ABSTRACT
Malaria in pregnancy can cause serious adverse outcomes for the mother and the fetus. However, little is known about the effects of submicroscopic infections (SMIs) in pregnancy, particularly in areas where Plasmodium falciparum and Plasmodium vivax cocirculate. A cohort of 187 pregnant women living in Puerto Libertador in northwest Colombia was followed longitudinally from recruitment to delivery. Malaria was diagnosed by microscopy, reverse transcription-quantitative PCR (RT-qPCR), and placental histopathology. Gestational age, hemoglobin concentration, VAR2CSA-specific IgG levels, and adhesion-blocking antibodies were measured during pregnancy. Statistical analyses were performed to evaluate the impact of SMIs on birth weight and other delivery outcomes. Twenty-five percent of women (45/180) were positive for SMIs during pregnancy. Forty-seven percent of infections (21/45) were caused by P. falciparum, 33% were caused by P. vivax, and 20% were caused by mixed Plasmodium spp. Mixed infections of P. falciparum and P. vivax were associated with lower gestational age at delivery (P = 0.0033), while other outcomes were normal. Over 60% of women had antibodies to VAR2CSA, and there was no difference in antibody levels between those with and without SMIs. The anti-adhesion function of these antibodies was associated with protection from SMI-related anemia at delivery (P = 0.0086). SMIs occur frequently during pregnancy, and while mixed infections of both P. falciparum and P. vivax were not associated with a decrease in birth weight, they were associated with significant risk of preterm birth. We propose that the lack of adverse delivery outcomes is due to functional VAR2CSA antibodies that can protect pregnant women from SMI-related anemia.
KEYWORDS: malaria, submicroscopic, pregnancy, VAR2CSA, antibodies, Plasmodium falciparum, Plasmodium vivax, Colombia, anemia
INTRODUCTION
Malaria in pregnancy (MiP) poses a significant risk to the mother and the fetus and can lead to adverse pregnancy outcomes (1). Pregnant women are particularly vulnerable to infection with Plasmodium falciparum, which can sequester in the placenta and cause placental malaria, maternal anemia, preterm birth (PTB), and low-birth-weight (LBW) infants (1). In sub-Saharan Africa, the risk of these outcomes is heightened in primigravid women infected with P. falciparum whereas multigravid women develop pregnancy-specific immunity from previous exposure to MiP (1, 2).
Acquisition of antibodies against the P. falciparum protein, VAR2CSA, is a key immune mechanism against MiP. VAR2CSA belongs to the P. falciparum EMP1 (PfEMP1) family and is the main parasite ligand that mediates placental binding of infected erythrocytes (IEs) to chondroitin sulfate A (CSA) on the surface of syncytiotrophoblasts and in the intervillous spaces (3). Pregnant women acquire antibodies to VAR2CSA following exposure to MiP, usually in a parity-dependent manner (1). These antibodies can block adhesion of IEs to CSA in vitro (4, 5) and are associated with protection from placental malaria and other adverse delivery outcomes (6, 7).
Most of our knowledge of MiP stems from research in sub-Saharan Africa in areas of high P. falciparum transmission. Far less is known about the impact of other Plasmodium species on MiP outcomes. Plasmodium vivax infection during pregnancy was associated with adverse outcomes in studies from Thailand and Indonesia (8, 9); however, studies in Latin America reported varying results. One study in Colombia demonstrated that P. vivax infection during pregnancy was associated with lower birth weight (10), while in another study, also in Colombia, no changes in mean birth weight, gestational age, or hemoglobin levels at delivery were observed (11). In studies from Brazil, Bolivia, Peru, and Venezuela, P. vivax was associated with anemia, reduced birth weight, and histological changes in the placenta (12–15).
Although some studies showed that P. vivax parasites can cytoadhere to placental tissue ex vivo (16, 17), this is not considered a pathogenic mechanism of P. vivax infection (18, 19). One of the characteristics of P. vivax that may contribute to more benign birth outcomes is the typically low parasitemia. Plasmodium infections are often undetectable by microscopy and only detected using molecular diagnostics and are therefore considered submicroscopic infections (SMIs). The application of molecular diagnostics has revealed a high prevalence of SMIs, particularly in lower-transmission settings such as Latin America (20).
SMIs are also frequently detected in pregnant women in sub-Saharan Africa and were associated with adverse outcomes in several cross-sectional (21–24) and longitudinal studies (25, 26). However, only a few studies have examined the effects of SMIs in regions where P. falciparum and P. vivax cocirculate. P. falciparum and P. vivax SMIs at delivery were associated with poor outcomes in one study from Papua New Guinea (PNG) (27) but not in studies conducted in Colombia and India (10, 28). In a multicenter study of pregnant women in Colombia, Guatemala, Brazil, India, and PNG, submicroscopic P. falciparum and P. vivax infections were not associated with either maternal anemia or LBW (29). These findings warrant further investigation to determine whether pregnant women with SMIs are at risk for adverse clinical outcomes and to identify possible immune mechanisms, including the role of VAR2CSA antibodies, in SMIs in pregnancy. We describe the first prospective longitudinal study conducted in Latin America to determine the prevalence of SMIs in pregnancy and characterize the host anti-VAR2CSA antibody response to SMIs. Our primary objective was to compare the birth weights in infants born of pregnancies complicated by SMI to those from pregnancies with no SMI. Secondary clinical outcomes of interest included preterm birth, babies small for gestational age (SGA), and maternal anemia. We examined antibody levels against VAR2CSA and functional inhibition of parasite binding in vitro among women with an SMI as predictors of clinical outcomes of interest.
RESULTS
Study cohort.
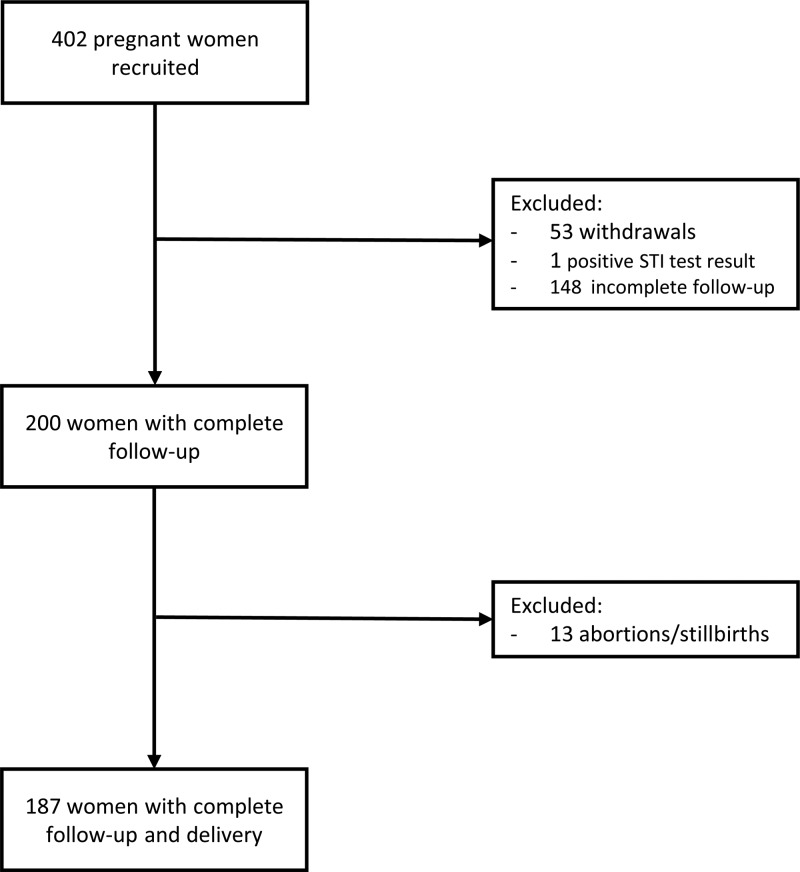
Of the 402 women recruited into the study, 187 women participated through to delivery (Fig. 1). A total of 148 women were lost to follow-up: 16 women delivered in their homes, 19 women delivered in their local villages, 98 women delivered at a distant regional hospital, 5 women delivered when study staff were unavailable, and 10 women were lost to follow-up without any known reason. Of note, because of the focus on SMI, seven women were excluded from downstream data analysis due to a smear-positive Plasmodium result by microscopy. Characteristics of the 180 pregnant women included in the analysis are presented in Table 1. Most women were recruited during their second trimester (median, 19 weeks gestation; interquartile range [IQR], 15 to 25), and the median number of antenatal visits (ANVs) attended by women was 2.2, ranging from 1 to 5 visits prior to delivery. There were no significant differences in characteristics at enrollment between women with and without an SMI in pregnancy.
