Abstract
Objective:
The present study is an exploration of the dynamic changes of plasma mitochondrial deoxyribonucleic acid (mtDNA) and inflammatory level in patients with acute myocardial infarction (MI).
Methods:
Thirty-eight patients with acute MI and 33 control participants were included in the study. Blood samples were collected on admission, 12 hours post-percutaneous coronary intervention (PCI), 24 hours post-PCI, and 48 hours post-PCI. White blood cell (WBC) count and C-reactive protein (CRP) level were determined. Plasma was isolated from whole blood. Plasma mtDNA was measured using real-time polymerase chain reaction, and tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were measured using enzyme-linked immunosorbent assay kits. Bivariate correlation analysis was used to find correlation between plasma mtDNA and inflammatory level on admission.
Results:
Plasma mtDNA was significantly higher in patients with acute MI than controls on admission (p<0.01). Plasma mtDNA decreased significantly after PCI treatment (p=0.01). WBC count, TNF-α, IL-6 and CRP showed similar pattern: elevation after onset of acute MI and contraction after PCI treatment (p<0.05). Positive correlations between plasma mtDNA and WBC count (r=0.435; p<0.001), TNF-α (r=0.538; p<0.001), IL-6 (r=0.518; p<0.001), and CRP (r=0.524; p<0.001) were identified.
Conclusion:
Plasma mtDNA elevated after onset of acute MI and positive correlation was observed between plasma mtDNA and inflammatory level, suggesting that mtDNA may play a key role in inflammatory responses in patients with acute MI.
Keywords: acute myocardial infarction, inflammatory responses, mitochondrial DNA
Introduction
Cardiovascular diseases are still the leading cause of mortality in the world, especially in Western societies. Myocardial infarction (MI), one of the acute coronary syndromes, is a major disease with high morbidity and mortality (1). When atherosclerosis of coronary arteries is present, rupture of vulnerable plaques partially or completely occludes coronary arteries, which activates platelets and leads to formation of thrombus (2). Percutaneous coronary intervention (PCI) developed rapidly once invented and became the first-line therapy for acute MI (3). It is widely known that inflammatory responses occur after MI in order to repair the heart. However, excessive inflammatory responses in circulation may lead to arrhythmia, death, recurrence of MI or heart failure (4, 5).
Mitochondrial deoxyribonucleic acid (mtDNA) is a pro-inflammatory agent that can provoke systemic inflammatory responses to many pathological conditions (6). Mitochondria are considered endosymbiotic bacteria, which evolved from saprophytic bacteria to endosymbionts to organelles (7). MtDNA contains the CpG motif and activates neutrophils, initiating inflammatory responses (8). When cells are damaged or mitochondria is insulted, mtDNA will be released into the extracellular matrix or circulation, which causes local or systemic inflammatory responses (9).
Given the pro-inflammatory character of mtDNA, present authors postulated that mtDNA may play a role in initiation of post-MI inflammatory responses. C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and white blood cell count (WBC) were measured to evaluate inflammatory level of systemic inflammatory responses. Relationship between plasma mtDNA level and systemic inflammatory level was also analyzed. The present study aimed to identify a new target for eliminating excessive post-MI inflammatory responses, which could have protective effect for the heart and other organs.
Method
Patients
Thirty-eight patients who were admitted to the Cardiovascular Department of West China Medical Center of Sichuan University with acute MI between January 2014 and July 2014 were included in the study. Acute MI, including ST-segment elevated MI and non ST-segment elevated MI, was diagnosed according to European Society of Cardiology and American College of Cardiology and American Heart Association updated guidelines (10–12). Medical history of endocarditis, neurological disease, psychiatric disease, infectious disease, previous transmural infarction, shock, or hematological disease was excluding criterion. Thirty-three patients with first onset of angina within 24 hours whose angiography was negative were regarded as controls. Written, informed consent was provided by all patients. All participant information was collected on admission, including basic information, laboratory data, and medications used. The study was conducted following the principles of the Declaration of Helsinki and was approved by the Research Ethics Committee.
