ABSTRACT
Cryptococcal species vary in capsule and cell size, thermotolerance, geographic distribution, and affected populations. Cryptococcus gattii sensu stricto and C. deuterogattii affect mainly immunocompetent hosts; however, C. bacillisporus, C. decagattii, and C. tetragattii cause infections mainly in immunocompromised hosts. This study aimed to compare the capacities of different species of the C. gattii species complex to induce cytokines and antimicrobial molecules in human peripheral blood mononuclear cells (PBMCs). Cryptococcus bacillisporus and C. deuterogattii induced the lowest levels of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 among the five species of the C. gattii complex. Cryptococcus deuterogattii induced higher levels of IL-22 than those induced by C. tetragattii and the environmental species C. flavescens. In addition, C. bacillisporus and C. gattii sensu stricto proliferated inside human monocyte-derived macrophages after 24 h of infection. All Cryptococcus species were able to generate reactive oxygen species (ROS) in human PBMCs, with C. bacillisporus and C. deuterogattii being more efficient than the other species. In conclusion, C. bacillisporus and C. deuterogattii induce lower levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 and higher ROS levels than those induced by the other species. Species of the Cryptococcus gattii complex have different abilities to induce cytokine and ROS production by human PBMCs.
KEYWORDS: Cryptococcus, Cryptococcus gattii, cryptococcosis, cytokines, proinflammatory
INTRODUCTION
Cryptococcosis is a systemic fungal disease caused by the basidiomycetous yeasts belonging to the Cryptococcus neoformans/C. gattii species complexes (1). The infection is acquired from the environment by inhalation of desiccated yeast cells or basidiospores, which establish either an asymptomatic latent infection or pneumonia and meningoencephalitis (2). The taxonomy of the polyphyletic genus Cryptococcus was recently revised (1, 3, 4). The two C. neoformans varieties were raised to the species level, as C. neoformans sensu stricto (formerly C. neoformans var. grubii) and C. deneoformans (formerly C. neoformans var. neoformans) (1). In addition, the five genotypes within the C. gattii species complex were raised to the species level, as C. gattii sensu stricto (AFLP4/VGI), C. bacillisporus (AFLP5/VGIII), C. deuterogattii (AFLP6/VGII), C. tetragattii (AFLP7/VGIV), and C. decagattii (AFLP10/VGIV) (1). Although the taxonomic revision was established after >15 years of debate, part of the community prefers the use of the term “species complex” (5) despite the accumulating evidence for the revised taxonomy (6).
Species discrimination has clinical and epidemiological importance, as it has been observed that there are differences in disease presentation and in susceptibility profiles for antifungal drugs (1). Infections by members of the C. gattii species complex mainly cause pneumonia, meningoencephalitis, skin lesions, pulmonary or cerebral cryptococcomas, and central nervous system (CNS) disease associated with neurological sequelae. These infections typically require prolonged antifungal therapy (7–11). In contrast, severe meningitis is the main infection caused by C. neoformans sensu lato (12). In addition, the species C. gattii sensu stricto and C. deuterogattii affect mainly immunocompetent hosts (1, 13), whereas C. bacillisporus, C. decagattii, and C. tetragattii are more commonly found in immunocompromised hosts, mostly HIV/AIDS patients, similar to C. neoformans sensu lato (1, 7, 14, 15). However, fatal cryptococcosis by C. bacillisporus in immunocompetent hosts has been reported in the literature (16, 17).
In addition, phenotypic diversity supports the differences among the species within the C. gattii species complex. Capsule and cell sizes vary: C. gattii sensu stricto (AFLP4/VGI) has the largest capsules but smaller cells than those of other species, whereas C. deuterogattii (AFLP6/VGII) has the largest cells but smaller capsules (18). All species have the ability to grow at 25, 30, and 35°C, but they have various levels of tolerance to 37°C (1, 18). Cryptococcus deuterogattii (AFLP6/VGII) has the highest thermotolerance at 37°C, while C. gattii sensu stricto (AFLP4/VGI), C. bacillisporus (AFLP/VGIII), and C. tetragattii (AFLP7/VGIV) have less growth at 37°C than at 30°C (1, 18, 19). There are no significant differences in tolerance to oxidative (19) or osmotic (18) stresses among species.
An important aspect of cryptococcal infections is how Cryptococcus species evade the host immune system and establish infection. To reach the CNS, cryptococcal cells use paracytosis to move between tight junctions of the brain endothelium (20), transcytosis to move directly through the endothelial cells (21), and hitchhiking within phagocytes (22, 23). Typically, these fungi avoid killing by host phagocytic cells due to virulence factors, such as the polysaccharide capsule, melanin, and urease production, in addition to other traits, such as the ability to escape phagocytosis by inducing nonlytic exocytosis and by producing titan cells (24–26).
Cryptococcus gattii sensu lato is also able to disturb the inflammatory process, inducing low levels of proinflammatory cytokine tumor necrosis factor alpha (TNF-α) production in vitro (27). The cryptococcal capsule components glucuronoxylomannan and galactoxylomannan dampen inflammation by suppressing the NF-κB pathway and blocking surface antigen recognition (27, 28). In consequence, dendritic cell maturation is compromised, leading to release of TNF-α, interleukin-12 (IL-12), and IL-23 at low levels and to decreased major histocompatibility complex class II molecule expression. Thus, suboptimal T-cell responses and weak proinflammatory responses are induced, inhibiting adequate cryptococcal clearance (27–29).
Most knowledge about the immune response patterns against Cryptococcus species is derived from studies of C. neoformans species complex isolates performed with animal models and human cells (30–34). However, the interest in studying immune response patterns against the members of the C. gattii species complex has increased recently (28, 29, 35–38) due to evidence of genetic diversity, phenetic differences, and epidemiologic particularities among the species (1, 18, 39–41). Based on these aspects, the present study hypothesized that the five species within the C. gattii complex have different abilities to induce cytokine production in human cells, a crucial step for the activation of host defense. In addition, macrophage infection and antimicrobial molecules were evaluated.
RESULTS
Cryptococcus bacillisporus and C. deuterogattii induced lower levels of TNF-α, IL-1β, and IL-6 than those induced by the other species.