FIG 1.
Flow chart of pregnant women recruited into the study.
TABLE 1.
Characteristics based on submicroscopic malaria infection in pregnant women included in the study
| Parameter | No. of women assessed (n = 180) | Value for the parameter by PCR result |
P valuef | |
|---|---|---|---|---|
| PCR positive (n = 45)e | PCR negative (n = 135) | |||
| Characteristics at enrollment | ||||
| Median age (yr [IQR]) | 21 (19–28) | 22 (19–28) | 0.96 | |
| Median height (cm [IQR]) | 157 (152–160) | 156 (152–161) | 0.88 | |
| Median weight (kg [IQR]) | 53 (50–62) | 55 (50–62) | 0.62 | |
| Gravidity (no. of subjects [%]) | ||||
| Primigravid | 16 (35.6) | 40 (29.6) | 0.46 | |
| Secundigravid | 11 (24.4) | 31 (23) | 0.84 | |
| Multigravid | 18 (40) | 64 (44.4) | 0.49 | |
| Median gestational age (weeks [IQR]) | 171 | 21 (14–24) | 19 (15–25) | 0.75 |
| Median Hb level (g/dl [IQR]) | 167 | 11.6 (10.7–12.6) | 11.8 (11–12.55) | 0.71 |
| Use of bed nets (no. [%]) | 179 | 25 (55.6) | 63 (46.7) | 0.31 |
| Delivery outcomes | ||||
| Mean wt of newborn (g [± SD]) | 177 | 3,220 ± 510 | 3,259 ± 451 | 0.63 |
| LBW babies (no. [%])a | 5 (11.1) | 6 (4.4) | 0.15 | |
| Mean gestational age (weeks [± SD])b | 171 | 38.5 ± 2.1 | 38.9 ± 2.1 | 0.25 |
| Preterm births (no. [%])c | 8 (17.7) | 16 (11.9) | 0.32 | |
| SGA births (no. [%]) | 177 | 5 (11.1) | 14 (10.4) | 0.59 |
| Anemia (no. [%])c | 164 | 17 (37.7) | 30 (22.2) | 0.11 |
| Median Apgar score (range)d | 174 | 8.5 (5–9) | 8.6 (4–10) | 0.20 |
LBW, low birth weight, defined as <2,500 g.
Gestational age was determined by ultrasound.
Preterm birth, <37 weeks. Anemia, Hb level of <11 g/dl.
The Apgar index was determined after the first minute of birth.
Only women positive for an SMI were included in the analysis.
Calculated by Fisher's exact test, unpaired t test, or Mann-Whitney test.
Prevalence of MiP.
Malaria diagnosis by microscopy and molecular tests were performed on all samples collected at enrollment, during subsequent antenatal visits, and at delivery. Of the 180 women included in the data analysis, 25% (n = 45) were positive for an SMI at least once during pregnancy. Forty-seven percent (21/45) of these women were infected with P. falciparum, 33% (15/45) were infected with P. vivax, and 20% (9/45) were infected with mixed infections, which included eight simultaneous infections (positive for both P. falciparum and P. vivax within the same blood sample) and one sequential infection (positive for P. falciparum and P. vivax at different times within pregnancy).
The dynamics of infection during the course of pregnancy are detailed in Table 2, which presents the number of positive samples at enrollment, during follow-up, and at delivery in all pregnant women (n = 1,111 total samples). Sixteen women (8.6%, 16/187) had a positive malaria diagnosis at enrollment (4 P. falciparum infections, 11 P. vivax infections, and 1 mixed species infection), another 29 infections were diagnosed during follow-up (12 P. falciparum infections, 13 P. vivax infections, 4 mixed species infections), and 12 infections were detected at delivery (6 P. falciparum, 4 P. vivax, and 2 mixed species). In nine women, multiple samples were positive for Plasmodium by reverse transcription-quantitative PCR (RT-qPCR) (eight by the same species, one mixed infection); however, the parasite DNA levels were too low for genotyping to discriminate between new, chronic, or relapse infections (in the case of P. vivax).
TABLE 2.
Malaria prevalence by microscopy, qPCR, and histopathology in all samples in the study
| Diagnostic method and infection typea | No. of positive samples/total no. of samples (%) at: |
||
|---|---|---|---|
| Enrollment | Follow-up | Delivery | |
| Peripheral microscopy | |||
| P. falciparum | 0/187 (0) | 1/368 (0.3) | 0/187 (0) |
| P. vivax | 5/187 (2.7) | 1/368 (0.3) | 0/187 (0) |
| Mixed infection | 0/187 (0) | 0/368 (0) | 0/187 (0) |
| Peripheral RT-qPCR | |||
| P. falciparum | 4/187 (2.1) | 12/368 (3.3) | 6/187 (3.2) |
| P. vivax | 11/187 (5.9) | 13/368 (3.5) | 4/187 (2.1) |
| Mixed infection | 1/187 (0.5) | 4/368 (1.1) | 2/187 (1.1) |
| Placental microscopy | |||
| P. falciparum | NAb | NA | 0/182 (0) |
| P. vivax | NA | NA | 0/182 (0) |
| Mixed infection | NA | NA | 0/182 (0) |
| Placental RT-qPCR | |||
| P. falciparum | NA | NA | 4/182 (2.2) |
| P. vivax | NA | NA | 2/182 (1.1) |
| Mixed infection | NA | NA | 3/182 (1.6) |
| Placental histopathology | |||
| Acute infection | NA | NA | 0/187 (0) |
| Chronic infection | NA | NA | 0/187 (0) |
| Past infection | NA | NA | 12/187 (6.4) |
Infection types were defined as follows: mixed infection, simultaneous infection with both P. falciparum and P. vivax; acute infection, the presence of parasites; chronic infection, the presence of parasites and hemozoin; past infection, the presence of hemozoin only.
NA, not available.
Placental malaria was not observed in this population by microscopy; however, placental blood from nine women was positive by RT-qPCR. Based on histopathology, active placental infections were not observed among the 180 women, but hemozoin was detected in the placentas of 12 women (6.7%), indicating a past Plasmodium infection in pregnancy. This was further confirmed by RT-qPCR; 6 of the 12 women were positive by RT-qPCR at an earlier time in pregnancy (two P. falciparum infections, one P. vivax infection, and three mixed infections). In an analysis to investigate whether SMIs during pregnancy were associated with placental malaria (past placental infection by histopathology or placental SMI by RT-qPCR), we observed no significant association between SMIs during pregnancy and presence of hemozoin in the placenta (P = 0.19) or submicroscopic placental infection by RT-qPCR (P = 0.08).
Clinical outcomes at delivery.
Overall, few differences were observed in the delivery outcomes of pregnant women who were infected with submicroscopic malaria during pregnancy compared to outcomes for those who were not (Table 1). The mean birth weight (± standard deviation [SD]) of infants born to mothers with an SMI was 3,220 g ± 510 g, and it was 3,259 g ± 451 g among those without an SMI (difference of means, 38.4 g; 95% confidence interval [CI], −121 to 198; P = 0.63).
