects (2), which include hepatotoxicity, nephrotoxicity, cardiotoxicity and so on (3-5). Although the underlying mechanisms concerning the antitumor effects of CDDP are relatively well known, the cellular and molecular mechanisms concerning its cardiotoxic effects are still unclear (6).
CDDP-induced cardiotoxic effects have been observed through serious changes in electrocardiography and arrhythmias, which include ventricular arrhythmias, supraventricular tachycardia, atrial fibrillation, occasional sinus bradycardia, and occasional complete atrioventricular block (6-10). These cardiotoxic (and other potentially unknown) problems eventually lead to congestive heart failure and sudden cardiac death (11). Clinical approaches to improve CDDP-induced cardiac toxicity have also met little achievement (12, 13). Therefore, it has become necessary to develop a therapy to limit these CDDP-induced toxicities as they still remain as a major challenge despite several clinical interventions (11).
Curcumin (diferuloylmethane, CMN) is a biologically active and natural dietary polyphenol component extracted from turmeric rhizomes, Curcuma longa Linn, a plant species found in Asian subcontinents. It is now used in several countries including the United States, India, Japan, Korea, Thailand, China, Turkey, South Africa, Nepal, and Pakistan as a dietary supplement and in traditional herbal medicine (14, 15). The protective effects of CMN against various diseases such as cancer and autoimmune, neurological, metabolic, lung, liver, and cardiovascular diseases (CVDs) have been attributed to its antioxidant, antimicrobial, anti-inflammatory, anticancer, and antiangiogenic activities (14-17). It inhibits cancer cell survival and proliferation and induces apoptosis without promoting the development of side effects (14, 18). Although, the molecular basis for the CMN effects is not fully understood, this polyphenol has been suggested to have various promising therapeutic properties, particularly antitumor activity through multiple mechanisms (18).
Carotenoids belong to the teraterpene family, which is found in plants, algae, photosynthetic bacteria, fungi, and animals (19). They are a group of naturally occurring lipid-soluble compounds that have strong antioxidant effects in humans and animals. Over 700 naturally occurring carotenoids have been identified so far. Beta-carotene (BC) is one of the isoprenoid compounds named carotenoids. BC, a well-known natural antioxidant and precursor of vitamin A, is responsible for the dark green or yellow-orange color of fruits and vegetables and plays a key role in photosynthesis (19, 20). BC is used in the prevention and treatment of many diseases that develop with the participation of oxidative stress (mainly cancer and cardiovascular abnormalities) because of its free radical scavenging properties (21). However, it may also act as a tissue-damaging pro-oxidant depending on the physiologic environment (22). However, in vitro studies have shown the potent antioxidant activity of BC; thus, it protects biological systems against CDDP-induced damage (20).
The aim of the present study was to examine the protective role of CMN and BC on CDDP-induced cardiotoxicity by investigating the effects of CDDP, CMN, and BC on (a) antioxidant and oxidative stress indicators such as malondialdehyde (MDA) and superoxide dismutase (SOD) antioxidant enzyme namely catalase (CAT), (b) inflammatory cytokines like interleukin-1 beta (IL-1β), interleukin-6 (IL- 6), and tumor necrosis factor-alpha (TNF-α), and (c) histopathological and immunohistochemical alterations in rat cardiac tissues.
Methods
Chemicals and drugs
CDDP, CMN, and BC were purchased from Sigma–Aldrich (Saint. Louis, MO, USA). Cisplatin was administered intraperitoneally (ip); CMN and BC were dissolved in sesame oil and given by gavage. All the solutions were prepared freshly before the experiment.
Animals
This study was performed at the Experimental and Clinical Research Centre of Erciyes University. All applications were performed under veterinarian control in accordance with the Universal Declaration of International Animal Rights after approval was received from Erciyes University, Clinical Research Ethics Committee (Approval protocol no. EUHADYEK-16/124).
A total of 49 8-week-old healthy adult female Wistar albino rats weighing between 190 and 250 gm were included. The rats were housed in separate clean plastic cages at a constant temperature of 24°C-26°C and humidity of 40%-60% with a constant 12 h light/dark cycle. Food and water were provided ad libitum. All treatments were started after almost 1 week of stabilization from arrival. The use of animals and the experimental protocol were approved by the Institutional Animal Care and Use Committee, and animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals of Research Council.
Treatment and experimental design
CDDP was dissolved in normal saline and injected at single doses of 5 mg/kg/week, which is well documented to induce cardiotoxicity in rats (23, 24). CMN was dissolved in sesame oil and was administered as 200 mg/kg BW (25). BC was dissolved in sesame oil and was administered as 100 mg/kg BW (26).
The rats were equally and randomly divided into seven groups with seven rats in each group as follows:
Group I (Control): rats did not receive any treatment until the end of the study.
Group II (sesame oil): rats received only sesame oil (1 mg/kg BW)
Group III (CDDP): rats received a single dose injection of CCDP two times as once a week (5 mg/kg BW/week, ip)
Group IV (BC): rats treated with BC (100 mg/kg BW) orally.
Group V (CDDP+BC): rats pretreated with BC for 30 min before CDDP injection, then received CDDP (5 mg/kg/week, ip)
Group VI (CMN): rats orally treated with CMN (200 mg/kg BW)
Group VII (CDDP+CMN): rats pretreated with CMN (200 mg/kg) for 30 min before CDDP injection, then received CDDP (5 mg/kg/week, ip).
All treatments were applied at the same time of day. The second administration was done 1 week after the first administration and the same injection and ip and/or gavage procedures as in the first administration were applied to the experimental groups for the second time.
