Abstract
Objective:
Coronary heart disease (CHD) is the most common cause of death worldwide. This study aimed to validate the association of the rs964184 polymorphism with the CHD risk and included 874 CHD patients and 776 controls.
Methods:
rs964184 polymorphism genotyping was performed using Tm-shift polymerase chain reaction.
Results:
A strong association of the rs964184 polymorphism with CHD was found (genotype: X2=14.365, p=0.001; allele: X2=14.191, p=1.67×10–4; power=0.965). Gender analysis revealed a significant association only in males (genotype: X2=12.387, p=0.002; allele: X2=12.404, p=4.32×10–4; OR=1.467, 95% CI=1.185–1.817, power=0.945). Age and gender analyses revealed significant associations of the rs964184 polymorphism with CHD in males between the ages of 55 and 65 years (genotype: X2=10.070, p=0.007; allele: X2=10.077, p=0.002; OR=1.706, 95% CI=1.224–2.377, power=0.996) and in females older than 65 years (genotype: X2=9.462, p=0.009; allele: X2=9.560, p=0.002; OR=2.112, 95% CI=1.308–3.412, power=0.994). Further subgroup analysis suggested that rs964184 genotypes were significantly associated with TG levels in the patients (r=0.191, adjusted p=1.05 ×10–5) and controls (r=0.101, adjusted p=0.026).
Conclusion:
Our results indicate that both gender and age have great impacts on the association of the rs964184 polymorphism with CHD among Chinese.
Keywords: coronary heart disease, SNPs, BUD13-ZNF259, rs964184, age, gender
Introduction
Coronary heart disease (CHD), which is mainly caused by atherosclerosis, has become the leading cause of death worldwide (1). Although the prevalence of CHD is now decreasing in high-income countries, most deaths caused by CHD occur in low- and middle-income countries (2). The development of CHD is a very complex process. Its risk factors include age, gender, family history, dyslipidemia, hypertension, diabetes, cigarette smoking history, physical inactivity, and even psychosocial issues (3-5). A twin study has established that gender factors play an extremely important role in CHD (6). The interaction between environmental and genetic factors contributes to the morbidity of CHD (7, 8).
A recent genome-wide association study (GWAS) found a significant association of the BUD13-ZNF259 rs964184 polymorphism with triglyceride (TG) levels (9). Another GWAS identified that the rs964184 polymorphism was strongly associated with low-density lipoprotein cholesterol (LDL-C) levels in Chinese school-age children (10). High LDL-C levels are risk factors for CHD (11). BUD13-ZNF259 is located on 11q23.3 and encodes Zinc finger protein (ZPR1), which is a cytoplasmic zinc finger protein that interacts with tyrosine kinase receptors in quiescent mammalian cells (12). ZPR1 transcription is upregulated in the brain tissues of mice fed a high-fat diet (13). The high expression of BUD13-ZNF259 in neuronal cells can lead to increased H2O2-induced cell death, suggesting that a high-fat diet enhances ZPR1 mRNA expression and might increase vulnerability to oxidative stress (13). We hypothesize that the BUD13-ZNF259 rs964184 polymorphism as a quantitative locus of TG increases the vulnerability of coronary artery endothelial cells to both oxidative stress and inflammatory response after damage. Furthermore, elevated serum TG levels are independent risk factors for CHD (5). The BUD13-ZNF259 rs964184 polymorphism has been shown to affect lipid levels in the blood and thus increase the risk of CHD (14).
Our previous association study in 290 patients and 197 controls found that the rs964184-G allele of BUD13-ZNF259 was a moderate risk factor for CHD in females [p=0.05, odd ratio (OR)=1.49, 95% confidential interval (CI)=1.00–2.22] (15). The aim of the present study was to confirm our previous findings in much larger samples: 874 CHD patients and 776 controls.
Methods
Sample collection
The present retrospective case–control study included 874 CHD patients (625 males and 249 females) and 776 controls (424 males and 352 females). All subjects were Han Chinese and were randomly recruited between May 2008 and July 2015 from Ningbo First Hospital in Zhejiang Province. Approximately 1754 CHD patients and controls were examined by standardized coronary angiography on the basis of the Seldinger technique (16); at least two independent cardiologists were involved in adjudication. The controls were chosen from patients whose vascular stenosis rates were less than 50% in every coronary artery. The CHD patients included patients with a history of prior angioplasty or coronary artery bypass surgeries. Of them, 93 were excluded because of cardiomyopathy, congenital heart, liver or kidney disease. A total of 1661 blood samples from the CHD patients and controls were collected by the same investigator and were stored in 3.2% citrate sodium-treated tubes at –80°C. All blood samples were used for genotyping. Approximately 11 blood samples were not analyzed because of technical problems. Finally, a total of 1650 samples were used for genotyping. Our study was approved by the Institutional Review Board. Written information consent was obtained from all individuals.