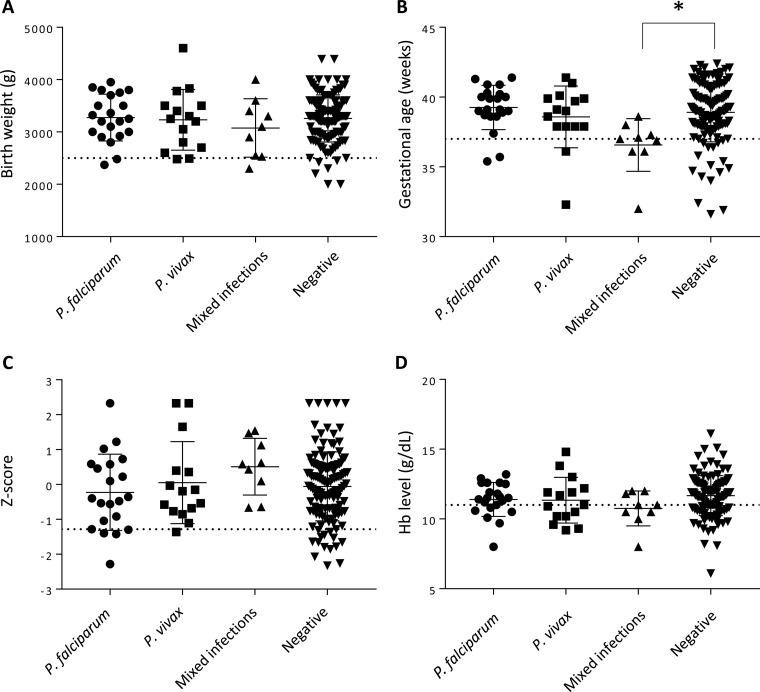
Monoinfection with different species of Plasmodium was not associated with decreases in birth weight, gestational age, weight-for-age Z-scores, and maternal hemoglobin (Hb) levels at delivery (Fig. 2). However, we observed that babies born to mothers who had a mixed infection during pregnancy had a significantly lower gestational age at birth than those of uninfected mothers (mean difference, 2.3 weeks; 95% CI, 35 to 38; P = 0.0033) (Fig. 2D). In a multivariable linear regression model adjusting for maternal age and parity as significant independent covariates, mixed infections remained a significant predictor of gestational age at delivery (P = 0.0074). In terms of relative risk (RR), mothers who were exposed to both P. falciparum and P. vivax during pregnancy were three times more likely (P = 0.005) to deliver a preterm infant (<37 weeks gestational age) than mothers who were not infected during pregnancy (Table 3). In the nine women with a mixed infection, we investigated whether placental malaria affected the risk for PTB. No significant association was observed with PTB and past placental infection (presence of hemozoin by placental histopathology) (P = 0.69; 95% CI, −3.1 to 4.5).
FIG 2.
Submicroscopic malaria infections during pregnancy are not generally associated with changes in outcomes at delivery. Infant birth weight (A), gestational age at delivery (B), Z-scores of babies to determine small-for-gestational-age (SGA) (C), and maternal hemoglobin (Hb) levels at delivery (D) are shown. Solid horizontal lines indicate the means for each group, error bars indicate the standard deviations, and dotted horizontal lines represent the thresholds for low birth weight (2,500 g), preterm birth (37 weeks), SGA (10th percentile), and anemia (11 g/dl). *, P = 0.0033 (Mann-Whitney test).
TABLE 3.
Risk factors associated with adverse delivery outcomes
| Infection type | Small for gestational age |
Low birth wt |
Preterm birth |
Maternal anemia |
||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| Submicroscopic infection | ||||||||
| None | 1 | 1 | 1 | 1 | ||||
| P. falciparum | 1.57 (0.59–4.17) | 0.36 | 1.93 (0.54–6.90) | 0.31 | 0.73 (0.19–2.88) | 0.66 | 0.71 (0.28–1.76) | 0.46 |
| P. vivax | 0.80 (0.20–3.13) | 0.75 | 1.93 (0.54–6.90) | 0.31 | 0.76 (0.19–3.02) | 0.70 | 1.13 (0.53–2.43) | 0.74 |
| Both speciesa | NAb | 2.43 (0.35–16.82) | 0.30 | 3.40 (1.45–7.97) | 0.005 | 1.37 (0.55–3.37) | 0.50 | |
| Timing of infection | ||||||||
| None | 1 | 1 | 1 | 1 | ||||
| 1st trimester | NA | NA | NA | 0.80 (0.14–4.46) | 0.80 | |||
| 2nd trimester | 0.56 (0.08–3.91) | 0.56 | 1.49 (0.19–11.55) | 0.70 | 0.53 (0.08–3.74) | 0.53 | 0.64 (0.22–1.83) | 0.40 |
| 3rd trimester | 1.26 (0.49–3.22) | 0.63 | 2.71 (0.81–9.05) | 0.11 | 1.70 (0.76–3.78) | 0.20 | 1.06 (0.61–1.86) | 0.83 |
| Gravidity | ||||||||
| Multigravid | 1 | 1 | 1 | 1 | ||||
| Primigravid | 1.38 (0.87–2.17) | 0.17 | 1.63 (0.91–2.91) | 0.10 | 1.00 (0.57–1.75) | 0.99 | 0.99 (0.60–1.66) | 0.99 |
Includes both simultaneous (detected in the same blood sample) and sequential (at different times within pregnancy) infections of P. falciparum and P. vivax.
NA, not available (zero patients based on exposure; unable to calculate relative risk).
In women with repeated parasite positivity by RT-qPCR (n = 9), we did not observe any significant association with LBW (P = 1.0), PTB (P = 0.83), SGA (P = 0.45), or maternal anemia at delivery (P = 0.51) compared to results for women with only one positive sample during pregnancy.
VAR2CSA-specific antibodies.
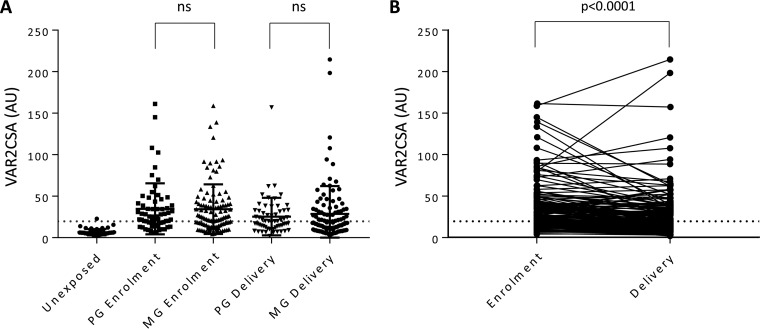
We hypothesized that the lack of adverse birth outcomes following monospecies SMI, particularly with P. falciparum, could be attributed to protective antibodies in pregnant women. To test this hypothesis, we measured VAR2CSA-specific IgG and observed that 65% (117/180) of women had antibodies at enrollment (Fig. 3A). No significant difference was observed in the antibody levels between women with an SMI compared to levels in healthy women (P = 0.69). In a regression analysis to test if antibody levels were associated with clinical outcomes at delivery, no significant association was observed between VAR2CSA-specific IgG levels and birth weight (P = 0.58), gestational age (P = 0.67), SGA (P = 0.58), or maternal Hb levels at delivery (P = 0.54).
FIG 3.
Anti-VAR2CSA antibody levels are independent of parity. (A) Anti-VAR2CSA antibody levels were measured by ELISA at inclusion and at delivery in sera from Colombian primigravid (PG) and multigravid (MG) women, and levels were compared to those of a Colombian malaria-unexposed control group. (B) Matched antibody levels measured at enrollment and at delivery in individual subjects. Horizontal lines indicate the means for each group, error bars indicate SDs, and dotted horizontal lines mark the cutoff for seropositivity (AU, 19.7). ns, not significant.
Antibody levels in primigravid and multigravid women were similar (P = 0.76), as were the proportions of primigravid (64%) and multigravid (65%) women with antibodies, consistent with our previous observations in pregnant women from this region (30). The proportion of women seropositive for anti-VAR2CSA antibodies was similar at enrollment and delivery (P = 0.67); however, anti-VAR2CSA antibody levels decreased from enrollment to delivery by a mean of 7.2 arbitrary units (AU) (P < 0.0001) in all women (Fig. 3B). In a subanalysis by gravidity, the anti-VAR2CSA antibody levels of primigravid and multigravid women had a mean decrease of 8.2 AU (P < 0.001) and 6.8 AU (P < 0.0001) from enrollment to delivery, respectively.