Sampling
After 5 days from the second administration, rats were intraperitoneally anesthetized with a mixture of 50 mg/kg BW of ketamine hydrochloride 10% (Ketalar; Eczacıbaşı İlaç Sanayi ve Ticaret AŞ, Lüleburgaz, Turkey) and 10 mg/kg BW of xylazine hydrochloride 2% (Alfazyne; Alfasan International BV, Woerden, Netherlands). A middle-line incision was made in the chest, and the cardiac tissues were quickly removed and separated from the surrounding tissues and washed twice with an ice-cold phosphate-buffered saline solution (pH: 7.4). Samples from the left ventricle were collected and fixed in 10% buffered neutral formalin solution for histopathological and immunohistochemical examinations. The other cardiac tissues were stored at −80°C to determine the level of MDA, CAT, and SOD. Then, all the rats were immediately sacrificed by exsanguination.
ELISA analysis
Cardiac tissues collected for enzyme-linked immunosorbent assay (ELISA) analyses were stored at −80°C until analyses were performed, at which time, the cardiac tissues were removed to room temperature and left to warm for 15 min and homogenized on ice-cold according to the procedures of the ELISA kit. After homogenization, the cardiac tissues were centrifuged at +4°C for 30 min at 12.000 rpm; the supernatants were then aliquoted and prepared for ELISA procedures.
Determination of MDA content
To investigate the cardiac oxidative damage caused by the induction of CDDP, the levels of MDA, a marker of lipid peroxidation were measured in the heart using a commercially available ELISA kit (MDA Elabscience® ELİSA Kit Catalog no. E-EL-0060, USA) according to the manufacturer’s instructions. The MDA level was assessed by measuring thiobarbituric-acid reactive substances at 540 nm and was expressed as nmol/g tissue for cardiac tissue homogenates.
Determination of CAT activity
The CAT activity was assayed using a commercially available assay kit (CAT Elabscience® ELISA Kit Catalog no. E-EL-R2456, USA) according to the manufacturer’s instructions. This kit uses the peroxidase function of catalase to determine the enzyme activity. The method is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of H2O2. Absorbance was read at 540 nm using a plate reader. The CAT activity was expressed as U/g for cardiac tissue homogenates.
Determination of SOD activity
The SOD activity was measured in accordance with the protocol supplied by commercially available SOD assay kit (SOD Elabscience® ELİSA Kit Catalog no. E-EL-R1424, USA). The assay used a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical, and the absorbance was read at 450 nm using an ELISA plate reader. The SOD activity was expressed as U/g protein for cardiac tissue homogenates.
Quantitative immunohistochemistry
The quantification of the IR intensity in both control and treated groups was included in the analysis. The cardiac tissues were taken from random ten of the visual fields for each section at an original magnification ×40. The IL-1β, IL-6, and TNF-α IR intensities for each cardiac sections were calculated with the mean of IR intensities using Image J software.
Histopathological evaluation
Tissue samples were fixed in 10% buffered neutral formalin solution and placed in fixation solution for 48 h and in water for one night. Next, tissue specimens were dehydrated by passing through increasing concentrations of alcohol, cleared by passing through xylol, and finally embedded in paraffin blocks. Paraffin sections of 5-µm slice thickness were cut from each specimen and placed on poly-lysine coated slides. Slides were deparaffinized, rehydrated through a graded series of ethanol, and rinsed in distilled water. The specimens were stained with haematoxylin–eosin (H&E) stain to evaluate the tissue morphology and integrity.
Immunohistochemistry
Immunohistochemical staining was performed using the avidin–biotin method to determine the differences in immunoreactivity (IR) intensity of IL-1β, IL-6, and TNF-α. Briefly, 5-µm thick sections were rinsed in deionized water, and antigen retrieval was performed by incubation in 0.01 M sodium citrate buffer (pH: 6.0) at 300 W for 10 min and cooling to room temperature for 20 min. The sections were incubated in 3% H2O2 for 10 min and rinsed in phosphate-buffered saline (PBS). Anti-Polyvalent HRP kit (Thermo Scientific, USA) was used for the following steps. To reduce nonspecific staining, sections were pretreated with normal block serum for 20 min. After sections were incubated with the primary antibodies IL-1 (1: 100, NBP1-19-775, Littleton, USA), IL-6 (1: 100, EPP14346, Hubei, China), and TNF-α antibody (1: 50, sc.25280; Santa Cruz Biotechnology, Santa Cruz, CA) were incubated overnight at 4°C in a humidified chamber. After washing thrice for 5 min in PBS, sections were incubated with the biotinylated secondary antibodies for 15 min. After washing in PBS, 3, 3 P-diaminobenzidine tetrahydrochloride (DAB) (Lab vision, UltraVision detection system Large volume DAB substrate system, TA-125-HD) was added as chromogen for 3-5 min at room temperature. After washing with deionized water, sections were counterstained with Gill’s hematoxylin and mounted using a clear mounting medium (00-8010; Zymed Laboratories Inc. South San Francisco, CA). The specificity of the immunoreaction was confirmed by incubation with PBS instead of the primary antibodies.
Statistical analysis
Data were expressed as either median (min-max) values or mean standard deviation (SD) depending upon the overall variable distribution. A computer program SPSS Version 15.0 (SPSS Inc, Chicago, Illinois, USA) was used for statistical analysis. Normally distributed data were analyzed by one-way analysis of variance (ANOVA) test followed by Tukey post-hoc test. Non-normally distributed data were compared by nonparametric Kruskal-Wallis H test followed by Dunn-Bonferroni test among the groups. Differences were considered to be significant at p<0.05.
Results
Biochemical findings
Table 1 summarizes the results of the biochemical parameters of oxidative stress, namely MDA, SOD activity, and antioxidant enzymes namely SOD and CAT activity in rat cardiac tissues in each experimental group.
Table 1.
