Abstract
The molecular, neurobiological, and physical health impacts of child maltreatment are well established, yet mechanistic pathways remain inadequately defined. Telomere length (TL) decline is an emerging molecular indicator of stress exposure with definitive links to negative health outcomes in maltreated individuals. The multiple confounders endemic to human maltreatment research impede the identification of causal pathways. This study leverages a unique randomized, cross-foster, study design in a naturalistic translational nonhuman primate model of infant maltreatment. At birth, newborn macaques were randomly assigned to either a maltreating or a competent control mother, balancing for sex, biological mother parenting history, and social rank. Offspring TL was measured longitudinally across the first 6 months of life (infancy) from peripheral blood. Hair cortisol accumulation was also determined at 6, 12, and 18 months of age. TL decline was greater in animals randomized to maltreatment, but also interacted with biological mother group. Shorter TL at 6 months was associated with higher mean cortisol levels through 18 months (juvenile period) when controlling for relevant covariates. These results suggest that even under the equivalent social, nutritional, and environmental conditions feasible in naturalistic translational nonhuman primate models, early adverse caregiving results in lasting molecular scars that foreshadow elevated health risk and physiologic dysregulation.
An estimated 683,000 children were victims of abuse and neglect in 2015 in the United States, with those in the first year of life having the highest rate of victimization (Stoltenborgh, Bakermans-Kranenburg, Alink, & van IJzendoorn, 2015; US Department of Health and Human Services, Administration for Children and Families, and Children’s Bureau, 2015, 2017). Early maltreatment, in both human studies and studies in preclinical animal models, is a strong and established risk factor for negative cognitive, physiologic, immunologic, behavioral, and physical health consequences (Drury, Gonzalez, & Sanchez, 2015; Sanchez et al., 2007). Specific effects include alterations in the functioning of the stress response systems, particularly the hypothalamus–pituitary–adrenal (HPA) axis with changes to both baseline/diurnal and reactive patterns reported across mammalian species (Avishai-Eliner, Yi, Newth, & Baram, 1995; Drury, Gonzalez, et al., 2015; Howell et al., 2013; Ladd, Huot, Thrivikraman, Nemeroff, & Plotsky, 2004; MacMillan et al., 2009; McLaughlin et al., 2015; Sanchez, 2006; Sanchez et al., 2010; Sanchez, McCormack, & Howell, 2015; Stanton, Gutierrez, & Levine, 1988). The patterns and direction of changes in human studies is less consistent than preclinical animal models, perhaps reflective of the complex environmental confounders in human studies. Particularly in relation to measurements of the stress hormone cortisol, comparisons across studies can be challenging due to methodological differences (e.g., time of day, diurnal measurements vs. single time point, or timing/severity of the stressor for reactive measurements), use of saliva compared to plasma, and expected developmental changes that together may, in part, explain these inconsistent findings. One innovative approach to cortisol measurement that addresses these limitations and captures chronic exposure is the use of cumulative cortisol levels in hair samples that reflect the overall cortisol produced during weeks or months prior to sampling that is embedded in growing hair (Davenport, Tiefenbacher, Lutz, Novak, & Meyer, 2006; Meyer, Novak, Hamel, & Rosenberg, 2014; Russell, Koren, Rieder, & Van Uum, 2012).
Nonhuman primate (NHP) models, particularly rhesus macaques (Macaca mulatta), represent an ecologically valid child maltreatment model system. Macaques are close phylogenetic relatives of humans exhibiting similar parenting behaviors with strong mother–infant bonds and, unfortunately, maltreatment (Maestripieri, 1999; Maestripieri & Carroll, 1998; Sanchez, 2006). Macaques develop approximately four times faster than humans, enhancing the feasibility of longitudinal studies. In rodents and humans, early adverse caregiving is associated with alterations in emotional and stress regulation, brain development, physical growth, and epigenetics (Asok, Bernard, Roth, Rosen, & Dozier, 2013; Drury, Gonzalez, et al., 2015; Drury et al., 2012; Gee et al., 2013; McLaughlin et al., 2015; Petrullo, Mandalaywala, Parker, Maestripieri, & Higham, 2016). Studies in rhesus monkeys mirror those findings demonstrating elevated cortisol levels associated with increased emotional reactivity, altered immune function, larger amygdala volumes, and changes in corticolimbic tracts (Howell et al., 2013, 2014; Petrullo et al., 2016; Sanchez, 2006).
Recent studies in humans have also found cellular impacts of child maltreatment, specifically changes in chromosomal telomere length (TL), a molecular marker of biological aging and cellular stress (Humphreys et al., 2016; Shalev et al., 2012; Tyrka et al., 2010). Telomeres represent the evolutionarily conserved aglet cap on each chromosome that prevents DNA loss. Telomeres shorten with age and are affected by oxidative stress, inflammation, and DNA damage, yet also serve as critical regulators of chromosome structure and epigenetic regulation of gene expression (Ye, Renault, Jamet, & Gilson, 2014). Childhood adversity has been associated with shorter TL in cross-sectional studies as well as accelerated TL loss (Drury et al., 2012; Humphreys et al., 2016; Shalev et al., 2012; Tyrka et al., 2010). Shorter TL has also been linked with poor health outcomes, including mental illness and obesity, which are independently associated with maltreatment (Darrow et al., 2016; Epel, 2009; Haycock et al., 2014; Mundstock et al., 2015; Savolainen, Eriksson, Kajantie, Lahti, & Räikkönen, 2015; Wojcicki et al., 2015). TL has also been associated with cortisol levels in offspring of depressed mothers, and with stress-induced activations in the autonomic nervous system and the HPA axis in young children, indicating that physiologic indicators of stress, psychopathology risk, and molecular changes may be correlated (Gotlib et al., 2015; Kroenke et al., 2011). Taken together, the existing body of research provides support for the hypothesis that TL is both a biological indicator (i.e., sensitive to stress hormones) and/or a mediator of stress exposure and a harbinger of future health risk including altered regulation of stress response systems (Choi, Fauce, & Effros, 2008; Kroenke et al., 2011; Lopizzo et al., 2017; Steptoe, Hamer, Lin, Blackburn, & Erusalimsky, 2016).
Substantial overlap in telomere biology exists between humans and macaques, including overall sequence homology and the tissue-specific expression of telomerase (Broer et al., 2013; Gardner et al., 2007). A significant age-related decline and sex differences in TL was reported, similar to humans, with females having longer initial telomeres and greater TL attrition than males (Smith et al., 2011). Longitudinal TL has only been examined in a small baboon study (n = 4) where TL declined rapidly during the first year of life, stabilizing between 50 and 70 weeks (Baerlocher, Rice, Vulto, & Lansdorp, 2007). No previous NHP studies have examined the effect of early caregiving or early life stress/adversity on TL.
Child maltreatment, both in humans and in NHPs, spans generations with individuals exposed to maltreatment at greater risk of maltreating their own offspring. Genetic and epigenetic factors putatively contribute to this transgenerational transmission, creating challenges for studies seeking to define the impact of maltreatment separate from inherent biological risk. This study leveraged an established naturally occurring NHP maltreatment model, with reported prevalence rates of 2%–5% (Maestripieri & Carroll, 1998; Sanchez, 2006) in group-living rhesus that is operationalized by early life physical abuse and maternal rejection associated with infant distress (Maestripieri, 1999; Sanchez, 2006). Mothers repeat these maltreating behaviors with subsequent offspring with maltreatment running in rhesus families, transmitted across generations through the maternal line both experientially and biologically (Maestripieri, 2005; Maestripieri & Carroll, 1998). In the current study, all infants were cross-fostered and randomly assigned to either competent mothers or mothers with a history of maltreating previous offspring (Howell et al., 2017). Cross-fostering all monkeys permits disentangling the impact of the actual caregiving experience (nurture) from the influence of heritable factors related to the biological mother (nature; Franklin et al., 2010; Huizinga et al., 2006; Maestripieri, 2005; McCormack, Newman, Higley, Maestripieri, & Sanchez, 2009).
