ABSTRACT
“Candidatus Liberibacter asiaticus” is the causative bacterium associated with citrus greening disease. “Ca. Liberibacter asiaticus” is transmitted by Diaphorina citri more efficiently when it is acquired by nymphs rather than adults. Why this occurs is not known. We compared midguts of D. citri insects reared on healthy or “Ca. Liberibacter asiaticus”-infected citrus trees using quantitative PCR, confocal microscopy, and mitochondrial superoxide staining for evidence of oxidative stress. Consistent with its classification as propagative, “Ca. Liberibacter asiaticus” titers were higher in adults than in nymphs. Our previous work showed that adult D. citri insects have basal levels of karyorrhexis (fragmentation of the nucleus) in midgut epithelial cells, which is increased in severity and frequency in response to “Ca. Liberibacter asiaticus.” Here, we show that nymphs exhibit lower levels of early-stage karyorrhexis than adults and are refractory to the induction of advanced karyorrhexis by “Ca. Liberibacter asiaticus” in the midgut epithelium. MitoSox Red staining showed that guts of infected adults, particularly males, experienced oxidative stress in response to “Ca. Liberibacter asiaticus.” A positive correlation between the titers of “Ca. Liberibacter asiaticus” and the Wolbachia endosymbiont was observed in adult and nymph midguts, suggesting an interplay between these bacteria during development. We hypothesize that the resistance of the nymph midgut to late-stage karyorrhexis through as yet unknown molecular mechanisms benefits “Ca. Liberibacter asiaticus” for efficient invasion of midgut epithelial cells, which may be a factor explaining the developmental dependency of “Ca. Liberibacter asiaticus” acquisition by the vector.
KEYWORDS: “Candidatus Liberibacter asiaticus,” Diaphorina citri; quantitative real-time PCR; Wolbachia; apoptosis; confocal microscopy; endosymbionts; midgut; oxidative stress; vector biology
INTRODUCTION
The bacterium “Candidatus Liberibacter asiaticus” is a vector-borne, phloem-restricted plant pathogen associated with “citrus greening” disease, the most economically important and devastating disease affecting citrus production worldwide. “Ca. Liberibacter asiaticus” is currently unculturable and is thus referenced as “Candidatus.” Citrus greening is present from Asia to the Indian subcontinent and neighboring islands, as well as Saudi Arabia, Africa, South America, and North America (1, 2). In 1995, the official name of the disease became huanglongbing (HLB), or “yellow dragon disease” in Chinese (1). Although first reported in Southern China in 1919, HLB was likely established in India much earlier (3). “Ca. Liberibacter asiaticus” was discovered in Florida in 2005, 1 year after it was discovered in the Western Hemisphere in Brazil (4). As of 2015, 90% of Florida acreage had HLB, while 80% of trees in citrus operations were “Ca. Liberibacter asiaticus” positive, leading to an average yield loss of 41% below pre-HLB levels (5). Management tactics for HLB in Florida primarily rely on controlling the Asian citrus psyllid (ACP) Diaphorina citri Kuwayama vector populations (6). Currently, there is no cure available, and preventive measures are expensive and prolong the life of individual trees for a few years at most. Prevention focuses on early detection of infected insects and plants, removal of infected material, heavy application of insecticides to kill the vectors, heat shocking, nutritional supplements, and quarantining healthy plants under nets (1, 7, 8). These methods are not especially effective, long-lasting, or cost-effective. Thus, it is important to find a means of controlling bacterial transmission by the insect vector.
D. citri has a complex microbiome involving a variety of microbial endosymbionts, including “Candidatus Carsonella rudii,” “Candidatus Profftella armatura,” and Wolbachia pipientis. All three species of bacteria are maternally inherited through the reproductive tract, although their distribution in the insect varies. The W. pipientis strain found in D. citri was phylogenetically described by Saha et al. in 2012 (9) and is genetically distinct from previously characterized W. pipientis strains in other insects. Whereas “Ca. Carsonella rudii” and “Ca. Profftella armatura” are enclosed in a specialized organ called a bacteriome (10), Wolbachia is known to reside in several tissues of psyllids (and other insects) (11), including those where, in D. citri, “Ca. Liberibacter asiaticus” has also been found, such as the fat body, salivary glands, ovaries, nervous system, tracheal cells, and midgut (12–14). We have previously observed Wolbachia colocalizing in the same cells of the gut as “Ca. Liberibacter asiaticus,” though in distinct subcellular locations involving little overlap (14). Wolbachia titers were found to be more variable in the guts of “Ca. Liberibacter asiaticus”-infected adult insects (14). The precise roles of these endosymbionts in the acquisition and transmission of “Ca. Liberibacter asiaticus” by D. citri are not known.
Successful acquisition and transmission of “Ca. Liberibacter asiaticus” by D. citri starts with ingestion of the bacteria through the food canal in the insect stylets as it feeds from the phloem of infected trees (15, 16). Once “Ca. Liberibacter asiaticus” has entered the food canal, it travels to the gut lumen, from where it can then make its way into the hemolymph through the gut wall. From the hemolymph, “Ca. Liberibacter asiaticus” can enter the salivary glands, at which point D. citri individuals can transmit “Ca. Liberibacter asiaticus” to a new susceptible plant (12, 13, 17). Not all D. citri insects reared on “Ca. Liberibacter asiaticus”-positive plants can acquire the bacteria and become infected. The psyllid is considered infected once “Ca. Liberibacter asiaticus” has invaded the midgut epithelium, hemolymph, or other tissues. Evidence supports the idea that “Ca. Liberibacter asiaticus” replicates in D. citri (16, 18). Here, we make the following distinctions. If “Ca. Liberibacter asiaticus” can be detected within the insect by PCR-based methods, the psyllid is considered infected and denoted “Ca. Liberibacter asiaticus”+. Therefore, D. citri raised on “Ca. Liberibacter asiaticus”+ plants but not tested directly for the bacteria are considered exposed, and the exposed group can contain a mixture of “Ca. Liberibacter asiaticus”+ and “Ca. Liberibacter asiaticus”− insects.
Vector competency in D. citri is developmentally regulated in a way that is not yet defined at the molecular level. Conflicting results have been reported regarding the persistence of “Ca. Liberibacter asiaticus” within D. citri following acquisition as adults (19). Adult D. citri may need to feed for longer periods on infected plants to maintain a high level of “Ca. Liberibacter asiaticus” infection (17, 20), while D. citri insects that acquire “Ca. Liberibacter asiaticus” as nymphs are better vectors of the bacteria when they become adults. Over 60% of the psyllids exposed to “Ca. Liberibacter asiaticus” as nymphs tend to acquire “Ca. Liberibacter asiaticus,” while a much lower proportion of psyllids exposed to “Ca. Liberibacter asiaticus” as adults tend to acquire it (17, 18, 20). In two studies conducted in Japan and Florida (17, 18), only D. citri insects that acquired “Ca. Liberibacter asiaticus” as nymphs were able to transmit “Ca. Liberibacter asiaticus” to healthy citrus plants, although some other studies showed that adults can acquire and transmit the bacterium at lower rates than nymphs (19). Once acquired, however, “Ca. Liberibacter asiaticus” can be harbored by adults throughout their life spans (17, 21). Additionally, a proteome study by Ramsey et al. suggests that nymphs show an attenuated proteome response to being reared on trees infected by “Ca. Liberibacter asiaticus” compared to adults (22).
Acquisition and transmission differences between adults and nymphs have significant implications regarding epidemiology and control of HLB and this economically important citrus bacterium. We previously investigated morphological abnormalities in the D. citri gut associated with exposure to “Ca. Liberibacter asiaticus”-infected trees and revealed complex phenotypes of nuclear DNA fragmentation in midgut epithelial cells associated with apoptosis (15). Rearing insects on “Ca. Liberibacter asiaticus”-infected trees was correlated with an increase in karyorrhexis (fragmentation of the nucleus) and the expression of apoptosis protein markers in the midgut epithelium of adults (14). Consistent with these results, a quantitative dual transcriptome and proteome analysis of excised D. citri guts demonstrated that “Ca. Liberibacter asiaticus”-exposed guts showed signatures of mitochondrial disturbance, including downregulation of the enzymes in the tricarboxylic acid (TCA) cycle (14). Apoptosis, or programmed cell death (23), is a highly regulated process of cell death that is important for regulation of development and immunity in multicellular organisms. Apoptosis has been reported in insects and in various areas of the insect body. In Culex pipiens pipiens, the vector of West Nile virus, it occurs in salivary glands and, like the psyllid, in the midgut (24). In Anopheles, the vector of several Plasmodium species, it occurs in the gut epithelial cells (reviewed in reference 25). By targeting the mechanisms driving the exacerbated nuclear DNA fragmentation and resulting cellular apoptosis in “Ca. Liberibacter asiaticus”-exposed D. citri midguts, there is potential to open new avenues by which to attack the bacterium-vector interaction and eliminate successful transmission of “Ca. Liberibacter asiaticus.”
In this study, we tested whether D. citri nymphs respond to “Ca. Liberibacter asiaticus”-infected trees in the same manner as adult insects in the midgut epithelium by quantifying and visualizing “Ca. Liberibacter asiaticus” and Wolbachia bacteria in excised guts and whole insects using a combination of molecular and cell biology approaches. We hypothesize that nymphs are better at acquiring “Ca. Liberibacter asiaticus” than adults because of an attenuated apoptotic response in the nymph midgut. We hypothesize that this attenuated response may have been favored by selection for the establishment of the endosymbionts, such as Wolbachia, within the insect. This hypothesis is consistent with the growing body of literature showing that the evolution of the immune system in hemipteran insects is adapted to maintain chronic infections of mutualistic endosymbionts (reviewed in reference 26).
RESULTS
Quantitative PCR (qPCR) of “Ca. Liberibacter asiaticus” in whole bodies and guts of individual D. citri insects showed higher variability in adults than in nymphs.
In a population that has a variable rate of infection by a pathogen, individuals—unless tested specifically for pathogen presence—must be referred to as either exposed or nonexposed due to the uncertainty of pathogen presence. This is the case with D. citri raised on healthy or “Ca. Liberibacter asiaticus”-infected citrus plants. Throughout this report, samples are referred to as either exposed or nonexposed unless they have been tested individually for “Ca. Liberibacter asiaticus.” Once individuals are confirmed to contain “Ca. Liberibacter asiaticus,” they are called “Ca. Liberibacter asiaticus”+. Confirmation of the presence of “Ca. Liberibacter asiaticus” works under the distinction that samples with cycle threshold (Ct) values of <35 are infected while those with values of >35 have an uncertain infection level, as described in Materials and Methods. Thus, our analyses of samples that were “Ca. Liberibacter asiaticus”+ were of samples with Ct values of <35. The Ct values for all the samples can be found in Fig. S1 and S2 in the supplemental material.