Blood samples collection
Blood samples were collected in 2 ethylenediaminetetraacetic acid-coated blood collection tubes on admission (T1), 12 hours post-PCI (T2), 24 hours post-PCI (T3), and 48 hours post-PCI (T4). One sample was used for routine blood test and C-reactive protein (CRP) assay, and results were reported by the hospital division of clinical hematology. The second sample was centrifuged at 1000 rpm/min for 15 minutes at 4°C, and supernatant was collected as plasma without touching the pellet or the bottom of the tube. Samples of plasma were stored in -80°C freezer for DNA isolation and enzyme-linked immunosorbent assay (ELISA).
DNA isolation and rt-PCR for mtDNA
Whole plasma DNA was isolated from plasma using DNeasy Blood and Tissue Kit (#69504; Qiagen, N.V., Hilden, Germany). Briefly, 50 μL plasma samples were added to 50 μL phosphate-buffered saline and centrifuged at 16000 g for 15 minutes at 4°C. A total of 90 μL of supernatant was collected. The next procedures were carefully performed according to kit manufacturer’s protocol. At the final step, 200 μL elution buffer was added to resolve DNA.
MtDNA levels were measured with SYBR (Thermo Fisher Scientific, Waltham, MA, USA) green dye-based real-time polymerase chain reaction (rt-PCR) assay using ABI PRISM 7300 sequence detection system (Applied Biosystems, Foster City, CA, USA). Primer sequence was human NADH dehydrogenase 1 gene (mtDNA): forward 5’-CGAGCAGTAGCCCAAACAAT-3’ and reverse 5’-TGTGATAAGGGTGGAGAGGTT-3’. Plasmid DNA with complementary DNA sequence for human mtDNA was obtained from OriGene Technologies (SC101172; Rockville, MD, USA). Concentrations of plasma mtDNA were converted to copy number via DNA copy number calculator (http://cels.uri.edu/gsc/cndna.html; University of Rhode Island Genomics and Sequencing Center, Kingston, RI, USA). Plasmid DNA were diluted in 10-fold serial dilutions and measured as standard curve (13).
All samples were measured with standards at the same time. Plasma mtDNA levels were recorded in copies per microliter of plasma according to the following formula:
c=Q * VDNA / VPCR * 1 / Vext
C represents the concentration of plasma mtDNA (copies/μL); Q means quantity of DNA measured by rt-PCR; VDNA is total volume of plasma DNA solution obtained from extraction, 200 μL in our study. VPCR means volume of plasma DNA solution for rt-PCR, 1 μL in present study, and Vext is volume of plasma used for extraction, 50 μL in this study.
Measurement of cytokines
Plasma TNF-α, and IL-6 were measured with ELISA kit (Solarbio Science&Technology Co., Ltd., Beijing, China). All procedures followed standard protocols (included in ELISA kits). Spectrophotometry was used to detect intensity of transmitted light. Data was expressed in picogram per mL.
Statistical analysis
All descriptive data were shown as mean±SD and analyzed with SPSS statistical software version 20.0 for Windows (SPSS, Inc., Chicago, IL, USA) and Prism 5 for Windows (GraphPad Software, Inc., San Diego, CA, USA). Normality of distribution of continuous variables was assessed using Kolmogorov-Smirnov test. Comparisons between groups were carried out using Student’s t-test according to normality of distribution. Multiple comparisons were analyzed with two-way analysis of variance followed by Bonferroni’s test. Pearson’s correlation coefficient test (two-tailed) was conducted. A p<0.05 was considered statistically significant.
Results
Baseline information
Patient basic information, laboratory data, and medications are presented in Table 1. All patients underwent coronary angiography and infarct-related artery was successfully treated. Mean age of acute MI patients was 54.8±14.3 years and that of controls (25 males, 7 females) was 59.3±12.7 years (p>0.05). Baseline level on admission of glucose, serum creatinine, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, hemoglobin and hematocrit for both acute MI patients and controls are also presented in Table 1.
Table 1.