NHP maltreatment models are uniquely able to experimentally control for the plethora of factors obfuscating child maltreatment, including, parental substance use and psychopathology, poor nutrition, and socioeconomic status and violence in the neighborhood/school. These factors likely interact bidirectionally, and synergistically, to contribute to negative outcomes by directly affecting the child and by influencing the parents’ buffering capacity. The cross-domain and persistent consequences of child maltreatment, in addition to the staggering economic cost of maltreatment, highlight the importance of understanding the underlying biological mechanisms. The ecological validity of the NHP maltreatment model, homology in telomere dynamics (Gardner et al., 2007), and evidence of accelerated TL shortening in human studies (Shalev et al., 2012; Tyrka et al., 2010) support our hypothesis that early adverse caregiving will be associated with accelerated TL loss across the first 6 months of life, equivalent to the infant and toddler human stages. We further explored how early caregiving experience interacted with heritable factors by testing the contribution of the expected “biological” maternal caregiving to infant TL. Given evidence of sex differences in both maltreatment outcomes and telomere dynamics, sex was examined as a moderator (De Bellis & Keshavan, 2003; Gardner et al., 2014; Samplin, Ikuta, Malhotra, Szeszko, & DeRosse, 2013). Finally, to test the ability of TL to predict future health risks, we examined the association between TL and cumulative hair cortisol levels through 18 months of age (juvenile and prepubertal period).
Method
Subjects
Forty-three rhesus monkeys were studied from birth through 18 months of age as part of a larger study examining biobehavioral outcomes following maltreatment (Howell et al., 2017; McCormack et al., 2015). Twenty-one infants were raised by control mothers (10 male, 11 female) and 22 were raised by maltreating mothers (14 males, 8 females; Table 1). Monkeys were born and housed at the Yerkes National Primate Research Center Field Station, in Lawrenceville, Georgia. Subjects lived with their mothers in large social groups consisting of 75–150 adult females, their subadult and juvenile offspring, and 2–3 adult males. This social complexity allowed a balanced distribution of social dominance ranks (high, medium, and low social status), in addition to sex, across experimental caregiving groups. Social troops were housed in 100× 100 ft outdoor compounds, with access to climate-controlled indoor areas. Standard high-fiber, low-fat monkey chow (Purina Mills Int., Lab Diets, St. Louis, MO) and seasonal fruits and vegetables were provided twice daily, in addition to enrichment items. Water was available ad libitum. All the procedures were in accordance with the Animal Welfare Act and the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and approved by the Emory Institutional Animal Care and Use Committee.
Table 1.
Demographics (n = 43)
| Foster Mother Caregiving | Biological Mother Caregiving
|
|||
|---|---|---|---|---|
| Maltreatment
|
Control
|
|||
| Male | Female | Male | Female | |
| Maltreatment | 5 | 5 | 9 | 3 |
| Control | 3 | 6 | 7 | 5 |
| Total | 8 | 11 | 16 | 8 |
Note: The number of animals in each group, divided by the sex of the infant, and foster and biological mother caregiving groups. Bold values indicate those monkeys with matched biological and foster mother caregiving (n = 22), and italic values indicate mismatched maternal caregiving (n = 21).
Cross-fostering
Infants were randomly assigned at birth to be fostered to mothers with either a history of nurturing maternal care (control) or mothers with histories of infant maltreatment (maltreatment) according to described protocols (Howell et al., 2017). Mothers were counterbalanced for social dominance rank and selected from different matrilines (i.e., subjects were unrelated). On the day of birth, newborn monkeys were placed with an unrelated foster mother within 5 min of initial separation from the biological mother (a small number of infants were cross-fostered within 48–72 hr of birth) resulting in a high rate of adoption success (Howell et al., in press; Maestripieri, 2005). Of the 21 infants fostered by control dams, 12 were the biological offspring of control females (7 males, 5 females), and 9 were born to maltreating females (3 males, 6 females). Of the 22 infants raised by maltreating dams, 12 were biological offspring of control females(9 males, 3 females), and 10 were born to maltreating females (5 males, 5 females).
Behavioral observations of mother–infant interactions for identification of maternal maltreatment
Characterization of mother–infant interactions and caregiving, beginning at birth, occurred across the first 3 postnatal months. Behavioral observations were collected in each mother–infant pair by experienced coders (interobserver reliability > 90% agreement, Cohen κ > 0.8) from observation towers using established ethograms and procedures (Altmann, 1962; Howell et al., 2017, in press; Maestripieri & Carroll, 1998; McCormack et al., 2009, 2015; McCormack, Sanchez, Bardi, & Maestripieri, 2006). Each observation was 30 min long and performed on 5 separate days per week in the first month, 2 days/week in the second month, and once/week in the third month, resulting in 32 observations (16 hr) per mother–infant pair. This observation schedule best documents early maternal care received by the infant as the frequency of physical abuse is highest in the first month and is rarely observed beyond the third month of life in this species (Maestripieri & Carroll, 1998; McCormack et al., 2006). Observations occurred between 7:00 and 11:00 a.m., when monkeys are most active. Maltreatment was defined as the comorbid exposure of the infant to physical abuse (at least three instances of violent maternal behaviors [dragging, crushing against the ground, sitting/stepping on, throwing, or roughly grooming the infant], which result in infant’s pain and distress) and rejection, defined as the mother preventing contact/access to her nipple/ventrum by pushing the infant away, blocking her chest, or twisting her torso away. Both abuse and rejection result in high levels of infant distress and elevations of stress hormones (Howell et al., 2013, 2017, in press; Maestripieri, Jovanovic, & Gouzoules, 2000; McCormack et al., 2006, 2015; Sanchez, 2006). Control mothers never exhibited these behaviors.
Telomere length
DNA for TL analyses was obtained from whole blood collected at 2 days, 2 weeks, and 1, 3, and 6 months of age and stored in EDTA tubes at −80 °C. DNA was extracted from the peripheral blood mononuclear cells using standard procedures. DNA was sent to Dr. Drury’s laboratory for evaluation of DNA quality and telomere analyses. The average relative TL as represented by the telomere repeat copy number to single gene (albumin) copy number (T/S) ratio, was determined with monochrome multiplex quantitative real-time polymerase chain reaction and a BioRad CFX96 using established procedures (Drury, Esteves, et al., 2015; Drury et al., 2014). All samples were performed in triplicate on a single plate, and each plate was duplicated with each sample in a different well position resulting in six replicates per individual per time point. All time points from an individual were run on the same duplicate plates to minimize plate-to-plate variability. Intraplate and interplate coefficients of variations (CV) were calculated to ensure uniformity of sample length estimates (CV ≤ 5%). All plates contained a 7-point standard curve from 0.0313 to 2 ng DNA, using the same pooled male and female purchased rhesus macaque genomic DNA (Biochain Institute, San Francisco, CA). Polymerase chain reaction efficiencies for telomere and albumin reactions were required to be between 90% and110% and not differ from each other by more than 10%. Coefficients of variance (CV) were calculated to ensure uniformity of T/S ratios within a triplicate (CV ≤ 10%) and between plates (CV ≤ 6%). Final T/S ratio for each sample and each time point was determined by the average of the triplicates on both plates. Although 14 samples initially failed CV requirements for all replicates and all time points simultaneously within an individual, these samples were repeated and no samples failed quality control twice. The final CV for all samples was 2.34%.