Quantitative-PCR analysis of individual “Ca. Liberibacter asiaticus”-exposed and nonexposed insects revealed that among “Ca. Liberibacter asiaticus”-exposed samples, whole-body 5th-instar nymphs were more often (70%) “Ca. Liberibacter asiaticus”+ than whole-body adults (58.6%) (Table 1), while all nonexposed samples did not contain the bacteria (see Table S1 in the supplemental material). For a psyllid to become “Ca. Liberibacter asiaticus”+, it must ingest phloem sap from a “Ca. Liberibacter asiaticus”-infected citrus plant. We tested guts (collected from the same mixed-age colony as whole-body samples, but the colony was 1 month older) from individual “Ca. Liberibacter asiaticus”-exposed psyllids for “Ca. Liberibacter asiaticus” presence and found that nymph guts were classified “Ca. Liberibacter asiaticus”+ 60% of the time while adult guts were classified “Ca. Liberibacter asiaticus”+ 86.2% of the time based on the qPCR results (Table 1). These results show that age may be correlated with the ability to detect “Ca. Liberibacter asiaticus” in the gut, but the difference here was statistically insignificant. All nonexposed guts did not test positive for “Ca. Liberibacter asiaticus” (see Table S1 in the supplemental material).
TABLE 1.
Percentages of “Ca. Liberibacter asiaticus”-exposed D. citri samples that contained “Ca. Liberibacter asiaticus” and Wolbachia
| Samplea | Stage | Bacterium | % with Ct |
Mean copy no.b | |
|---|---|---|---|---|---|
| <35 | >35 | ||||
| Whole body | Nymph (5th instar) | “Ca. Liberibacter asiaticus” | 70.0 | 30.0 | 744.3 |
| Wolbachia | 100.0 | 0.0 | 20,115.6 | ||
| Adult | “Ca. Liberibacter asiaticus” | 58.6 | 41.4 | 539.0 | |
| Wolbachia | 100.0 | 0.0 | 14,829.8 | ||
| Gut | Nymph (5th instar) | “Ca. Liberibacter asiaticus” | 60.0 | 40.0 | 331.1 |
| Wolbachia | 100.0 | 0.0 | 118,160.2 | ||
| Adult | “Ca. Liberibacter asiaticus” | 86.2 | 13.8 | 1,846.1 | |
| Wolbachia | 100.0 | 0.0 | 723,056.0 | ||
Each sample was tested for both “Ca. Liberibacter asiaticus” and Wolbachia titers. For each life stage, 30 samples were used, and each sample had three technical replicates, which were averaged for a final Ct value. The samples with an average technical-replicate Ct value of <35 contributed to the percent infection rate (e.g., for the whole-body nymphs tested, 21/30 samples had Ct values of <35, for a 70% “Ca. Liberibacter asiaticus” infection rate). The individuals used for guts and whole bodies were taken from separate generations of the same colonies. Exposed nymphs and adults were fed on “Ca. Liberibacter asiaticus”-infected citrus trees for one or more generations.
The mean copy number is the average of individual sample copy numbers with Ct values of <35 using qPCR. Cycle threshold values were converted to copy numbers using a standard curve.
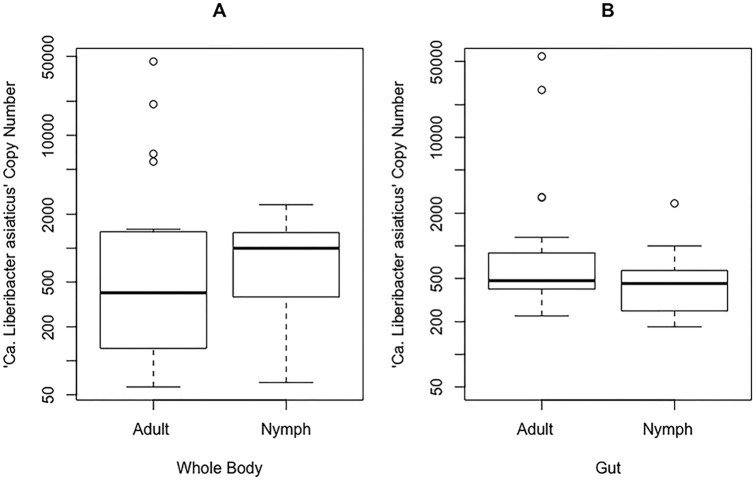
The Mann-Whitney U statistical test for a difference between two unpaired, nonnormal groups was performed in R (function, wilcox.test, where W is the Mann-Whitney U test statistic), as well as the F test for sample variance (function, var.test, where F is the F-test statistic for the ratio of variances), on “Ca. Liberibacter asiaticus” copy numbers (a measure of total target sequence in a sample or the titer of the bacteria) calculated from each sample's qPCR Ct value (Table 1). When we compared adults to nymphs, the average titers of “Ca. Liberibacter asiaticus”+ whole bodies were not different (W = 143; P = 0.13), a result we also found when we compared “Ca. Liberibacter asiaticus”+ guts of adults to those of nymphs (W = 290; P = 0.22) (Fig. 1). However, as shown in Fig. 1 by the outliers, there was greater sample variance among “Ca. Liberibacter asiaticus”+ adults than among nymphs at the whole-body (F = 2.91; P = 0.02) and gut (F = 131.20; P ≪ 0.05) levels. This variance among adult samples is a phenotype of “Ca. Liberibacter asiaticus” infection in D. citri (Fig. 1). Additionally, we compared the average “Ca. Liberibacter asiaticus” titers in whole bodies and guts and found that in “Ca. Liberibacter asiaticus”+ nymphs, whole bodies were not different from guts (W = 216; P = 0.67), while “Ca. Liberibacter asiaticus”+ adults did show a difference between whole bodies and guts (W = 143; P = 0.025) (Fig. 1).
FIG 1.
Individual “Ca. Liberibacter asiaticus” copy number distribution for all “Ca. Liberibacter asiaticus”+ adult and nymph whole-body (A) and gut (B) D. citri samples. When adults and nymphs were compared using the Mann-Whitney U test in R, they were not statistically different (W = 143, P = 0.13 and W = 290, P = 0.22, respectively). For adults, there is a significant difference between panels A and B at the 0.05 confidence interval (W = 143; P = 0.03). For nymphs, there is no significant difference between panels A and B (W = 216; P = 0.67). The individual “Ca. Liberibacter asiaticus” copy numbers were calculated from Ct values using a standard curve; the scale is logarithmic. The circles are outliers, the whiskers represent minimum and maximum values, and the tops and bottoms of the boxes represent the 75th and 25th quantiles, respectively. The thick horizontal lines are the median values.
Quantitative PCR of Wolbachia in whole bodies and guts of individual D. citri nymphs and adults revealed 100% infection in all sample types.
In our previous gut analysis (14), the titer of Wolbachia was more variable in “Ca. Liberibacter asiaticus”-exposed D. citri gut samples. In this study, we analyzed “Ca. Liberibacter asiaticus”-exposed gut and whole-body samples that had been used for the “Ca. Liberibacter asiaticus” quantification shown in Table 1, in addition to the nonexposed samples (see Table S1 in the supplemental material), for the additional presence of Wolbachia. Wolbachia was found in 100% of samples tested, including all “Ca. Liberibacter asiaticus”+ and nonexposed samples.
(i) Variance in Wolbachia titer is greater in nymphs than in adults for “Ca. Liberibacter asiaticus”+ samples.
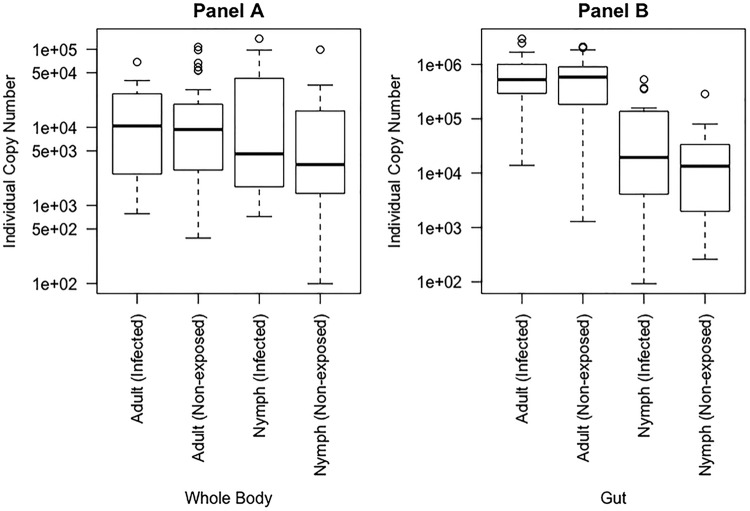
We ran the Mann-Whitney U test and the F test of sample variance to find differences between adults and nymphs in both “Ca. Liberibacter asiaticus”+ and nonexposed samples relative to the mean and variance of the Wolbachia titer. We found that the average titers of Wolbachia in nonexposed whole bodies were the same for adults and nymphs (W = 554; P = 0.13). The average titers of Wolbachia in “Ca. Liberibacter asiaticus”+ whole bodies were also the same for adults and nymphs (W = 202; P = 0.96). However, unlike the consistent variance phenotype observed for the “Ca. Liberibacter asiaticus” titer in Fig. 1, the variance in the titer of Wolbachia in “Ca. Liberibacter asiaticus”+ whole bodies was greater for nymphs than for adults (F = 0.23; P < 0.05) (Fig. 2A).
FIG 2.
Sample distribution of individual Wolbachia copy numbers for whole bodies (A) and guts (B) of “Ca. Liberibacter asiaticus”+ and nonexposed samples of nymph and adult D. citri insects. Samples referenced as infected are “Ca. Liberibacter asiaticus”+. All the samples were found to contain Wolbachia. (A) For both nonexposed and exposed individuals, adults were not significantly different from nymphs (W = 554, P = 0.13 and W = 202, P = 0.96, respectively). (B) For both nonexposed and infected insects, adults and nymphs were significantly different (W = 847, P ≪ 0.05 and W = 419, P ≪ 0.05, respectively). Whole-body and gut distributions are shown in separate graphs due to differences in sample collection times; note the 10-fold difference shown on the y axis. This translates into significant differences between all whole-body and gut comparisons, including adults (nonexposed, W = 853, P ≪ 0.05; infected, W = 451, P ≪ 0.05) and nymphs (nonexposed, W = 568, P = 0.08; infected, W = 9, P ≪ 0.05). The P values were generated using the Mann-Whitney U test in R. The circles are outliers, the whiskers represent minimum and maximum values, and the tops and bottoms of the boxes represent the 75th and 25th quantiles, respectively. The thick horizontal lines are the median values.