SNP genotyping
Human genomic DNA was separated from peripheral blood samples using a nucleic acid extraction automatic analyzer (Lab-Aid 820, Xiamen, China), and all DNA samples were stored in TE buffer. Genotyping was done by Tm-shift polymerase chain reaction (PCR) (17). The sequences of the primers were 5’-gcgggcagggcggcTCACCATCTGATGTACTGTTTTCCTc-3’ (AS1 primer), 5’-gattaccgTCACCATCTGATGTACTGTTTTCCTg-3’ (AS2 primer), and 5’-TTCATGGAACTTGAAGTCTAGTGGGGA-3’ (common primer). PCR amplification was performed on ABI GeneAmp® PCR System 9700 Dual 384-Well Sample Block Module (Applied Biosystems, Foster City, CA). The PCR process included an initial denaturation stage at 95°C for 30 s, followed by 40 cycles of 95°C for 30 s, 59°C for 30 s, 72°C for 30 s, and a final extension stage at 72°C for 5 min. Then, melting curve analysis was conducted on a Roche LightCycler 480® fluorescence quantitative PCR instrument (Roche, Basel, Switzerland). The process included using temperatures of 95°C of 15 s and 60°C of 30 s and then increasing the temperature by 0.11°C/s to 95°C. Melting curve data were acquired by Air borne software provided by Roche automatic clustering based on fluorescence intensity analysis (18).
Statistical analysis
Hardy–Weinberg equilibrium (HWE) was analyzed according to Arlequin software Version 3.5 (Bern, Switzerland) and p>0.05 was considered to be in HWE. The differences in the genotype and allele frequencies between the CHD patients and controls were calculated by CLUMP22 software (Denmark Hill, London, UK) with 10,000 Monte Carlo simulations. Power analysis was performed by Power and Sample Size Calculation software, Version 3.0.43 (Nashville, TN, USA). The odds ratio (OR) and their 95% confidence intervals (95% CIs) were calculated using PASW Statistics 18.0 software (SPSS Inc., NY, USA). If the expected frequency for 2×2 and 2×3 chi-square and 2×2 tables was over 25%, Fisher’s exact test can be used. Correlation analysis was used Pearson test and performed on both PASW and R statistical software (Stanford, California, USA). Two-sided p-value of <0.05 was considered to be statistically significant.
Results
The clinical and demographic details of the patients and controls [including smoking, hypertension, diabetes, and total cholesterol, high-density lipoprotein cholesterol (HDL-C), LDL-C, and TG levels] have been described in our previous studies (19, 20). A case–control comparison of genotype and allele frequencies for the rs964184 polymorphism is presented in Table 1. No departure of HWE was observed in the patients and controls. Our results revealed a strong association of the rs964184 polymorphism with the risk of CHD at the genotype and allele levels (genotype, X2=14.365, df=2, p=0.001; allele, X2=14.191, df=1, p=1.67×10–4). The frequency of the rs964184-G allele was significantly higher in the patients than in the controls (25.9% vs. 20.4%; p=1.67×10–4, OR=1.368, 95% CI=1.162–1.611).
Table 1.
Genotype and allele distribution of rs964184*
| Gender | Group | Genotype (counts) | X2 | P (df=2) | HWE | Allele (n,%) | X2 | P (df=1) | OR (95% CI) | Power | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GC | CC | G | C | ||||||||||
| All | Cases | |||||||||||||
| (n=874) | 56 | 341 | 477 | 0.724 | 453 (25.9) | 1295 (74.1) | ||||||||
| Controls | ||||||||||||||
| (n=776) | 32 | 252 | 492 | 14.365 | 0.001 | 1.000 | 316 (20.4) | 1236 (79.6) | 14.191 | 1.67’10–4 | 1.368 (1.162 -1.611) | 0.965 | ||
| Males | Cases | |||||||||||||
| (n=625) | 40 | 238 | 347 | 1.000 | 318 (25.4) | 932 (74.6) | ||||||||
| Controls | ||||||||||||||
| (n=424) | 15 | 130 | 279 | 12.387 | 0.002 | 1.000 | 160 (18.9) | 688 (81.1) | 12.404 | 4.32’10–4 | 1.467 (1.185-1.817) | 0.945 | ||
| Females | Cases | |||||||||||||
| (n=249) | 16 | 103 | 130 | 0.523 | 135 (27.1) | 363 (72.9) | ||||||||
| Controls | ||||||||||||||
| (n=352) | 17 | 122 | 213 | 4.190 | 0.123 | 1.000 | 156 (22.2) | 548 (77.8) | 3.894 | 0.049 | 1.306 (1.001-1.704) | 0.504 | ||
P value less than or equal to 0.05 is in bold. 95%CI-95% confidence interval; HWE-hardy Weinberg equilibrium; OR-odds ratio
Breakdown analysis by gender showed a significant association in males (genotype: X2=12.387, df=2, p=0.002; allele: X2=12.404, df=1, p=4.32×10–4). In contrast, no significant association between the patients and controls was observed in females at the genotype level (p>0.05), although there is a much weaker association at the allele level in females (X2=3.894, df=1, p=0.049) than in males. We hypothesized that the inconsistent results in females between the genotype and allele levels results from the inheritance models. A further test showed that there was a strong association between the CHD patients and controls in the dominant model (Table 2, GG+GC vs. CC: X2=13.209, df=1, p=2.81×10–4; OR=1.442, 95% CI=1.183–1.757). Moreover, a stratification test by gender showed a significant effect in the dominant model (GG+GC vs. CC) for both males and females (Table 2; males: X2=11.098, df=1, p=0.001, OR=1.542, 95% CI=1.194–1.990; females: X2=4.103, df=1, p=0.043, OR=1.403, 95% CI=1.011–1.947).