Hair cortisol
Approximately one square inch of hair was shaved from the back of the head just above the foramen magnum (nuccal area) at birth, and the hair that grew in that area was shaved again at 6, 12, and 18 months of age for all animals. Thus, at each time point the samples assayed included all cortisol incorporated into the growing hair shaft between the collection times. Hair samples were processed and assayed using previously described protocols (Meyer et al., 2014). Briefly, each sample was weighed, washed twice in isopropanol to remove external contamination, ground to a fine powder, and then extracted with methanol overnight. The methanol was evaporated, the residue was redissolved in assay buffer, and then cortisol was measured using the Salimetrics (Carlsbad, CA) enzyme immunoassay kit (Cat. No. 1-3002) according to the manufacturer’s directions. Intra- and interassay coefficients of variation were <10%. Two animals had only one time point, 18 had two time points, and 23 had three time points.
Data analyses
Descriptive statistics characterized the sample overall and assessed the normality of TL data. Spearman correlation coefficients compared crude relationships between TL across time and the relationship with hair cortisol levels. Cumulative mean hair cortisol level through 18 months was determined from the mean (the sum divided by the number of time points cortisol was measured) of the cortisol measured at 6, 12, and 18 months of age as not all subjects had valid cortisol results at every time point. Linear mixed models examined TL trajectory across the first 6 months of life (2 weeks and 1, 3, and 6 months) accounting for the nonindependence of observations (repeated measures nested within the individual). Time (e.g., the age at which DNA was collected) was used as a time-varying independent variable across which an individual’s TL was modeled. Mixed modeling explores systematic differences in the rate of change and allows for the examination of key variables (e.g., caregiving experience/foster group) on differences in TL trajectory. We conducted a multilevel mixed-effects regression model to generate an intraclass correlation coefficient, using an empty base model, to assess the degree of clustering of TL within an individual. Foster mother caregiving group was then included as a predictor of TL trajectory. Covariates included infant sex, biological mother caregiving group, maternal social dominance rank, gravida (number of pregnancies), parity (number of life births with offspring raised past 6 months, to control for maternal experience), and maternal age. Given the apparent nonlinear pattern of TL trajectory, models also included a quadradic factor, which remained significant and was therefore included in the full model. Only significant covariates were included in final models. Analyses tested the interaction between sex and foster group, and sex and biological group, and the final model included the three-way interaction. Post hoc exploratory analyses, acknowledging the limitations due to sample size, were conducted within each sex and included the interaction between biological and foster mother caregiving group. Sensitivity analyses were performed where each time point was sequentially removed to assess whether effects were driven by particular ages, and analyses were also run excluding each individual animal. Exploratory analyses testing the mismatch hypothesis were conducted. A dichotomous dummy variable was created that was defined as either “matched” (foster and biological group matched, i.e., both control or both maltreating) or “mismatched” (biological and foster group status differed, i.e., maltreating biological with control foster mom; control biological with maltreating foster mom). Models again tested the interaction of match/mismatch group with sex and then subsequently within each sex separately. Significance was defined as p < .05. No correction for multiple testing was performed. Linear regression was used to test the relationship between cumulative hair cortisol levels through 18 months and TL including relevant covariates.
Results
Demographics
The final sample consisted of 43 monkeys (Table 1). Analyses were performed on the entire data set, acknowledging that not all time points were available for all monkeys. Mean maternal age was 10.01 years, mean gravida was 4.6, and mean parity was 4.1. No significant differences existed in maternal age, parity, or gravida between caregiving groups (by foster or biological condition). A total of 170 unique TL measurements were available across the five time points. The number of time points per individual was not significantly different by caregiving group, sex of the infant, or rank. The number of independent measurements at each time point varied from 27 (2-week time point) to 43 (3- and 6-month time points) individuals. Four subjects had three time points, the remaining had either four or five time points, with all subjects having TL at 6 months of age. Analyses excluding each monkey individually did not alter significant findings. Analyses excluding animals with cortisol at only one time point also did not alter results.
Base model
As expected, TL was highly correlated across time (age), and there was a significant effect of time on TL so that, overall, TL shortened (b =−0.01, p <.0001; Figure 1). TL at 6 months of age was crudely associated with accumulative mean cortisol through 18 months of age (Table 2). Maternal age, parity, and gravida were not significant predictors and did not alter findings. Not accounting for any covariates there was a significant effect of time.
Figure 1.
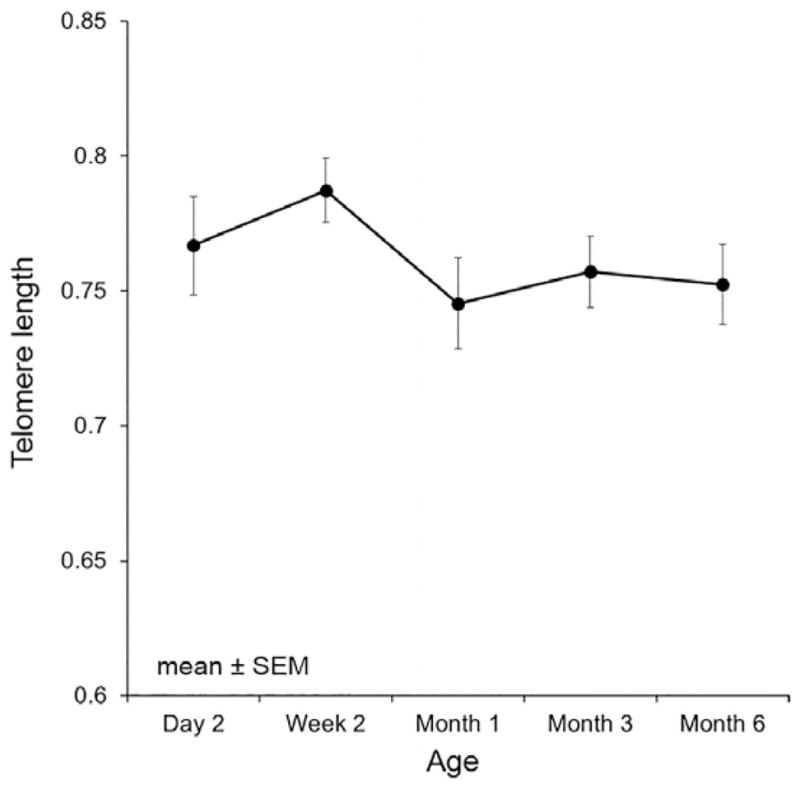
Mean telomere length and standard error of all subjects over time demonstrating a nonlinear decline in telomere length over time.
Table 2.
Telomere length correlation over time
| Telomere Length | 2 Days | 2 Weeks | 1 Month | 3 Months | 6 Months | Mean Cortisol |
|---|---|---|---|---|---|---|
| 2 days | 1 | |||||
| 2 weeks | .397 | 1 | ||||
| 1 month | .921**** | .670** | 1 | |||
| 3 months | .684**** | .631*** | .666**** | 1 | ||
| 6 months | .660*** | .501* | .548** | .698**** | 1 | |
| Mean cortisol | −.21 | −.01 | −.01 | −.24 | −.35* | 1 |
Note: Raw correlation of telomere length measurement across time and mean cortisol exposure from birth through 18 months.
p < .05.
p < .01.
p < .001.
p < .0001.