Comparison of the oxidative/antioxidant status in cardiac tissue of each experimental group
| Parameters | Experimental Groups | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Control mean±SD | Sesame oil mean±SD | CDDP mean±SD | BC mean±SD | CDDP+BC mean±SD | CMN mean±SD | CDDP+CMN mean±SD | ||
| MDA | 29.239±0.91a | 28.562±1.09a | 33.274±1.35b | 30.383±2.13a,b | 31.528±1.24a,b | 27.701±1.60a | 28.748±1.12a,b | 0.187 |
| (nmol/g tissue) | ||||||||
| SOD | 51.815±1.39a | 51.021±3.02a | 27.197±2.56c | 45.441±3.05a,b | 30.335±3.42c,d | 43.263±3.46a,b,d | 36.460±3.21b,d | 0.000 |
| (U/g tissue) | ||||||||
| CAT | 62.089±3.46a | 53.849±1.65a | 28.018±4.82c | 48.550±2.17b | 35.771±3.50c,d,e,f | 45.258±3.45b,d | 42.575±4.72b,d,e | 0.000 |
| (U/g tissue) | ||||||||
CDDP - Cisplatin; BC - beta-carotene; CMN - curcumin; MDA - malondialdehyde; CAT - catalase; SOD - superoxide dismutase
Values are expressed as mean±SD for seven rats in each group. One-way ANOVA test was also used for comparisons, Tukey test was applied for multiple comparisons. Similar letters on the same line indicate similarity between groups, whereas different letters indicate differences between groups. P<0.05 was considered significant
The MDA levels were significantly higher in the CDDP group than in the control (p=0.025), sesame oil (p=0.018) and CMN (p=0.035) groups. Those levels were found to be much lower in the CDDP+CMN and CDDP+BC groups than in the CDDP group, which was not statistically significant (p>0.05) (Table 1).
The SOD activity in the CDDP group was significantly lower than that in the control (p=0.04), sesame oil (p=0.004), BC (p=0.004), CMN (p=0.004), and CDDP+CMN (p=0.025) groups, but this decrease was not statistically significant in the CDDP+BC group (p>0.05). Also, the SOD levels in the CDDP+BC group were significantly lower than those in the control (p=0.004), sesame oil (p=0.004), and BC (p=0.025) groups. As for the CDDP+CMN group, the SOD levels were significantly lower than those in the control (p=0.004), sesame oil (p=0.016), and BC (p=0.025) groups. However, the cardiac SOD levels in the groups treated with only BC or CMN did not significantly differ with those in the control and sesame oil groups (p>0.05) (Table 1).
The CAT activity in the cardiac tissue was significantly impaired by the induction of CDDP. The CAT activity in the CDDP group was significantly lower than that in the control (p=0.004), sesame oil (p=0.004), CMN (p=0.006), BC (p=0.006), and CDDP+CMN (p=0.006) groups, but this decrease was not statistically significant except in the CDDP+BC group (p>0.05). Also, the CAT levels in the CDDP+BC group were significantly lower than those in the control (p=0.004), sesame oil (p=0.004), and BC (p=0.016) groups. As for the CDDP+CMN group, the CAT levels were significantly lower than those in the control (p=0.010), sesame oil (p=0.016), and CDDP (p=0.006) groups. Meanwhile, in the control and sesame oil groups, the CAT activities were significantly higher than those in the BC and CMN groups (p=0.016 for each). Antioxidant treatment with BC (p=0.006) and especially with CMN (p=0.004) demonstrated a tendency to increase the CAT activity and this increase was statistically significant in the CDDP+CMN group (Table 1).
Immunohistochemical findings IL-1β expression
Sections stained with IL-1β primary antibody are represented in Table 2 and Figure 1. Diffuse and cytoplasmic stainings were assessed in the myocardial tissue slides. Some of the myocardial fibers have demonstrated moderate expressions of IL-1β in the control (Fig. 1a) and sesame oil (Fig. 1a) groups. In only the CDDP group (Fig. 1c), the expression of antiapoptotic protein IL-1β was higher than the control (Fig. 1a), sesame oil (Fig. 1b), BC (Fig. 1d) and CMN (Fig. 1f) groups (p=0.001 for each). The IL-1β IR intensity in the CDDP+BC group (Fig. 1e) was similar to that in the CDDP group (Fig. 1c), but was significantly higher than in the control (Fig. 1a), sesame oil (Fig. 1b), CMN (Fig. 1f), and BC (Fig. 1d) groups (p=0.001 for each). Meanwhile, in the CDDP+CMN group (Fig. 1g), the IL-1 β IR intensity was higher than that in both control (Fig. 1a) and sesame oil (Fig. 1b) groups (p=0.05 for each). However, tissue samples of the CDDP+CMN group (Fig. 1g) exhibited lower IR intensity than both the CDDP+BC (Fig. 1e) (p=0.017) and CDDP (Fig. 1c) (p=0.001) groups. Furthermore, in the samples from only BC (Fig. 1d) and CMN (Fig. 1f) administered groups, there were moderate expressions of IL-1β (p>0.05) (Table 2).
Table 2.
Comparison of the TNF-α, IL-1β, and IL-6 levels in cardiac tissue of each experimental group
| Parameters | Experimental Groups | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Control median (25%-75%) | Sesame oil median (25%-75%) | CDDP median (25%- 75%) | BC median (25%-75%) | CDDP+BC median (25%-75%) | CMN median (25%-75%) | CDDP+CMN median (25%-75%) | ||
| IL-1β | 79.31 | 81.95 | 89.26 | 81.51 | 88.54 | 80.50 | 85.02 | 0.000 |
| (68.10-90.72)a | (72.43-88.51)a | (84.86-99.52)b | (68.17-89.61)a,c | (76.94-98.71)b | (73.61-95.28)a | (69.60-93.67)c | ||
| IL-6 | 59.65 | 64.40 | 74.98 | 59.74 | 62.90 | 62.57 | 58.92 | 0.000 |
| (47.60-68.60)a | (47.40-76.19)a | (52.35-99.63)b | (50.32-69.51)c | (56.57-67.00)d | (47.85-78.25)c | (50.77-69.80)d | ||
| TNF-α | 61.29 | 61.00 | 70.59 | 68.49 | 70.23 | 66.70 | 59.55 | 0.000 |
| (48.59-73.68)a | (50.34-77.04)a | (55.00-79.99)b | (52.33-83.30)b | (61.02-78.82)b | (59.43-76.91)a,b | (53.18-78.37)a | ||
CDDP - cisplatin; BC - beta-carotene; CMN - curcumin; TNF-α - tumor necrosis factor-alpha; IL-1β - interleukin-1 beta; IL-6 - interleukin-6
Data are expressed as median (min.-max.) for seven rats in each group. Kruskal-Wallis analysis was used to compare the groups. Dunn-Bonferroni test was applied for multiple comparisons. Similar letters on the same line indicate similarity between groups, whereas different letters indicate differences between groups. P<0.05 was considered significant.