Table 2.
Genetic testing under the dominant and recessive models*
| Gender | Group | Dominant | X2 | P (df=1) | OR (95% CI) | Power | Recessive | X2 | P (df=1) | OR (95% CI) | Power |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GG+GC CC | GG GC+CC | ||||||||||
| All | Cases | ||||||||||
| (n=874) | 397 477 | 56 818 | |||||||||
| Controls | |||||||||||
| (n=776) | 284 492 | 13.209 | 2.81’10–4 | 1.442 (1.183-1.757) | 0.999 | 32 744 | 4.246 | 0.039 | 1.592 (1.019-2.485) | 0.787 | |
| Males | Cases | ||||||||||
| (n=625) | 278 347 | 40 585 | |||||||||
| Controls | |||||||||||
| (n=424) | 145 279 | 11.098 | 0.001 | 1.542 (1.194-1.990) | 0.997 | 15 409 | 4.166 | 0.041 | 1.864 (1.016-3.420) | 0.670 | |
| Females | Cases | ||||||||||
| (n=249) | 119 130 | 16 233 | |||||||||
| Controls | |||||||||||
| (n=352) | 139 213 | 4.103 | 0.043 | 1.403 (1.011-1.947) | 0.817 | 17 335 | 0.716 | 0.397 | 1.353 (0.670-2.733) | 0.300 |
P value less than or equal to 0.05 is in bold. 95%CI-95% confidence interval; OR - odds ratio
In Table 3, subgroup analysis by the age of the individuals showed a significant difference between the rs964184 polymorphism and the risk of CHD in individuals between the ages of 55 and 65 years (genotype: X2=8.056, df=2, p=0.018; allele: X2=7.876, df=1, p=0.005; OR=1.411, 95% CI=1.109–1.795, power=0.802) and in individuals older than 65 years (genotype: X2=8.426, df=2, p=0.015; allele: X2=8.377, df=1, p=0.004; OR=1.605, 95% CI=1.163–2.215, power=0.835). As age and gender are two important risk factors for CHD, we further investigate the effect of gender in each age subgroup. As shown in Table 4, a strong association of the rs964184 polymorphism with CHD in males between the ages of 55 and 65 years (genotype: X2=10.070, df=2, p=0.007; allele: X2=10.077, df=1, p=0.002; OR=1.706, 95% CI=1.224–2.377) and in females older than 65 years (genotype: X2=9.462, df=2, p=0.009; allele: X2=9.560, df=1, p=0.002; OR=2.112, 95% CI=1.308–3.412). In Table 5, further analysis suggested significant associations in the dominant model (GG+GC vs. CC) for males between the ages of 55 and 65 years (X2=7.643, df=1, p=0.006, OR=1.748, 95% CI=1.175–2.600) and for females older than 65 years (X2=7.467, df=1, p=0.006, OR=2.218, 95% CI=1.247–3.945).
Table 3.