When we compared Wolbachia titers in adult and nymph guts, we found that the average titer in nonexposed guts was greater for adults than for nymphs (W = 847; P ≪ 0.05). The average titer of Wolbachia in “Ca. Liberibacter asiaticus”+ guts was also greater for adults than for nymphs (W = 419; P ≪ 0.05). Additionally, we found that, like the “Ca. Liberibacter asiaticus” variance phenotype, the variance in the titer of Wolbachia in “Ca. Liberibacter asiaticus”+ guts was greater in adults than in nymphs (F = 24.52; P ≪ 0.05) (Fig. 2B).
(ii) The Wolbachia titer is greater in guts than in whole bodies in “Ca. Liberibacter asiaticus”+ adults and nymphs.
We ran the Mann-Whitney U test on Wolbachia Ct values. When we looked more specifically at adults and compared guts to whole bodies, we found that the average titer of Wolbachia in nonexposed adults was at least 10 times greater in guts than in whole bodies (W = 853; P ≪ 0.05). However, there was no difference in the average Wolbachia titer between guts and whole bodies in nonexposed nymphs (W = 568; P = 0.08) (Fig. 2). When we looked at the average titer of Wolbachia in “Ca. Liberibacter asiaticus”+ adults, we found that the Wolbachia titer was much greater in guts than in whole bodies (W = 451; P ≪ 0.05) (Fig. 2). Unlike nonexposed nymphs, the average titer of Wolbachia in “Ca. Liberibacter asiaticus”+ nymphs was also much greater in guts than in whole bodies (W = 9; P ≪ 0.05). The gut samples were collected approximately 1 month after the whole-body samples. These results show that the Wolbachia titer may increase within a colony over time in “Ca. Liberibacter asiaticus”+ adult and nymph insects, but not in nonexposed insects.
A correlation analysis of “Ca. Liberibacter asiaticus” and Wolbachia copy numbers shows a positive relationship.
To test whether there is a correlation between “Ca. Liberibacter asiaticus” and Wolbachia titers, we ran a Pearson product-moment correlation analysis (function, cor.test, where cor is the correlation coefficient) on “Ca. Liberibacter asiaticus”+ samples to compare paired “Ca. Liberibacter asiaticus” copy numbers to Wolbachia copy numbers. Whole-body adults (see Fig. S3A in the supplemental material), guts of adults (see Fig. S3C in the supplemental material), and guts of nymphs (see Fig. S3D in the supplemental material) showed a positive correlation between “Ca. Liberibacter asiaticus” and Wolbachia titers (P < 0.05, cor = 0.76; P < 0.05, cor = 0.56; and P < 0.05, cor = 0.77, respectively). Only whole-body nymphs (see Fig. S3B in the supplemental material) did not show a correlation between “Ca. Liberibacter asiaticus” and Wolbachia titers (P = 0.49; cor = 0.16). With our current data, it is impossible to determine if “Ca. Liberibacter asiaticus” increases in response to Wolbachia, or vice versa, but only that a correlation exists. This positive correlation in titer is likely not an artifact of DNA extraction efficiency, as the DNA was measured and standardized prior to qPCRs.
Midguts of “Ca. Liberibacter asiaticus”-exposed and nonexposed D. citri insects show quantifiable differences in nuclear phenotypes.
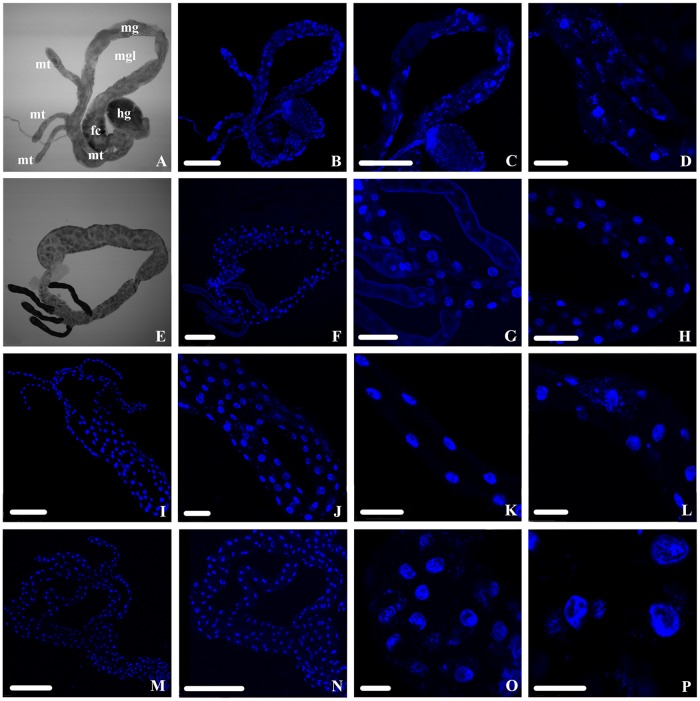
We extracted 40 guts from four groups comprising “Ca. Liberibacter asiaticus”-exposed and nonexposed adults and nymphs (a total of 160 guts) and stained each gut with DAPI (4′,6′-diamidino-2-phenylindole) to visualize the epithelial cell nuclear DNA. The guts were stained only for DAPI and not for the presence of “Ca. Liberibacter asiaticus,” so all the guts were referenced as exposed, not “Ca. Liberibacter asiaticus”+. After imaging the midguts, we described four classes of nuclear DNA organization that represented increasing levels of nuclear fragmentation (Fig. 3). Class 0 represented nuclear DNA that was seen in a semicircular shape, often appearing as a solid blue dot with a continuous smooth edge. Three levels followed this healthy/normal phenotype. Class 1 nuclei were characterized by mild deformation from the rounded appearance of class 0, often with small breaks in the whole. Class 2 nuclei were severely deformed and exhibited clear dispersal of nuclear DNA from the mostly circular phenotype of classes 0 and 1. Class 3 nuclei were the most fragmented phenotype observed, making identification of the location of a DAPI-stained nucleus difficult, as the DNA appeared to have been spread within the cytoplasm. The frequency of each fragmentation class differed across adults and nymphs, exposed and nonexposed (Fig. 4), and we quantified the DAPI-stained nuclei in each fragmentation class (Fig. 5 and 6).
FIG 3.
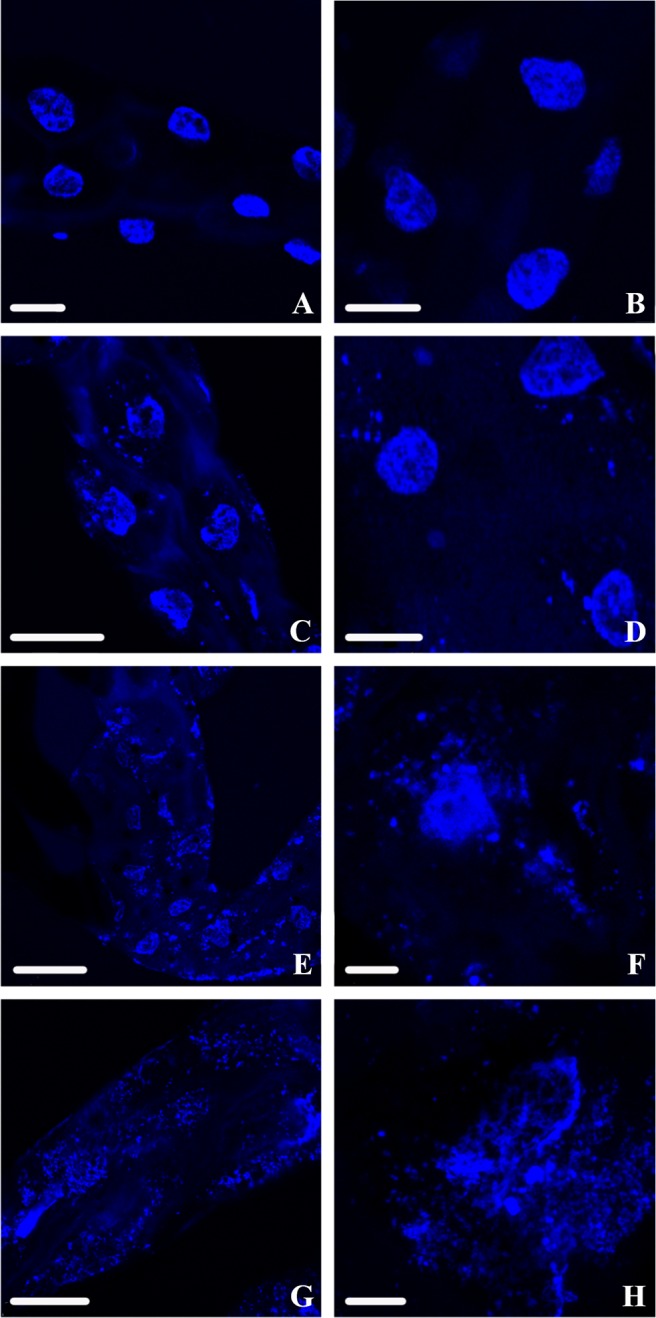
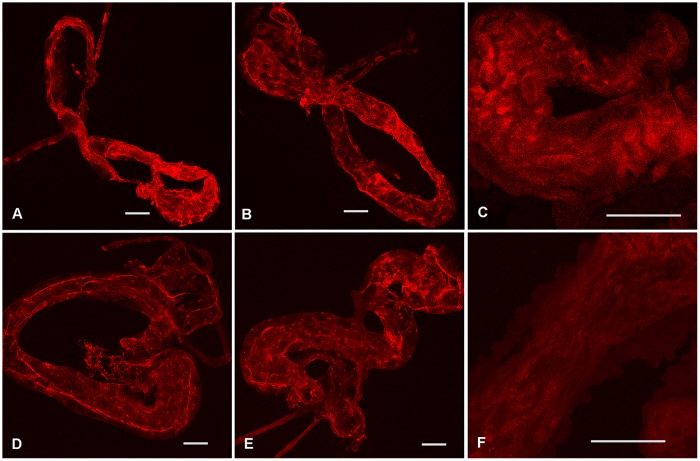
DAPI-stained nuclear DNA of D. citri midguts viewed using confocal microscopy. (A and B) Class 0 (normal) nuclear DNA phenotypes are often solid blue circles and are distinguished by a cohesive edge with semicircular shape. (C and D) Class 1 nuclear DNA phenotypes are irregularly shaped, with the edges tending to show mild breakage or splintering. (E and F) Class 2 nuclear DNA phenotypes still show a noticeable density of DNA reminiscent of the circular phenotype from class 0 but are fragmenting from all points and beginning to disperse. (G and H) Class 3 nuclear DNA phenotypes are highly fragmented with no intact cohesion remaining to suggest that there was a nucleus, as the DNA appears to have spread throughout the cytoplasm. Scale bars: 25 μm (A, B, and G), 50 μm (C), 75 μm (D and E), and 10 μm (F and H).