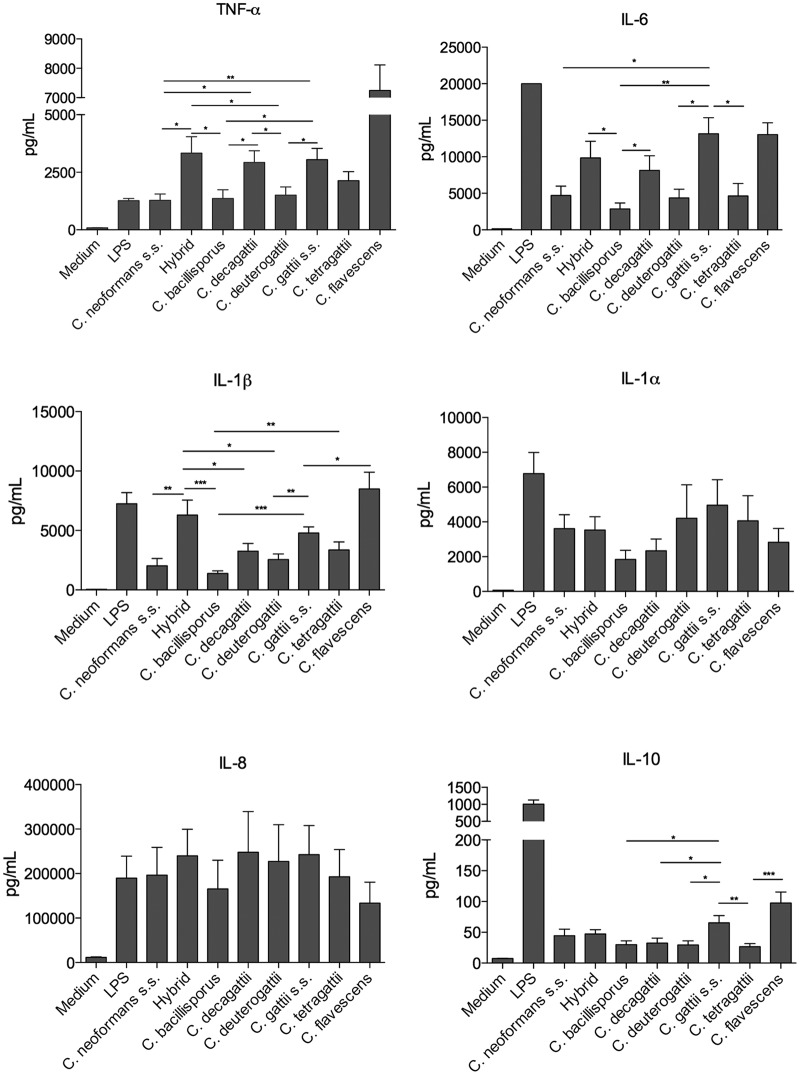
The quantitative concentrations of cytokines produced by human peripheral blood mononuclear cells (PBMCs) from healthy volunteers after stimulation with different Cryptococcus species are shown in Fig. 1. C. neoformans sensu stricto, C. bacillisporus, and C. deuterogattii induced lower levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 than those induced by the other species. Cryptococcus gattii sensu stricto and the control species C. flavescens induced higher levels of IL-10 than those observed with the other species, and no differences in IL-8 levels were observed.
FIG 1.
Monocyte-derived cytokine production induced by members of the C. gattii species complex. Human PBMCs (n = 6) from healthy volunteers (5 × 105 cells/ml) were stimulated with LPS (10 ng/ml) or live yeast forms of Cryptococcus species (2.5 × 106 cells/ml). After 24 h of incubation, TNF-α, IL-1α, IL-1β, IL-6, IL-8, and IL-10 production in supernatants was determined by ELISA. Mean values with SEM for three independent experiments done in duplicate are presented. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. s.s., sensu stricto.
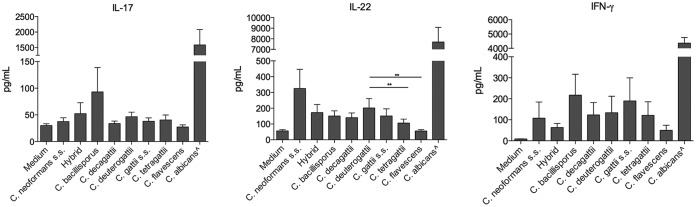
Cryptococcus deuterogattii induced higher levels of IL-22 than those induced by C. tetragattii and C. flavescens.
A comparison of quantitative T-cell-derived cytokine production stimulated by Cryptococcus species is shown in Fig. 2. Cryptococcus species induced low levels of gamma interferon (IFN-γ) production by human PBMCs, without significant differences among the species. The same was observed for IL-17, with all species tested inducing small amounts of IL-17. For IL-22, C. bacillisporus, C. decagattii, C. deuterogattii, and C. gattii sensu stricto induced similar levels. However, C. deuterogattii induced higher levels than those induced by C. tetragattii (P < 0.01) and C. flavescens (P < 0.01).
FIG 2.
T-cell-derived cytokine production induced by members of the C. gattii species complex. Human PBMCs (n = 6) from healthy volunteers (5 × 105 cells/ml) were stimulated with LPS (10 ng/ml), live yeast forms of Cryptococcus species (2.5 × 105 cells/ml), or heat-killed Candida albicans (1 × 107 cells/ml), as a positive control. After 7 days of incubation, IL-17, IL-22, and IFN-γ production in supernatants was determined by ELISA. Mean values (n = 6) with SEM for three independent experiments done in duplicate are presented. *, P ≤ 0.05; **, P ≤ 0.01. ∧, Candida albicans.
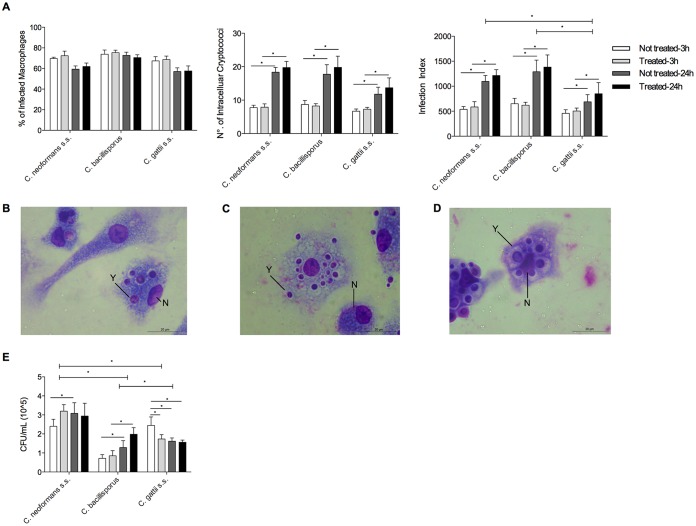
Cryptococcus bacillisporus and C. gattii sensu stricto proliferated inside macrophages after 24 h of infection.
Human primary granulocyte-macrophage colony-stimulating factor (GM-CSF)-derived macrophages were used to evaluate the rates of phagocytosis of three Cryptococcus species. Cryptococcus neoformans sensu stricto was included as a control; C. bacillisporus and C. gattii sensu stricto were included because the first induced low and the latter large amounts of proinflammatory cytokines. The rates of phagocytosis for macrophages infected with C. neoformans sensu stricto, C. bacillisporus, and C. gattii sensu stricto are shown in Fig. 3. After infection, 60 to 80% of the total number of macrophages were infected with cryptococci. No significant differences in the percentage of infection were observed after 3 or 24 h of incubation. After preactivation of macrophages with IFN-γ (50 U/ml), no effect on phagocytosis rates was observed (Fig. 3A), and no differences in numbers of intracellular cryptococci per preactivated or nonpreactivated macrophage were found. However, after 24 h, there was a significant increase in the number of intracellular C. neoformans, C. bacillisporus, or C. gattii sensu stricto organisms per macrophage (P < 0.05), independent of the absence or presence of IFN-γ. The infection index after 24 h was lower for C. gattii sensu stricto (P < 0.05) than for C. neoformans and C. bacillisporus in preactivated and nonpreactivated macrophages. Photomicrographs of macrophages infected with different cryptococcal species are presented in Fig. 3B to D.
FIG 3.