Comparison of genotype and allele frequencies between cases and controls by age*
| Age | Group | Genotype(counts) | X2 | P (df=2) | HWE | Allele (n,%) | X2 | P (df=1) | OR (95% CI) | Power | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GC | CC | G | C | |||||||||
| 55< | Cases | ||||||||||||
| (n=186) | 7 | 70 | 109 | 0.404 | 84 (22.6) | 288 (77.4) | |||||||
| Controls (n=236) | 10 | 77 | 149 | 1.156 | 0.561 | 1.000 | 97 (20.6) | 375 (79.4) | 0.509 | 0.476 | 1.128 (0.811-1.569) | 0.111 | |
| 55-65 | Cases | ||||||||||||
| (n=361) | 27 | 149 | 185 | 0.796 | 203 (28.1) | 519 (71.9) | |||||||
| Controls (n=357) | 15 | 125 | 217 | 8.056 | 0.018 | 0.641 | 155 (21.7) | 559 (78.3) | 7.876 | 0.005 | 1.411 (1.109-1.795) | 0.803 | |
| >65 | Cases | ||||||||||||
| (n=327) | 22 | 122 | 183 | 0.768 | 166 (25.4) | 488 (74.6) | |||||||
| Controls (N=183) | 7 | 50 | 126 | 8.426 | 0.015 | 0.446 | 64 (17.5) | 302 (82.5) | 8.377 | 0.004 | 1.605 (1.163-2.215) | 0.835 | |
P value less than or equal to 0.05 is in bold. 95%CI-95% confidence interval; HWE-Hardy Weinberg equilibrium; OR-odds ratio
Table 4.
Comparison of genotype and allele frequencies between cases and controls by age as well as gender*
| Age | Gender | Group | Genotype (counts) | X2 | P (df=2) | HWE | Allele (n,%) | X2 | P (df=1) | OR (95%CI) | Power | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GC | CC | G | C | ||||||||||
| 55< | Males | Cases | ||||||||||||
| (n=149) | 7 | 60 | 82 | 0.511 | 74 (24.8) | 224 (75.2) | ||||||||
| Controls | ||||||||||||||
| (n=159) | 5 | 53 | 101 | 2.418 | 0.299 | 0.802 | 63 (19.8) | 255 (80.2) | 2.242 | 0.134 | 1.337 (0.914-1.957) | 0.562 | ||
| Females | Cases | |||||||||||||
| (n=37) | 0 | 10 | 27 | 1.000 | 10 (13.5) | 64 (86.5) | ||||||||
| Controls | ||||||||||||||
| (n=77) | 5 | 24 | 48 | 2.976 | 0.226 | 0.504 | 34 (22.1) | 120 (77.9) | 2.354 | 0.125 | 0.552 (0.256-1.188) | 0.588 | ||
| 55-65 | Males | Cases | ||||||||||||
| (n=262) | 22 | 103 | 137 | 0.648 | 147 (28.1) | 377 (71.9) | ||||||||
| Controls | ||||||||||||||
| (n=172) | 5 | 54 | 113 | 10.070 | 0.007 | 0.803 | 64 (18.6) | 280 (81.4) | 10.077 | 0.002 | 1.706 (1.224-2.377) | 0.996 | ||
| Females | Cases | |||||||||||||
| (n=99) | 5 | 46 | 48 | 0.215 | 56 (28.3) | 142 (71.7) | ||||||||
| Controls | ||||||||||||||
| (n=185) | 10 | 71 | 104 | 1.759 | 0.415 | 0.842 | 91 (24.6) | 279 (75.4) | 0.915 | 0.339 | 1.209 (0.819-1.785) | 0.275 | ||
| >65 | Males | Cases | ||||||||||||
| (n=214) | 11 | 75 | 128 | 1.000 | 97 (22.7) | 331 (77.3) | ||||||||
| Controls | ||||||||||||||
| (n=93) | 5 | 23 | 65 | 3.216 | 0.200 | 0.151 | 33 (17.7) | 153 (82.3) | 1.882 | 0.170 | 1.359 (0.876-2.108) | 0.492 | ||
| Females | Cases | |||||||||||||
| (n=113) | 11 | 47 | 55 | 0.826 | 69 (30.5) | 157 (69.4) | ||||||||
| Controls | ||||||||||||||
| (n=90) | 2 | 27 | 61 | 9.462 | 0.009 | 1.000 | 31(17.2) | 149(82.8) | 9.560 | 0.002 | 2.112 (1.308-3.412) | 0.994 | ||
P value less than or equal to 0.05 is in bold. 95%CI-95% confidence interval; HWE-Hardy Weinberg equilibrium; OR-odds ratio
Table 5.