FIG 4.
DAPI-stained nuclear DNA phenotypic diversity across “Ca. Liberibacter asiaticus”-exposed and nonexposed D. citri adults and nymphs visualized using confocal microscopy. (A to D) Exposed adults. (E to H) Nonexposed adults (I to L). Exposed nymphs. (M to P) Nonexposed nymphs. mt, Malpighian tubules; mg, midgut; mgl, midgut loop; hg, hindgut; fc, filter chamber. Scale bars: 250 μm (A, B, C, E, F, I, M, and N), 50 μm (D, K, and L), 100 μm (G and J), 75 μm (H), and 25 μm (O and P).
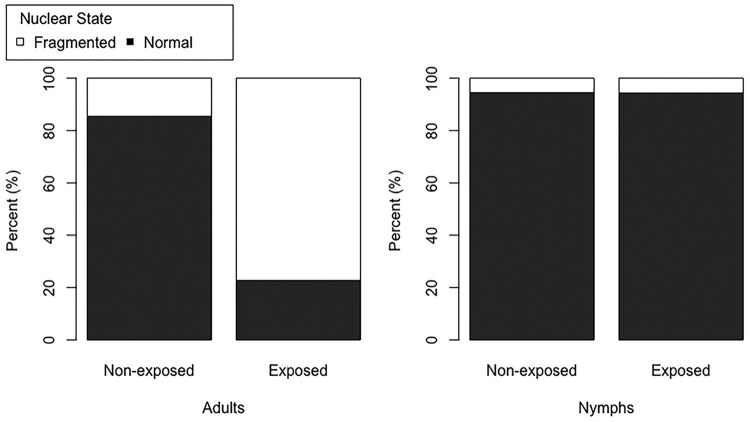
FIG 5.
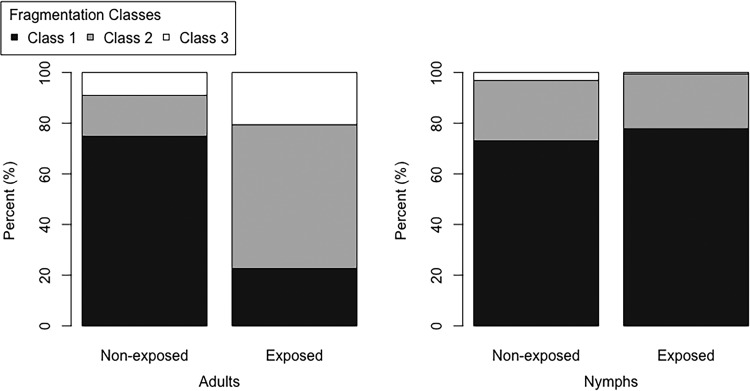
Percentages of nuclear DNA in the midguts of “Ca. Liberibacter asiaticus” exposed and nonexposed adult and 5th-instar nymph D. citri insects showing a normal (black) or fragmented (white) phenotype. These samples are referenced as “Ca. Liberibacter asiaticus” exposed rather than “infected” because they were stained with DAPI to observe nuclear DNA phenotypes and not the “Ca. Liberibacter asiaticus” probe. Among adults, the percentages fragmented were significantly different between nonexposed and exposed insects (W = 1,558; P ≪ 0.05), while the difference in nymphs was not significant (W = 670; P = 0.21). Among nonexposed samples, adults and nymphs had significantly different numbers of fragmented DAPI-stained nuclei (W = 2,195.5; P = 0.0001). Among exposed samples, adults and nymphs also had significantly different numbers of fragmented DAPI-stained nuclei (W = 3,024; P ≪ 0.05). The P values were generated using the Mann-Whitney U test in R.
FIG 6.
Percentages of nuclear DNA in the midgut of “Ca. Liberibacter asiaticus”-exposed and nonexposed adult and 5th-instar nymph D. citri insects showing the three fragmentation classes. These samples are referenced as “Ca. Liberibacter asiaticus” exposed rather than infected because they were stained only for DAPI to observe nuclear DNA phenotypes, not for the “Ca. Liberibacter asiaticus” fluorescent probe, as described in Materials and Methods. Among adults, nonexposed and exposed insects were significantly different for classes 2 and 3 (W = 1,180.5, P ≪ 0.05 and W = 1,034.5, P ≪ 0.05, respectively) but not different for class 1 (W = 586.5; P = 0.69). Among nymphs, nonexposed and exposed insects were not significantly different for classes 2 and 3 (W = 288, P = 0.63 and W = 255.5, P = 0.65, respectively), while class 1 was significantly different (W = 384; P = 0.009). P values were generated using the Mann-Whitney U test in R.
Broadly, we found that both “Ca. Liberibacter asiaticus”-exposed and nonexposed nymphal midguts exhibited mostly class 0 (healthy/normal) nuclear DNA organization, with very low frequencies of the other three fragmentation classes (Fig. 4I to P). Among adults, the nuclear DNA phenotypes were more variable. Whereas DAPI-stained nuclei of nymphs were either healthy (class 0) or in fragmentation class 1, DAPI-stained nuclei of adults exhibited either fragmentation class 0 or 1 to 3. Nonexposed adults, like nymphs, were mostly composed of the class 0/healthy phenotype (Fig. 4E to H). Exposed adults were the most divergent. Unlike nymphs, and unlike nonexposed adults, the exposed adult nuclear DNA exhibited almost entirely fragmentation phenotypes 1 to 3, with very few DAPI-stained nuclei appearing as class 0/healthy (Fig. 4A to D).
The increased frequency of fragmented nuclear DNA in the midgut is correlated with the longest exposure to “Ca. Liberibacter asiaticus”-infected citrus plants, as the most extreme examples of nuclear DNA fragmentation (classes 2 and 3) occur almost entirely in the midguts of exposed adults (Fig. 6) that were raised for several generations on infected plants. The Mann-Whitney U test for differences in two populations verified the visual results we obtained (where the P value threshold for significance was <0.05). Exposed nymphs were not significantly different from nonexposed nymphs (W = 670; P = 0.21), while exposed adults were very different (W = 1,558; P ≪ 0.05), having much higher numbers of nuclei in classes 1 to 3 in their midguts than nonexposed adults (Fig. 5). Among nonexposed samples, the numbers of adult fragmented nuclei were significantly different from those of nymphs (W = 2,195.5; P = 0.0001), showing a larger amount of nuclear fragmentation in nonexposed adult midguts than in nymphs. Among exposed samples, adults also had significantly more fragmented nuclei than nymphs (W = 3024; P ≪ 0.05) (Fig. 5). When we looked more closely at the fragmentation categories, excluding the healthy (class 0) nuclei (Fig. 6), we saw that among adults, nonexposed insects showed significantly lower numbers of nuclei in both fragmentation classes 2 and 3 than exposed insects (W = 1,180.5, P ≪ 0.05 and W = 1,034.5, P ≪ 0.05, respectively) but no difference for class 1 (W = 586.5; P = 0.69) (Fig. 6). Among nymphs, nonexposed insects were not significantly different from exposed nymphs for both fragmentation classes 2 and 3 (W = 288, P = 0.63 and W = 255.5, P = 0.65, respectively), while a significantly greater number of nuclei fell within class 1 for the exposed insects (W = 384; P = 0.009) (Fig. 6).
Oxidative stress in adult guts is significantly different between “Ca. Liberibacter asiaticus”-exposed and nonexposed males, but not females.
In two experiments using age-synchronized D. citri adult males and females (1 to 2 weeks after adult emergence) reared throughout their lives on “Ca. Liberibacter asiaticus”− or “Ca. Liberibacter asiaticus”+ plants, dissected and unfixed midguts were treated with MitoSox Red and examined with confocal microscopy. MitoSox Red is a fluorogenic dye that is specifically targeted to mitochondria and reacts with the superoxide produced in mitochondria to yield a highly fluorescent oxidation product (27–30). Image J was used to quantify the red fluorescence intensity in the whole gut in each case so that treatments could be statistically analyzed as described in Materials and Methods. A significantly larger proportion of the gut area from “Ca. Liberibacter asiaticus”-exposed adult males had higher fluorescence intensity than those from nonexposed males, with pixel values of ≥100 (P = 0.046), but the difference in fluorescence intensity between the guts of nonexposed and exposed females was not significant (Table 2 and Fig. 7). Also, there was no significant difference in fluorescence intensity between nonexposed males and females (P = 0.14), but the guts of exposed males had significantly greater areas with brighter fluorescence than guts from exposed females (Table 2) (P = 0.00005). Quantitative real-time PCR results for whole bodies (excluding the midgut) of psyllids from these experiments indicated that the percentages of infected adults were 90% and 85% in males and females, respectively, with mean CT values of 29.95 for males and 31.04 for females. There was no significant difference in this regard between the two sexes (P = 0.226). The above-mentioned results indicate a significant increase in cellular superoxide production by mitochondria in the guts of “Ca. Liberibacter asiaticus”-exposed/infected adult males compared to those from nonexposed/uninfected adult males, while a similar effect was not observed in the females.
TABLE 2.
Quantitative analysis of MitoSox Red-treated midguts to compare oxidative stress in the gut mitochondria between “Ca. Liberibacter asiaticus”+ and nonexposed psyllid adult males and females
| Psyllid sex | “Ca. Liberibacter asiaticus” exposure | Mean gut area (%) with a fluorescence threshold of ≤100 | No. observed | P value (t test) |
|---|---|---|---|---|
| Male | Infected | 31.03 ± 3.15 | 24 | 0.046 |
| Nonexposed | 22.54 ± 2.67 | 22 | ||
| Female | Infected | 13.94 ± 2.05 | 24 | 0.10 |
| Nonexposed | 18.03 ± 1.35 | 26 |
FIG 7.
MitoSox Red-treated midguts from adult male psyllids were reared either on “Ca. Liberibacter asiaticus”-infected citrus plants (A to C) or on healthy plants (D to F), showing larger areas of brighter fluorescence, indicating higher oxidative activity of mitochondria, in the guts of infected males than in those from nonexposed males. Scale bars, 100 μm.