Infection with P. falciparum in pregnancy typically results in boosting of VAR2CSA-specific antibodies (7, 31). We therefore analyzed antibody levels in SMI samples with a positive diagnosis by RT-qPCR and in samples collected in subsequent ANVs or at delivery to test whether SMIs boosted VAR2CSA-specific antibodies. There was no significant boosting of antibody levels following Plasmodium infection, irrespective of the species (P = 0.66, P = 0.21, P = 0.25, for P. falciparum, P. vivax, and mixed infections, respectively).
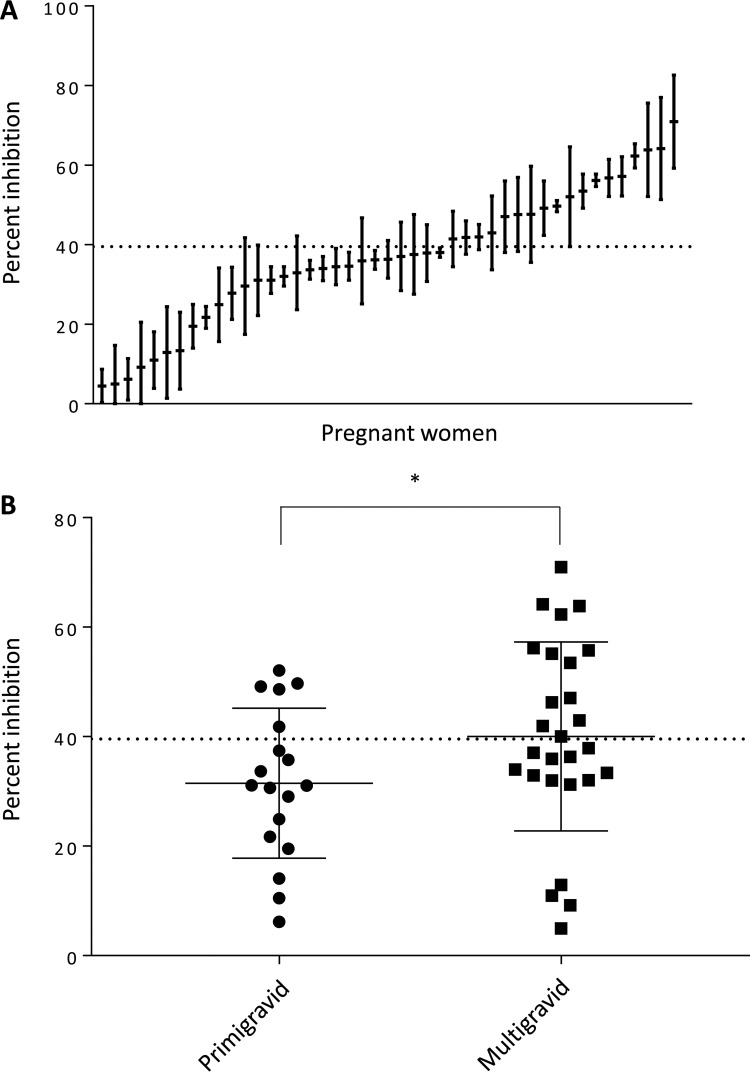
To determine whether the high frequency of anti-VAR2CSA antibodies at enrollment contributed to positive pregnancy outcomes despite SMI during pregnancy, we measured the functional activity of antibodies at enrollment in the 45 women with SMIs. Antibody function was assessed using an inhibition of binding assay (IBA), which measures the ability of antibodies to block binding of P. falciparum CS2 IEs to CSA in vitro. Parasites were selected to express VAR2CSA and bound strongly to CSA in the presence of pooled sera from unexposed Colombians, which served as a negative control. The percent inhibition of individual sera from malaria-exposed women was determined relative to this unexposed pool. Samples that inhibited IE binding to CSA by >39.1% were considered functional. Of the 45 women with an SMI, 40% (18/45) had functional antibodies at enrollment (Fig. 4A); 28% (5/18) were primigravid, and 48% (13/27) were multigravid (Fig. 4B). A significant difference in the levels of inhibition was observed between primigravid and multigravid women (31.5% versus 40%; mean difference, 8.5%; P = 0.049).
FIG 4.
CSA adhesion-blocking antibodies are observed in sera from Colombian pregnant women infected with submicroscopic malaria. (A) Binding inhibition profile for pregnant women infected by Plasmodium spp. (n = 45). The horizontal line indicates the means of three replicate measurements, error bars indicate SDs, and the horizontal dotted line represents the cutoff for inhibition (39.1%). (B) Inhibitory activity of antibodies from primigravid and multigravid women at enrollment. The horizontal dotted line represents the cutoff for inhibition (39.1%). *, P = 0.049 (Mann-Whitney test).
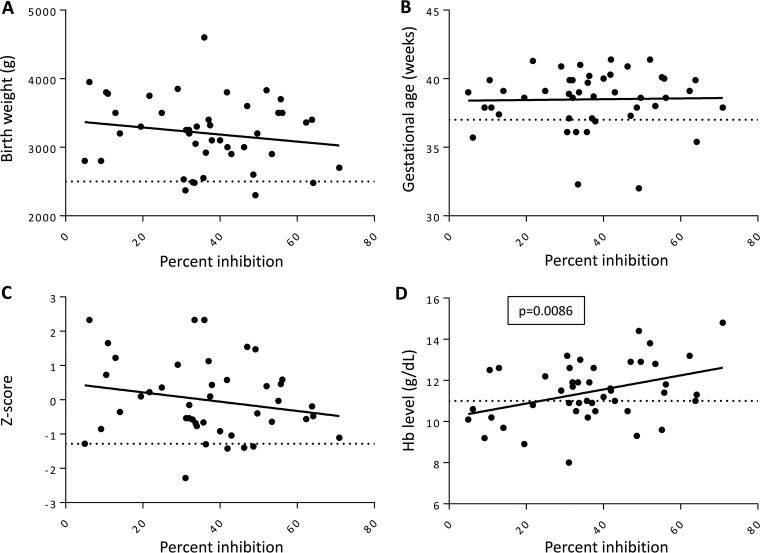
We next performed linear regression analysis to test if inhibitory antibody function correlated with clinical outcomes (Fig. 5). No significant association was observed between the percent inhibition and birth weight (P = 0.85) (Fig. 5A), gestational age (P = 0.80) (Fig. 5B), or SGA (P = 0.33) (Fig. 5C). However, in a multivariable linear regression model adjusting for the potential confounding effects of covariates, inhibitory antibody function was a significant independent predictor of higher maternal Hb levels at delivery (P = 0.0086) (Fig. 5D). Furthermore, both primigravid and multigravid women who did not have functional antibodies were at a significantly higher risk for anemia (Hb of <11 g/dl) (RR, 1.87; 95% CI, 1.13 to 2.79; P = 0.014) (Table 4).
FIG 5.
CSA adhesion-blocking antibodies correlate with hemoglobin levels at delivery but not with other delivery outcomes in women with a submicroscopic infection. Graphs represent linear regression analysis of the association of inhibitory antibodies and birth weight (A), gestational age at delivery (B), Z-scores of SGA (C), and maternal hemoglobin levels at delivery (D). Horizontal dotted lines indicate thresholds for low birth weight (2,500 g), preterm birth (37 weeks), SGA (10th percentile), and anemia (11 g/dl). The P value was determined by linear regression.
TABLE 4.
Relative risk of delivery outcomes in women without CSA adhesion-blocking antibodies compared to those of women with functional antibodies
| Outcome | Relative riska | 95% CI | P value |
|---|---|---|---|
| Low birth wt | 1.08 | 0.19–5.93 | 0.93 |
| Preterm birth | 0.59 | 0.13–2.65 | 0.49 |
| Small for gestational age | 1.62 | 0.36–7.29 | 0.53 |
| Maternal anemia | 1.87 | 1.13–2.79 | 0.014 |
Relative risk of women without functional antibodies versus that of women with functional antibodies (reference group). Functional inhibition was defined as seropositivity (AU ≥ 19.7) with a percent inhibition of ≥39.1%.