Genetic testing under the dominant and recessive models by age and gender*
| Age | Gender | Group | Dominant | X2 | P (df=1) | Power | OR (95%CI) | Recessive | X2 | P (df=1) | OR (95%CI) | Power |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rs964184 | GG+GC CC | GG GC+CC | ||||||||||
| Males | Cases | |||||||||||
| (n=149) | 67 82 | 7 142 | ||||||||||
| 55< | Controls | |||||||||||
| (n=159) | 58 101 | 2.298 | 0.130 | 0.573 | 1.423 (0.901-2.246) | 5 154 | 0.496 | 0.481 | 1.518 (0.471-4.892) | 0.170 | ||
| Females | Cases | |||||||||||
| (n=37) | 10 27 | 0 37 | ||||||||||
| Controls | ||||||||||||
| (n=77) | 29 48 | 1.256 | 0.262 | 0.349 | 0.613 (0.260-1.448) | 5 72 | 2.513 | 0.113 | NA | NA | ||
| 55-65 | Males | Cases | ||||||||||
| (n=262) | 125 137 | 22 240 | ||||||||||
| Controls | ||||||||||||
| (n=172) | 59 113 | 7.643 | 0.006 | 0.977 | 1.748 (1.175-2.600) | 5 167 | 5.364 | 0.021 | 3.062 (1.137-8.247) | 0.928 | ||
| Females | Cases | |||||||||||
| (n=99) | 51 48 | 5 94 | ||||||||||
| Controls | ||||||||||||
| (n=185) | 81 104 | 1.550 | 0.213 | 0.421 | 1.364 (0.836-2.226) | 10 175 | 0.016 | 0.899 | 0.931 (0.309-2.803) | 0.051 | ||
| >65 | Males | Cases | ||||||||||
| (n=214) | 86 128 | 11 203 | ||||||||||
| Controls | ||||||||||||
| (n=93) | 28 65 | 2.821 | 0.093 | 0.666 | 1.560 (0.927-2.625) | 5 88 | 0.007 | 0.932 | 0.954 (0.322-2.826) | 0.054 | ||
| Females | Cases | |||||||||||
| (n=113) | 58 55 | 11 102 | ||||||||||
| Controls | ||||||||||||
| (n=90) | 29 61 | 7.467 | 0.006 | 0.975 | 2.218 (1.247-3.945) | 2 88 | 4.717 | 0.030 | 4.745 (1.024-21.989) | 0.886 |
P value less than or equal to 0.05 is in bold. NA-not applicable. 95%CI-95% confidence interval; OR-odds ratio
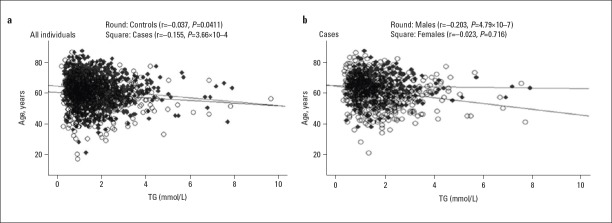
In Figure 1, TG levels were showed to associated with rs964184 genotypes (patients: r=0.191, adjusted p=1.05×10–5; controls: r=0.101, adjusted p=0.026). Moreover, a significant reverse correlation was observed between age and TG levels (Fig. 2, Panel A; patients: r=–0.155, p=3.66×10–4; controls: r=–0.0.037, p=0.411). Breakdown analysis by gender showed that a significant association only existed in male patients (Fig. 2, Panel B; males: r=–0.203, p=4.79×10–7, females: r=0.023, p=0.716). TG levels reduced with increasing age.
Figure 1.
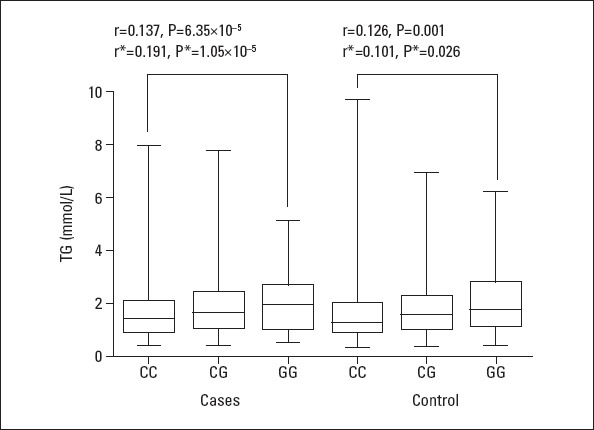
Correlation between TG levels and rs964184 genotypes*
*: indicates that cigarette smokers were excluded. TG – triglyceride
Figure 2.
Breakdown correlation between TG levels and age by gender in the patients and controls
A-Correlation between TG levels and age in the subgroups of the patients and controls; B-Correlation between TG levels and age in the subgroup of male and female patients. TG - triglyceride
Discussion
In the present study, which contained a higher number of samples than our previous study, we wanted to confirm the association of the rs964184 polymorphism and the CHD risk. Our results revealed a significant association between the BUD13-ZNF259 rs964184 polymorphism and CHD. In addition, we investigated the effect of age and gender on the association of this polymorphism with CHD.