Macrophage infection indexes and cryptococcal viability. Primary human GM-CSF-derived macrophages (n = 6) (2 × 105 cells/ml), pretreated or not with IFN-γ (50 U/ml), were infected with one of three cryptococcal species (1 × 106 cells/ml). After 3 and 24 h, the lysates of cells diluted 100× in PBS were seeded onto Sabouraud dextrose medium and incubated for 48 to 72 h, and the numbers of CFU were determined. (A) Primary GM-CSF-derived macrophages (2 × 105 cells/ml) were infected with cryptococcal species (1 × 106 cells/ml) on coverslips under the conditions described above. After the incubation time, cells were stained, and the percentage of infected macrophages, number of intracellular cryptococci per macrophage, and infection index were determined. (B to D) C. neoformans sensu stricto (B), C. bacillisporus (C), and C. gattii sensu stricto (D) internalized by macrophages. (E) Total numbers of live cryptococcal cells recovered from macrophages after 3 and 24 h of incubation. N, macrophage nucleus; Y, yeast cell. *, P ≤ 0.05.
The phagocytosed cryptococci were recovered by macrophage lysis, and their viability was investigated by CFU counting (Fig. 3E). The numbers of viable Cryptococcus neoformans sensu stricto and C. bacillisporus organisms were higher after 24 h than after 3 h of infection for nonpreactivated macrophages. For preactivated macrophages, there was an increase in the number of viable cells only for C. bacillisporus after 24 h of infection compared to 3 h of infection. In contrast, C. gattii sensu stricto had more viability than that of C. bacillisporus 3 h after infecting nonpreactivated macrophages. Nevertheless, the viability of C. gattii decreased from 3 h to 24 h. Comparing the viabilities among species, C. neoformans had more viability than C. bacillisporus and C. gattii sensu stricto after 24 h of infection in preactivated or nonpreactivated macrophages (P < 0.05).
Macrophage cytokine production was measured in the supernatants 3 and 24 h after infection. After 3 h of infection, there was no production of IL-1β, IL-6, and IL-10 by macrophages. All tested cryptococcal species induced IL-8 and TNF-α production in macrophages after 24 h; C. gattii sensu stricto induced higher levels of IL-8 than those induced by C. bacillisporus (P < 0.05) (see Fig. S1 in the supplemental material). Preactivated macrophages with IFN-γ produced higher levels of TNF-α after 24 h of infection with C. neoformans sensu stricto than those produced by nonpreactivated macrophages infected with C. neoformans sensu stricto (P < 0.05); however, no differences were found among other species.
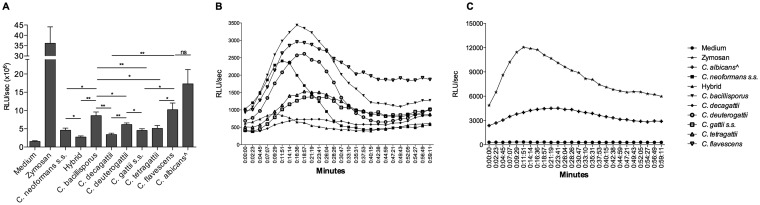
Cryptococcus bacillisporus and C. deuterogattii induced higher levels of ROS production than those induced by the other species.
All species within the C. gattii complex induced smaller amounts of reactive oxygen species (ROS) than those seen with Candida albicans. Cryptococcus decagattii, C. gattii sensu stricto, C. tetragattii, and an interspecies hybrid (C. neoformans sensu stricto × C. gattii sensu stricto) showed the lowest levels of ROS. On the other hand, human PBMCs produced high levels of ROS when stimulated with C. bacillisporus, C. deuterogattii, and C. flavescens, with peak ROS production at 11 to 19 min (Fig. 4B).
FIG 4.
ROS production induced by cryptococcal species. Human PBMCs (1 × 106 cells/ml) from healthy volunteers were stimulated with zymosan (positive control; 1 mg/ml), live yeast forms of Cryptococcus species (5 × 106 cells/ml), or heat-killed Candida albicans (as a control; 1 × 107 cells/ml), and ROS production was measured by chemiluminescence assay. Data represent mean RLU (n = 6 [in quadruplicate]) for two independent experiments, with data collected every 2 min 23 s for 1 h. (A) Integral levels of PBMC ROS production induced by controls and Cryptococcus species. *, P ≤ 0.05; **, P ≤ 0.01; ns, not significant. (B) Kinetics of PBMC ROS production induced by Cryptococcus species. (C) Kinetics of PBMC ROS production induced by controls. ∧, Candida albicans.
The mRNA expression levels of inducible nitric oxide synthase (iNOS) and the antimicrobial peptides β-defensin 2 and cathelicidin were measured quantitatively (Fig. S2). Cryptococcus neoformans sensu stricto downregulated and the C. gattii species complex did not change iNOS expression (Fig. S2A). Similarly, no change in β-defensin 2 mRNA expression was detected (Fig. S2B). Overall, the interspecies hybrid, C. bacillisporus, C. decagattii, and C. gattii sensu stricto downregulated the expression of cathelicidin in human PMBCs in vitro (Fig. S2C). The main results found are summarized in Fig. 5 and Table 1.
FIG 5.
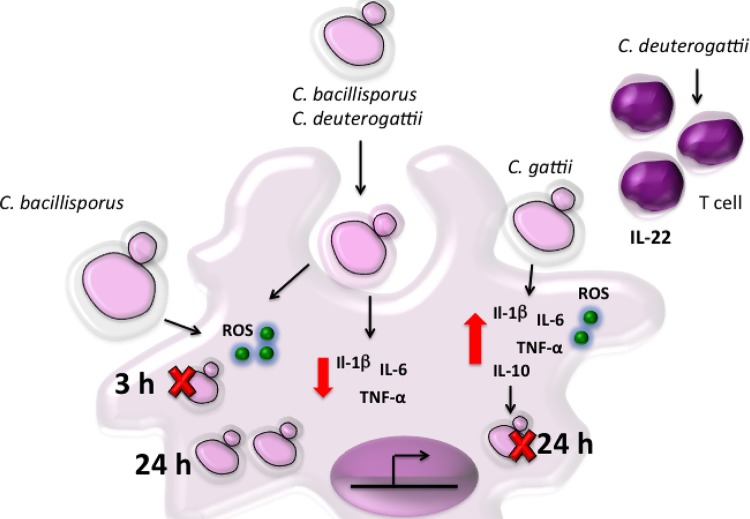
Representative scheme of cytokine profile and antimicrobial molecule production in human PBMCs induced by cryptococcal species. C. bacillisporus and C. deuterogattii induced low levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6, and C. deuterogattii also induced IL-22. In contrast, C. gattii sensu stricto induced higher levels of TNF-α, IL-1β, IL-6, and IL-10 than those induced by the other species. ROS are important for controlling C. bacillisporus infection in the beginning, but nonkilled C. bacillisporus cells are able to proliferate inside macrophages. Cryptococcus gattii sensu stricto infection control may be mediated by the combination of ROS and proinflammatory cytokine production. Red arrow, inhibition; dark blue arrow, induction.
TABLE 1.
Summary of observed data
| Species, host | Regulationa |
|||||
|---|---|---|---|---|---|---|
| Cytokines | IL-22 | IL-10 | ROS | Cathelicidin | Proliferation in macrophages | |
| C. gattii sensu stricto, immunocompetent host | ↑ | ↑ | ↓ | ↓ | ↓ | |
| C. deuterogattii, immunocompetent host | ↓ | ↑ | ↑ | |||
| C. bacillisporus, immunocompromised host | ↓ | ↑ | ↑ | |||
| C. decagattii, immunocompromised host | ↑ | ↓ | ||||
| C. tetragattii, immunocompromised host | ↑ | ↓ | ||||
| C. neoformans, immunocompromised host | ↓ | |||||
| C. flavescens, environmental host | ↑ | ↑ | ||||
↓, downregulation; ↑, upregulation.