Baseline information
| Variable | Acute MI patients n=38 | Controls n=32 |
|---|---|---|
| Age, years | 54.8±14.3 | 59.3±12.7 |
| Male, % | 31 (81.6%) | 25 (78.1%) |
| BMI, kg/m2 | 24.3±4.2 | 23.9±3.6 |
| Hypertension, % | 21 (55.3%) | 17 (53.1%) |
| Current smoker, % | 11 (28.9%) | 9 (28.1%) |
| Inferior/posterior infarction, % | 20 (52.6%) | – |
| Anterior infarction, % | 18 (47.4%) | – |
| Laboratory data | ||
| Glucose | 133.4±30.8 | 131.8±34.7 |
| Serum creatinine, μmol/L | 0.6±0.2 | 0.6±0.3 |
| Total cholesterol, mg/dL | 207.1±44.6 | 203.3±39.5 |
| HDL, mmol/L | 1.1±0.3 | 1.1±0.2 |
| LDL, mmol/L | 3.1±0.6 | 3.0±0.5 |
| TG, mmol/L | 1.7±0.4 | 1.7±0.3 |
| Hemoglobin, g/L | 125.3±14.1 | 131.3±15.7 |
| Hematocrit, % | 38.2±3.4 | 38.3±3.3 |
| Medications | ||
| Statins, % | 33 (86.8%) | 26 (81.3%) |
| ACEIs/ARBs, % | 23 (60.5%) | 14 (43.7%) |
| β-blockers, % | 34 (89.5%) | 16 (50.0%) |
| CCB, % | 9 (23.7%) | 6 (5.2%) |
| Nitrates, % | 25 (65.8%) | 15 (46.9%) |
| Aspirin, % | 32 (84.2%) | 25 (78.1%) |
ACEI - angiotensin converting enzyme inhibitor; ARB - angiotensin receptor blocker; BMI - body mass index; CCB - calcium channel blocker; HDL - high-density lipoprotein; LDL - low-density lipoprotein; MI - myocardial infarction; TG - triglycerides. Comparison performed using Student’s t-test. All descriptive data presented as mean±SD
Dynamic changes of plasma mtDNA
Plasma mtDNA of all samples was examined using rt-PCR and results are provided in Figure 1. On admission, plasma mtDNA level was significantly higher in acute MI patients than in controls (478±106 copies/μL vs. 157±97 copies/μL; p<0.01). Dynamic changes after PCI in acute MI patients were further illustrated: Plasma mtDNA was highest on admission, decreased quickly at T2 reading, and gradually returned to normal at T3 and T4 (p=0.01). Data indicated that plasma mtDNA elevated after acute MI, and that after PCI treatment, plasma mtDNA decreased remarkably and was normal level within 48 hours.
Figure 1.
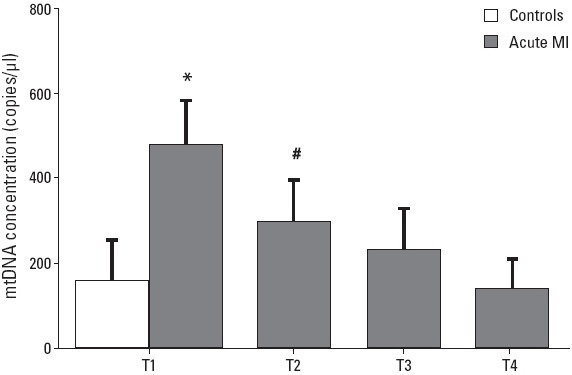
Dynamic changes of plasma mtDNA in patients with acute myocardial infarction (MI) and controls. Peak level of plasma mtDNA was found on admission in patients with acute MI compared with controls (P<0.01). After PCI treatment, plasma mtDNA decreased significantly and returned to normal level at 48 hours post-PCI (P=0.01). *P<0.01 vs. controls; #P=0.01 vs. T1
Inflammatory levels in acute MI patients and controls
WBC count, TNF-α, IL-6 and CRP were evaluated to represent inflammatory levels in acute MI patients and controls. As shown in Figure 2, significantly higher WBC count, and TNF-α, IL-6 and CRP levels were found in acute MI patients compared with controls (p<0.05). In acute MI patients, all inflammatory parameters decreased from T1 to T4, and no significant difference was defined compared with controls at T4 (p>0.05). Our results demonstrated presence of inflammatory responses after onset of acute MI, and after PCI treatment, just like change observed in plasma mtDNA, all inflammatory parameters decreased.
Figure 2.