Overall model
The full model converged in seven iterations with a decrease in the intraclass correlation coefficient to 0.52. A significant three-way interaction between foster mother group, biological mother group, and sex was detected (b = −0.26, p = .004). In the full model time, the quadratic effect of time, infant sex, and biological mother were independent predictors, as were all two-way interactions (Table 3). The model remained robust to the inclusion of rank, gravida, parity, and age for both biological and foster mothers (data not shown).
Table 3.
Full model and within male and female infants
| Model 1: Interaction
|
Model 2: Males
|
Model 3: Females
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |
| Intercept | 0.78 | <.0001 | [0.6, 0.96] | 0.75 | <.0001 | [0.65, 0.85] | 0.79 | <.0001 | [0.70, 0.9] |
| Time | −0.03 | .21 | [−0.05, 0.01] | 0.003 | .86 | [−0.03, 0.04] | −0.04 | .09 | [−0.1, 0.0009] |
| Time2 | 0.003 | .24 | [−0.002, 0.008] | −0.001 | .7 | [−0.006, 0.004] | 0.008 | .08 | [−0.0001, 0.02] |
| Sex | −0.006 | .9 | [−0.1, 0.09] | ||||||
| Foster mom | −0.2 | .1 | [−0.40, 0.04] | −0.05 | .34 | [−0.15, 0.05] | 0.08 | .04 | [0.001, 0.17] |
| Biological mother | 0.03 | .75 | [−0.18, 0.24] | 0.03 | .54 | [−0.07, 0.12] | 0.03 | .5 | [−0.06, 0.1] |
| Sex×Foster Mom | 0.13 | .03 | [0.007, 0.26] | ||||||
| Sex ×Bio Mom | −0.002 | .97 | [−0.13, 0.12] | ||||||
| Foster Mom×Bio Mom | 0.3 | .03 | [0.02, 0.58] | 0.04 | .51 | [−0.08, 0.17] | −0.2 | .001 | [−0.34, −0.09] |
| Foster Mom×Bio Mom ×Sex | −0.27 | .004 | [−0.45, −0.09] | ||||||
Note: Models exploring the interaction between foster mother caregiving group, biological mother caregiving group. and sex of the infant on telomere length trajectory across the first 6 months of life.
Sensitivity analyses
Analyses with the full model were run sequentially removing each time point of TL collection. Removal of any single time point did not result in loss of significance for any direct effects or interactions, supporting the sensitivity of the analysis (data not shown). As with the base model, inclusion of maternal age, parity, and gravida did not influence results.
Within-sex analyses
Given evidence of sex differences in the telomere literature (Barrett & Richardson, 2011; Drury et al., 2014), and a significant Sex×Foster Group interaction effect in our model, post hoc analyses examined direct and interaction effects within males and females separately. For females, foster mother care-giving (b = −0.08, p = .04) and the Foster Mom×Biological Mom interaction effect were all significant predictors of TL trajectory (b = −0.20, p = .001; Table 3). In the males, none of the variables were significant predictors of TL trajectory.
Match/mismatch caregiving environment analyses
Post hoc analyses directly tested the mismatch hypothesis using dummy coded dichotomous variables. In this model, sex interacted with match/mismatch caregiving status (b = −0.07, p =.03). Analyses within sex revealed a significant effect on TL trajectory but only for females (b = −0.08, p = .004) and not males (b = −0.01, p = .52).
Association with cortisol
Mean cortisol levels through 18 months of age were significantly higher in animals reared by maltreating mothers (maltreating M = 117.34 pg/mg, SD = 29); control M = 92 pg/mg, SD = 16). Foster care group remained a significant predictor of mean cortisol levels even after accounting for biological mother caregiving group, sex, and the interaction between foster mother group and biological mother group (Table 4). Given the crude association between TL at 6 months and mean cortisol through 18 months of age (Table 2), we next tested whether TL at 6 months contributed further variance to the relation between early caregiving exposure and mean cortisol. When TL at 6 months was included in the model predicting mean cortisol, the r2 increased (.39 to .48). TL was a significant, independent predictor of mean cortisol levels. Even with including TL in the model, all other significant covariates remained significant, including the interaction between foster mother status and biological mother status. Sex was not a significant predictor, and given the small sample size, further stratification by sex was not warranted.
Table 4.
Predictors of mean total cortisol through 18 months
| Model R2 = .39
|
|||
|---|---|---|---|
| β | p | 95% CI | |
| Intercept | 138.3 | <.0001 | [112, 164.7] |
| Foster mom | −43.8 | .0005 | [−66.8, −20.8] |
| Sex | −2.5 | .74 | [−17.8, 12.8] |
| Bio mother | −29.5 | .006 | [−49.9, −9.2] |
| Foster Mom ×Bio Mom | 32.2 | .03 | [3.1, 61.2] |
Note: Model testing the prediction of mean cortisol level by foster mother group, biological mother group and sex of the infant.
Discussion
This is the first study to examine the impact of early caregiving differences on TL trajectory from birth through the juvenile period in NHPs, leveraging a powerful randomized, cross-foster, study design in a naturalistic translational macaque model of infant maltreatment. Overall, there was a general nonlinear decline of TL across the first 6 months of life consistent with studies suggesting that the first years of life in humans are associated with shortening of TL (Baerlocher et al., 2007; Frenck, Blackburn, & Shannon, 1998). We observed a complex interaction between caregiving experience (“nurture”: foster mother), biological inheritance (“nature”: biological mother), and infant sex. Exposure to early maternal maltreatment was associated with accelerated TL decline, particularly in females born to control biological mothers and in males born to maltreating biological mothers. Similar to the impact of early caregiving on TL trajectory, mean cumulative cortisol levels through 18 months of age were significantly higher in animals reared by a maltreating mother compared to a control mother. Our findings support the increasing body of literature linking early childhood maltreatment, altered physiologic regulation, and accelerated TL shortening across the life course (Kananen et al., 2010; Shalev et al., 2012; Tyrka et al., 2010).
The observed effect on TL persisted despite all monkeys receiving balanced nutrition, appropriate health care, and living in highly similar environments. The effects of childhood maltreatment, when confounded by poor nutrition, lower socioeconomic status, and other components of toxic stress, result in magnified negative outcomes throughout the life course. Our findings suggest that, beyond these potentially synergistic risk factors, there remains a significant direct impact of early adverse caregiving at the cellular level in primates (Hackman, Farah, & Meaney, 2010; Shonkoff, Garner, & Committee on Psychosocial Aspects of Child and Family Health, 2011). These findings indicate that the first months of life in macaques, similar to the first years in humans, are a very vulnerable developmental window when disruption in caregiving, however brief, can result in persistent changes with long-term physiological implications (Humphreys et al., in press; Nelson, Fox, & Zeanah, in press; Wachs, Georgieff, Cusick, & McEwen, 2014).