DISCUSSION
In this study, we demonstrate that species of the C. gattii complex present different abilities to induce cytokines in human cells. Cryptococcus bacillisporus and C. deuterogattii induced lower levels of production of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 by human PBMCs than those induced by the other species. Cryptococcus gattii sensu stricto induced higher levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 and higher levels of the anti-inflammatory cytokine IL-10 than those observed with other species of the C. gattii complex. The interspecies hybrid, C. decagattii, and C. gattii sensu stricto induced high levels of the proinflammatory cytokines TNF-α and IL-6. All cryptococcal species induced low levels of IFN-γ and IL-17 compared to those induced by another medically important yeast, Candida albicans, used as a positive control.
The decrease of proinflammatory cytokines may favor an environment that disturbs maturation and epitope presentation by dendritic cells, leading to suboptimal levels of the Th1 and Th17 responses, which are protective against cryptococcosis and provide fungal clearance (29, 30, 42, 43). IL-1α and IL-1β are important in the host defense against fungal infections, activating innate immune cells and modulating adaptive immunity (44, 45). In addition, high levels of the proinflammatory cytokines IL-6, IL-8, IFN-γ, and TNF-α in serum have been associated with cryptococcal meningitis survival in HIV patients (46). Schoffelen and colleagues observed that heat-killed C. gattii species complex members induced higher concentrations of the proinflammatory cytokines IL-1β, IL-6, TNF-α, and IL-17/22 than those seen with the C. neoformans species complex (47). However, they did not investigate differences among species of the C. gattii complex. Only C. deuterogattii induced significant production of IL-22, with clear differences among the species. IL-22 is a proinflammatory cytokine that promotes antimicrobial molecule production by epithelial cells and may have a protective role against infections (48), including cryptococcal infections. Meningitis caused by members of the C. gattii species complex in non-HIV-infected patients has been associated with low levels of the proinflammatory cytokines IFN-γ, TNF-α, and IL-6 and high levels of the anti-inflammatory cytokine IL-10 (49). In the present study, C. gattii sensu stricto and the predominantly environmental species C. flavescens induced high levels of IL-10, while other species of the C. gattii complex induced low levels of IL-10. According to Angkasekwinai and coworkers, C. gattii sensu stricto and C. deuterogattii downregulate pulmonary chemokine expression, leading to a failure to mount protective immunity in immunocompetent hosts (29) and thus contributing to the disease process. It may be that differences in immune responses or clinical aspects of immunocompetent hosts preferentially infected by C. gattii sensu stricto or C. deuterogattii are due to the differential levels of cytokines induced in PBMCs, as we showed that these species lead to production of different amounts of cytokines.
Our results showed that all cryptococcal species induced low levels of IFN-γ, and the use of IFN-γ in macrophages preactivation did not improve the phagocytosis rate or reduce the infection, even for C. neoformans sensu stricto. However, this cytokine increased TNF-α production in C. neoformans sensu stricto-infected human macrophages (see Fig. S1 in the supplemental material). Ikeda-Dantsuji and colleagues also showed that preactivation with IFN-γ did not promote phagocytosis of C. deuterogattii but that IFN-γ increased the phagocytic rate of C. neoformans sensu stricto (50). Since IFN-γ has been associated with a good prognosis for cryptococcosis (46, 51, 52), these findings may be important for understanding the pathogenesis of cryptococcosis. In our experiments, IFN-γ did not significantly increase the TNF-α production of macrophages after infection with C. bacillisporus or C. gattii sensu stricto, which would affect the efficiency of macrophages at controlling cryptococcal growth. Wang and colleagues observed low levels of serum IFN-γ in immunocompetent patients with pulmonary cryptococcosis, and after antifungal treatment the serum levels of IFN-γ increased and the protective inflammatory response was restored. They suggested that cryptococcal infection may suppress the immune system and that its elimination helps to establish the immune system again (52). In addition, the use of IFN-γ in combination with standard therapy has been related to successful fungal clearance, resolution of symptoms, and restoration of immunological parameters (51, 53). Improvements in general condition were also observed among non-HIV/nontransplant patients after administration of IFN-γ as adjuvant therapy (54). Meningitis caused by C. gattii sensu lato in non-HIV-infected patients has been associated with low levels of IFN-γ and other proinflammatory cytokines in parallel with high levels of the anti-inflammatory cytokine IL-10 (49). Thus, according to in vivo results, IFN-γ is important for controlling cryptococcal disease. The results obtained in in vitro experiments in the present study may have been affected by the assay conditions, such as the IFN-γ concentration and the time of macrophage exposure to this cytokine. Despite that possibility, for PBMCs, no differences in IFN-γ induction could be detected among the species of the C. gattii species complex.
Cryptococcus bacillisporus induced the highest production of ROS, induced small amounts of cytokines, and downregulated cathelicidin compared to those observed with the other species. In contrast, C. gattii sensu stricto induced low levels of ROS and high levels of cytokines and downregulated cathelicidin expression. We speculate that ROS are important for controlling C. bacillisporus infection during the first hours of infection, as fewer viable C. bacillisporus cells than C. gattii sensu stricto cells were recovered after 3 h of infection. However, after the initial killing by ROS, the nonkilled C. bacillisporus cells were able to proliferate inside macrophages, which might explain the increased number of intracellular cryptococci after 24 h of infection (Fig. 3E). It might be that the production of cytokines by the different Cryptococcus species does not correlate with the virulence of the particular species. However, the combination of cytokine production and ROS production may be important in the host-microbe interaction that finally results in control of the microorganism (in this case C. bacillisporus). Although C. gattii sensu stricto had more viable cells after 3 h of infection, we observed a decrease in viable intracellular cryptococci after 24 h. We speculate that nonlytic exocytosis may have happened, as cryptococcal cells were observed outside macrophages after 24 h of incubation (27). For this species, the combination of ROS and proinflammatory cytokines may increase the capacity of macrophages to control infection. The environmental species C. flavescens induced high concentrations of ROS and cytokine production by human PBMCs, suggesting that the host immune response is effective at eliminating this nonhuman pathogen; this may explain the small number of cases of cryptococcosis caused by this species (55). However, C. neoformans and C. deuterogattii induced small amounts of cytokines and large amounts of ROS in the first hour of infection, showing the ability to escape and survive inside macrophages, in accordance with previous studies (56, 57–61). Hole and coworkers observed that ROS produced by human plasmacytoid dendritic cells are required for C. neoformans sensu stricto growth inhibition but are not the only mechanism used to control C. neoformans sensu stricto (62). The control of the interspecies hybrid, C. gattii sensu stricto, and C. decagattii may be mediated by cytokine pathways in an ROS-independent manner. These species induced high concentrations of proinflammatory cytokines (TNF-α, IL-6, and IL-1β) which are essential for protection against cryptococcosis (43, 44, 46). According to our results, neither iNOS nor β-defensin 2 seems to be involved in C. gattii species complex infection control, but other antimicrobial molecules might be involved.