DISCUSSION
We present the results of a prospective longitudinal study of MiP in Latin America to assess the impact of SMIs in pregnancy on delivery outcomes. We observed a high frequency of submicroscopic MiP (25%, 45/180). We did not detect a statistically significant difference in birth weights between infants born of pregnancies complicated by submicroscopic MiP. Of note, our study had adequate statistical power (80%) to detect a clinically meaningful difference in birth weights (250 g), and the mean difference observed in our cohort was 38 g, based on the 95% CI. We can therefore exclude an effect of SMI on infant birth weight in this Colombian cohort. However, in a secondary analysis looking at other delivery outcomes, women with mixed P. falciparum and P. vivax SMIs were at increased risk of PTB. According to the WHO Global Survey on maternal and perinatal health (32), patent malaria infection in pregnancy is not a risk factor for PTB in Latin America. However, the number of malaria cases in Latin America reported in that study was low, and the data were not stratified based on the species of Plasmodium. Our findings reveal a novel association of P. falciparum and P. vivax mixed SMIs and adverse birth outcomes that is particularly relevant to regions outside Africa. While this result has not been reported previously in longitudinal studies of MiP, it is in keeping with findings from nonpregnant populations that mixed P. falciparum and P. vivax infections can have deleterious outcomes, including anemia in infants in Papua, Indonesia (33), and severe malaria in children in PNG (34). The mechanism underlying the pathogenesis of mixed infections is not known.
The women in our study had no other negative delivery outcomes associated with submicroscopic malaria. Our data are consistent with the findings of a multicenter study, which included Colombia, that submicroscopic P. falciparum and P. vivax infections had no significant association with LBW or maternal anemia (29). In contrast, several studies in Africa reported associations between SMIs and placental malaria, anemia, PTB, and LBW (21–26). In our study, women with SMIs were not treated with antimalarials as RT-qPCR was performed retrospectively, and no intermittent preventive treatment in pregnancy (IPTp) program is available in the study communities, suggesting that these SMIs resolved naturally during the course of pregnancy. Our data suggest that women had immunity that facilitated parasite clearance and prevented sequestration of IEs in the placenta. Antibodies that blocked adhesion of IEs to CSA were associated with reduced risk of anemia in women exposed to SMIs during pregnancy, suggesting a role for these antibodies in opsonization of IEs (35). Opsonizing antibodies to variant surface antigens were associated with reduced risk of anemia in pregnant women in Malawi, suggesting that this is an important immune mechanism in pregnancy.
Antibodies that protect women from adverse outcomes in pregnancy predominantly target the VAR2CSA surface antigen (1, 2). A majority of the women in our cohort were positive for anti-VAR2CSA antibodies at enrollment (65%). Furthermore, VAR2CSA antibody levels were independent of parity, and 64% of primigravid women were seropositive at enrollment. These results are consistent with our previous observations in this region that antibodies to the DBL5ε domain of VAR2CSA were not parity dependent (30). Furthermore, we observed similar levels of antibodies to DBL5ε in nonpregnant populations (men and children), as well as antibodies to the DBL3X domain and the minimal CSA-binding domain, ID1-ID2. This prior work, in conjunction with the data presented here, suggests that in this population and malaria transmission area, antibodies to the full-length VAR2CSA protein may be acquired outside of pregnancy. One hypothesis is that the antibodies arose against an antigen from P. vivax and cross-reacted with VAR2CSA. This is supported by our findings that pregnant women, men, and children who were infected with only P. vivax had antibodies that cross-reacted with the DBL5ε, DBL3X, and ID1-ID2 domains of VAR2CSA with similar frequencies (30).
Our study has certain limitations. First, the final sample size was affected by the loss to follow-up in the early stages of the study. Nevertheless, our study was adequately powered not to reject the null hypothesis of our primary objective to test whether SMIs during pregnancy are significantly associated with birth weight at delivery. Additionally, not all women attended monthly antenatal visits, and infections may have been missed during follow-up.
It is estimated that 4.3 million pregnancies are at risk of malaria in Latin America (36). Our results with molecular diagnostics confirm the high prevalence of submicroscopic MiP in Colombia despite a reduction in the overall burden of malaria in recent years (37). This study sheds light on the clinical outcomes of SMIs with P. falciparum, P. vivax, and mixed infections in pregnancy in this lower-transmission region and identifies an important role for host immunity in protecting pregnant women from SMI-related anemia.
MATERIALS AND METHODS
Ethics.
All participants provided voluntary consent and were free to withdraw from the study at any time. If the participant was less than 18 years of age, a parent or legal guardian provided informed consent. Ethical approval was obtained by the Health Research Ethics Board at the University of Alberta in Canada (Pro00041720) and the Comité de Ética of Instituto de Investigaciones Médicas of Universidad de Antioquia in Colombia (009-2013, 002-2015, and 009-2016). Pools of control sera from primigravid and multigravid women were obtained from Benin (protocol no. 21/CER/ISBA/13) and Uganda (protocol HDREC368).
Sample size calculation.
Our primary statistical analysis was a comparison by t test of the birth weights of infants born of pregnancies complicated by SMI versus those without evidence of infection. Using a standard sample size calculation in R (R Core Team, version 3.1.2; R package pwr, version 1.2-1 [https://github.com/heliosdrm/pwr]) with 80% power, alpha (α) level of significance of 0.05, assuming a prevalence of SMI of 45% (10), and a normal mean birth weight (standard deviation [SD]) of 3,230 g (473 g). Among Colombian newborns (3), a sample size of 115 mother-infant pairs was needed to detect a clinically significant difference of 250 g in birth weight between groups.
Study design and study site.
The study was performed in the municipality of Puerto Libertador in the Department of Córdoba (7°53′17″N, 75°40′18″W). Pregnant women were recruited at the antenatal care clinic and followed until delivery at the local hospital. At each antenatal visit, women had a clinical exam and were tested for Plasmodium sp. infection by thick blood smear. Samples were tested by reverse-transcription real-time quantitative PCR (RT-qPCR) retrospectively.
The population of Puerto Libertador was 44,694 in 2013 and 49,179 in 2016; 63% of the population resided in rural areas. The municipality is located within the Urabá-Altos Sinú-San Jorge-Bajo Cauca region, which accounts for 60% of all malaria cases in Colombia (38, 39). Epidemiologic characteristics of this region were described previously (38, 40, 41). Briefly, the intensity of transmission in this region is low and stable (two rainy seasons, two dry seasons) (38), with cocirculation of both P. falciparum and P. vivax in a ratio of 2:1 (39). The mean parasite index, measured as the number of malaria cases/1,000 inhabitants, of Puerto Libertador was 13.29 in 2000 to 2016 (39). The entomological inoculation rate in this municipality ranges from 3.5 to 4.8 infective bites per person per year (42). The most widely distributed Anopheles species are A. nuneztovari, A. albimanus, and A. darlingi (43).
Inclusion and exclusion criteria.
Consenting pregnant women were included in the study regardless of age or parity. Exclusion criteria were the following: residence outside the rural area of Puerto Libertador; renal, heart or respiratory disease; hepatic cirrhosis, sexually transmitted infections, behavioral disorder, seizures in the last 24 h prior to enrollment, jaundice, generalized edema, or any other chronic disease; antimalarial treatment within the 2 weeks prior to the time of enrollment; high-risk pregnancy by pathologies other than gestational malaria; missing samples from enrollment or delivery, miscarriage, or stillborn infant.
Sample and data collection.