“Ca. Liberibacter asiaticus” and Wolbachia localization patterns in guts of D. citri nymphs and adults are distinct.
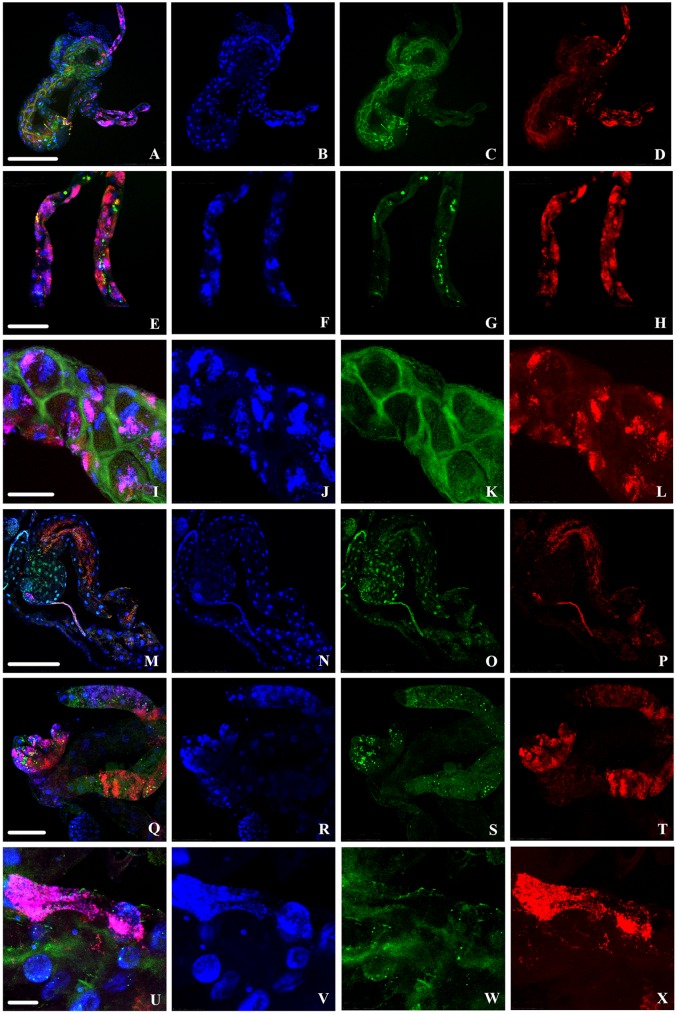
To visually study the intracellular localization of “Ca. Liberibacter asiaticus” and Wolbachia, we conducted an experiment separate from the previous DAPI quantification effort. We analyzed a total of 30 “Ca. Liberibacter asiaticus”+ adult guts and 20 “Ca. Liberibacter asiaticus”+ nymph guts for the locations of the two target bacteria, using a specific fluorescent oligonucleotide probe for each bacterium, as well as DAPI staining to aid in visualizing the bacteria relative to the gut cell nuclei. Previously, we found four subcellular localizations of “Ca. Liberibacter asiaticus” in the gut (14), which were also observed in this experiment. The signal from the “Ca. Liberibacter asiaticus” probe was observed in all areas of the gut, localizing (i) to the apical, basal, and lateral plasma membranes of the midgut cells, (ii) in punctate spots in the cytoplasm around the DAPI-stained nuclear DNA, (iii) in association with filamentous actin around the outside of the gut, and (iv) within the region occupied by the DAPI-stained nuclear DNA. The signal from the Wolbachia fluorescence in situ hybridization (FISH) probe was observed in a punctate distribution across the entire gut, though in varying densities, which often created dense patches of probe fluorescence. The localization patterns and bacterial probe densities observed in the midguts can be seen in Fig. 8.
FIG 8.
Localization of “Ca. Liberibacter asiaticus” (green) and Wolbachia (red) in infected D. citri gut cells with DAPI staining of nuclei (blue). Infected adult guts (A to L) and infected nymph guts (M to X) were stained using FISH. (A, E, I, M, Q, and U) Overlays of “Ca. Liberibacter asiaticus,” Wolbachia, and nuclei. Rows 1 to 3 (from the top) are progressively enlarged images of adult gut cells, and rows 4 to 6 are progressively enlarged images of nymph gut cells. Scale bars: 250 μm (A to D and M to P), 75 μm (E to H and Q to T), 50 μm (I to L), and 25 μm (U to X).
(i) “Ca. Liberibacter asiaticus” and Wolbachia FISH signals rarely overlap in “Ca. Liberibacter asiaticus”+ adults.
In “Ca. Liberibacter asiaticus”+ adult guts, “Ca. Liberibacter asiaticus” and Wolbachia were distributed along both the midgut and Malpighian tubules (Fig. 8A to L). While we did not quantify cellular phenotypes or “Ca. Liberibacter asiaticus” and Wolbachia localization in the Malpighian tubules, association of both Wolbachia and “Ca. Liberibacter asiaticus” along their entirety raises questions as to the function of the Malpighian tubules in the tritropic interaction, namely, whether the bacteria gain benefits from manipulating nutrient exchange or whether the tubules are used as a site of disposal for the bacteria.
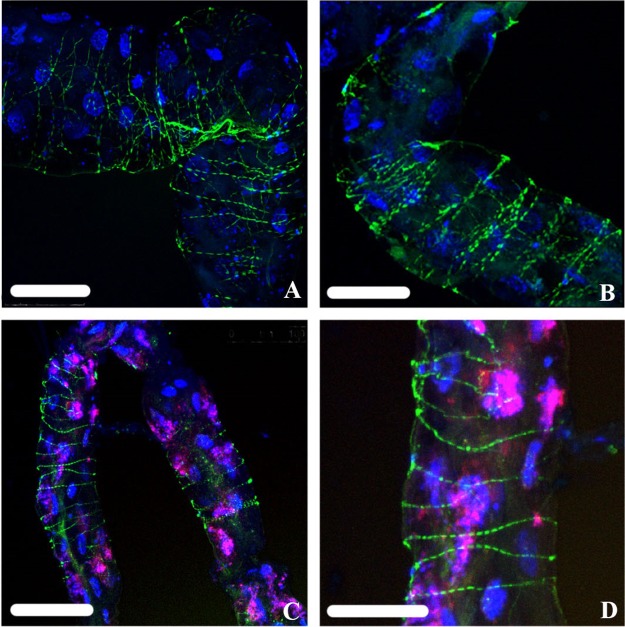
Of the 30 adult guts analyzed for localization of “Ca. Liberibacter asiaticus,” 8 showed the “Ca. Liberibacter asiaticus” probe in a pattern akin to that of phalloidin staining of actin along filaments wrapped around the gut (Fig. 9A and B), a comparison consistent with previous observations (14). Using phalloidin staining, we independently visualized the filamentous actin apparently wrapping the gut (Fig. 9C and D). Four of the 30 guts showed “Ca. Liberibacter asiaticus” in association with the nucleus, and in all 30 guts, “Ca. Liberibacter asiaticus” localization was also seen along the apical, basal, and lateral plasma membranes (Fig. 8I to K). The Wolbachia probe signal could be consistently found in both “Ca. Liberibacter asiaticus”+ and “Ca. Liberibacter asiaticus”− guts (see Fig. S4 in the supplemental material), appearing as a dense congregation of smaller particles that inhabit the cytoplasm around the nucleus in variable densities (Fig. 8D, H, L, P, T, and X; see Fig. S4 in the supplemental material). Relative to nuclear DNA fragmentation classes, the “Ca. Liberibacter asiaticus” probe signal appeared to localize along the apical, basal, and lateral cell membranes of the most fragmented DAPI-stained nuclei (Fig. 8I to K). While both Wolbachia and “Ca. Liberibacter asiaticus” have been found in the cytoplasm of the same midgut cells, they rarely are found to overlap spatially in the cytoplasm, as evidenced by a lack of FISH probe signal overlap. In Fig. 8A, E, and I, we can clearly see that Wolbachia appears to fill the spaces that are devoid of “Ca. Liberibacter asiaticus.” In “Ca. Liberibacter asiaticus”+ adults, especially, Wolbachia is more widespread in the gut than it is in nymphs.
FIG 9.
“Ca. Liberibacter asiaticus” (green) (A and B) stained using a fluorescently labeled “Ca. Liberibacter asiaticus”-specific oligonucleotide probe was found to localize strikingly similarly to filamentous actin (green) (C and D) stained with fluorescently conjugated phalloidin. DAPI-stained nuclear DNA is shown in blue (A to D), and FISH probe staining of Wolbachia is shown in red/pink (A and B). Scale bars: 100 μm (A), 50 μm (B and D), and 75 μm (C).
(ii) “Ca. Liberibacter asiaticus” localization in nymph guts is random relative to Wolbachia localization.
Three of the “Ca. Liberibacter asiaticus” subcellular localizations reported by Kruse et al. (14) in adult midguts were seen in the midguts of “Ca. Liberibacter asiaticus”+ nymphs (Fig. 8). Among the 20 nymph guts analyzed, all expressed association with the apical, basal, and lateral plasma membrane. Nine of the 20 additionally showed the “Ca. Liberibacter asiaticus” probe in association with the nucleus, and none showed association with the actin filament network that was observed wrapping around the gut in the case of adults (Fig. 8C, G, K, O, S, and W). When “Ca. Liberibacter asiaticus” appeared to associate with the nucleus, the DAPI-stained nuclear DNA appeared relatively healthy, falling under fragmentation classes 0 and 1 (Fig. 8M to O). When “Ca. Liberibacter asiaticus” associated with the cytoplasm or with the cell membranes, DAPI-stained nuclei often displayed fragmentation classes 2 and 3 (Fig. 8Q to S), and the “Ca. Liberibacter asiaticus” nuclear association phenotype was completely absent (Fig. 8Q to S and U to W). Wolbachia was observed scattered about the gut in patches that did not seem to have any association with the localization of “Ca. Liberibacter asiaticus” (unlike adults) but appeared generally grouped around the nucleus (Fig. 8M to X).
DISCUSSION
Our results provide a snapshot of the effect that “Ca. Liberibacter asiaticus” exposure has on D. citri nymph midgut epithelial cells. The results, which show no “Ca. Liberibacter asiaticus”-induced karyorrhexis in nymph midguts, are in stark contrast to the effect that “Ca. Liberibacter asiaticus” has on adult midgut tissues. A parallel can be drawn between “Ca. Liberibacter asiaticus” invasion of adult midgut epithelial cells and invasion of Plasmodium species into the midgut of the mosquito (genus Anopheles) vector. In the mosquito, midgut invasion by the parasite causes cellular damage that activates a cascade of responses leading to apoptosis. More than 80% of the invading parasites are destroyed by the mosquito immune response during the process. Reactive oxygen species (ROS), which are toxic to the Plasmodium cells, are released (reviewed in reference 25). We hypothesize that a similar response occurs in adult D. citri insects in response to “Ca. Liberibacter asiaticus” and that nymphs do not mount this response.