In conclusion, C. bacillisporus and C. deuterogattii induced low levels of production of the proinflammatory cytokines TNF-α, IL-1β, and IL-6, and C. deuterogattii induced more IL-22 than that induced by C. tetragattii. In contrast, C. gattii sensu stricto stimulated high levels of production of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 and high levels of the anti-inflammatory cytokine IL-10. Despite ROS being important for controlling C. bacillisporus infection at the beginning of infection, nonkilled C. bacillisporus cells are able to proliferate inside macrophages, maintaining the infection. On the other hand, for C. gattii sensu stricto, the combination of ROS and proinflammatory cytokine production may be involved in infection control. Overall, the species within the C. gattii complex have different abilities to induce cytokine production and ROS by human PBMCs. We have to be aware that host variability is an important factor in determining disease processes, and we cannot exclude the possibility that differences in exposure time or concentrations of fungi or the variation in the host defense systems of the human donors may have affected the outcomes of the comparison experiments. Our results with regard to differences in cytokine and ROS production in response to the members of the C. gattii species complex might contribute to the understanding of the cryptococcosis disease process.
MATERIALS AND METHODS
Cryptococcus isolates.
Eight cryptococcal strains were obtained from the Westerdijk Fungal Biodiversity Institute (Utrecht, the Netherlands). The details of these isolates are provided in Table 2. The yeast isolates were grown on Sabouraud dextrose agar plates, and a suspension of each isolate was prepared in sterile phosphate-buffered saline (PBS), pH 7.4 (1,000 × g, 10 min, 10°C). The cell viability was checked by dilution in 0.01% trypan blue in PBS, and cells were counted by use of a hemocytometer. Cryptococcus neoformans sensu stricto was used as a control instead of C. deneoformans because it is predominant worldwide and is related to the majority of cryptococcosis cases caused by C. neoformans species complex infection (39, 64).
TABLE 2.
Details of cryptococcal strains used to conduct human PBMC and macrophage stimulation
| Isolate code | Code(s) in other collection(s) | Species | Mating serotype | Genotype | Origin | Reference (source)a |
|---|---|---|---|---|---|---|
| CBS 10515 | H99, WM 04.15 | C. neoformans sensu stricto | αA | AFLP1/VNI | Clinical (USA) | 1 (CBS) |
| CBS 10085 | WM148 | C. neoformans sensu stricto | AFLP1/VNI | Clinical (Australia) | ||
| CBS 996 | C. neoformans sensu stricto | AFLP1/VNI | Clinical (Argentina) | |||
| CBS 10496 | LSPQ#308 | C. neoformans sensu stricto × C. gattii sensu stricto | A × B | AFLP1/VNI × AFLP4/VGI | Clinical (Canada) | 63 (CBS) |
| CBS 10081 | WM161, TP 0689, D1.13H | C. bacillisporus | αB | AFLP5/VGIII | Clinical (USA) | 1 (CBS) |
| CBS 6993 | C. bacillisporus | AFLP5/VGIII | Clinical (USA) | |||
| CBS 8755 | C. bacillisporus | AFLP5/VGIII | Environmental (Colombia) | |||
| CBS 11687 | IHEM14941, IHEM14941S | C. decagattii | AFLP10/VGIV | Clinical (Mexico) | 1 (CBS) | |
| IHEM14941W | C. decagattii | AFLP10 | Clinical (Mexico) | |||
| CBS 10082 | WM 178, IFM 50894 | C. deuterogattii | αB | AFLP6/VGII | Clinical (Australia) | 1 (CBS) |
| CBS 6956 | C. deuterogattii | AFLP6/VGII | Clinical (USA) | |||
| CBS 10090 | C. deuterogattii | AFLP6/VGII | Clinical (Greece) | |||
| CBS 10078 | WM 179, H33.1, MH56 | C. gattii sensu stricto | αB | AFLP4/VGI | Clinical (Australia) | 1 (CBS) |
| CBS 919 | C. gattii sensu stricto | AFLP4/VGI | Clinical (USA) | |||
| CBS 6290 | C. gattii sensu stricto | AFLP4/VGI | Clinical (Democratic Republic of Congo) | |||
| CBS 10101 | WM 779, IFM 50896 | C. tetragattii | αC | AFLP7/VGIV | Veterinary (South Africa) | 1 (CBS) |
| B5472 | C. tetragattii | AFLP7/VGIV | Clinical (India) | |||
| B5478 | C. tetragattii | AFLP7/VGIV | Clinical (India) | |||
| CBS 8645 | DBVPG 7166 | C. flavescens | Clinical (Greece) | CBS | ||
| UC820 | ATCC MYA-3573 | Candida albicans | Clinical (USA) | ATCC |
CBS, Westerdijk Fungal Biodiversity Institute (http://www.westerdijkinstitute.nl); ATCC, American Type Culture Collection (https://www.lgcstandards-atcc.org).
Candida isolate.
Heat-killed Candida albicans ATCC MYA-3573 (UC 820) diluted in sterile PBS was used as a positive control for cytokine stimulation.
PBMC isolation and stimulation.
Buffy coats from healthy donors were obtained after written informed consent (Sanquin Blood Bank, Nijmegen, the Netherlands). Human peripheral blood mononuclear cell (PBMC) isolation was performed by dilution of blood in PBS and differential density centrifugation over Ficoll-Paque density gradient medium (GE Healthcare, Uppsala, Sweden). Cells were washed three times in sterile PBS and then suspended and cultured in RPMI 1640 medium (Gibco-Life Technologies, Carlsbad, CA, USA) supplemented with 10 mM pyruvate, 10 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, Saint Louis, MO, USA). Subsequently, cells were counted in a Coulter ZH counter (Beckman Coulter, Fullerton, CA, USA) and adjusted to 5 × 106 cells/ml. Thereafter, 100-μl aliquots of PBMCs (5 × 105 cells) were added to a round-bottomed 96-well plate and incubated with either 100 μl live Cryptococcus (2.5 × 106 cells/ml) isolate, 100 μl of ultrapure Escherichia coli lipopolysaccharide (LPS) (O111:B4; Sigma-Aldrich) (10 ng/ml), or 100 μl of RPMI medium alone at 37°C and 5% CO2. All stimulations were performed in medium containing 10% human serum, which was obtained from a serum pool from healthy volunteers. After 24 h or 7 days, supernatants were collected and stored at −20°C until they were used for assays. After 24 h, the cell monolayers were collected by adding 100 μl of 0.5% Triton X-100 to measure intracellular cytokine production and 200 μl of TRIzol and were stored at −80°C until mRNA extraction.
Cytokine measurements.
Monocyte-derived TNF-α, IL-6, IL-1β, IL-8, and IL-10 levels in culture supernatants after 24 h of incubation were determined by use of commercial enzyme-linked immunosorbent assay (ELISA) kits (Sanquin, Amsterdam, the Netherlands [IL-6, IL-8, and IL-10], and R&D Systems, Minneapolis, MN, USA [TNF-α and IL-1β]), and intracellular IL-1α protein in cell lysates collected by use of Triton X-100 was measured by another ELISA (R&D Systems) according to the manufacturer's instructions. In preliminary experiments, we determined that 24 h was the time of maximum levels of monocyte-derived cytokine production for each species (data not shown). The levels of T-cell-derived cytokines IFN-γ, IL-17, and IL-22 in culture supernatants after 7 days of incubation were determined using ELISA kits (Sanquin [IFN-γ] and R&D Systems [IL-17 and IL-22]). Results are presented in picograms per milliliter.
Macrophage infection assay.