Figure 1.
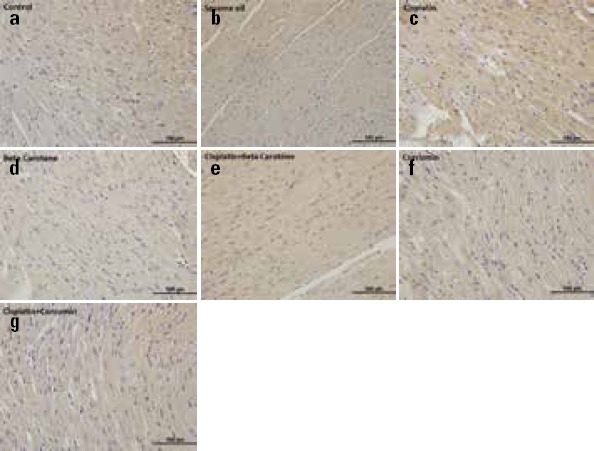
Representative photomicrographs of cardiac immunostaining for IL-1β in experimental groups. (a) Control group (b) Sesame oil group demonstrated weak IL-1β immunoreaction (c) Cisplatin group showing dense IL-1β expression (d) Only β-carotene treated group (e) cisplatin+β-carotene group showing mild IL-1β expression (f) Only curcumin-treated group (g) cisplatin+curcumin group treated group showing mild staining of IL-1β in myocardial fibers. IL-1β immunohistochemical staining of heart samples from the experimental groups (Original magnification=40x, Scale bar=100 µm)
IL-6 expression
We confirmed the expression of IL-6 by immunohistochemistry (Table 2 and Fig. 2). Immunostaining of IL-6 expressions demonstrated moderate staining in the control (Fig. 2a) and sesame oil (Fig. 2b) groups compared with the CDDP group (Fig. 2c). The IR intensity of IL-6 in the CDDP group was significantly higher (Fig. 2c) than that in the control (Fig. 2a), sesame oil (Fig. 2b), BC (Fig. 2d), CDDP+BC (Fig. 2e) and CMN (Fig. 2f), CDDP+CMN (Fig. 2g) groups (p=0.001 for control, sesame oil, BC, CMN, and CDDP+CMN groups; p=0.006 for CDDP+BC group). In other words, the IR intensity of IL-6 was significantly decreased in the CDDP+CMN (Fig. 2g) and CDDP+BC (Fig. 2e) groups compared with the CDDP group (p<0.01). However, IL-6 IR showed no significant change between the BC (Fig. 2d) and CMN (Fig. 2f) groups (p>0.05). Also, the IL-6 expression was reduced in the CDDP+CMN group (Fig. 2g) compared with the CDDP+BC group (Fig. 2e), but the result was not statistically significant (p>0.05) (Table 2).
Figure 2.
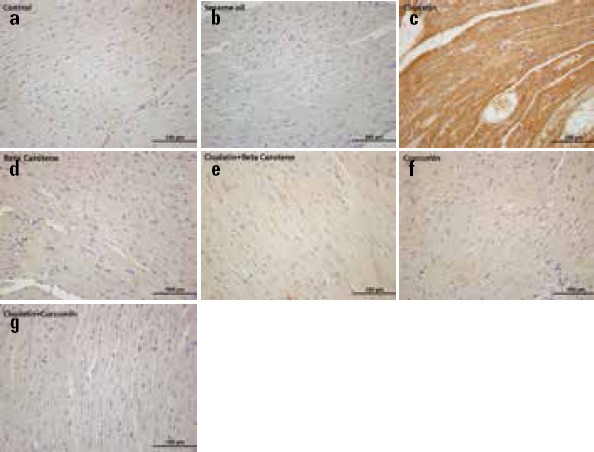
Representative photomicrographs of cardiac immunostaining for IL-6 in experimental groups. (a) Control group (b) Sesame oil group demonstrated weak IL-6 immunoreaction (c) Cisplatin group showing more denser IL-6 expression (d) Only β-carotene treated group (e) cisplatin+ β-carotene group showing mild IL-6 expression like (f) Only curcumin-treated group and (g) cisplatin+ curcumin group. IL-6 immunohistochemical staining of heart samples from the experimental groups (Original magnification=40x, Scale bar=100 µm)
TNF-α expression
Regarding TNF-α IR, the values were significantly lower in the control (Fig. 3a) and sesame oil (Fig. 3b) groups than in the CDDP group (Fig. 3c) (p=0.001 for each) Also, the IR intensity of TNF-α in the CDDP+CMN group (Fig. 3g) was significantly lower than that in the CDDP group (Fig. 3c) (p=0.001); the IR intensity of TNF-α in the CDDP+BC group (Fig. 3e) was significantly higher than that in the control group (Fig. 3a) (p=0.001). In the CDDP+CMN group (Fig. 3g), the TNF-α IR was significantly lower than that in both CDDP+BC (Fig. 3e) (p=0.001) and BC (Fig. 3d) (p=0.005 for each) groups. However, in the BC group (Fig. 3d) TNF-α IR was significantly higher than that in both control (Fig. 3a) (p=0.008) sesame oil (Fig. 3b) groups (p=0.015 for each). Also, in the CDDP+BC group (Fig. 3e), TNF-α IR was significantly higher than that in the sesame oil (Fig. 3b) group (p=0.001). TNF-α IR did not manifest increased immunostaining patterns only in the BC (Fig. 3d) and CMN (Fig. 3f) groups (p>0.05). The expression intensity of TNF-α was almost never reduced in the CDDP+BC group (Fig. 3e), whereas it showed a statistically significant decrease in the CDDP+CMN group (Fig. 3g) compared with the CDDP+BC group (Fig. 3e) (p=0.001) (Table 2).