The question remains as to why exposed nymphs, but not adults, show such low levels of fragmentation when both are raised on “Ca. Liberibacter asiaticus”-infected plants. The difference in the numbers of fragmented nuclei in exposed and nonexposed nymph guts is minute, a result that stands in stark contrast to the adult guts, which have a pronounced difference in the number of fragmented nuclei between exposed and nonexposed guts (Fig. 5). One hypothesis to explain the difference between the adult and nymph midgut responses, which is also supported by proteomics analysis (22), is that nymphs have an attenuated immune response at the molecular level compared to adults. A dampened immune response may provide a window for “Ca. Liberibacter asiaticus” establishment in close coordination with the bacterial endosymbionts. D. citri nymphs have been shown to be much better “Ca. Liberibacter asiaticus” acquirers and transmitters than adults (20). Hemipterans are known to have a reduced or missing immunodeficiency (IMD) pathway relative to other insects. It is well established in the literature that the immune responses of many insects are highly adapted to allow the maintenance of bacterial endosymbionts (26, 31–33). D. citri has supplemented its nutritive and defensive arsenals with the bacterial endosymbionts “Ca. Carsonella ruddii” and “Ca. Profftella armatura,” respectively, and Wolbachia (10). There is evidence that endosymbiont titers increase over the life span of D. citri (34). Though Chu et al. suggest that there is no competitive exclusion between “Ca. Liberibacter asiaticus” and the endosymbionts, they do not exclude the possibility that “Ca. Liberibacter asiaticus” advantageously includes itself early in the D. citri life span. Wolbachia is found in the gut and other areas, while “Ca. Profftella armatura” and “Ca. Carsonella ruddii” are localized solely in the bacteriome (10). However, “Ca. Profftella armatura” and “Ca. Carsonella ruddii” may still interact with “Ca. Liberibacter asiaticus” throughout the life of its infection, or at least they did at one point in the life history of D. citri. Nakabachi and colleagues found phylogenetic evidence that the “Ca. Liberibacter” bacteria share an amino acid transporter (LysE) gene with “Ca. Profftella armatura” (35), which was acquired by horizontal gene transfer. With the LysE gene available in the “Ca. Liberibacter” genome, it is likely that “Ca. Liberibacter asiaticus” steals needed nutrients from its vector. Furthermore, D. citri infection with “Ca. Liberibacter asiaticus” induces “Ca. Profftella armatura” to alter the production of a small polyketide called diaphorin and a structurally related diaphorin molecule, along with the “Ca. Profftella armatura” proteins involved in polyketide biosynthesis (41). Additionally, Wolbachia was recently shown to encode a small secreted protein that suppresses expression of the holin promoter in the bacteriophage of “Ca. Liberibacter asiaticus” in vitro, suggesting that Wolbachia may play a role in suppressing the phage's lytic cycle when “Ca. Liberibacter asiaticus” infects D. citri (36), a result that throws the Wolbachia endosymbiont into unclear waters regarding its role as help or hindrance to the insect. Our data show a 100% infection rate by Wolbachia in D. citri, which has also been published elsewhere, and a positive correlation between Wolbachia and “Ca. Liberibacter asiaticus” titers. In contrast to the model in which Wolbachia may cooperate with another bacterium within an insect, a study performed on the mosquito Aedes aegypti showed that Wolbachia infection leads to a reduction in the densities of four different pathogens via a proposed innate immune system priming method whereby the presence of Wolbachia activates the insect's basal immune response to better defend against an invading pathogen (37). Ye and colleagues (37) showed that immune priming may occur only where Wolbachia-host associations are relatively recent. Our data are consistent with a model in which “Ca. Liberibacter asiaticus” was also once an endosymbiont of the insect, cooperating closely with the immune system and microbial community of D. citri, including Wolbachia. Of course, these observations are purely correlative, and direct experiments testing the roles of the D. citri immune system and the endosymbionts in “Ca. Liberibacter asiaticus” infection must be done in the future.
Our data are not yet able to adequately address the puzzle emerging from this model as to why adult midgut epithelial cells would react to “Ca. Liberibacter asiaticus” as a foreign invader. The indirect effects of the “Ca. Liberibacter asiaticus”-infected host plant on D. citri may also contribute to the observed phenotypic changes in midguts of exposed insects. Chemical ecology, the study of how plants interact with their herbivores and bacterial symbionts, tells us that when plants are being attacked, whether by a bacterium like “Ca. Liberibacter asiaticus” or by a herbivore feeding on plant tissues, the plant responds chemically. This volatile plant response affects nearby plants, as well as any insect on or near the plant, in ways that are still being discovered. “Ca. Liberibacter asiaticus” has been shown to produce effector proteins that induce apoptosis in plant cells (38). Nuclear DNA fragmentation and ROS production could simply be the adult psyllid's response to feeding on a plant that is chemically defending itself or otherwise interacting with “Ca. Liberibacter asiaticus” as opposed to a direct response to “Ca. Liberibacter asiaticus” presence in the psyllid midgut. Distinguishing between the effects of plant and bacteria on the psyllid gut and related microbiome is one of the more pressing avenues for future research.
Fluorescent imaging of both “Ca. Liberibacter asiaticus” and Wolbachia in the psyllid gut (Fig. 8) showed that the two bacteria may reside within the same cell but rarely colocalize within the same area of a cell (consistent with Kruse et al. [14]). The two bacteria colocalize less in adults than in nymphs. Since exposed nymphs and adults were both fed on “Ca. Liberibacter asiaticus”-infected plants all their lives (starting with early nymphal instars), this suggests that the longer the insect has been infected with “Ca. Liberibacter asiaticus,” the less colocalization there is with Wolbachia within each gut cell. From the perspective of “Ca. Liberibacter asiaticus,” these results suggest it is most beneficial to be acquired first by nymphs, as opposed to adults, before the symbionts (Wolbachia) can become well established in the insect body. Although our study is by no means exhaustive, our analysis shows that “Ca. Liberibacter asiaticus” often resides in the actin filament network in the midgut but that this localization occurs infrequently in nymphs (we did not observe it at all). The function of these actin filament structures in gut biology and “Ca. Liberibacter asiaticus” interaction should be a focus of future research.
Damage caused by the “Ca. Liberibacter asiaticus” bacterium in midguts may be a precursor to the DAPI-stained nuclear DNA fragmentation we observed in “Ca. Liberibacter asiaticus”+ adults. Killiny et al. (39) addressed whether “Ca. Liberibacter asiaticus” directly manipulates its vector by studying the “Ca. Liberibacter asiaticus” genome. They found that the bacteria can both induce production of and take up energetic nucleotides, such as ATP, from its vector using ATP translocase, thus enabling “Ca. Liberibacter asiaticus” to fulfill its own energy needs. Additionally, an analysis of the gut proteome and transcriptome by Kruse et al. found a concerted downregulation of D. citri proteins involved in the TCA cycle and mitochondrial function (14). Side effects of ATP redirection and mitochondrial disruption may include oxidative stress, such as damage to proteins, lipids, and DNA directly (40). Our finding of increased cellular superoxide production by mitochondria in the guts of infected over noninfected males—a result not observed in females—supports the hypothesis that “Ca. Liberibacter asiaticus”+ adult gut cells are increasingly energetically stressed compared to nonexposed adult guts (41). Additionally, this result emphasizes the importance of collecting metadata at all stages of experiments when testing individuals. While levels of nuclear disruption are significantly increased in exposed adult D. citri insects, it would be useful to compare this disruption between known infected individual males and females to further distinguish the effects of sex on the response to “Ca. Liberibacter asiaticus” infection. D. citri populations infected with “Ca. Liberibacter asiaticus” show a higher net reproductive rate than their noninfected conspecifics (42), although their nymphal development rate and adult survival are reduced. These results highlight a difference in the immune responses to “Ca. Liberibacter asiaticus” of male and female insects. Taken together, these results may indicate that the relationship between female D. citri insects and “Ca. Liberibacter asiaticus” may be more cooperative than that of males at the molecular level and that these distinct types of molecular interactions have complex consequences for the life history traits of D. citri.
MATERIALS AND METHODS
“Ca. Liberibacter asiaticus,” insect, and host plant sources and preparation.
“Ca. Liberibacter asiaticus”-uninfected D. citri insects were collected from two cages containing mixed-age populations continuously maintained on Madam Vinous sweet orange [Citrus sinensis (L.) Osbeck] at 22.8°C to 26.7°C, with a 14-h light/10-h dark photoperiod and 70 to 80% relative humidity. “Ca. Liberibacter asiaticus”-exposed D. citri insects were raised throughout the nymphal and adult stages on “Ca. Liberibacter asiaticus”-positive Madam Vinous sweet orange under the same conditions as the uninfected colonies. Both healthy and infected plants were tested monthly by pooling three leaves from each plant and then running qPCR to determine the “Ca. Liberibacter asiaticus” titer. Psyllid colonies were originally established in 1999 from a field population collected at the U.S. Horticultural Research Laboratory experimental farm at Fort Pierce, FL. The plants were infected using graft inoculation from a Florida strain of “Ca. Liberibacter asiaticus” maintained by David Hall at the USDA-ARS Horticultural Research Laboratory in Fort Pierce, FL. The same infected mother plant was used to graft inoculate all the trees used in these experiments.
DNA extraction and qPCR assays.
Whole-body adult exposed (n = 30), and nonexposed (n = 30), and 5th-instar nymph exposed (n = 30) and nonexposed (n = 30) D. citri insects were collected to determine the “Ca. Liberibacter asiaticus” infection rate in D. citri populations using qPCR detection of “Ca. Liberibacter asiaticus” 16S rRNA. Individual whole-body DNA extraction, as well as individual gut dissection and subsequent DNA extraction, was carried out using a protocol described previously (43) with modification of the lysis buffer to include only 5 μl 1 M Tris (pH 8), 1 μl 0.5 M EDTA, 50 μl proteinase K (20 mg/ml), and 939 μl double-distilled H2O (ddH2O). After adding 40 μl lysis buffer to each sample and incubating at 65°C for 15 min and then at 95°C for 10 min, followed by 2 min of centrifugation at 14,000 rpm, the supernatant was collected as DNA. The supernatant obtained was tested for total nucleotide content using a nanodrop spectrophotometer. All samples were standardized to equal concentrations of total nucleic acid content so that all reaction wells in the qPCR plate contained the same concentration of total nucleic acids. This standardization reduced the effects of DNA extraction efficiency bias during qPCR amplification.