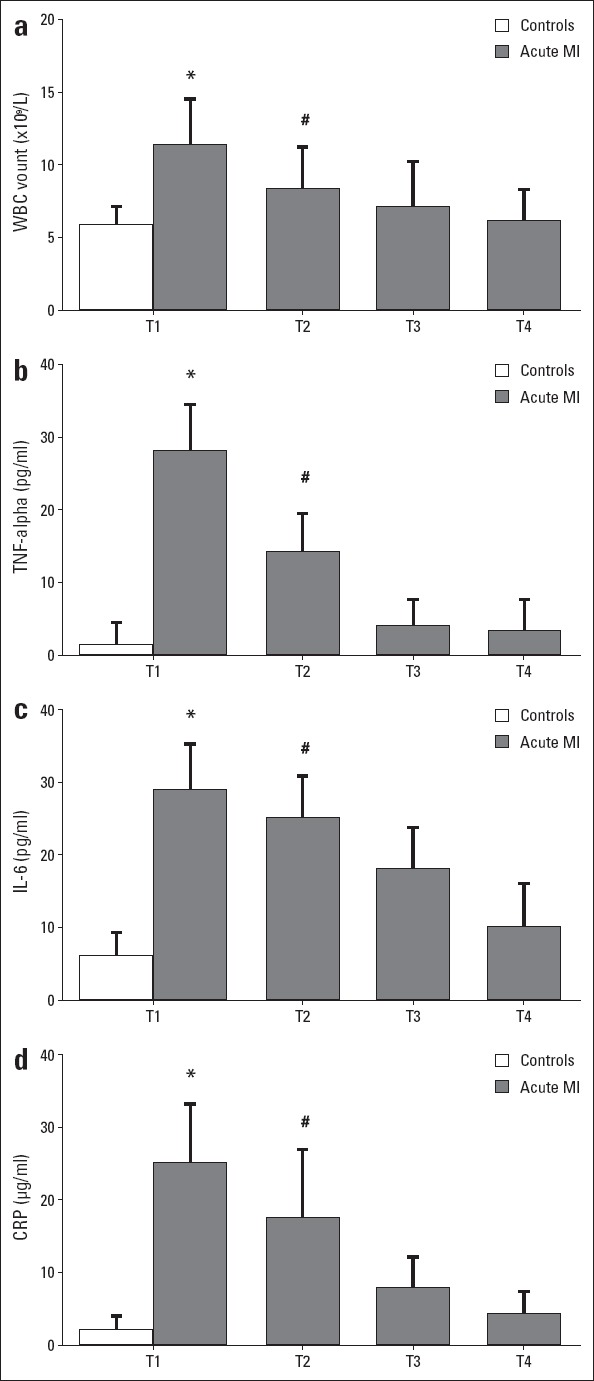
Dynamic changes of inflammatory markers in patients with acute myocardial infarction (MI) and controls. (a–d) white blood cell count, tumor necrosis factor-alpha, interleukin-6, and C-reactive protein level elevated on admission in patients with acute MI compared with controls (P<0.05). After PCI treatment, all inflammatory markers decreased significantly and returned to normal (P<0.05). *P<0.05 vs. controls; #P<0.05 vs. T1
Correlations between plasma mtDNA and inflammatory parameters on admission
Bivariate correlation analysis was used to establish correlation between plasma mtDNA and each inflammatory parameter on admission. Amazingly, positive correlation was identified between plasma mtDNA and WBC (r=0.435; p<0.001), TNF-α (r=0.538; p<0.001), IL-6 (r=0.518; p<0.001), and CRP (r=0.524; p<0.001). These interesting data showed tight relationship between mtDNA and inflammatory parameters, which might aid understanding of pro-inflammatory role of mtDNA.
Discussion
The present study demonstrated dynamic changes of plasma mtDNA and inflammatory levels during acute MI treated with PCI. We identified that plasma mtDNA rose significantly in acute MI compared with controls and decreased after PCI treatment. Inflammatory level was represented by WBC count, TNF-α, IL-6, and CRP levels, which all increased after acute MI and decreased after PCI treatment. Interestingly, positive correlation between plasma mtDNA and inflammatory level on admission was seen using bivariate correlation analysis.
Increasing number of studies have demonstrated that levels of inflammatory markers are positively correlated with adverse cardiovascular events, a result of elevated inflammatory levels following the acute MI (14). It has been presented that acute myocardial necrosis and impaired matrix can cause activation of inflammatory signaling pathways and production of pro-inflammatory cytokines via release of endogenous damage-associated molecular patterns (DAMPs) (5). WBC count has been reported as predictor of death in patients with acute MI (15). Infiltration of neutrophils to coronary plaques and infarcted myocardium was reported, causing damage through secretion of matrix-degrading enzyme and reactive oxygen species (16).