CHD patients often have abnormal lipid phenotypes, including high LDL-C and low HDL-C as well as TG levels (4, 21). The BUD13-ZNF259 rs964184 polymorphism has been shown to be associated with the risk of dyslipidemia (22, 23). This polymorphism could influence blood lipid levels and increase the risk of cardiovascular (24) and cerebrovascular (17) diseases. Ueyama et al. (25) found that the rs964184-G allele was significantly associated with increases in serum TG levels and decreases in serum HDL-C levels in metabolic syndrome (MetS) patients from Japan (26). Mirhafez et al. (27) suggested that the CG+GG genotypes of the rs964184 polymorphism were associated with increased serum TG and LDL-C levels in MetS patients from northeastern Iran. Zhang et al. (28) showed that patients with the rs964184-CC genotype had the lowest TG level and that those with the rs964184 GG genotype had the highest serum TG level. In the present study, we confirmed a significant association of the rs964184 polymorphism with TG levels (Fig. 2). In addition, we observed a significant inverse correlation between age and TG levels, suggesting that the older patients lave lower TG levels. This result seems to be in contrast with current knowledge that the risk of CHD increases along with increased TG levels and aging (21). We speculate that these phenomena are caused by medicines taken by the patients and controls. Normally, older patients (patients and controls) tend to have a longer history of taking medicines that may reduce TG levels to a lower extent. An epidemiologic study has shown that the incidence of CHD was eight to nine times greater in men and women who aged 55–64 years than in young patients (29). The death rate due to CHD increased quickly in patients aged 55 years and more and was higher in patients aged 65 years or more than in young patients (30). Other studies have suggested that these rates were beginning to level off in younger age groups (31, 32). Scientists have confirmed that genetic polymorphisms may play an important role in the pathogenesis of early onset CHD (33, 34). Here we demonstrated significant differences between the rs964184 polymorphism and CHD in patients aged 55–65 years and those older than 65 years; thus suggested that aging is a robust risk factor for CHD (35).
Epidemiologic evidence has shown that men have higher cardiovascular disease morbidity and mortality rates than women (36). Among conventional risk factors, diabetes mellitus and hyperlipidemia affected women more, while smoking was found to affect men more (37). Along with the decreased secretion of testosterone and the accumulative effect of smoking and weight gain, middle-aged men have higher onset rates of CHD (38). Women with late menopause and women who used postmenopausal estrogens had a significantly lower risk of CHD than those with early menopause and those who never used estrogen; this finding provided evidence that estrogen is a protective factor against CHD (39). In the present study, further analysis by gender showed a significant association of age with TG levels only in males.
CHD is a dynamic inflammatory disease caused by atherosclerosis (40). BUD13-ZNF259 encodes ZPR1 that interacts with tyrosine kinase receptors (17). A study has shown that the inhibition of spleen tyrosine kinase (Syk) attenuates atherogenesis in mice and reduces the atherogenesis inflammatory process and plaque development (41). The activation of Syk may play an important role in the development of atherosclerosis (42). Using Syk as a target to block the pathway of inflammasomes may be a new anti-atherosclerotic treatment (43). Although there is a lack of evidence supporting the relationship between ZPR1 and Syk, ZPR1 might be associated with atherosclerosis as well as CHD because of its interaction with tyrosine kinase receptors.
Study limitations
Our study had great power to detect a the relative association of the rs964184 polymorphism with CHD risk among Han Chinese. However, the current study has some limitations. First, patients who had no significant vasoconstriction seen during their angiography (<50%) were considered as controls. Their degree of vasoconstriction might be reduced due to a spontaneous re-channelization. Therefore, healthy controls should be included in future studies. Second, gene–gene and gene–disease interactions should be considered. Third, only one SNP was checked in terms of its association with CHD. Other genetic polymorphisms in selected genes may be functional markers for the risk of CHD.
Conclusions
Our results indicate that both gender and age have a great impact on the association of the rs964184 polymorphism with CHD among Han Chinese. Specifically, the rs964184 polymorphism may increase 70.6% of CHD risk in males between 55 and 65 years, and 111.2% of CHD risk in females older than 65. This gender–age interaction in the association of the BUD13-ZNF259 rs964184 polymorphism with CHD may be worth being validated in other populations.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – X.C.; Design – S.D.; Supervision – H.Y., H.J.; Fundings – S.D., Y.H.; Materials – X.X., N.W.; Data collection &/or processing – X.X., N.W., D.J., H.J., X.C., D.D.; Analysis &/or interpretation – L.H., L.X.; Literature search – Y.H.; Writing – Y.L.; Critical review – X.C.