Figure 3.
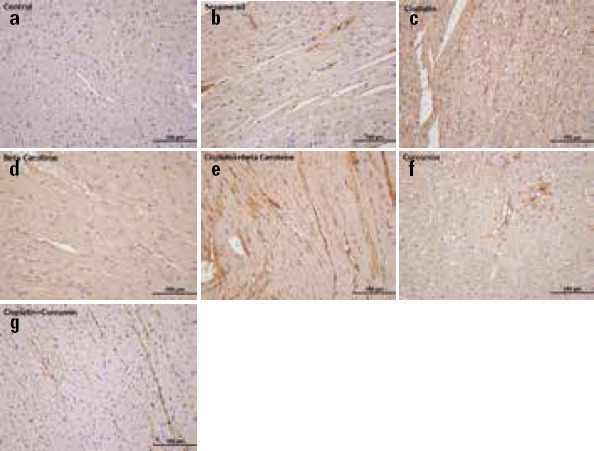
Representative photomicrographs of cardiac immunostaining for TNF-α in experimental groups. (a) TNF-α immunoreactivity was weak in the control group (b) Sesame oil group. (c) TNF-α immunoreactivity intensity of myocardial fibers in cisplatin group was higher than the control group (d) TNF-α immunoreactivity of only β-carotene treated group (e) cisplatin+β-carotene group was lower when the compared to cisplatin group (f) Only curcumin-treated group (g) cisplatin+ curcumin group showed TNF-α immunoreactivity decreased in the cardiac tissues compared to cisplatin group. TNF-α immunohistochemical staining of heart samples from the experimental groups (Original magnification=40x, Scale bar=100 µm)
Histological findings
The general architecture of the cardiac tissues from animals in the control group appeared as regularly arranged cardiac myofibers that had several myocytes consisting mainly of single ovoid and centrally located nuclei (Fig. 4a). The histology of the heart of the sesame oil group was similar to that of the control group (Fig. 4b). In the CDDP groups, normal cardiac architecture was not observed. Microscopic examination of the heart after CDDP administration showed several instances of muscle fiber damage and cytoplasmic vacuolization, hemorrhage, and interstitial edema on section profiles compared with control group (Fig. 4c). However, similar histologic findings such as cytoplasmic vacuolization, hemorrhage, and edema at a more moderate level were observed in only the BC (Fig. 4d) and CMN-treated (Fig.4f) groups. Interestingly, both CDDP+BC (Fig. 4e) and CDDP+CMN (Fig. 4g) groups showed an improved histological appearance compared with CDDP group. Histopathological changes in the CDDP group were not significantly obvious according to these groups.
Figure 4.
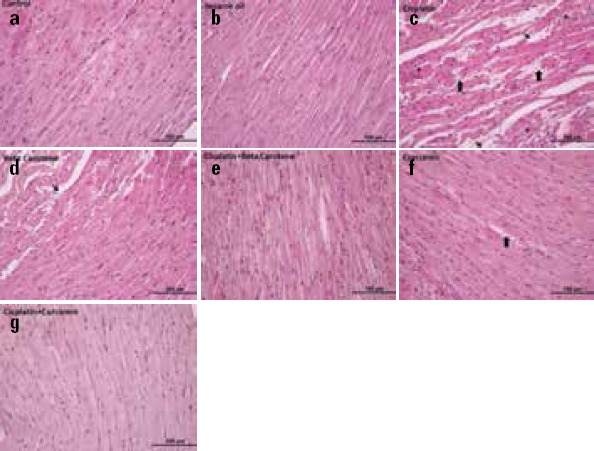
The morphology of the heart tissue. The images were representatives the H&E–stained sections of heart from experimental groups (a) Sagittal sections of the rat cardiac tissue showing the normal structure in control group (b) Only sesame oil treated group (cc) Cisplatin-injured cardiac tissue obtained from cisplatin group (d) Only β-carotene treated group (e) The cardiac tissue obtained from the cisplatin+ β-carotene group treated with β-carotene (f) Only curcumin-treated group (g) The cardiac tissue obtained from cisplatin+ curcumin group treated with curcumin (Original magnification=40x, Scale bar=100 µm) Thick arrow, hemorrhage Arrow, interstitial edema Star, muscle fiber damage and cytoplasmic vacuolization
Discussion
Cellular protection against the toxic effects of chemotherapeutic agents is considered as an essential target and is the objective of several studies on dietary antioxidants supplementation. Several medicinal plants and/or essential oils may be used as chemopreventive agents because they contain natural antioxidant active constituents and also prevent the generation of free radicals that may influence the progression of oxidative damage induced by chemotherapeutic agents (3, 4, 18, 27). The present study was conducted to assess the effect of the chronic supplementation of CMN and BC on CDDP-induced cardiotoxicity, to evaluate oxidative stress markers and antioxidant molecules, including MDA, SOD, and CAT, and proinflammatory cytokines like IL-1β, IL-6, and TNF-α in rat cardiac tissues. Additionally, we evaluated the histological and immunohistochemical alterations as well as biochemical and proinflammatory parameters. To the best of our knowledge, there is no report showing that both CMN and BC ameliorated CDDP-induced cardiotoxicity in rats.