Primary monocytes were obtained by hyperosmotic Percoll (Sigma-Aldrich) density gradient centrifugation of PBMCs. Afterward, cells were washed once with PBS and seeded in tissue culture plates at 37°C and 5% CO2 in the presence of RPMI medium supplemented with 10% human pooled serum and GM-CSF (50 ng/ml) (R&D Systems). After 6 days of differentiation, macrophages were harvested by use of cold PBS and adjusted to 5 × 106 cells/ml. Thereafter, 200-μl aliquots of macrophage suspension (2 × 105 cells/ml) were added to 24-well plates, with or without glass coverslips. Macrophages were left to adhere for 30 min and preincubated with or without recombinant human IFN-γ (rhIFN-γ) (50 U/ml; Boehringer Ingelheim, Alkmaar, the Netherlands) for 1 h. Thereafter, cultures were infected with 1 × 106 (multiplicity of infection [MOI] = 5:1) live yeast forms of C. neoformans sensu stricto, C. bacillisporus, or C. gattii sensu stricto. After 3 h, noninternalized fungi were washed off, the medium was replaced, and cultures were incubated for 24 h. For wells without coverslips, after the incubation time (3 h or 24 h), macrophages were lysed with water and mechanical lysis, diluted 100× in PBS, and seeded onto Sabouraud dextrose medium. Plates were incubated for 48 to 72 h at 37°C for CFU quantification. For wells with coverslips, cells were fixed, stained with Giemsa stain (Merck Millipore, Billerica, MA, USA), and analyzed by light microscopy (magnification, ×1,000) to determine the infection index. Three hundred cells were analyzed, and the percentage of infected cells and the mean number of intracellular cryptococci per infected cell were determined. The infection index was calculated as follows: infection index = percentage of infected macrophages × mean number of intracellular cryptococci per macrophage.
ROS measurement.
Human PBMCs (1 × 106 cells/ml) were suspended in Hanks' balanced salt solution and exposed to different concentrations of live yeast forms of Cryptococcus species (5 × 106 cells/ml). Heat-killed C. albicans and zymosan (1 mg/ml) (InvivoGen, Toulouse, France) were used as positive controls. ROS formation was measured by a chemiluminescence assay using luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) (5 mM; Sigma-Aldrich). The luminometer measured chemiluminescence in the integration mode at 37°C for 1 h after luminol was added. Results are presented as numbers of relative light units (RLU) per second.
mRNA expression by qPCR.
RNA isolation was carried out as reported previously (65). RNA was precipitated with isopropanol and washed with 75% ethanol, followed by reconstitution in RNase-free water. Subsequently, RNA was reverse transcribed into cDNA by use of an iScript kit (Bio-Rad, Hercules, CA, USA). Diluted cDNA was used for quantitative real-time PCR (qPCR) analysis, which was done by using a StepOne Plus sequence detection system (Applied Biosystems, Foster City, CA, USA) with SYBR green master mix (Applied Biosystems). Primer sequences (see Table S1 in the supplemental material) for inducible nitric oxide synthase (iNOS), β-defensin 2, and cathelicidin were obtained from the Harvard Primerbank database. Primers were purchased from Biolegio (Nijmegen, the Netherlands). The mRNA analysis was done by the 2−ΔΔCT method, and the results were normalized against those for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene.
Statistical analysis.
Data are given as mean values with standard errors of the means (SEM). The Mann-Whitney U test for unpaired, nonparametric data was used to compare differences in cytokine production between two groups. Two-way analysis of variance (ANOVA) with Bonferroni's multiple-comparison test was used when more than two groups were compared. GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA) was used to tabulate and analyze the data.
Ethics statement.
The donation of blood by healthy volunteers was performed after written consent and was approved by the Ethical Committee of the Arnhem-Nijmegen Region, the Netherlands.
Supplementary Material
ACKNOWLEDGMENTS
We thank CNPq for financial support (F.R.-D. and L.A.B.J.) (grant 465771/2014-9 via the INCT [National Institute of Science and Technology], Brazil, for strategies in host-pathogen interaction). F.R.-D. is a research fellow of CNPq. CAPES and CNPq, Brazil, financially supported the work of P.F.H. and J.C.D.S. M.G.N. was supported by an ERC consolidator grant (grant 310372) and a POC-FUSE grant of the Romanian National Agency for Scientific Research.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00958-17.
REFERENCES
- 1.Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, Falk R, Parnmen S, Lumbsch HT, Boekhout T. 2015. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol 78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Velagapudi R, Hsueh Y-P, Geunes-Boyer S, Wright JR, Heitman J. 2009. Spores as infectious propagules of Cryptococcus neoformans. Infect Immun 77:4345–4355. doi: 10.1128/IAI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X-Z, Wang Q-M, Göker M, Groenewald M, Kachalkin AV, Lumbsch HT, Millanes AM, Wedin M, Yurkov AM, Boekhout T, Bai F-Y. 2015. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol 81:85–147. doi: 10.1016/j.simyco.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X-Z, Wang Q-M, Theelen B, Groenewald M, Bai F-Y, Boekhout T. 2015. Phylogeny of tremellomycetous yeasts and related dimorphic and filamentous basidiomycetes reconstructed from multiple gene sequence analyses. Stud Mycol 81:1–26. doi: 10.1016/j.simyco.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, Bicanic TA, Castañeda E, Chang YC, Chen J, Cogliati M, Dromer F, Ellis D, Filler SG, Fisher MC, Harrison TS, Holland SM, Kohno S, Kronstad JW, Lazera M, Levitz SM, Lionakis MS, May RC, Ngamskulrongroj P, Pappas PG, Perfect JR, Rickerts V, Sorrell TC, Walsh TJ, Williamson PR, Xu J, Zelazny AM, Casadevall A. 2017. The case for adopting the “species complex” nomenclature for the etiologic agents of cryptococcosis. mSphere 2:e00357-. doi: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagen F, Lumbsch HT, Arsic Arsenijevic V, Badali H, Bertout S, Billmyre RB, Bragulat MR, Cabañes FJ, Carbia M, Chakrabarti A, Chaturvedi S, Chaturvedi V, Chen M, Chowdhary A, Colom M-F, Cornely OA, Crous PW, Cuétara MS, Diaz MR, Espinel-Ingroff A, Fakhim H, Falk R, Fang W, Herkert PF, Ferrer Rodríguez C, Fraser JA, Gené J, Guarro J, Idnurm A, Illnait-Zaragozi M-T, Khan Z, Khayhan K, Kolecka A, Kurtzman CP, Lagrou K, Liao W, Linares C, Meis JF, Nielsen K, Nyazika TK, Pan W, Pekmezovic M, Polacheck I, Posteraro B, de Queiroz Telles F, Romeo O, Sánchez M, Sampaio A, Sanguinetti M, Sriburee P, Sugita T, Taj-Aldeen SJ, Takashima M, Taylor JW, Theelen B, Tomazin R, Verweij PE, Wahyuningsih R, Wang P, Boekhout T. 2017. Importance of resolving fungal nomenclature: the case of multiple pathogenic species in the Cryptococcus genus. mSphere 2:e00238-. doi: 10.1128/mSphere.00238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrnes EJ, Bartlett KH, Perfect JR, Heitman J. 2011. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes Infect 13:895–907. doi: 10.1016/j.micinf.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SC-A, Slavin MA, Heath CH, Playford EG, Byth K, Marriott D, Kidd SE, Bak N, Currie B, Hajkowicz K, Korman TM, McBride WJH, Meyer W, Murray R, Sorrell TC, Australia and New Zealand Mycoses Interest Group (ANZMIG)-Cryptococcus Study. 2012. Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis 55:789–798. doi: 10.1093/cid/cis529. [DOI] [PubMed] [Google Scholar]