References
- 1.Franco M, Cooper RS, Bilal U, Fuster V. Challenges and opportunities for cardiovascular disease prevention. Am J Med. 2011;124:95–102. doi: 10.1016/j.amjmed.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broeckel U, Hengstenberg C, Mayer B, Holmer S, Martin LJ, Comuzzie AG, et al. A comprehensive linkage analysis for myocardial infarction and its related risk factors. Nat Genet. 2002;30:210–4. doi: 10.1038/ng827. [DOI] [PubMed] [Google Scholar]
- 4.Lim S, Park YM, Sakuma I, Koh KK. How to control residual cardiovascular risk despite statin treatment:focusing on HDL-cholesterol. Int J Cardiol. 2013;166:8–14. doi: 10.1016/j.ijcard.2012.03.127. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary:heart disease and stroke statistics--2010 update:a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 6.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, De Faire U. Heritability of death from coronary heart disease:a 36-year follow-up of 20 966 Swedish twins. J Intern Med. 2002;252:247–54. doi: 10.1046/j.1365-2796.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 7.Sing CF, Stengard JH, Kardia SL. Genes, environment, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:1190–6. doi: 10.1161/01.ATV.0000075081.51227.86. [DOI] [PubMed] [Google Scholar]
- 8.Hasanaj Q, Wilson BJ, Little J, Montazeri Z, Carroll JC Screening CETiGi. Family history:impact on coronary heart disease risk assessment beyond guideline-defined factors. Public Health Genomics. 2013;16:208–14. doi: 10.1159/000353460. [DOI] [PubMed] [Google Scholar]
- 9.Braun TR, Been LF, Singhal A, Worsham J, Ralhan S, Wander GS, et al. A replication study of GWAS-derived lipid genes in Asian Indians:the chromosomal region 11q23.3 harbors loci contributing to triglycerides. PLoS One. 2012;7:e37056. doi: 10.1371/journal.pone.0037056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Y, Xi B, Zhao X, Cheng H, Hou D, Wu L, et al. Common genetic variants associated with lipid profiles in a Chinese pediatric population. Hum Genet. 2013;132:1275–85. doi: 10.1007/s00439-013-1332-1. [DOI] [PubMed] [Google Scholar]
- 11.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment:prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 12.Gangwani L. Deficiency of the zinc finger protein ZPR1 causes defects in transcription and cell cycle progression. J Biol Chem. 2006;281:40330–40. doi: 10.1074/jbc.M608165200. [DOI] [PubMed] [Google Scholar]
- 13.Nogusa Y, Yanaka N, Sumiyoshi N, Takeda K, Kato N. Expression of zinc finger protein ZPR1 mRNA in brain is up-regulated in mice fed a high-fat diet. Int J Mol Med. 2006;17:491–6. [PubMed] [Google Scholar]
- 14.Waterworth DM, Ricketts SL, Song K, Chen L, Zhao JH, Ripatti S, et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010;30:2264–76. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Zhou J, Huang S, Huang Y, Le Y, Jiang D, et al. An association study between genetic polymorphisms related to lipoprotein-associated phospholipase A(2) and coronary heart disease. Exp Ther Med. 2013;5:742–50. doi: 10.3892/etm.2013.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique:50 years on. Lancet. 2005;366:1407–9. doi: 10.1016/S0140-6736(05)66878-X. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Zhao J, Wang Z, Li K, Nie S, Gao F, et al. Association study of BUD13-ZNF259 gene rs964184 polymorphism and hemorrhagic stroke risk. Int J Clin Exp Med. 2015;8:22503–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Nie S, Zhou S, Li K, Sun J, Zhao J, et al. PPARD rs2016520 polymorphism and circulating lipid levels connect with brain diseases in Han Chinese and suggest sex-dependent effects. Biomed Pharmacother. 2015;70:7–11. doi: 10.1016/j.biopha.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Ye H, Zhou A, Hong Q, Tang L, Xu X, Xin Y, et al. Positive Association between APOA5 rs662799 Polymorphism and Coronary Heart Disease:A Case-Control Study and Meta-Analysis. PLoS One. 2015;10:e0135683. doi: 10.1371/journal.pone.0135683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Ye H, Hong Q, Xu X, Jiang D, Xu L, et al. Association of CDKN2BAS polymorphism rs4977574 with coronary heart disease:a case-control study and a meta-analysis. Int J Mol Sci. 2014;15:17478–92. doi: 10.3390/ijms151017478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aslibekyan S, Goodarzi MO, Frazier-Wood AC, Yan X, Irvin MR, Kim E, et al. Variants identified in a GWAS meta-analysis for blood lipids are associated with the lipid response to fenofibrate. PLoS One. 2012;7:e48663. doi: 10.1371/journal.pone.0048663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aung LH, Yin RX, Wu DF, Wang W, Liu CW, Pan SL. Association of the variants in the BUD13-ZNF259 genes and the risk of hyperlipidaemia. J Cell Mol Med. 2014;18:1417–28. doi: 10.1111/jcmm.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe S, Tokoro F, Matsuoka R, Arai M, Noda T, Watanabe S, et al. Association of genetic variants with dyslipidemia. Mol Med Rep. 2015;12:5429–36. doi: 10.3892/mmr.2015.4081. [DOI] [PubMed] [Google Scholar]