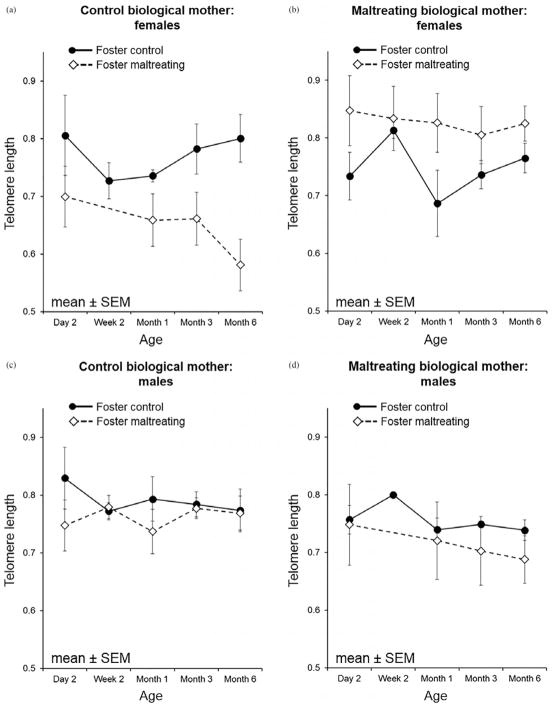
Our observed sex difference is comparable to human (Barrett & Richardson, 2011; Drury et al., 2012; Gardner et al., 2014) and rodent findings (Asok et al., 2013). In females, the interaction between foster mother group and expected biological caregiving is consistent with the adaptive calibration model or mismatch theory (Del Giudice, Ellis, & Shirtcliff, 2011; Hostinar & Gunnar, 2013), which suggests ancestral experiences guide the developmental trajectory of the offspring (or even future generations) to match that expected environment. Postnatal environmental changes/mismatches with ancestral environment, either positive or negative, necessitate recalibration of physiological systems from the programmed pattern, that while adaptive, come at a biological cost, including increased oxidative stress and cellular metabolism, factors captured by telomere dynamics (Drury, 2015; Epel, 2009). In males, however, telomere attrition is more in line with a cumulative risk model. Consistent with that model, males with the highest combined risk in our study (i.e., born to and raised by maltreating mothers) exhibited the greatest telomere loss. These sex differences fit with both biological and evolutionary factors and theories. There is greater maternal energy/metabolic investment in sons compared to daughters in rhesus monkeys and other NHP species (Schino, Cozzolino, & Troisi, 1999; Small & Smith, 1984). Males are larger at birth, have accelerated physical growth, spend more time in ventral contact with mothers, and are fed maternal milk with a higher energy density (Bercovitch, Widdig, & Nürnberg, 2000; Hinde, 2009; Hinde & Spencer-Booth, 1967). These differences have been explained in the literature as likely arising from the different evolutionary and social roles of macaque females and males in a matriarchal society with strict matrilineal social dominance ranks (Bercovitch et al., 2000; Suomi, 2005). Males are expected to leave the natal group after they reach puberty, forming bachelor groups that search for and reproduce in a different troop. The greater maternal investment in infant sons (Bercovitch et al., 2000; Hinde & Spencer-Booth, 1967) would ensure enhanced reproductive success due to male dispersal and explain the exacerbation of the negative effects from a “double-hit” (biological risks plus an adverse caregiving experience) in males. Female macaques, however, remain within the matrilineal social group for their entire lives (Suomi, 2005) and depend on strong alliances within their family/kinship to maintain their status in the social hierarchy. As such, the highest biological cost for females would be expected with a mismatched postnatal environment that would require unanticipated adaptation to a challenging (maltreating) environment for which females from biological competent care lineages (biological control group), evolutionarily, may not be prepared. The finding that daughters born to maltreating biological mothers have longer TL than their sons supports that females are prenatally preparing for a more stressful existence.
While there is a substantial body of research documenting TL and the trajectory of TL across development as reflective of exposure to a range of adversity and stressors, the utility of TL as a predictor of negative health trajectories and the association between TL and other biological indicators of maltreatment remains insufficiently tested. Altered regulation and function of the HPA axis and its downstream hormone effector, cortisol, have been associated with maltreatment and early adverse caregiving across species, with notable discongruities and developmental moderators (Bernard, Hostinar, & Dozier, 2015; Cicchetti & Rogosch, 2001; Gunnar, Fisher, & Early Experience, Stress, and Prevention Network, 2006; Koss, Hostinar, Donzella, & Gunnar, 2014; McLaughlin et al., 2015; Petrullo et al., 2016). The links between TL and cortisol have also been reported (Tomiyama et al., 2012) with the predominant modeling hypothesizing increased cortisol would be associated with shorter TL potentially due to increased oxidative stress and the resulting metabolic damage to DNA, particularly telomere caps (Haussmann, Longenecker, Marchetto, Juliano, & Bowden, 2012). Lymphocytes exposed to cortisol have been found, in cell culture, to exhibit decreased telomerase, offering another mechanism through which these markers may be related (Choi et al., 2008). In our study, despite relations between TL and mean cumulative cortisol through 18 months, cumulative hair cortisol through 6 months of age did not contribute significantly to TL decline across the first 6 months. Including hair cortisol through 6 months of age in our full models and those within each sex also did not indicate an independent or interactive impact of cortisol on TL trajectory.
An alternative model, and one supported by our findings, suggests that TL, an index of cellular aging, instead of being a consequence of altered regulation of the HPA axis, is predictive of prolonged HPA activation. Our data suggest that the links between TL and cortisol are not simply temporally aligned as TL at 6 months contributed additional significant variance in the model explaining cumulative cortisol through 18 months. While this study does not permit causal pathway analyses, it is intriguing to hypothesize that rapid cellular aging, indicated by shorter TL, during the first months of life (equivalent to the infant through toddler periods in humans) is reflective of decreased physiologic plasticity and/ or flexibility, in this case leading to potentially higher activation, and less variability, of the HPA axis with resulting increased chronic cortisol levels. Our results suggest that additional studies that go beyond measuring the concurrent correlation between TL and other markers of the stress response system to instead explore predictive trajectories are needed to better understand the underlying mechanisms and long-term physiological consequences. However, these pathways, and the role of cellular aging in the persistence of altered biological systems, may differ by sex and have a heritable component.
Despite strengths, there are also limitations to this study, the main one being the limited sample size, although large for a macaque project. Therefore, we underscore the need for cautious interpretation of these findings and the need to replicate these results in future studies, with a larger sample size or, at the very least, additional longitudinal measurements. All monkeys were randomized to an alternative care-giver prohibiting direct comparison to monkeys raised by their biological mothers, either control or maltreating. As this study already included a large number of animals for a macaque study, we were not able to add these additional control groups, but future studies ought to examine how maltreatment versus nurturing mothering influences TL and cortisol in the absence of an early change in the biological caregiving environment. Not all time points were available on all monkeys, and the overall number of monkeys is small in comparison to human studies. To address this limitation, all analyses were run randomly excluding each monkey and also excluding each independent time point. These sensitivity analyses did not lead to any significant differences in outcomes. Nevertheless, this remains the largest study in any species with more than two measurements of TL within an individual during the first years of life (Baerlocher et al., 2007; Frenck et al., 1998). While macaques are matriarchal, another limitation is that we did not obtain data on the biological father. Given evidence of both heritability and paternal influences on TL, future studies that examine how maternal and paternal TL are related to offspring TL attrition, as well as studies that examine transgenerational effects accounting for both maternal and paternal exposure, are needed (Eisenberg, 2011; Honig et al., 2015; Küffer, Maercker, & Burri, 2014; Stindl, 2016). Hair cortisol in NHP models remains a relatively new methodology, but there has been validation work performed in macaques regarding the expected range and the relationship between hair cortisol levels and how they compare to both baseline and chronic stress serum cortisol levels, despite lacking sensitivity in studies of dynamic fluctuations of HPA axis diurnal and stress reactive cortisol (Davenport, Lutz, Tiefenbacher, Novak, & Meyer, 2008; Meyer & Novak, 2012). To our knowledge this remains the first study in any species to examine hair cortisol and TL.