Venous blood samples (2 ml) were obtained at each antenatal visit by venipuncture. In addition to peripheral blood, placental blood (2 ml) was collected at delivery by cutting a 2-mm-deep incision on the maternal side of the placenta and collecting blood with a blunt syringe. Thick smears were prepared for all blood samples by staining with Giemsa and read by a trained microscopist. All microscopists were trained and underwent proficiency testing. Proficiency testing was performed at the beginning of the study for external quality assurance. Parasitemia was determined by thick smear and calculated by counting the number of parasites per 200 leukocytes, based on a mean count of 8,000 leukocytes per microliter of blood. Samples were considered negative if no parasites were detected in at least 200 high-power (×1,000) fields. Women who tested positive by microscopy were treated according to Colombian health guidelines (artemether-lumefantrine or quinine-clindamycin for P. falciparum infections and chloroquine for P. vivax infections). Serum was separated from whole blood by centrifugation and stored at −20°C for downstream analyses.
At each antenatal visit, women completed a physical examination and a questionnaire to collect the following data: age, weight, height, demographic information, history of pregnancies, history of malaria, bed net usage, drug/medication history, and symptoms (e.g., presence of fever, cough, chills, diarrhea, bleeding, or other symptoms). Hemoglobin (Hb) levels were measured using a HemoCue Hb 201+ system (HemoCue, Sweden) according to the manufacturer's protocol. Gestational age was determined by ultrasound, which was performed at the hospital by an obstetrician on 181/187 women. Twenty-six (14%) women had an ultrasound during the first trimester, 128 (71%) women had one during the second trimester, and 27 (15%) women had an ultrasound during the third trimester. Birth weight and Apgar score (measured 1 min and 5 min postpartum) were determined at delivery.
Diagnosis of SMIs by RT-qPCR and placental malaria by histopathology.
Total nucleic acid was extracted from whole blood (peripheral blood and placental blood) using a MagMAX 96 DNA Multi-Sample kit (Applied Biosystems, Foster City, CA, USA). Samples were first screened by real-time PCR (qPCR) using primers and probes that detect a conserved region of the 18S rRNA gene from all species of Plasmodium (44). Briefly, 5 μl of template was added to a 20-μl reaction mix containing 1× TaqMan Universal PCR master mix (ThermoFisher, USA), 0.2 μM each primer, and 0.05 μM probe. qPCR was performed on an ABI 7500 Fast Real-Time PCR machine (Applied Biosystems, Foster City, CA, USA) with the following thermal profile: 15-min activation step at 95°C, followed by 45 cycles of 15 s of denaturation at 95°C and 1 min of annealing/extension at 60°C.
Plasmodium species was determined by reverse transcription qPCR (RT-qPCR) using species-specific primers and probes for P. falciparum and P. vivax according to a recently published method (45). Total nucleic acid template (5 μl) was added to a 5-μl reaction mixture containing 1× TaqMan Fast Virus 1-Step master mix (Thermo Fisher), 0.8 μM each primer, and 0.2 μM probe. Reactions were run with the following thermal profile: 20-s reverse transcription (RT) step at 50°C, 20-s activation step at 95°C, and 40 cycles of 3 s of denaturation at 95°C and 30 s of annealing/extension at 60°C. Samples with a cycle threshold (CT) of ≤40 were considered positive. Only samples positive in both the genus assay and species assay were considered positive by the molecular test (qPCR and RT-qPCR).
Placental tissue was collected immediately after delivery and processed at the Laboratorio de Dermatopatologia, Universidad de Antioquia. Methods for analysis were described elsewhere (19). Active infections were diagnosed by the presence of Plasmodium parasites, past infections were diagnosed by the presence of hemozoin only, and chronic infections were diagnosed by the presence of both hemozoin and Plasmodium parasites.
P. falciparum cultures.
P. falciparum CS2 parasites were cultured in vitro, as described previously (46). Parasites were selected on CSA (Sigma-Aldrich) to enrich for expression of VAR2CSA. Mature-stage parasites were purified using a VarioMACS according to the manufacturer's protocol (Miltenyl Biotec). Expression of VAR2CSA on the surface of IEs infected with P. falciparum CS2 was confirmed by flow cytometry using a rabbit anti-VAR2CSA polyclonal antibody (1:40 dilution) and detected using a goat anti-rabbit secondary conjugated to Alexa Fluor 647 (1:500 dilution; Life Technologies). Parasite DNA was stained with 5 μg/ml of 4′,6′-diamidino-2-phenylindole (DAPI). Surface labeling of DAPI-positive IEs was quantified by flow cytometry (Fortessa X20) and analyzed using FlowJo, version 7.6 (TreeStar).
Analysis of VAR2CSA antibodies by enzyme-linked immunosorbent assay (ELISA).
Full-length VAR2CSA recombinant protein (FCR3 allele) (0.5 μg/ml) was incubated with 100 μl of human serum (1:1,000 dilution) and detected with a horseradish peroxidase-conjugated anti-human IgG (1:6,000 dilution) (A0170; Sigma-Aldrich). IgG serum levels were expressed as optical density (OD) values read at 450 nm. OD values were calibrated for each plate and converted to arbitrary units (AU), as described previously (47). Seropositivity (cutoff, 19.7 AU) was defined as an AU of >2 SDs above the mean absorbance using 20 serum samples from Canadian residents with no history of travel to areas of malaria endemicity as negative controls. A pool of serum samples from multigravid women from Uganda served as a positive control. AUs of primigravid and multigravid women were compared to the values for malaria-unexposed Colombian individuals (mean AU, 4.6).
Static inhibition of IE binding to CSA.
The ability of antibodies to interfere with CSA adhesion of IEs was assessed by a modified static inhibition of binding assay (IBA) protocol as reported previously (30). Briefly, petri dishes (Falcon) were coated overnight at 4°C with 10 individual spots of 20 μl of phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and soluble CSA (sCSA) (50 μg/ml; Sigma-Aldrich). Spots were blocked with 3% BSA in 1× PBS for 1 h at 37°C. CSA-selected mature-stage CS2 IEs were adjusted to 20% parasitemia in suspension and blocked in a 3% BSA-RPMI medium buffer for 30 min at room temperature. IEs were incubated with sCSA (500 μg/ml) or serum (1:3 dilution) for 30 min at room temperature, added to the plate, and incubated for 15 min at room temperature. Unbound cells were washed with 24 ml of 1× PBS on a plate rocker for 10 min, fixed with 1.5% glutaraldehyde in 1× PBS, and stained with 5% filtered Giemsa. The entire area of each spot was imaged using an EVOS FL Auto instrument (Life Technologies) with a 4× objective, and the number of bound IEs was quantified using ImageJ (NIH). Serum samples were run in triplicate across three separate plates within the same experiment. A pool of sera from 50 unexposed Colombians from Medellín was used as a negative control and was included on two spots on each plate to control for variable binding of IEs across the plate. Five replicates of sCSA were run in parallel as a positive inhibition control for every experiment. The threshold for functional inhibition was based on the mean count of parasites bound to the plate from all replicates of the Colombian unexposed pool (across all plates and experiments, n = 29 plates) minus 2 SDs, divided by the mean count of parasites bound to the plate from all replicates of the Colombian unexposed pool [percent inhibition = 1 − (mean − 2 SDs/mean) × 100]. Based on these criteria, samples that inhibited binding of IEs to CSA by ≥39.1% were considered positive for functional inhibition. Functional inhibition per sample was calculated using the counts of bound parasites following the formula: percent inhibition = ([binding control − test sample]/[binding control − sCSA control]) × 100. The pool of sera from the unexposed population served as the binding control on each plate.
Statistical analyses.