TNF-α and IL-6 were found to have elevated rapidly in patients with acute coronary syndrome (ACS). It was fully studied that TNF-α and IL-6 can act as predictors of mortality (17–19). CRP, as hot inflammatory marker, was found to be strong predictor of adverse outcome in patients with ACS (20, 21). All of the above suggested that WBC count and other inflammatory markers elevated in acute MI and inflammatory levels in patients with acute MI could influence recovery. In the present study, we found elevated WBC count, TNF-α, IL-6, and CRP, representing inflammatory responses after acute MI. All inflammatory markers subsequently decreased after PCI and were almost normal at 48 hours post-PCI.
It has been documented that plasma mtDNA released after trauma surgery was correlated with invasiveness and complexity of surgery (22). As mentioned above, plasma mtDNA can act as pro-inflammatory agent, a fact that has been noted and studied in many different conditions (23–25). Recently, Qin et al. (26) found plasma mtDNA during cardiopulmonary bypass surgery. Positive correlation between plasma mtDNA and peak post-CPB inflammatory responses was identified. It is fully understood that plasma mtDNA can cause inflammatory responses and create a sepsis-like state (7). It has also been reported that intravenous injection of mtDNA into mice can result in systemic inflammation and multiple organ failures (27). In the present study, after onset of acute MI, we identified significantly increased plasma mtDNA compared with control patients. After PCI treatment, gradual decrease of plasma mtDNA was demonstrated. Further, correlation between plasma mtDNA and inflammatory level on admission was analyzed and positive correlation was observed. Given the pro-inflammatory properties of mtDNA and correlation between plasma mtDNA and inflammatory level in patients with acute MI, we believe that mtDNA was released into the circulation after onset of acute MI and, at least partially, responsible for post-MI inflammation.
Study limitations
Although present study found some genuinely interesting results, it is still a preliminary study, and further large-scale and well-designed clinical investigations are needed to confirm the association between mtDNA and post-acute MI inflammatory status. Give effects of inflammation on prognosis of acute MI, more outcomes and prognostic values should be included in future studies to explore effects of mtDNA. In addition, our study provided some novel associations through bivariate correlation analysis. However, they could not be proven under clinical conditions, which calls for further animal work to study the detailed mechanism between plasma mtDNA and post-acute MI inflammation. Another limitation is that due to the small study population, we didn’t perform subgroup analysis.
Conclusion
The present study demonstrated release of mtDNA into circulation and inflammatory level in patients with acute MI. We also first revealed positive correlation between plasma mtDNA and inflammatory level, suggesting that plasma mtDNA may play a critical role in inflammatory responses in patients with acute MI. This study has provided novel clinical observations for further mechanism study and raised a promising target for eliminating inflammatory responses in patients with acute MI and improving outcomes.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 8150036, No. 81370413, No. 81500213) and the Natural Science Foundation of Sichuan Province (2013FZ0089, 2015-HM01-00032-SF).
Footnotes
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – W.M.; Design – C.Q., J.G., Supervision – W.M.; Funding – W.M.; Materials – Q.H.; Data collection &/or processing – R.L., F.X., H.Q.; Analysis and/or interpretation – C.Q.; Literature review – J.G.; Writing – C.Q.; Critical review – W.M.