Our finding of accelerated cellular aging may have broader relevance to NHP models, particularly when modeling diseases associated with aging, such as obesity, atherosclerosis, cardiovascular disease, HIV, and immune senescence (Didier et al., 2016; Fyhrquist, Saijonmaa, & Strandberg, 2013; Getz & Reardon, 2012; Policicchio, Pandrea, & Apetrei, 2016; Vaughan & Mattison, 2016). Early caregiving experiences represent an important, often not captured, source of individual variation in many NHP studies. Although nursery rearing has long been established to alter immune function (Coe, Lubach, Schneider, Dierschke, & Ershler, 1992; Lubach, Coe, & Ershler, 1995; Provençal et al., 2012), our data suggests that other caregiving differences can introduce additional confounds. Maltreatment may be less commonly recorded in the animal’s health records and may not even be detected in primate breeding facilities that do not study the phenomenon unless it is severe. Future studies, as well as colony management strategies, should consider effect moderation by early social experiences.
This study indicates that molecular changes following early infant maltreatment persist despite equivalent health, nutrition, and other environmental resources. Further, our findings suggest that TL early in childhood is predictive of future HPA axis function (i.e., cortisol levels), even after accounting for the impact of early caregiving and genetic factors. The implications are obvious. The increasing evidence that TL trajectories are both harbingers of future health (Denham, O’Brien, & Charchar, 2016; Prescott et al., 2016) and indicators of cumulative stress/adversity exposure (Bateson, 2016), when coupled with our results, highlight the paramount importance of early interventions specifically targeting the parent–child relationship for children exposed to, or at risk of, maltreatment. Failure to provide very young children with a nurturing stable caregiver, even with attention to adequate health, nutrition, and safe physical environments, is likely inadequate to fully mitigate risk across socioemotional and health outcomes.
Figure 2.
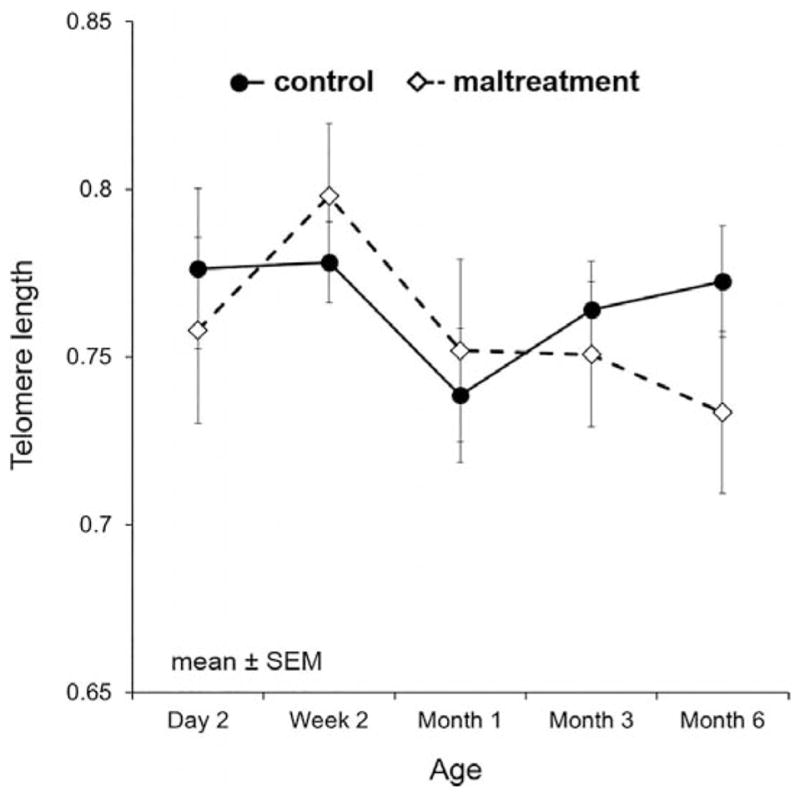
Mean telomere length of all subjects over time with standard error bars with groups separated by foster mother status: control and maltreating mothers.
Figure 3.
Models separated by biological mother status and infant sex. (a, b) The trajectory of telomere length (TL) in females separated out by biological mother status indicating the greatest impact on TL trajectory in females born to control biological mothers but reared by maltreating mothers. (c, d) The effects in males where the impact on TL trajectory is in males born to maltreating mothers who were also raised by maltreating mothers.
Table 5.
Predictors of mean total cortisol through 18 months including telomere length at 6 months of age
| Model 1 Interaction R2 = .48
|
|||
|---|---|---|---|
| β | p | 95% CI | |
| Intercept | 200.9 | <.0001 | [142.1, 259.8] |
| Telomere length at 6 months | −83.6 | .02 | [−155.03, 112.3] |
| Foster mom | −43.2 | .0003 | [−64.7, −21.6] |
| Sex | −4.2 | .58 | [−19.8, 11.4] |
| Bio mom | −31.9 | .002 | [−51.1, −12.6] |
| Foster Mom ×Bio Mom | 36.8 | .01 | [9.3, 64.5] |
Note: Model testing the prediction of mean cortisol level by foster mother group, biological mother group, and sex of the infant including effect of TL at 6 months.
Table 6.
Effect of biological mother
| Model 1: Maltreating Biomom
|
Model 2: Control Biomom
|
|||||
|---|---|---|---|---|---|---|
| β | p | 95% CI | β | p | 95% CI | |
| Time | −0.004 | .55 | [−0.02, 0.009] | −0.014 | .0004 | [−0.02, −0.007] |
| Sex | 0.09 | .008 | [0.03, 0.17] | −0.14 | .0011 | [−0.22, −0.05] |
| Foster mom | 0.15 | .07 | [−0.01, 0.33] | −0.12 | .13 | [−0.27, 0.03] |
| Sex×Foster Mom | −0.12 | .02 | [−0.22, −0.02] | 0.12 | .02 | [0.01, 0.24] |
Note: Models demonstrating sex differences in the impact of biological compared to foster mother group and sex within each biological mother group.
Acknowledgments
Funding for this work was obtained from the Tulane National Primate Center Pilot Grant (to K.B.), Tulane Oliver Fund (to S.D.), NIH Grant R01MH101533 (to S.D.), NIH/NIMH Grants MH078105 (to Megan Gunnar; to M.M.S. Project 4) and MH015755 (Institutional NRSA, to Dante Cicchetti; B.R.H., mentee), and Office of Research Infrastructure Programs/OD Grant OD11132 (Yerkes National Primate Research Center Base Grant, formerly RR000165). We thank Anne Glenn, Christine Marsteller, Dora Guzman, and the staff at the Yerkes National Primate Research Center Field Station for the excellent technical support and animal care provided during these studies. The funders had no role in review design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not represent the official views of the NIMH or the NIH. The Yerkes National Primate Research Center is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International. The authors have no conflicts of interest or relevant disclosures.