Graphs and figures were generated using Prism, version 7 (GraphPad). To investigate the impact of SMIs specifically, data from women who were positive for malaria by microscopy (n = 7) were excluded from the data analyses. Categorical variables were compared using a chi-square test or Fisher's exact test, as appropriate. For comparisons between continuous variables, a D'Agostino-Pearson omnibus normality test was used to determine whether data followed a normal distribution, and comparisons were made using parametric tests (Student's t test or one-way analysis of variance [ANOVA]) or nonparametric tests (Mann-Whitney or Kruskal-Wallis for unpaired comparisons and Wilcoxon matched-pairs test for paired comparisons), as appropriate. Associations between risk factors and adverse birth outcomes were quantified using relative risk with 95% binomial confidence intervals. Classification of infants as small for gestational age (SGA) followed published methodology (48) and was specifically adapted to our Colombian population based on the birth weight of term infants in Colombia (40 weeks of gestation). Infants below the 10th percentile were defined as SGA (Z-score of <−1.28). A linear mixed-effects model was used to study the effect of SMIs during pregnancy on the levels of anti-VAR2CSA antibodies. We used R (R Core Team, version 3.1.2) and lme4 to perform a linear mixed-effects analysis of antibody levels as a function of gestational age and exposure to malaria. As fixed effects, we entered gestational age and SMI as a time-dependent covariate into the model. We modeled intercepts for each individual as random effects. Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. The P value for the hypothesis (effect of SMI on antibody levels) was obtained by a likelihood ratio test of the full model (including SMI) against the model without SMI. Linear regression was used to test the association between functional antibodies and birth outcomes. We used multivariable linear regression models to verify associations between key predictor variables (maternal age, parity, maternal hemoglobin concentration at enrollment, malaria infection, timing [trimester] of infection, antibody levels [AU], and inhibitory antibodies [percent inhibition]) and key outcomes, coded as continuous variables (gestational age at delivery, weight-for-gestational age Z-score, birth weight, and maternal hemoglobin concentration at delivery). We used the R package MASS (49) and the function “step” to perform backward stepwise selection of variables for inclusion in multivariable linear regression models.
ACKNOWLEDGMENTS
We are grateful to the pregnant women and field staff for their participation in this work. We thank Momar Ndao, from the National Reference Centre for Parasitology, Montreal, Canada, for assisting with external quality assessment (EQA) for microscopy in Colombia. We thank Sandra Shokoples, Diana Gonzalez, Jahanara Rajwani, and Evelyn Medawar for assistance in the laboratory. CS2 parasites were obtained through the Malaria Research and Reference Reagent Resource Center (MR4) and were deposited by Stephen Rogerson. We also thank Michael Good for providing helpful feedback and comments on the manuscript.
We declare that we have no conflicts of interest.
This work was supported by the following: an Open Operating Grant from the Canadian Institutes of Health Research (CIHR), COLCIENCIAS (Colombia), the Universidad de Antioquia (Estrategia de Sostenibilidad 2014–2015; Colombia), a PhD scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC) Create Host-Parasite Interactions Network, and graduate scholarships from the University of Alberta to K.G.; a Frederick Banting and Charles Best Canada Graduate Scholarship from CIHR, a graduate scholarship from the NSERC, and graduate scholarships from the University of Alberta to C.M.; an Emerging Leaders of the Americas scholarship to O.A.; and a generous donation from D. Davis.
REFERENCES
- 1.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. 2007. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis 7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 2.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. 1998. Maternal antibodies block malaria. Nature 395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 3.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol 49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 4.Magistrado PA, Minja D, Doritchamou J, Ndam NT, John D, Schmiegelow C, Massougbodji A, Dahlback M, Ditlev SB, Pinto VV, Resende M, Lusingu J, Theander TG, Salanti A, Nielsen MA. 2011. High efficacy of anti DBL4ε-VAR2CSA antibodies in inhibition of CSA-binding Plasmodium falciparum-infected erythrocytes from pregnant women. Vaccine 29:437–443. doi: 10.1016/j.vaccine.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 5.Bigey P, Gnidehou S, Doritchamou J, Quiviger M, Viwami F, Couturier A, Salanti A, Nielsen MA, Scherman D, Deloron P, Tuikue Ndam N. 2011. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J Infect Dis 204:1125–1133. doi: 10.1093/infdis/jir499. [DOI] [PubMed] [Google Scholar]
- 6.Ndam NT, Denoeud-Ndam L, Doritchamou J, Viwami F, Salanti A, Nielsen MA, Fievet N, Massougbodji A, Luty AJ, Deloron P. 2015. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg Infect Dis 21:813–823. doi: 10.3201/eid2105.141626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuikue Ndam NG, Salanti A, Le-Hesran JY, Cottrell G, Fievet N, Turner L, Sow S, Dangou JM, Theander T, Deloron P. 2006. Dynamics of anti-VAR2CSA immunoglobulin G response in a cohort of Senegalese pregnant women. J Infect Dis 193:713–720. doi: 10.1086/500146. [DOI] [PubMed] [Google Scholar]
- 8.Nosten F, McGready R, Simpson JA, Thwai KL, Balkan S, Cho T, Hkirijaroen L, Looareesuwan S, White NJ. 1999. Effects of Plasmodium vivax malaria in pregnancy. Lancet 354:546–549. doi: 10.1016/S0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 9.Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Warikar N, Seal A, McGready R, Sugiarto P, Tjitra E, Anstey NM, Price RN. 2008. Adverse pregnancy outcomes in an area where multidrug-resistant plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis 46:1374–1381. doi: 10.1086/586743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arango EM, Samuel R, Agudelo OM, Carmona-Fonseca J, Maestre A, Yanow SK. 2013. Molecular detection of malaria at delivery reveals a high frequency of submicroscopic infections and associated placental damage in pregnant women from northwest Colombia. Am J Trop Med Hyg 89:178–183. doi: 10.4269/ajtmh.12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agudelo OM, Aristizabal BH, Yanow SK, Arango E, Carmona-Fonseca J, Maestre A. 2014. Submicroscopic infection of placenta by Plasmodium produces Th1/Th2 cytokine imbalance, inflammation and hypoxia in women from north-west Colombia. Malar J 13:122. doi: 10.1186/1475-2875-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh FK, Davison BB, Gamboa D, Hernandez J, Branch OH. 2010. Placental histopathologic changes associated with subclinical malaria infection and its impact on the fetal environment. Am J Trop Med Hyg 83:973–980. doi: 10.4269/ajtmh.2010.09-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souza RM, Ataide R, Dombrowski JG, Ippolito V, Aitken EH, Valle SN, Alvarez JM, Epiphanio S, Marinho CR. 2013. Placental histopathological changes associated with Plasmodium vivax infection during pregnancy. PLoS Negl Trop Dis 7:e2071. doi: 10.1371/journal.pntd.0002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brutus L, Santalla J, Schneider D, Avila JC, Deloron P. 2013. Plasmodium vivax malaria during pregnancy, Bolivia. Emerg Infect Dis 19:1605–1611. doi: 10.3201/eid1910.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Morales AJ, Sanchez E, Vargas M, Piccolo C, Colina R, Arria M, Franco-Paredes C. 2006. Pregnancy outcomes associated with Plasmodium vivax malaria in northeastern Venezuela. Am J Trop Med Hyg 74:755–757. [PubMed] [Google Scholar]
- 16.Chotivanich K, Udomsangpetch R, Suwanarusk R, Pukrittayakamee S, Wilairatana P, Beeson JG, Day NP, White NJ. 2012. Plasmodium vivax adherence to placental glycosaminoglycans. PLoS One 7:e34509. doi: 10.1371/journal.pone.0034509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho BO, Lopes SC, Nogueira PA, Orlandi PP, Bargieri DY, Blanco YC, Mamoni R, Leite JA, Rodrigues MM, Soares IS, Oliveira TR, Wunderlich G, Lacerda MV, del Portillo HA, Araujo MO, Russell B, Suwanarusk R, Snounou G, Renia L, Costa FT. 2010. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis 202:638–647. doi: 10.1086/654815. [DOI] [PubMed] [Google Scholar]