For qPCR, the standardized DNA was subjected to amplification using TaqMan reagents to test for the “Ca. Liberibacter asiaticus” titer. The probe sequence and the specific forward and reverse primer set used for the “Ca. Liberibacter asiaticus” 16S rRNA gene were as follows: forward primer, 5′-TCGAGCGCGTATGCAATACG-3′; reverse primer, 5′-GCGTTATCCCGTAGAAAAAGGTAG-3′; probe, 5′-FAM-AGACGGGTG/ZEN/AGTAACGCG-3′ (probe includes the 5′ 6-FAM fluorescent dye also known as fluorescein and the quencher ZEN with 3′ Iowa Black FQ). Quantitative PCR was performed under standard thermal conditions of 95°C for 10 min (1 cycle) and 95°C for 15 s and 60°C for 60 s (40 cycles). Separate qPCR assays, using the same sample DNA as for “Ca. Liberibacter asiaticus” detection, were carried out for detection of Wolbachia in D. citri individuals. SYBR green reagents and specific primer set sequences developed for the ftsZ gene (ftsZ-81) of the Wolbachia symbiont were as follows: forward primer, 5′-AGCAGCCAGAGAAGCAAGAG-3′, and reverse primer, 5′-TACGTCGCACACCTTCAAAA-3′ (44). Quantitative PCR was run under standard thermal conditions of 95°C for 20 s (1 cycle) and 95°C for 3 s and 60°C for 30 s (40 cycles). For both TaqMan and SYBR green assays, each biological replicate was run with three technical replicates, which were averaged to achieve a single Ct value for each sample. The qPCR results for “Ca. Liberibacter asiaticus” (16S rRNA) and Wolbachia (ftsZ) amplification were divided into two categories based on the Ct value. Samples with average Ct values below or equal to 35 were considered infected with “Ca. Liberibacter asiaticus,” while those with values above 35 were considered to have a Ct too high to determine infection status. This uncertain category comprised two groups of Ct values, those greater than 35 and those registered as undetermined, meaning the Ct values were greater than or equal to 40 (the highest number of cycles the qPCR machine records). The frequency histograms (see Fig. S1 and S2 in the supplemental material) of Ct values for all samples in each treatment discussed in Results above show the relative quantities of samples that were below a Ct value of 35 or above a Ct value of 35. Absolute quantification of microbial copy numbers was enabled by comparing Ct values from biological samples to Ct values from standard curves made using serial dilutions of synthetic plasmids containing the qPCR target region. Statistical analyses of Ct values and respective copy numbers for both “Ca. Liberibacter asiaticus” and Wolbachia were conducted using the Mann-Whitney U test (function, wilcox.test), the F test of sample variance (function, var.test), and Pearson's product-moment correlation (function, cor.test) in R (45).
DAPI staining of nuclear DNA for fragmentation quantification.
Guts from 40 “Ca. Liberibacter asiaticus”-exposed nymphs and 40 “Ca. Liberibacter asiaticus”-exposed adults, as well as 40 healthy (nonexposed) nymphs and 40 healthy (nonexposed) adults, were dissected in 1× phosphate-buffered saline (PBS). In total, 160 guts were stained for imaging. To detect nuclear DNA fragmentation phenotypes, nuclei were stained with 0.1 mg DAPI ml−1. The stained guts were mounted in hybridization buffer and viewed under a Leica TCS-SP5 confocal microscope (Leica Microsystems, Exton, PA, USA). The images were processed using Leica LAS-AF software (v. 2.6.0).
Quantification of nuclear fragmentation phenotypes.
Images of DAPI-stained, excised, and fixed D. citri guts were analyzed for evidence of nuclear DNA fragmentation phenotypes. Only the midgut loop was analyzed, excluding Malpighian tubules, to standardize the quantification efforts. To quantitatively compare the fragmentation levels and diversity across exposed and nonexposed adults and nymphs, each of the 160 images (a combination of projections and individual frames) of midguts was analyzed by two different people, who counted the DAPI-stained nuclei exhibiting each phenotype, categorized them, and then averaged their scores.
Statistical analyses were carried out using the Mann-Whitney U test in R (45) (function, wilcox.test) to initially compare nonfragmented nuclei (class 0) to total fragmented nuclei (classes 1, 2, and 3 combined) for each group of midguts (exposed and nonexposed adults and 5th-instar nymphs). Graphs constructed in R representing 100% of the nuclei counted provide a visual means to compare the percentages of nonfragmented nuclear DNA to total fragmented nuclear DNA (Fig. 5). Statistical analysis using the Mann-Whitney U test was run again in R to find statistically significant differences between the three fragmentation classes, excluding nonfragmented nuclei, for the same groups of midguts as before. Graphs constructed in R representing 100% of the fragmented nuclei (classes 1, 2, and 3) provide a visual means to compare only the nuclei that were fragmented (Fig. 6).
FISH for colocalization of “Ca. Liberibacter asiaticus” and Wolbachia relative to DAPI-stained nuclei.
Fifth-instar “Ca. Liberibacter asiaticus”+ nymphs (n = 20) and “Ca. Liberibacter asiaticus”+ adults (n = 30) were dissected in 1× PBS in preparation for FISH. FISH was performed as previously described by Kliot et al. (46). Samples were fixed in Carnoy's fixative (chloroform-ethanol-glacial acetic acid [6:3:1 {vol/vol/vol}]) for 5 min following gut dissection in 1× PBS and hybridized overnight in hybridization buffer (20 mM Tris-HCl, pH 8.0, 0.9 M NaCl, 0.01% [wt/vol] sodium dodecyl sulfate, 30% [vol/vol] formamide) containing 10 pmol fluorescent probes per milliliter. For specific targeting of Wolbachia and “Ca. Liberibacter asiaticus” 16S rRNA, DNA probes W2 (5′-Cy5-CTTCTGTGAGTACCGTCATTATC-3′) (11) and “Ca. Liberibacter asiaticus” (5′-Cy3-CATTATCTTCTCCGGCG-3′) (15) (Fig. 9) were used, respectively. Nuclear DNA was stained with 0.1 mg DAPI. The stained guts were mounted in hybridization buffer and viewed under a Leica TCS-SP5 (Leica Microsystems, Exton, PA, USA) confocal microscope. The images were processed using Leica LAS-AF software (v. 2.6.0). Specificity of detection was confirmed using no-probe and “Ca. Liberibacter asiaticus”-negative controls for all observations.
Phalloidin and DAPI staining of midguts.
The guts of nonexposed adults were dissected in 1× PBS and fixed in 4% paraformaldehyde in 1× PBS for 1 h. Then, the guts were incubated with 0.5 ng/μl fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma) diluted 1:1,000 in PBS containing 0.05% Tween 20 (PBST) for 30 min. The guts were then washed with PBST and stained with DAPI as described above. Phalloidin binds to filamentous actin.
MitoSox Red analysis.
Two experiments using midguts of D. citri male and female adults of similar relative age reared on “Ca. Liberibacter asiaticus”− and “Ca. Liberibacter asiaticus”+ plants were conducted. Guts dissected in PBS were treated (without fixation) in small glass dishes (20-mm diameter) with 5 μM MitoSox Red (ThermoFisher Scientific) for 20 min at 30°C, washed in PBS, mounted on slides in Fluoro-Gel (Electron Microscopy Sciences, Hatfield, PA), examined using a confocal laser scanning microscope (Zeiss LSM 510), and imaged using Zen 2009 SP2 (Zeiss software). Following gut dissections, the rest of the body (excluding the midgut) of each male or female was processed for qPCR to indicate the proportion of infected adults used. MitoSox Red reacts with the superoxide produced in mitochondria to yield a highly fluorescent oxidation product. Image J was used to quantify the fluorescence in each midgut so that treatments could be statistically analyzed. The images were converted to black and white. The black-and-white images were analyzed using the histogram function of Image J to obtain a count of the pixels with intensities from 0 to 255. The List function was used to table these data for export to Excel. The number of pixels in the image is the summation of all count values. The sum of counts for values 0 to 24 gives the number of pixels in the background. The balance is the number of pixels for the image of the entire gut. Next, the number of pixels with a value equal to or greater than 100 is determined. From this, the proportion of the gut's image area with a fluorescent intensity of ≥100 was calculated. Between 22 and 26 guts from each category (healthy and “Ca. Liberibacter asiaticus”+ males; healthy and “Ca. Liberibacter asiaticus”+ females) were examined and analyzed.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the California Citrus Research Board, grant numbers 5300-155 and 5300-163, and by USDA NIFA grant number 2015-70016-23028.
We acknowledge the Plant Cell Imaging Center at Boyce Thompson Institute and its funding sources at NSF (NSF DBI-0618969).
Mention of trade names or commercial products is solely to provide specific information and does not imply any recommendation or endorsement.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00889-17.