References
- Altmann S. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Annals of the New York Academy of Sciences. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Asok A, Bernard K, Roth T, Rosen J, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Development and Psychopathology. 2013 doi: 10.1017/s0954579413000011. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neuroscience Letters. 1995;192:49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerlocher G, Rice K, Vulto I, Lansdorp P. Longitudinal data on telomere length in leukocytes from newborn baboons support a marked drop in stem cell turnover around 1 year of age. Aging Cell. 2007;6:121–123. doi: 10.1111/j.1474-9726.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Barrett ELB, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell. 2011;10:913–921. doi: 10.1111/j.1474-9726.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- Bateson M. Cumulative stress in research animals: Telomere attrition as a biomarker in a welfare context? BioEssays. 2016;38:201–212. doi: 10.1002/bies.201500127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovitch F, Widdig A, Nürnberg P. Maternal investment in rhesus macaques (Macaca mulatta): Reproductive costs and consequences of raising sons. Behavioral Ecology and Sociobiology. 2000;48:1–11. [Google Scholar]
- Bernard K, Hostinar C, Dozier M. Intervention effects on diurnal cortisol rhythms of CPS-referred infants persist into early childhood: Preschool follow-up results of a randomized clinical trial. JAMA Pediatrics. 2015;169:112. doi: 10.1001/jamapediatrics.2014.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer L, Codd V, Nyholt D, Deelen J, Mangino M, Willemsen G, … de Geus E. Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. European Journal of Human Genetics. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain, Behavior, and Immunity. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch F. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Coe C, Lubach G, Schneider M, Dierschke D, Ershler W. Early rearing conditions alter immune responses in the developing infant primate. Pediatrics. 1992;90:505–509. [PubMed] [Google Scholar]
- Darrow S, Verhoeven J, Révész D, Lindqvist D, Penninx B, Delucchi K, … Mathews C. The association between psychiatric disorders and telomere length: A meta-analysis involving 14,827 persons. Psychosomatic Medicine. 2016;78:776–787. doi: 10.1097/PSY.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport M, Lutz C, Tiefenbacher S, Novak M, Meyer J. A rhesus monkey model of self-injury: Effects of relocation stress on behavior and neuroendocrine function. Biological Psychiatry. 2008;63:990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport M, Tiefenbacher S, Lutz C, Novak M, Meyer J. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- De Bellis M, Keshavan M. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews. 2003;27:103–117. doi: 10.1016/s0149-7634(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis B, Shirtcliff E. The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham J, O’Brien B, Charchar F. Telomere length maintenance and cardio-metabolic disease prevention through exercise training. Sports Medicine. 2016 doi: 10.1007/s40279-016-0482-4. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Didier E, MacLean A, Mohan M, Didier P, Lackner A, Kuroda M. Contributions of nonhuman primates to research on aging. Veterinary Pathology. 2016 doi: 10.1016/0300985815622974. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S. Unraveling the meaning of telomeres for child psychiatry. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54:539–540. doi: 10.1016/j.jaac.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S, Esteves K, Hatch V, Woodbury M, Borne S, Adamski A, Theall K. Setting the trajectory: Racial disparities in newborn telomere length. Journal of Pediatrics. 2015;166:1181–1186. doi: 10.1016/j.jpeds.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S, Gonzalez A, Sanchez M. When mothering goes awry: Challenges and opportunities for utilizing evidence across rodent, primate and human studies to better define the biological consequences of negative early caregiving. Hormones and Behavior. 2015;77:182–192. doi: 10.1016/j.ybeh.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S, Mabile E, Brett S, Esteves K, Jones E, Shirtcliff E, Theall K. The association of telomere length with family violence and disruption. Pediatrics. 2014;134:e128–e137. doi: 10.1542/peds.2013-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S, Theall K, Gleason M, Smyke A, De Vivo I, Wong J, … Nelson C. Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Molecular Psychiatry. 2012;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. An evolutionary review of human telomere biology: The thrifty telomere hypothesis and notes on potential adaptive paternal effects. American Journal of Human Biology. 2011;23:149–167. doi: 10.1002/ajhb.21127. [DOI] [PubMed] [Google Scholar]
- Epel ES. Telomeres in a life-span perspective: A new “psychobio-marker”? Current Directions in Psychological Science. 2009;18:6–10. [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, … Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Frenck RW, Blackburn E, Shannon K. The rate of telomere sequence loss in human leukocytes varies with age. Proceedings of the National Academy of Sciences. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nature Reviews Cardiology. 2013;10:274–283. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- Gardner J, Kimura M, Chai W, Durrani J, Tchakmakjian L, Cao X, … Skurnick J. Telomere dynamics in macaques and humans. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62:367–374. doi: 10.1093/gerona/62.4.367. [DOI] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, … Park J. Gender and telomere length: Systematic review and meta-analysis. Experimental Gerontology. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D, Humphreys K, Flannery J, Goff B, Telzer E, Shapiro M, … Tottenham N. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. Journal of Neuroscience. 2013;33:4584–4593. doi: 10.1523/jneurosci.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz G, Reardon C. Animal models of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I, LeMoult J, Colich N, Foland-Ross L, Hallmayer J, Joormann J, … Wolkowitz O. Telomere length and cortisol reactivity in children of depressed mothers. Molecular Psychiatry. 2015;20:615–620. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Fisher PA, Early Experience Stress, Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Hackman D, Farah M, Meaney M. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann M, Longenecker A, Marchetto N, Juliano S, Bowden R. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proceedings of the Royal Society Part B: Biological Sciences. 2012;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. British Medical Journal. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K. Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. American Journal of Human Biology. 2009;21:512–519. doi: 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- Hinde R, Spencer-Booth Y. The behaviour of socially living rhesus monkeys in their first two and a half years. Animal Behaviour. 1967;15:169–196. doi: 10.1016/s0003-3472(67)80029-0. [DOI] [PubMed] [Google Scholar]
- Honig L, Kang M, Cheng R, Eckfeldt J, Thyagarajan B, Leiendecker-Foster C, … Christensen K. Heritability of telomere length in a study of long-lived families. Neurobiology of Aging. 2015 doi: 10.1016/j.neurobiolaging.2015.06.017. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. The developmental effects of early life stress: An overview of current theoretical frameworks. Current Directions in Psychological Science. 2013;22:400–406. doi: 10.1177/0963721413488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B, Grand A, McCormack K, Shi Y, LaPrarie J, Maestripieri D, … Sanchez M. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: Relation to amygdala volume. Developmental Psychobiology. 2014;56:1735–1746. doi: 10.1002/dev.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B, McCormack K, Grand A, Zhang X, Maestripieri D, Hu D, Sanchez M. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: Associations with high cortisol during infancy. Biology of Mood & Anxiety Disorders. 2013;3:21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B, McMurray M, Guzman D, Nair G, Shi Y, McCormack K, … Sanchez M. Maternal buffering beyond glucocorticoids: Impact of early life stress on corticolimbic circuits that control infant responses to novelty. Social Neuroscience. 2017;12:50–64. doi: 10.1080/17470919.2016.1200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B, McMurray M, Guzman D, Nair G, Shi Y, McCormack K, … Sanchez M. Infant maltreatment and behavioral inhibition: Roles of maternal presence and prefrontal-amygdala connectivity. Social Neuroscience in press. [Google Scholar]