References
- 1.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–84. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 3.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 4.Gökdemir MT, Kaya H, Sögüt O, Kaya Z, Albayrak L, Taşkın A. The role of oxidative stress and inflammation in the early evaluation of acute non-ST-elevation myocardial infarction: an observational study. Anadolu Kardiyol Derg. 2013;13:131–6. doi: 10.5152/akd.2013.037. [DOI] [PubMed] [Google Scholar]
- 5.Fang L, Moore XL, Dart AM, Wang LM. Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol. 2015;12:305–12. doi: 10.11909/j.issn.1671-5411.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itagaki K, Kaczmarek E, Lee YT, Tang IT, Isal B, Adibnia Y, et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PloS one. 2015;10:e0120549. doi: 10.1371/journal.pone.0120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verschoor CP, Loukov D, Naidoo A, Puchta A, Johnstone J, Millar J, et al. Circulating TNF and mitochondrial DNA are major determinants of neutrophil phenotype in the advanced-age, frail elderly. Mol Immunol. 2015;65:148–56. doi: 10.1016/j.molimm.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Mittra I, Nair NK, Mishra PK. Nucleic acids in circulation: are they harmful to the host? J Biosci. 2012;37:301–12. doi: 10.1007/s12038-012-9192-8. [DOI] [PubMed] [Google Scholar]
- 10.StephanWindecker Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Rev Esp Cardiol. 2015;68:144. doi: 10.1016/j.rec.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–94. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 12.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction-executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10:e1001577. doi: 10.1371/journal.pmed.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.İpek G, Onuk T, Karataş MB, Güngör B, Atasoy I, Murat A, et al. Relationship between Neutrophil-to-Lymphocyte Ratio and Left Ventricular Free Wall Rupture in Acute Myocardial Infarction. Cardiology. 2015;132:105–10. doi: 10.1159/000431354. [DOI] [PubMed] [Google Scholar]
- 15.Nunez J, Nunez E, Bodi V, Sanchis J, Minana G, Mainar L, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–52. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost. 2011;106:591–9. doi: 10.1160/TH11-02-0096. [DOI] [PubMed] [Google Scholar]
- 17.Valgimigli M, Ceconi C, Malagutti P, Merli E, Soukhomovskaia O, Francolini G, et al. Tumor necrosis factor-alpha receptor 1 is a major predictor of mortality and new-onset heart failure in patients with acute myocardial infarction: the Cytokine-Activation and Long-Term Prognosis in Myocardial Infarction (C-ALPHA) study. Circulation. 2005;111:863–70. doi: 10.1161/01.CIR.0000155614.35441.69. [DOI] [PubMed] [Google Scholar]
- 18.Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–84. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- 19.Andrie RP, Becher UM, Frommold R, Tiyerili V, Schrickel JW, Nickenig G, et al. Interleukin-6 is the strongest predictor of 30-day mortality in patients with cardiogenic shock due to myocardial infarction. Crit Care. 2012;16:R152. doi: 10.1186/cc11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodi V, Sanchis J, Llacer A, Facila L, Nunez J, Pellicer M, et al. Multimarker risk strategy for predicting 1-month and 1-year major events in non-ST-elevation acute coronary syndromes. Am Heart J. 2005;149:268–74. doi: 10.1016/j.ahj.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 21.Bahadır A, Baltacı D, Türker Y, Türker Y, Iliev D, Öztürk S, et al. Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anatol J Cardiol. 2015;15:816–22. doi: 10.5152/akd.2014.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIlroy DJ, Bigland M, White AE, Hardy BM, Lott N, Smith DW, et al. Cell necrosis-independent sustained mitochondrial and nuclear DNA release following trauma surgery. J Trauma Acute Care Surg. 2015;78:282–8. doi: 10.1097/TA.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao H, Ye H, Sun Z, Shen X, Song Z, Wu X, et al. Circulatory mitochondrial DNA is a pro-inflammatory agent in maintenance hemodialysis patients. PloS one. 2014;9:e113179. doi: 10.1371/journal.pone.0113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkins HM, Carl SM, Weber SG, Ramanujan SA, Festoff BW, Linseman DA, et al. Mitochondrial lysates induce inflammation and Alzheimer's disease-relevant changes in microglial and neuronal cells. J Alzheimers Dis. 2015;45:305–18. doi: 10.3233/JAD-142334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YS, Kwak JW, Lee KE, Cho HS, Lim SJ, Kim KS, et al. Can mitochondrial dysfunction be a predictive factor for oxidative stress in patients with obstructive sleep apnea? Antioxid Redox Signal. 2014;21:1285–8. doi: 10.1089/ars.2014.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin C, Liu R, Gu J, Li Y, Qian H, Shi Y, et al. Variation of perioperative plasma mitochondrial DNA correlate with peak inflammatory cytokines caused by cardiac surgery with cardiopulmonary bypass. J Cardiothorac Surg. 2015;10:85. doi: 10.1186/s13019-015-0298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan L, Chen X, Sun T, Li Q, Zhang R, Zhang J, et al. Significance of Serum mtDNA concentration in lung injury induced by hip fracture. Shock. 2015;44:52–7. doi: 10.1097/SHK.0000000000000366. [DOI] [PubMed] [Google Scholar]