- 18.McGready R, Davison BB, Stepniewska K, Cho T, Shee H, Brockman A, Udomsangpetch R, Looareesuwan S, White NJ, Meshnick SR, Nosten F. 2004. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg 70:398–407. [PubMed] [Google Scholar]
- 19.Carmona-Fonseca J, Arango E, Maestre A. 2013. Placental malaria in Colombia: histopathologic findings in Plasmodium vivax and P. falciparum infections. Am J Trop Med Hyg 88:1093–1101. doi: 10.4269/ajtmh.12-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recht J, Siqueira AM, Monteiro WM, Herrera SM, Herrera S, Lacerda MVG. 2017. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar J 16:273. doi: 10.1186/s12936-017-1925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adegnika AA, Verweij JJ, Agnandji ST, Chai SK, Breitling LP, Ramharter M, Frolich M, Issifou S, Kremsner PG, Yazdanbakhsh M. 2006. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg 75:798–803. [PubMed] [Google Scholar]
- 22.Mayor A, Moro L, Aguilar R, Bardaji A, Cistero P, Serra-Casas E, Sigauque B, Alonso PL, Ordi J, Menendez C. 2012. How hidden can malaria be in pregnant women? Diagnosis by microscopy, placental histology, polymerase chain reaction and detection of histidine-rich protein 2 in plasma. Clin Infect Dis 54:1561–1568. doi: 10.1093/cid/cis236. [DOI] [PubMed] [Google Scholar]
- 23.Mockenhaupt FP, Rong B, Till H, Eggelte TA, Beck S, Gyasi-Sarpong C, Thompson WN, Bienzle U. 2000. Submicroscopic Plasmodium falciparum infections in pregnancy in Ghana. Trop Med Int Health 5:167–173. doi: 10.1046/j.1365-3156.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- 24.Mohammed AH, Salih MM, Elhassan EM, Mohmmed AA, Elzaki SE, El-Sayed BB, Adam I. 2013. Submicroscopic Plasmodium falciparum malaria and low birth weight in an area of unstable malaria transmission in Central Sudan. Malar J 12:172. doi: 10.1186/1475-2875-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottrell G, Moussiliou A, Luty AJ, Cot M, Fievet N, Massougbodji A, Deloron P, Tuikue Ndam N. 2015. Submicroscopic Plasmodium falciparum infections are associated with maternal anemia, premature births, and low birth weight. Clin Infect Dis 60:1481–1488. doi: 10.1093/cid/civ122. [DOI] [PubMed] [Google Scholar]
- 26.Cohee LM, Kalilani-Phiri L, Boudova S, Joshi S, Mukadam R, Seydel KB, Mawindo P, Thesing P, Kamiza S, Makwakwa K, Muehlenbachs A, Taylor TE, Laufer MK. 2014. Submicroscopic malaria infection during pregnancy and the impact of intermittent preventive treatment. Malar J 13:274. doi: 10.1186/1475-2875-13-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanisic DI, Moore KA, Baiwog F, Ura A, Clapham C, King CL, Siba PM, Beeson JG, Mueller I, Fowkes FJ, Rogerson SJ. 2015. Risk factors for malaria and adverse birth outcomes in a prospective cohort of pregnant women resident in a high malaria transmission area of Papua New Guinea. Trans R Soc Trop Med Hyg 109:313–324. doi: 10.1093/trstmh/trv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh N, Bharti PK, Singh MP, Singh R, Yeboah-Antwi K, Desai M, Udhayakumar V, Muniyandi M, Hamer DH, Wylie BJ. 2015. What is the burden of submicroscopic malaria in pregnancy in central India? Pathog Glob Health 109:30–38. doi: 10.1179/2047773215Y.0000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardaji A, Martinez-Espinosa FE, Arevalo-Herrera M, Padilla N, Kochar S, Ome-Kaius M, Botto-Menezes C, Castellanos ME, Kochar DK, Kochar SK, Betuela I, Mueller I, Rogerson S, Chitnis C, Hans D, Menegon M, Severini C, Del Portillo H, Dobano C, Mayor A, Ordi J, Piqueras M, Sanz S, Wahlgren M, Slutsker L, Desai M, Menendez C. 2017. Burden and impact of Plasmodium vivax in pregnancy: a multi-centre prospective observational study. PLoS Negl Trop Dis 11:e0005606. doi: 10.1371/journal.pntd.0005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gnidehou S, Doritchamou J, Arango EM, Cabrera A, Arroyo MI, Kain KC, Ndam NT, Maestre A, Yanow SK. 2014. Functional antibodies against VAR2CSA in nonpregnant populations from Colombia exposed to Plasmodium falciparum and Plasmodium vivax. Infect Immun 82:2565–2573. doi: 10.1128/IAI.01594-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowkes FJ, McGready R, Cross NJ, Hommel M, Simpson JA, Elliott SR, Richards JS, Lackovic K, Viladpai-Nguen J, Narum D, Tsuboi T, Anders RF, Nosten F, Beeson JG. 2012. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis 206:1612–1621. doi: 10.1093/infdis/jis566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogel JP, Lee AC, Souza JP. 2014. Maternal morbidity and preterm birth in 22 low- and middle-income countries: a secondary analysis of the WHO Global Survey dataset. BMC Pregnancy Childbirth 14:56. doi: 10.1186/1471-2393-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenangalem E, Karyana M, Burdarm L, Yeung S, Simpson JA, Tjitra E, Anstey NM, Poespoprodjo JR, Price RN, Douglas NM. 2016. Plasmodium vivax infection: a major determinant of severe anaemia in infancy. Malar J 15:321. doi: 10.1186/s12936-016-1373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I. 2008. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med 5:e127. doi: 10.1371/journal.pmed.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng G, Aitken E, Yosaatmadja F, Kalilani L, Meshnick SR, Jaworowski A, Simpson JA, Rogerson SJ. 2009. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. J Infect Dis 200:299–306. doi: 10.1086/599841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. 2010. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 7:e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO. 2016. World malaria report 2016. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1. [Google Scholar]
- 38.Rodriguez JC, Uribe GA, Araujo RM, Narvaez PC, Valencia SH. 2011. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz 106(Suppl 1):114–122. doi: 10.1590/S0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmona-Fonseca J, Agudelo OM, Arango EM. 2017. Asymptomatic plasmodial infection in Colombian pregnant women. Acta Trop 172:97–101. doi: 10.1016/j.actatropica.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Vallejo AF, Chaparro PE, Benavides Y, Alvarez A, Quintero JP, Padilla J, Arevalo-Herrera M, Herrera S. 2015. High prevalence of sub-microscopic infections in Colombia. Malar J 14:201. doi: 10.1186/s12936-015-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Instituto Nacional de Salud. 2012. Sistema de vigilancia en Salud Publica (SIVIGILA). Instituto Nacional de Salud, Bogota, Colombia: https://www.minsalud.gov.co/salud/Paginas/SIVIGILA.aspx. [Google Scholar]
- 42.Naranjo-Diaz N, Rosero DA, Rua-Uribe G, Luckhart S, Correa MM. 2013. Abundance, behavior and entomological inoculation rates of anthropophilic anophelines from a primary Colombian malaria endemic area. Parasit Vectors 6:61. doi: 10.1186/1756-3305-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahumada ML, Orjuela LI, Pareja PX, Conde M, Cabarcas DM, Cubillos EF, Lopez JA, Beier JC, Herrera S, Quinones ML. 2016. Spatial distributions of Anopheles species in relation to malaria incidence at 70 localities in the highly endemic northwest and south Pacific coast regions of Colombia. Malar J 15:407. doi: 10.1186/s12936-016-1421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K. 2004. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol 42:5636–5643. doi: 10.1128/JCM.42.12.5636-5643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavina K, Arango E, Larrotta CA, Maestre A, Yanow SK. 2017. A sensitive species-specific reverse transcription real-time PCR method for detection of Plasmodium falciparum and Plasmodium vivax. Parasite Epidemiol Control 2:70–76. doi: 10.1016/j.parepi.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 47.Cham GK, Kurtis J, Lusingu J, Theander TG, Jensen ATR, Turner L. 2008. A semi-automated multiplex high-throughput assay for measuring IgG antibodies against Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) domains in small volumes of plasma. Malar J 7:108. doi: 10.1186/1475-2875-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gulmezoglu AM, Merialdi M. 2011. A global reference for fetal-weight and birthweight percentiles. Lancet 377:1855–1861. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 49.Venables WN, Ripley BD. 2002. Modern applied statistics with S. Springer, New York, NY. [Google Scholar]