Some studies have been indicated that CDDP decreased antioxidant enzymes activities (CAT, SOD, glutathione S-transferase; GST, glutathione peroxidase; GSH-Px,) and increased MDA levels (1, 3, 5, 7). However, it has been demonstrated that CMN increased the reduced level of CAT, SOD, GST, and GSH and decreased the elevated level of MDA and lipid peroxidase in rat cardiac tissues against experimental-induced different cardiotoxicity models, except for CDDP-induced cardiotoxicity model in rats (16, 28). Regarding the CDDP-induced cardiotoxicity model, Uday Kiran et al. (29) documented that the elevated levels of MDA clearly indicate the myocardial damage with a significant reduction in the GSH and CAT levels in the CDDP-treated rat groups. However, BC-treated rats showed a significant decrease in lipid peroxidation in both prophylactic and curative groups compared with the CDDP group. Thus, they suggested that BC has a protective role against CDDP-induced cardiotoxicity because of its antioxidant and radical scavenging properties. Other studies have also reported a decrease in activities of GSH-Px and CAT and an increase in MDA levels in the cardiac tissues of CDDP- treated rats (1, 7, 27). In the current study, a significant increase in MDA level was observed in the cardiac tissues in the CDDP-treated group compared with the control, sesame, and CMN groups as adherence to previous studies (27, 30). A possible explanation for the enhancement of MDA concentration may be decreased formation of antioxidants in the CDDP-induced tissues, which in view of the augmented activity of reactive oxygen species allowed a consequent increase in MDA production (27). However, we noted that CDDP-induced elevation of MDA level was reversed by CMN treatment compared with the CDDP+CMN group, this reduction was not found statistically different. This decrease was also observed in the BC group, but was not significant. Our results are in line with the results of the previous studies (27, 30). Thus, our study confirmed the CDDP-induced oxidative stress by causing a marked increase in the MDA level in the cardiac tissue.
In terms of the SOD activity, it was marginally decreased in the cardiac tissues of CDDP- treated animals compared with other groups, expect for the CDDP+BC groups, in agreement with the literature (1, 5). On the other hand, CMN and BC significantly improved CDDP-induced reduction of the SOD activity in heart consistent with the results of Oz et al.’s (30) study in brain tissues.
Additionally, the CAT activities in the CDDP-treated group rats were lower than those in the control, sesame oil, CMN, BC, and CDDP+CMN groups. When SOD and CAT levels were compared between the CDDP and control groups, significant differences were observed. Our study showed that both CMN and BC were effective in increasing SOD and CAT levels. All these findings indicated the preventive effects of these antioxidants on the CDDP-induced cardiac injury.
Tumor cells exhibit an elevation in the constitutive production of several proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. They are important mediators of acute inflammation and play decisive roles at different stages of tumor development, including initiation, promotion, malignant conversion, invasion, and metastasis (31). It has also been demonstrated that myocyte necrosis and apoptosis can be linked to elevated proinflammatory cytokine levels, such as IL-1β, TNF-α, and interferon-γ (32). The CDDP treatment also enhances the TNF-α and IL-6 cytokine levels (3, 7, 17). Additionally, many studies reported that stimulation of the inflammatory response in vivo lead to exacerbated CDDP-induced cardiotoxicity (33), and the inhibition of TNF-α activity and deficiency of TNF-α have demonstrated protection from CDDP toxicity (34). Thus, the inhibition or suppression of proinflammatory cytokines during CDDP treatment could prevent the influx of macrophages and neutrophils and could limit the inflammatory process, thus protecting the heart against CDDP toxicity (11).
However, CMN inhibits the expression of various inflammatory cytokines such as TNF-α, ILs-1, 2, 6, 8, and 12, interferon γ, monocyte chemoattractant protein (MCP), and migration of inhibitory protein, oxidative stress markers, some other chemokines by multiple genes/pathways involved in apoptosis, cell invasion, and adhesion (35). Moreover, it has been postulated that CMN is a TNF-α expression blocker due to its direct union to TNF-α (36). In addition, CMN showed noticeable resistance to cardiac hypertrophy caused by banding of the aorta, and the progression of heart failure was decreased by NF-κB activation and monocyte chemoattractant protein-1 (MCP-1), IL-6, IL-1β, and TNF-α mRNA and protein expression induced by aortic banding (37). Also, Song et al. (38) demonstrated that CMN reduces the systemic and local expressions of NF-κB, IL-6, and IL-1β in the myocardium as evidenced by ameliorated symptoms of myocarditis. In a different study by Wang et al. (39), CMN downregulated the levels of proinflammatory cytokines in the ischemic heart, which is concomitant with reduced TNF-α and IL-6 levels, infarct size, and ameliorated ischemic injury. These studies suggest that CMN can restrain the expression or activity of proinflammatory cytokines to protect the heart (16). Besides, there are numerous studies demonstrating the association of IL-6 down-regulation and/or of IL-6 inhibition signaling with therapeutic effects of CMN suggesting a role for modulation of IL-6 in anti-inflammatory effects of CMN. So, CMN can be considered as a potential therapy against IL-6 involved pathologic status (40). In agreement with these results, we found that the TNF-α, IL-1β, and IL-6 expressions were significantly increased in the cardiac tissues of the CDDP-treated rats. Also, CMN significantly reduced the intensities of CDDP-induced increased TNF-α, IL-1β, and IL-6 immunoreactivity in accord with the results of Kumar et al. (17) in kidney tissues. Thus, our results suggested that CMN has a higher cardioprotective effect than BC in terms of TNF-α, IL-1β, and IL-6 expression intensities.
According to the histopathological findings, the CDDP-treated group showed prominent histopathological changes including muscle fiber damage, cytoplasmic vacuolization, hemorrhage, and interstitial edema in cardiac tissues in this study. Similar findings including severe myocardial degenerative changes associated with dark stained pyknotic nuclei and cardiac hypertrophy were previously observed after CDDP adminstration (3, 5, 7, 41).
CMN may effectively prevent tissue damage to the heart by decreasing the oxidative stress and restoring the antioxidant status (42). Kim et al. (43) reported that the cardioprotective effects of CMN on myocardial ischemia-reperfusion injury rat model could exhibit anti-inflammatory activities and inhibition of apoptosis that occurred in the cardiomyocytes. Also, there are several pieces of evidence suggesting that an increased intake of antioxidants, vitamins, ascorbic acid (vitamins C), tocopherols (vitamin E), and beta-carotene (vitamin A), ha some protective role in coronary heart disease and CVDs (29, 44). Although previous studies have indicated that BC was able to inhibit the growth of cancer cells (45, 46), it has been reported that lung cancer risk is increased in smokers and breast cancer risk is increased in women by high-dose BC consumption. Accordingly, it has adverse effects on the risk of death from CVDs mainly among smokers (45, 47). Thus, BC has both beneficial and potentially harmful effects in the cardiovascular system because of its antioxidant property. Several clinical studies investigated the cardiovascular effects of BC, but all these results are rather controversial (21). In the present study, it was found that CMN or BC exhibited counteraction against the mentioned changes associated with CDDP administration. Besides, normal myocardial morphology structure was observed in rats pretreated with CMN. According to our histopathological findings, BC and especially CMN have been found to possess cardioprotective activities.