- 9.Marques SA, Bastazini I, Martins ALGP, Barreto JA, Barbieri D'Elia MP, Lastória JC, Marques MEA. 2012. Primary cutaneous cryptococcosis in Brazil: report of 11 cases in immunocompetent and immunosuppressed patients: primary cutaneous cryptococcosis. Int J Dermatol 51:780–784. doi: 10.1111/j.1365-4632.2011.05298.x. [DOI] [PubMed] [Google Scholar]
- 10.Cicora F, Petroni J, Formosa P, Roberti J. 2015. A rare case of Cryptococcus gattii pneumonia in a renal transplant patient. Transpl Infect Dis 17:463–466. doi: 10.1111/tid.12371. [DOI] [PubMed] [Google Scholar]
- 11.Franco-Paredes C, Womack T, Bohlmeyer T, Sellers B, Hays A, Patel K, Lizarazo J, Lockhart SR, Siddiqui W, Marr KA. 2015. Management of Cryptococcus gattii meningoencephalitis. Lancet Infect Dis 15:348–355. doi: 10.1016/S1473-3099(14)70945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bicanic T. 2005. Cryptococcal meningitis. Br Med Bull 72:99–118. doi: 10.1093/bmb/ldh043. [DOI] [PubMed] [Google Scholar]
- 13.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, MacDougall L, Boekhout T, Kwon-Chung KJ, Meyer W. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A 101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer DJ, Billmyre RB, Filler EE, Voelz K, Pursall R, Mieczkowski PA, Larsen RA, Dietrich FS, May RC, Filler SG, Heitman J. 2014. Cryptococcus gattii VGIII isolates causing infections in HIV/AIDS patients in southern California: identification of the local environmental source as arboreal. PLoS Pathog 10:e1004285. doi: 10.1371/journal.ppat.1004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyazika TK, Hagen F, Meis JF, Robertson VJ. 2016. Cryptococcus tetragattii as a major cause of cryptococcal meningitis among HIV-infected individuals in Harare, Zimbabwe. J Infect 72:745–752. doi: 10.1016/j.jinf.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Walraven CJ, Gerstein W, Hardison SE, Wormley F, Lockhart SR, Harris JR, Fothergill A, Wickes B, Gober-Wilcox J, Massie L, Ku TS, Firacative C, Meyer W, Lee SA. 2011. Fatal disseminated Cryptococcus gattii infection in New Mexico. PLoS One 6:e28625. doi: 10.1371/journal.pone.0028625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illnait-Zaragozí MT, Ortega-Gonzalez LM, Hagen F, Martínez-Machin GF, Meis JF. 2013. Fatal Cryptococcus gattii genotype AFLP5 infection in an immunocompetent Cuban patient. Med Mycol Case Rep 2:48–51. doi: 10.1016/j.mmcr.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes KE, Dwyer C, Campbell LT, Carter DA. 2016. Species in the Cryptococcus gattii complex differ in capsule and cell size following growth under capsule-inducing conditions. mSphere 1:e00350-. doi: 10.1128/mSphere.00350-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson GR, Albert N, Hodge G, Wilson MD, Sykes JE, Bays DJ, Firacative C, Meyer W, Kontoyiannis DP. 2014. Phenotypic differences of Cryptococcus molecular types and their implications for virulence in a Drosophila model of infection. Infect Immun 82:3058–3065. doi: 10.1128/IAI.01805-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vu K, Tham R, Uhrig JP, Thompson GR, Na Pombejra S, Jamklang M, Bautos JM, Gelli A. 2014. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio 5:e01101-. doi: 10.1128/mBio.01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, Paul-Satyasee M, Kim KS, Kwon-Chung KJ. 2004. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun 72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. 2009. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun 77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santiago-Tirado FH, Onken MD, Cooper JA, Klein RS, Doering TL. 2017. Trojan horse transit contributes to blood-brain barrier crossing of a eukaryotic pathogen. mBio 8:e02183-. doi: 10.1128/mBio.02183-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coelho C, Bocca AL, Casadevall A. 2014. The intracellular life of Cryptococcus neoformans. Annu Rev Pathol 9:219–238. doi: 10.1146/annurev-pathol-012513-104653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicola AM, Robertson EJ, Albuquerque P, Derengowski LDS, Casadevall A. 2011. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. mBio 2:e00167-. doi: 10.1128/mBio.00167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okagaki LH, Nielsen K. 2012. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot Cell 11:820–826. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huston SM, Ngamskulrungroj P, Xiang RF, Ogbomo H, Stack D, Li SS, Timm-McCann M, Kyei SK, Oykhman P, Kwon-Chung KJ, Mody CH. 2016. Cryptococcus gattii capsule blocks surface recognition required for dendritic cell maturation independent of internalization and antigen processing. J Immunol 196:1259–1271. doi: 10.4049/jimmunol.1501089. [DOI] [PubMed] [Google Scholar]
- 28.Huston SM, Li SS, Stack D, Timm-McCann M, Jones GJ, Islam A, Berenger BM, Xiang RF, Colarusso P, Mody CH. 2013. Cryptococcus gattii is killed by dendritic cells, but evades adaptive immunity by failing to induce dendritic cell maturation. J Immunol 191:249–261. doi: 10.4049/jimmunol.1202707. [DOI] [PubMed] [Google Scholar]
- 29.Angkasekwinai P, Sringkarin N, Supasorn O, Fungkrajai M, Wang Y-H, Chayakulkeeree M, Ngamskulrungroj P, Angkasekwinai N, Pattanapanyasat K. 2014. Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect Immun 82:3880–3890. doi: 10.1128/IAI.01773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voelz K, Lammas DA, May RC. 2009. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun 77:3450–3457. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Meara TR, Holmer SM, Selvig K, Dietrich F, Alspaugh JA. 2013. Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. mBio 4:e00522-. doi: 10.1128/mBio.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohatgi S, Pirofski L. 2015. Host immunity to Cryptococcus neoformans. Future Microbiol 10:565–581. doi: 10.2217/fmb.14.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M, Xing Y, Lu A, Fang W, Sun B, Chen C, Liao W, Meng G. 2015. Internalized Cryptococcus neoformans activates the canonical caspase-1 and the noncanonical caspase-8 inflammasomes. J Immunol 195:4962. doi: 10.4049/jimmunol.1500865. [DOI] [PubMed] [Google Scholar]