- 24.Fu Q, Tang X, Chen J, Su L, Zhang M, Wang L, et al. Effects of Polymorphisms in APOA4-APOA5-ZNF259-BUD13 Gene Cluster on Plasma Levels of Triglycerides and Risk of Coronary Heart Disease in a Chinese Han Population. PLoS One. 2015;10:e0138652. doi: 10.1371/journal.pone.0138652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueyama C, Horibe H, Yamase Y, Fujimaki T, Oguri M, Kato K, et al. Association of FURIN and ZPR1 polymorphisms with metabolic syndrome. Biomed Rep. 2015;3:641–7. doi: 10.3892/br.2015.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naj AC, West M, Rich SS, Post W, Kao WH, Wasserman BA, et al. Association of scavenger receptor class B type I polymorphisms with subclinical atherosclerosis:the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Genet. 2010;3:47–52. doi: 10.1161/CIRCGENETICS.109.903195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirhafez SR, Avan A, Pasdar A, Khatamianfar S, Hosseinzadeh L, Ganjali S, et al. Zinc Finger 259 Gene Polymorphism rs964184 is Associated with Serum Triglyceride Levels and Metabolic Syndrome. Int J Mol Cell Med. 2016;5:8–18. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang LX, Sun Y, Liang Y, Li K, Chen Y, Gusanglamu, et al. Relationship between dyslipidemia and gene polymorphism in Tibetan population. Biomed Environ Sci. 2012;25:305–10. doi: 10.3967/0895-3988.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Konnov MV, Dobordginidze LM, Deev AD, Gratsiansky NA. [Own and Parental Predictors of Low Blood Level of High Density Lipoprotein Cholesterol in Offspring of Persons With Early Coronary Heart Disease] Kardiologiia. 2016;56:12–8. doi: 10.18565/cardio.2016.3.12-18. [DOI] [PubMed] [Google Scholar]
- 30.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update:a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin JB, Borden WB. Coronary heart disease in young adults. Curr Atheroscler Rep. 2012;14:140–9. doi: 10.1007/s11883-012-0226-3. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen MK. Early-onset Coronary Artery Disease Clinical and Hereditary Aspects. Dan Med J. 2017;64 [PubMed] [Google Scholar]
- 33.Bressler J, Folsom AR, Couper DJ, Volcik KA, Boerwinkle E. Genetic variants identified in a European genome-wide association study that were found to predict incident coronary heart disease in the atherosclerosis risk in communities study. Am J Epidemiol. 2010;171:14–23. doi: 10.1093/aje/kwp377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes MF, Saarela O, Stritzke J, Kee F, Silander K, Klopp N, et al. Genetic markers enhance coronary risk prediction in men:the MORGAM prospective cohorts. PLoS One. 2012;7:e40922. doi: 10.1371/journal.pone.0040922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinkuniene E, Petrulioniene Z, Laucevicius A, Ringailaite E, Laucyte A. [Prevalence of conventional risk factors in patients with coronary heart disease] Medicina (Kaunas) 2009;45:140–6. [PubMed] [Google Scholar]
- 36.Ginter E, Simko V. Women live longer than men. Bratisl Lek Listy. 2013;114:45–9. doi: 10.4149/bll_2013_011. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Tian F, Hu SY, Wang J, Zhang T, Chen YD, et al. [Characteristics of traditional risk factors and coronary lesions on coronary heart disease among different sex populations] Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:423–7. [PubMed] [Google Scholar]
- 38.Leibar Tamayo A, Astobieta Odriozola A, Garcia-Cruz E, Cordero Fort A, Romero Otero J. Testosterone and coronary artery disease. Arch Esp Urol. 2013;66:689–95. [PubMed] [Google Scholar]
- 39.Joakimsen O, Bonaa KH, Stensland-Bugge E, Jacobsen BK. Population-based study of age at menopause and ultrasound assessed carotid atherosclerosis:The Tromso Study. J Clin Epidemiol. 2000;53:525–30. doi: 10.1016/s0895-4356(99)00197-3. [DOI] [PubMed] [Google Scholar]
- 40.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–97. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilgendorf I, Eisele S, Remer I, Schmitz J, Zeschky K, Colberg C, et al. The oral spleen tyrosine kinase inhibitor fostamatinib attenuates inflammation and atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1991–9. doi: 10.1161/ATVBAHA.111.230847. [DOI] [PubMed] [Google Scholar]
- 42.Bijli KM, Fazal F, Minhajuddin M, Rahman A. Activation of Syk by protein kinase C-delta regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via tyrosine phosphorylation of RelA/p65. J Biol Chem. 2008;283:14674–84. doi: 10.1074/jbc.M802094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]