REFERENCES
- 1.Bove J. 2006. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 88:7–37. [Google Scholar]
- 2.Manjunath K, Halbert S, Ramadugu C, Webb S, Lee R. 2008. Detection of “Candidatus Liberibacter asiaticus” in Diaphorina citri and its importance in the management of citrus huanglongbing in Florida. Phytopathology 98:387–396. doi: 10.1094/PHYTO-98-4-0387. [DOI] [PubMed] [Google Scholar]
- 3.Gottwald T, da Graça J, Bassanezi R. 6 September 2007. Citrus huanglongbing: the pathogen and its impact. doi: 10.1094/php-2007-0906-01-rv. [DOI] [Google Scholar]
- 4.Teixeira D, Saillard C, Eveillard S, Danet J, da Costa P, Ayres A, Bove J. 2005. “Candidatus Liberibacter americanus”, associated with citrus huanglongbing (greening disease) in Sao Paulo State, Brazil. Int J Syst Evol Microbiol 55:1857–1862. doi: 10.1099/ijs.0.63677-0. [DOI] [PubMed] [Google Scholar]
- 5.Singerman A, Useche P. 2016. Impact of citrus greening on citrus operations in Florida. FE983. Food and Resource Economics Department, UF/IFAS Extension, Gainesville, FL: http://edis.ifas.ufl.edu/fe983. [Google Scholar]
- 6.Brlansky R, Rogers M. 2008. Citrus huanglongbing: understanding the vector-pathogen interaction for disease management. doi: 10.1094/APSnetFeature-2007-1207. [DOI] [Google Scholar]
- 7.Belasque JJ, Bassanezi R, Yamamoto P, Ayres A, Tachibana A, Violante A, Tank AJ, Giorgi FD, Tersi F, Menezes G, Dragone J, Jank RJ, Bové J. 2010. Lessons from Huanglongbing management in Sao Paolo States, Brazil. J Plant Pathol 92:285–302. [Google Scholar]
- 8.Qureshi J, Stansly P. 2007. Integrated approaches for managing the Asian citrus psyllid Diaphorina citri (Homoptera: Psyllidae) in Florida. Proc Fla State Hort Soc 120:110–115. [Google Scholar]
- 9.Saha S, Hunter W, Reese J, Morgan J, Marutani-Hert M, Huang H, Lindeberg M. 2012. Survey of endosymbionts in the Diaphorina citri metagenome and assembly of a Wolbachia wDi draft genome. PLoS One 7:e50067. doi: 10.1371/journal.pone.0050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakabachi A, Ueoka R, Oshima K, Teta R, Mangoni A, Gurgui M, Oldham N, van Echten-Deckert G, Okamura K, Yamamoto K, Inoue H, Ohkuma M, Hongoh Y, Miyagishima S, Hattori M, Piel J, Fukatsu T. 2013. Defensive bacteriome symbiont with a drastically reduced genome. Curr Biol 23:1478–1484. doi: 10.1016/j.cub.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Heddi A, Grenier A, Khatchadourian C, Charles H, Nardon P. 1999. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc Natl Acad Sci U S A 96:6814–6819. doi: 10.1073/pnas.96.12.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammar E, Shatters R, Lynch C, Hall D. 2011. Detection and relative titer of Candidatus Liberibacter asiaticus in the salivary glands and alimentary canal of Diaphorina citri (Hemiptera: Psyllidae) vector of citrus huanglongbing disease. Ann Entomol Soc Am 104:526–533. doi: 10.1603/AN10134. [DOI] [Google Scholar]
- 13.Ammar E, Shatters R, Hall D. 2011. Localization of Candidatus Liberibacter asiaticus, associated with citrus huanglongbing disease, in its psyllid vector using fluorescence in situ hybridization. J Phytopathol 159:726–734. doi: 10.1111/j.1439-0434.2011.01836.x. [DOI] [Google Scholar]
- 14.Kruse A, Fattah-Hosseini S, Saha S, Johnson R, Warwick E, Sturgeon K, Mueller L, MacCoss M, Shatters RJ, Cilia Heck M. 2017. Combining 'omics and microscopy to visualize interactions between the Asian citrus psyllid vector and the Huanglongbing pathogen Candidatus Liberibacter asiaticus in the insect gut. PLoS One 12:e0179531. doi: 10.1371/journal.pone.0179531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghanim M, Fattah-Hosseini S, Levy A, Cilia M. 2016. Morphological abnormalities and cell death in the Asian citrus psyllid (Diaphorina citri) midgut associated with Candidatus Liberibacter asiaticus. Sci Rep 6:33418. doi: 10.1038/srep33418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ammar E, Hall D, Shatters RJ. 2013. Stylet morphometrics and citrus leaf vein structure in relation to feeding behavior of the Asian citrus psyllid Diaphorina citri, vector of citrus huanglongbing bacterium. PLoS One 8:e59914. doi: 10.1371/journal.pone.0059914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ammar E, Ramos J, Hall D, Dawson W, Shatters RJ. 2016. Acquisition, replication and inoculation of Candidatus Liberibacter asiaticus following various acquisition periods on huanglongbing-infected citrus by nymphs and adults of the Asian citrus psyllid. PLoS One 11:e0159594. doi: 10.1371/journal.pone.0159594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue H, Ohnishi J, Ito T, Tomimura K, Miyata S, Iwanami T, Ashihara W. 2009. Enhanced proliferation and efficient transmission of Candidatus Liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Ann Appl Biol 155:29–36. doi: 10.1111/j.1744-7348.2009.00317.x. [DOI] [Google Scholar]
- 19.Grafton-Cardwell E, Stelinski L, Stansly P. 2013. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu Rev Entomol 58:413–432. doi: 10.1146/annurev-ento-120811-153542. [DOI] [PubMed] [Google Scholar]
- 20.Pelz-Stelinski K, Brlansky R, Ebert T, Rogers M. 2010. Transmission parameters for Candidatus Liberibacter asiaticus by Asian citrus psyllid (Hemiptera: Psyllidae). J Econ Entomol 103:1531–1541. doi: 10.1603/EC10123. [DOI] [PubMed] [Google Scholar]
- 21.Capoor SP, Rao DG, Viswanath SM. 1974. Greening disease of citrus in the Deccan Trap Country and its relationship with the vector, Diaphorina citri Kuwayama, p 43–49. Proceedings of the Sixth Conference of the International Organization of Citrus Virologists, Riverside, CA. [Google Scholar]
- 22.Ramsey J, Chavez J, Johnson R, Hosseinzadeh S, Mahoney J, Mohr J, Robison F, Zhong X, Hall D, MacCoss M, Bruce J, Cilia M. 2017. Protein interaction networks at the host-microbe interface in Diaphorina citri, the insect vector of the citrus greening pathogen. R Soc Open Sci 4:160545. doi: 10.1098/rsos.160545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmore S. 2007. Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaidyanathan R, Scott T. 2006. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis 11:1643–1651. doi: 10.1007/s10495-006-8783-y. [DOI] [PubMed] [Google Scholar]
- 25.Smith R, Barillas-Mury C. 2016. Plasmodium oocysts: overlooked targets of mosquito immunity. Trends Parasitol 32:979–990. doi: 10.1016/j.pt.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Ratzka C, Gross R, Feldhaar H. 2012. Endosymbiont tolerance and control within insect hosts. Insects 3:553–572. doi: 10.3390/insects3020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batandier C, Fontaine E, Keriel C, Leverve X. 2002. Determination of mitochondrial reactive oxygen species: methodological aspects. J Cell Mol Med 6:175–187. doi: 10.1111/j.1582-4934.2002.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudin A, Bimpong-Buta N, Vielhaber S, Elger C, Kunz W. 2004. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Fiskum G, Schubert D. 2002. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 30.Gauuan P, Trova M, Gregor-Boros L, Bocckino S, Crapo J, Day B. 2002. Superoxide dismutase mimetics: synthesis and structure-activity relationship study of MnTBAP analogues. Bioorg Med Chem 10:3013–3021. doi: 10.1016/S0968-0896(02)00153-0. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Hasegawa D, Kaur N, Kliot A, Pinheiro P, Luan J, Stensmyr M, Zheng Y, Liu W, Sun H, Xu Y, Luo Y, Kruse A, Yang X, Kontsedalov S, Lebedev G, Fisher T, Nelson D, Hunter W, Brown J, Jander G, Cilia M, Douglas A, Ghanim M, Simmons A, Wintermantel W, Ling K, Fei Z. 2016. The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol 14:110. doi: 10.1186/s12915-016-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglas A, Bouvaine S, Russell R. 2011. How the insect immune system interacts with an obligate symbiotic bacterium. Proc Biol Sci 278:333–338. doi: 10.1098/rspb.2010.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arp A, Hunter W, Pelz-Stelinski K. 2016. Annotation of the Asian citrus psyllid genome reveals a reduced innate immune system. Front Physiol 7:570. doi: 10.3389/fphys.2016.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu C, Gill T, Hoffman M, Pelz-Stelinski K. 2016. Inter-population variability of endosymbiont densities in the Asian citrus psyllid (Diaphorina citri Kuwayama). Microb Ecol 71:999–1007. doi: 10.1007/s00248-016-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakabachi A, Nikoh N, Oshima K, Inoue H, Ohkuma M, Hongoh Y, Miyagishima SY, Hattori M, Fukatsu T. 2013. Horizontal gene acquisition of Liberibacter plant pathogens from a bacteriome-confined endosymbiont of their psyllid vector. PLoS One 8:e82612. doi: 10.1371/journal.pone.0082612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain M, Fleites L, Gabriel D. 2017. A small Wolbachia protein directly represses phage lytic cycle genes in “Candidatus Liberibacter asiaticus” within psyllids. mSphere 2:e00171-17. doi: 10.1128/mSphereDirect.00171-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye Y, Woolfit M, Rances E, O'Neill S, McGraw E. 2013. Wolbachia-associated bacterial protection in the mosquito Aedes aegypti. PLoS Negl Trop Dis 7:e2362. doi: 10.1371/journal.pntd.0002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitino M, Armstrong C, Cano L, Duan Y. 2016. Transient expression of Candidatus Liberibacter asiaticus effector induces cell death in Nicotiana benthamiana. Front Plant Sci 7:982. doi: 10.3389/fpls.2016.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Killiny N, Hijaz F, Ebert T, Rogers M. 2017. A plant bacterial pathogen manipulates its insect vector's energy metabolism. Appl Environ Microbiol 83:e03005-16. doi: 10.1128/AEM.03005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brand M, Affourtit C, Esteves T, Green K, Lambert AJ, Miwa S, Pakay J, Parker N. 2004. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Ramsey J, Johnson R, Hoki J, Kruse A, Mahoney J, Hilf M, Hunter W, Hall D, Schroeder F, MacCoss M, Cilia M. 2015. Metabolic interplay between the Asian citrus psyllid and its Profftella symbiont: an Achilles' heel of the citrus greening insect vector. PLoS One 10:e0140826. doi: 10.1371/journal.pone.0140826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelz-Stelinski K, Killiny N. 2016. Better together: association with “Candidatus Liberibacter asiaticus” increases the reproductive fitness of its insect vector, Diaphorina citri (Hemiptera: Liviidae). Ann Entomol Soc Am 109:371–376. doi: 10.1093/aesa/saw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frohlich DR, Torres-Jerez I, Bedford ID, Markham PG, Brown JK. 1999. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol Ecol 8:1683–1691. [DOI] [PubMed] [Google Scholar]
- 44.Guidolin A, Cônsoli F. 2013. Molecular characterization of Wolbachia strains associated with the invasive Asian citrus psyllid Diaphorina citri in Brazil. Microb Ecol 65:475–486. doi: 10.1007/s00248-012-0150-7. [DOI] [PubMed] [Google Scholar]
- 45.R Core Team. 2017. R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 46.Kliot A, Kontsedalov S, Lebedev G, Brumin M, Cathrin P, Marubayashi J, Skaljac M, Belausov E, Czosnek H, Ghanim M. 2014. Fluorescence in situ hybridizations (FISH) for the localization of viruses and endosymbiotic bacteria in plant and insect tissues. J Vis Exp 84:e51030. doi: 10.3791/51030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.