- 34.Davis MJ, Eastman AJ, Qiu Y, Gregorka B, Kozel TR, Osterholzer JJ, Curtis JL, Swanson JA, Olszewski MA. 2015. Cryptococcus neoformans-induced macrophage lysosome damage crucially contributes to fungal virulence. J Immunol 194:2219–2231. doi: 10.4049/jimmunol.1402376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright L, Bubb W, Davidson J, Santangelo R, Krockenberger M, Himmelreich U, Sorrell T. 2002. Metabolites released by Cryptococcus neoformans var. neoformans and var. gattii differentially affect human neutrophil function. Microbes Infect 4:1427–1438. doi: 10.1016/S1286-4579(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 36.Cheng P-Y, Sham A, Kronstad JW. 2009. Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infect Immun 77:4284–4294. doi: 10.1128/IAI.00628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson JF, Johnston SA. 2015. Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis. Fungal Genet Biol 78:76–86. doi: 10.1016/j.fgb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urai M, Kaneko Y, Ueno K, Okubo Y, Aizawa T, Fukazawa H, Sugita T, Ohno H, Shibuya K, Kinjo Y, Miyazaki Y. 2016. Evasion of innate immune responses by the highly virulent Cryptococcus gattii by altering capsule glucuronoxylomannan structure. Front Cell Infect Microbiol 5:101. doi: 10.3389/fcimb.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cogliati M. 2013. Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: an atlas of the molecular types. Scientifica 2013:675213. doi: 10.1155/2013/675213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigues J, Fonseca FL, Schneider RO, Godinho RM, Firacative CC, Maszewska K, Meyer W, Schrank A, Staats C, Kmetzsch L, Vainstein MH, Rodrigues ML. 2015. Pathogenic diversity amongst serotype C VGIII and VGIV Cryptococcus gattii isolates. Sci Rep 5:11717. doi: 10.1038/srep11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souto ACP, Bonfietti LX, Ferreira-Paim K, Trilles L, Martins M, Ribeiro-Alves M, Pham CD, Martins L, dos Santos W, Chang M, Brito-Santos F, Santos DCS, Fortes S, Lockhart SR, Wanke B, Melhem MSC, Lazéra MS, Meyer W. 2016. Population genetic analysis reveals a high genetic diversity in the Brazilian Cryptococcus gattii VGII population and shifts the global origin from the Amazon rainforest to the semi-arid desert in the northeast of Brazil. PLoS Negl Trop Dis 10:e0004885. doi: 10.1371/journal.pntd.0004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, Olszewski MA. 2009. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol 175:2489–2500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Eastman AJ, Flaczyk A, Neal LM, Zhao G, Carolan J, Malachowski AN, Stolberg VR, Yosri M, Chensue SW, Curtis JL, Osterholzer JJ, Olszewski MA. 2016. Disruption of early tumor necrosis factor alpha signaling prevents classical activation of dendritic cells in lung-associated lymph nodes and development of protective immunity against cryptococcal infection. mBio 7:e00510-. doi: 10.1128/mBio.00510-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Netea MG, Simon A, van de Veerdonk F, Kullberg B-J, Van der Meer JWM, Joosten LAB. 2010. IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog 6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, Cramer RA, Obar JJ. 2015. IL-1α signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog 11:e1004625. doi: 10.1371/journal.ppat.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddiqui AA, Brouwer AE, Wuthiekanun V, Jaffar S, Shattock R, Irving D, Sheldon J, Chierakul W, Peacock S, Day N, White NJ, Harrison TS. 2005. IFN-γ at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol 174:1746–1750. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 47.Schoffelen T, Illnait-Zaragozi M-T, Joosten LAB, Netea MG, Boekhout T, Meis JF, Sprong T. 2013. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PLoS One 8:e55579. doi: 10.1371/journal.pone.0055579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. 2010. IL-17 and IL-22: siblings, not twins. Trends Immunol 31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Brouwer AE, Siddiqui AA, Kester MI, Sigaloff KCE, Rajanuwong A, Wannapasni S, Chierakul W, Harrison TS. 2007. Immune dysfunction in HIV-seronegative, Cryptococcus gattii meningitis. J Infect 54:e165–e168. doi: 10.1016/j.jinf.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda-Dantsuji Y, Ohno H, Tanabe K, Umeyama T, Ueno K, Nagi M, Yamagoe S, Kinjo Y, Miyazaki Y. 2015. Interferon-γ promotes phagocytosis of Cryptococcus neoformans but not Cryptococcus gattii by murine macrophages. J Infect Chemother 21:831–836. doi: 10.1016/j.jiac.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Netea MG, Brouwer AE, Hoogendoorn EH, Van der Meer JWM, Koolen M, Verweij PE, Kullberg BJ. 2004. Two patients with cryptococcal meningitis and idiopathic CD4 lymphopenia: defective cytokine production and reversal by recombinant interferon-gamma therapy. Clin Infect Dis 39:e83–e87. doi: 10.1086/425121. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Zeng Y, Luo W, Xie X, Li S. 2015. The role of Cryptococcus in the immune system of pulmonary cryptococcosis patients. PLoS One 10:e0144427. doi: 10.1371/journal.pone.0144427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pappas PG, Bustamante B, Ticona E, Hamill RJ, Johnson PC, Reboli A, Aberg J, Hasbun R, Hsu HH. 2004. Recombinant interferon-γ1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis. J Infect Dis 189:2185–2191. doi: 10.1086/420829. [DOI] [PubMed] [Google Scholar]
- 54.Lomes NR, Melhem MSDC, Szeszs MW, Martins MDA, Buccheri R. 2016. Cryptococcosis in non-HIV/non-transplant patients: a Brazilian case series. Med Mycol 54:669–676. doi: 10.1093/mmy/myw021. [DOI] [PubMed] [Google Scholar]
- 55.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect 20(Suppl 3):76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 56.Bojarczuk A, Miller KA, Hotham R, Lewis A, Ogryzko NV, Kamuyango AA, Frost H, Gibson RH, Stillman E, May RC, Renshaw SA, Johnston SA. 2016. Cryptococcus neoformans intracellular proliferation and capsule size determines early macrophage control of infection. Sci Rep 6:21489. doi: 10.1038/srep21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldmesser M, Tucker S, Casadevall A. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol 9:273–278. doi: 10.1016/S0966-842X(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 58.Ma H, Hagen F, Stekel DJ, Johnston SA, Sionov E, Falk R, Polacheck I, Boekhout T, May RC. 2009. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A 106:12980–12985. doi: 10.1073/pnas.0902963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coelho C, Souza ACO, Derengowski LDS, de Leon-Rodriguez C, Wang B, Leon-Rivera R, Bocca AL, Gonçalves T, Casadevall A. 2015. Macrophage mitochondrial and stress response to ingestion of Cryptococcus neoformans. J Immunol 194:2345–2357. doi: 10.4049/jimmunol.1402350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Mueller M, Wormley FL Jr. 2015. STAT1 signaling within macrophages is required for antifungal activity against Cryptococcus neoformans. Infect Immun 83:4513–4527. doi: 10.1128/IAI.00935-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trevijano-Contador N, Rueda C, Zaragoza O. 2016. Fungal morphogenetic changes inside the mammalian host. Semin Cell Dev Biol 57:100–109. doi: 10.1016/j.semcdb.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Hole CR, Leopold Wager CM, Mendiola AS, Wozniak KL, Campuzano A, Lin X, Wormley FL Jr. 2016. Antifungal activity of plasmacytoid dendritic cells against Cryptococcus neoformans in vitro requires expression of Dectin-3 (CLEC4D) and reactive oxygen species. Infect Immun 84:2493–2504. doi: 10.1128/IAI.00103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagen F, Illnait-Zaragozi M-T, Bartlett KH, Swinne D, Geertsen E, Klaassen CHW, Boekhout T, Meis JF. 2010. In vitro antifungal susceptibilities and amplified fragment length polymorphism genotyping of a worldwide collection of 350 clinical, veterinary, and environmental Cryptococcus gattii isolates. Antimicrob Agents Chemother 54:5139–5145. doi: 10.1128/AAC.00746-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E, IberoAmerican Cryptococcal Study Group. 2003. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis 9:189–195. doi: 10.3201/eid0902.020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.