In the current study, CDDP-induced obvious cardiac injuries were demonstrated by biochemical, histopathological, and immunohistochemical alterations. These alterations included decreased CAT and SOD activities, increased MDA level, and elevated immunoreactivity intensity of IL-1β, IL-6 and TNF-α. Histopathologically, CDDP-induced severe myocardial degenerative changes were observed. Consequently, results of this study confirmed certain cardioprotective effects of CMN and BC on CDDP-induced cardiotoxicity in isolated rats. The antioxidative properties of CMN and BC have shown beneficial influence on ameliorating effects of chronic CDDP treatment, as the consequence of oxidative damage, by means of improvement of oxidative status and attenuation of morphological changes in isolated rat tissue. These results could recommend CMN and BC supplementation as a potential protection against CDDP-induced cardiotoxicity.
Study limitations
Our study had some limitations. Cardiac injury biomarkers such as creatine kinase (CK), creatine kinase isoenzyme MB (CK-MB), lactate dehydrogenase (LDH) activities, plasma cardiac troponin I (cTnI) concentration, and glutathione (GSH) contents could not be evaluated due to the limited facilities in our study. In addition, we could not estimate other oxidative stress parameters such as tissue antioxidant capacity, total oxidative status, and oxidative stress index levels.
Conclusion
The results of the current study highlight the significant impact of histopathological, biochemical, and cytokine changes in cardiotoxicity induced by CDDP treatment. Furthermore, our findings show that the use of CMN or BC before CDDP may be effective for protection against cardiac damage in CDDP-treated animals. However, CMN has a more remarkable ameliorative effect against the CDDP-induced cardiotoxicity than BC. Hence, CMN may be an excellent choice for cardioprotection against CDDP-induced cardiotoxicity.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.C., A.Y.; Design – A.B., A.Y.; Supervision – A.C., Ö.Ö.G., T.M.Ö.; Fundings – B.Y., M.Ü., T.M.Ö.; Materials – Ö.Ö.G., B.Y., M.Ü., A.Y.; Data collection &/or processing – A.B., A.C., B.Y., A.Y.; Analysis &/or interpretation – A.C., B.Y., M.Ü., A.Y.; Literature search – A.B.; Writing – A.B., Ö.Ö.G., A.Y.; Critical review – A.B., A.Y.
References
- 1.Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009;47:1176–83. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdellatief SA, Galal AA, Farouk SM, Abdel-Daim MM. Ameliorative effect of parsley oil on cisplatin-induced hepato-cardiotoxicity: A biochemical, histopathological, and immunohistochemical study. Biomed Pharmacother. 2017;86:482–91. doi: 10.1016/j.biopha.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim A, Eldaim MA, Abdel-Daim MM. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology. 2016;68:1039–48. doi: 10.1007/s10616-015-9860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Awady el SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011;650:335–41. doi: 10.1016/j.ejphar.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 6.Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol. 2010;37:460–5. doi: 10.1111/j.1440-1681.2009.05323.x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Majed AA, Sayed-Ahmed AA, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Shabanah OA. Propionyl-L-carnitine prevents the progression of cisplatin induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol Res. 2006;53:278–86. doi: 10.1016/j.phrs.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Raja W, Mir MH, Dar I, Banday MA, Ahmad I. Cisplatin induced paroxysmal supraventricular tachycardia. Indian J Med Paediatr Oncol. 2013;34:330–2. doi: 10.4103/0971-5851.125262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: chemotherapy-induced arrhythmia. Europace. 2009;11:1579–86. doi: 10.1093/europace/eup300. [DOI] [PubMed] [Google Scholar]
- 10.Ozcan T, Cirit A, Kiykim A. Recurrent complete atrioventricular block during cisplatin infusion: a case report. J Clinic Experiment Cardiol. 2011;2:151. [Google Scholar]
- 11.Dugbartey GJ, Peppone LJ, de Graaf IA. An integrative view of cisplatin-induced renal and cardiac toxicities: Molecular mechanisms, current treatment challenges and potential protective measures. Toxicology. 2016;371:58–66. doi: 10.1016/j.tox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2900–9. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 13.van Laar M, Feltbower RG, Gale CP, Bowen DT, Oliver SE, Glaser A. Cardiovascular sequelae in longterm survivors of young people's cancer: a linked cohort study. Br J Cancer. 2014;110:1338–41. doi: 10.1038/bjc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandal S. Curcumin, a promising anti-cancer threapeutic: It's bioactivity and development of drug delivery vehicles. Int J Drug Res Tech. 2016;6:43–57. [Google Scholar]
- 15.Prasad S, Gupta S, Tyagi A, Aggarwal B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol Adv. 2014;32:1053–64. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Jiang S, Han J, Li T, Xin Z, Ma Z, Di W, et al. Curcumin as a potential protective compound against cardiac diseases. Pharmacol Res. 2017;119:373–83. doi: 10.1016/j.phrs.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P, Sulakhiya K, Barua CC, Mundhe N. TNF-α, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats. Mol Cell Biochem. 2017;431:113–22. doi: 10.1007/s11010-017-2981-5. [DOI] [PubMed] [Google Scholar]