- Huizinga D, Haberstick B, Smolen A, Menard S, Young S, Corley R, … Hewitt J. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. Biological Psychiatry. 2006;60:677–683. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Esteves K, Zeanah C, Fox N, Nelson C, Drury S. Accelerated telomere shortening: Tracking the lasting impact of early institutional care at the cellular level. Psychiatry Research. 2016;246:95–100. doi: 10.1016/j.psychres.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K, Gleason M, Drury S, Murphy D, Nelson C, Fox N, Zeanah C. Effects of early deprivation and current placements on psychopathology in early adolescence: Follow up of a randomized clinical trial. Lancet Psychiatry in presss. [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvusaari J, Lonnqvist J, Peltonen L, … Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLOS ONE. 2010;5:e10826. doi: 10.13‘71/journal.-pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, Hostinar CE, Donzella B, Gunnar MR. Social deprivation and the HPA axis in early development. Psychoneuroendocrinology. 2014;50:1–13. doi: 10.1016/j.psyneuen.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Epel E, Adler N, Bush NR, Obradović J, Lin J, … Boyce WT. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosomatic Medicine. 2011;73:533–540. doi: 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küffer A, Maercker A, Burri A. Transgenerational effects of PTSD or traumatic stress: Do telomeres reach across the generations? Trauma and Treatment. 2014;3:8. [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and miner-alocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological Psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lopizzo N, Tosato S, Begni V, Tomassi S, Cattane N, Barcella M, … Pariante C. Transcriptomic analyses and leukocyte telomere length measurement in subjects exposed to severe recent stressful life events. Translational Psychiatry. 2017;7:e1042. doi: 10.1038/tp.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubach G, Coe C, Ershler W. Effects of early rearing environment on immune-responses of infant Rhesus monkeys. Brain, Behavior, and Immunity. 1995;9:31–46. doi: 10.1006/brbi.1995.1004. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, … Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the youth mood project. Biological Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. The biology of human parenting: Insights from nonhuman primates. Neuroscience & Biobehavioral Reviews. 1999;23:411–422. doi: 10.1016/s0149-7634(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proceedings of the National Academy of Sciences. 2005;102:9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Carroll K. Risk factors for infant abuse and neglect in group-living rhesus monkeys. Psychological Sciences. 1998;9:143. [Google Scholar]
- Maestripieri D, Jovanovic T, Gouzoules H. Crying and infant abuse in rhesus monkeys. Child Development. 2000;71:301–309. doi: 10.1111/1467-8624.00145. [DOI] [PubMed] [Google Scholar]
- McCormack K, Howell B, Guzman D, Villongco C, Pears K, Kim H, … Sanchez M. The development of an instrument to measure global dimensions of maternal care in rhesus macaques (Macaca mulatta) American Journal of Primatology. 2015;77:20–33. doi: 10.1002/ajp.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K, Newman T, Higley J, Maestripieri D, Sanchez M. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K, Sanchez M, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Developmental Psychobiology. 2006;48:537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- McLaughlin K, Sheridan M, Tibu F, Fox N, Zeanah C, Nelson C. Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences. 2015;112:5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Novak M. Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153:4120–4127. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Novak M, Hamel A, Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized Experiments. 2014;24:e50882. doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundstock E, Sarria E, Zatti H, Mattos Louzada F, Kich Grun L, Herbert Jones M, … Barbé-Tuana F. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity. 2015;23:2165–2174. doi: 10.1002/oby.21183. [DOI] [PubMed] [Google Scholar]
- Nelson C, Fox N, Zeanah C. Romania’s abandoned children: Deprivation, brain development and the struggle for recovery. Cambridge, MA: Harvard University Press; in press. [Google Scholar]
- Petrullo L, Mandalaywala T, Parker K, Maestripieri D, Higham J. Effects of early life adversity on cortisol/salivary alpha-amylase symmetry in free-ranging juvenile rhesus macaques. Hormones and Behavior. 2016 doi: 10.1016/j.yhbeh.2016.05.004. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policicchio B, Pandrea I, Apetrei C. Animal models for HIV cure research. Frontiers in Immunology. 2016;7:12. doi: 10.3389/fimmu.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J, Karlson E, Orr E, Zee R, De Vivo I, Costenbader K. A prospective study investigating prediagnostic leukocyte telomere length and risk of developing rheumatoid arthritis in women. Journal of Rheumatology. 2016;43:282–288. doi: 10.3899/jrheum.150184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provençal N, Suderman M, Guillemin C, Massart R, Ruggiero A, Wang D, … Côté S. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. Journal of Neuroscience. 2012;32:15626–15642. doi: 10.1523/JNEUROSCI.1470-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Samplin E, Ikuta T, Malhotra A, Szeszko P, DeRosse P. Sex differences in resilience to childhood maltreatment: Effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. Journal of Psychiatric Research. 2013;47:1174–1179. doi: 10.1016/j.jpsychires.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Alagbe O, Felger J, Zhang J, Graff A, Grand A, … Miller A. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Molecular Psychiatry. 2007;12:895–897. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- Sanchez M, McCormack K, Grand A, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Developmental Psychopathology. 2010;22:45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, McCormack K, Howell B. Social buffering of stress responses in nonhuman primates: Maternal regulation of development of emotional regulatory brain circuits. Social Neuroscience. 2015 doi: 10.1080/17470919.2015.1087426. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen K, Eriksson J, Kajantie E, Lahti J, Räikkönen K. Telomere length and hypothalamic–pituitary–adrenal axis response to stress in elderly adults. Psychoneuroendocrinology. 2015;53:179–184. doi: 10.1016/j.psyneuen.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Schino G, Cozzolino R, Troisi A. Social rank and sex-biased maternal investment in captive Japanese macaques: Behavioural and reproductive data. Folia Primatologica. 1999;70:254–263. doi: 10.1159/000021704. [DOI] [PubMed] [Google Scholar]
- Shalev I, Moffitt T, Sugden K, Williams B, Houts R, Danese A, … Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: A longitudinal study. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.32. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood Adoption, Dependent Care & Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2011;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Small M, Smith D. Sex differences in maternal investment by Macaca mulatta. Behavioral Ecology and Sociobiology. 1984;14:313–314. [Google Scholar]
- Smith D, Mattison J, Desmond R, Gardner J, Kimura M, Roth G, … Aviv A. Telomere dynamics in rhesus monkeys: No apparent effect of caloric restriction. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:1163–1168. doi: 10.1093/gerona/glr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behavior Neuroscience. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Lin J, Blackburn E, Erusalimsky J. The longitudinal relationship between cortisol responses to mental stress and leukocyte telomere attrition. Journal of Clinical Endocrinology and Metabolism. 2016 doi: 10.1210/jc.2016-3035. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stindl R. The paradox of longer sperm telomeres in older men’s testes: A birth-cohort effect caused by transgenerational telomere erosion in the female germline. Molecular Cytogenetics. 2016;9:1. doi: 10.1186/s13039-016-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenborgh M, Bakermans-Kranenburg M, Alink L, van IJzendoorn M. The prevalence of child maltreatment across the globe: Review of a series of meta-analyses. Child Abuse Review. 2015;24:37–50. [Google Scholar]
- Suomi S. Mother-infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Human Development. 2005;48:67–79. [Google Scholar]
- Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, Chan J, … Epel E. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiology & Behavior. 2012;106:40–45. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Administration for Children and Families, and Children’s Bureau. Child maltreatment 2013. Washington, DC: Author; 2015. Retrieved from http://www.acf.hhs.gov/sites/default/files/cb/cm2013.pdf. [Google Scholar]
- US Department of Health and Human Services, Administration for Children and Families, and Children’s Bureau. Child maltreatment 2015. Washington, DC: Author; 2017. Retrieved from http://www.acf.hhs.gov/programs/cb/research-data-technology/statistics-research/child-maltreatment. [Google Scholar]
- Vaughan K, Mattison J. Obesity and aging in humans and non-human primates: A mini-review. Gerontology. 2016 doi: 10.1159/000445800. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs TD, Georgieff M, Cusick S, McEwen BS. Issues in the timing of integrated early interventions: Contributions from nutrition, neuroscience, and psychological research. Annals of the New York Academy of Sciences. 2014;1308:89–106. doi: 10.1111/nyas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcicki J, Heyman M, Elwan D, Shiboski S, Lin J, Blackburn E, Epel E. Telomere length is associated with oppositional defiant behavior and maternal clinical depression in Latino preschool children. Translational Psychiatry. 2015;5:e581. doi: 10.1038/tp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Renault V, Jamet K, Gilson E. Transcriptional outcome of telomere signalling. Nature Reviews Genetics. 2014;15:491–503. doi: 10.1038/nrg3743. [DOI] [PubMed] [Google Scholar]