- 18.Park BH, Lim JE, Jeon HG, Seo SI, Lee HM, Choi HY, et al. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget. 2016;7:63870–86. doi: 10.18632/oncotarget.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol. 2017;174:1290–324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller L, Boehm V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules. 2011;16:1055–69. doi: 10.3390/molecules16021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csepanyi E, Czompa A, Haines D, Lekli I, Bakondi E, Balla G, et al. Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol Res. 2015;100:148–56. doi: 10.1016/j.phrs.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Siems W, Salerno C, Crifo C, Sommerburg O, Wiswedel I. Beta carotene degradation products-formation: toxicity and prevention of toxicity. Forum Nutr. 2009;61:75–86. doi: 10.1159/000212740. [DOI] [PubMed] [Google Scholar]
- 23.Rosic G, Srejovic I, Zivkovic V, Selakovic D, Joksimovic J, Jakovljevic V. The effects of N-acetylcysteine on cisplatin-induced cardiotoxicity on isolated rat hearts after short-term global ischemia. Toxicol Rep. 2015;2:996–1006. doi: 10.1016/j.toxrep.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taskin MI, Yay A, Adali E, Balcioglu E, Inceboz U. Protective effects of sildenafil citrate administration on cisplatin-induced ovarian damage in rats. Gynecol Endocrinol. 2015;31:272–7. doi: 10.3109/09513590.2014.984679. [DOI] [PubMed] [Google Scholar]
- 25.Eser A, Hizli D, Haltas H, Namuslu M, Kosus A, Kosus N, et al. Effects of curcumin on ovarian ischemia-reperfusion injury in a rat model. Biomed Rep. 2015;3:807–13. doi: 10.3892/br.2015.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aksak Karamese S, Toktay E, Unal D, Selli J, Karamese M, Malkoc I. The protective effects of beta-carotene against ischemia/reperfusion injury in rat ovarian tissue. Acta Histochem. 2015;117:790–7. doi: 10.1016/j.acthis.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Yüce A, Ateşşahin A, Ceribaşi AO, Aksakal M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol. 2007;101:345–9. doi: 10.1111/j.1742-7843.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 28.Abo-Salem OM, Harisa GI, Ali TM, El-Sayed el SM, Abou-Elnour FM. Curcumin ameliorates streptozotocin-induced heart injury in rats. J Biochem Mol Toxicol. 2014;28:263–70. doi: 10.1002/jbt.21562. [DOI] [PubMed] [Google Scholar]
- 29.Uday Kiran B, Sushma M, Prasad KVSRG, Uma Maheshwara Rao V, Jhansi Laxmi Bai D, Nisheetha V. Antioxidant and radical scavenging properties of β-Carotene on Cisplatin induced cardiotoxicity. Cardiol Angiol. 2015;4:98–106. [Google Scholar]
- 30.Oz M, Nurullahoglu Atalik KE, Yerlikaya FH, Demir EA. Curcumin alleviates cisplatin-induced learning and memory impairments. Neurobiol Learn Mem. 2015;123:43–9. doi: 10.1016/j.nlm.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–8. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 32.Rutschow S, Li J, Schultheiss HP, Pauschinger M. Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc Res. 2006;69:646–56. doi: 10.1016/j.cardiores.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 33.El-Sawalhi MM, Ahmed LA. Exploring the protective role of apocynin, a specific NADPH oxidase inhibitor, in cisplatin induced cardiotoxicity in rats. Chem Biol Interact. 2014;207:58–66. doi: 10.1016/j.cbi.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Benedetti G, Fredriksson L, Herpers B, Meerman J, y de Water B, de Graauw M. TNF-α-mediated NF-κB survival signaling impairment by cisplatin enhances JNK activation allowing synergistic apoptosis of renal proximal tubular cells. Biochem Pharmacol. 2013;85:274–86. doi: 10.1016/j.bcp.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin” : from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys. 2014;559:91–9. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Li HL, Liu C, de Couto G, Ouzounian M, Sun M, Wang AB, et al. Curcumin prevents and reverses murine cardiac hypertrophy. J Clin Invest. 2008;118:879–93. doi: 10.1172/JCI32865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Song Y, Ge W, Cai H, Zhang H. Curcumin protects mice from coxsackievirus B3-induced myocarditis by inhibiting the phosphatidylinositol 3 kinase/Akt/nuclear factor-κB pathway. J Cardiovasc Pharmacol Ther. 2013;18:560–9. doi: 10.1177/1074248413503044. [DOI] [PubMed] [Google Scholar]
- 39.Wang NP, Pang XF, Zhang LH, Tootle S, Harmouche S, Zhao ZQ. Attenuation of inflammatory response and reduction in infarct size by postconditioning are associated with downregulation of early growth response 1 during reperfusion in rat heart. Shock. 2014;41:346–54. doi: 10.1097/SHK.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 40.Ghandadi M, Sahebkar A. Curcumin: An effective inhibitor of interleukin-6. Curr Pharm Des. 2017;23:921–31. doi: 10.2174/1381612822666161006151605. [DOI] [PubMed] [Google Scholar]
- 41.Saleh RM, Awadin WF, Elseady YY, Waheish FE. Renal and cardiovascular damage induced by cisplatin in rats. Life Sci J. 2014;11:191–203. [Google Scholar]
- 42.Bashandy MA, Amin SA, Seleem H. Cardiotoxic effect of Chlorpromazine in adult male Albino rats and the possible Curcumin cardioprotection (histological, histochemical and immunohistochemical study) J Am Sci. 2012;8:888–97. [Google Scholar]
- 43.Kim SK, Park HJ, Joo SY, Hong MH, Kim KH, Hong YJ, et al. The protective effect of curcumin on myocardial ischemia-perfusion injury. Korean Circ J. 2008;38:353–59. [Google Scholar]
- 44.Zhang PY, Xu X, Li XC. Cardiovascular diseases: oxidative damage and antioxidant protection. Eur Rev Med Pharmacol Sci. 2014;18:3091–6. [PubMed] [Google Scholar]
- 45.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 46.Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88:372–83. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- 47.Misotti AM, Gnagnarella P. Vitamin supplement consumption and breast cancer risk: a review. Ecancermedicalscience. 2013;7:365. doi: 10.3332/ecancer.2013.365. [DOI] [PMC free article] [PubMed] [Google Scholar]


