ABSTRACT
Rats vary in their susceptibilities to Toxoplasma gondii infection depending on the rat strain. Compared to the T. gondii-susceptible Brown Norway (BN) rat, the Lewis (LEW) rat is extremely resistant to T. gondii. Thus, these two rat strains are ideal models for elucidating host mechanisms that are important for host resistance to T. gondii infection. Therefore, in our efforts to unravel molecular factors directing the protective early innate immune response in the LEW rat, we performed RNA sequencing analysis of the LEW versus BN rat with or without T. gondii infection. We identified three candidate small GTPase immunity-associated proteins (GIMAPs) that were upregulated (false discovery rate, 0.05) in the LEW rat in response to T. gondii infection. Subsequently, we engineered T. gondii-susceptible NR8383 rat macrophage cells for overexpression of LEW rat-derived candidate GIMAP 4, 5, and 6. By immunofluorescence analysis we observed that GIMAP 4, 5, and 6 in T. gondii-infected NR8383 cells each colocalized with GRA5, a parasite parasitophorous vacuole membrane (PVM) marker protein, suggesting their translocation to the PVM. Interestingly, overexpression of each candidate GIMAP in T. gondii-infected NR8383 cells induced translocation of LAMP1, a lysosome marker protein, to the T. gondii surface membrane. Importantly, overexpression of GIMAP 4, 5, or 6 individually inhibited intracellular T. gondii growth, with GIMAP 4 having the highest inhibitory effect. Together, our findings indicate that upregulation of GIMAP 4, 5, and 6 contributes to the robust refractoriness of the LEW rat to T. gondii through induction of lysosomal fusion to the otherwise nonfusogenic PVM.
KEYWORDS: Toxoplasma gondii resistance, Lewis rat, Brown Norway rat, small GTPase immunity-associated proteins, lysosomal fusion
INTRODUCTION
Toxoplasma gondii is a zoonotic apicomplexan protozoan estimated to have infected about a third of the world human population (1). The hallmark of T. gondii is its ability to induce long-term chronic infections through its interactions with the host, leading to conversion of the prolific tachyzoite stage to the quiescent bradyzoite parasite stage (2). Bradyzoites of T. gondii are not usually harmful in immunocompetent individuals, but in immunodeficient hosts they reconvert into cytolytic tachyzoites, resulting in reactivation of toxoplasmosis (3). Currently, there is no drug to eliminate bradyzoites in infected individuals, and the use of drugs against tachyzoites is limited by hypersensitivity and toxicity in humans. To date, no vaccine against T. gondii has been developed for humans.
Rats, like immunocompetent humans, develop a subclinical chronic infection but vary in their susceptibilities to T. gondii infection depending on the rat strain (4). Compared to the T. gondii-susceptible Brown Norway (BN) rat, the Lewis (LEW) rat is extremely resistant to T. gondii infection and has been demonstrated to inhibit proliferation of T. gondii within the parasitophorous vacuoles of infected peritoneal macrophages (5) and to rapidly kill both parasites and infected cells (5). Thus, because of the disparity in their responses to T. gondii infection, LEW and BN rats would serve as ideal models for dissecting the host molecular mechanisms that are important for host resistance to T. gondii infection, with the goal of identifying intervention strategies against the infection.
During T. gondii infection, gamma interferon (IFN-γ)-stimulated GTPases, namely, guanylate-binding proteins (GBPs) and immunity-related GTPases (IRGs), have been shown to limit the infection in humans (6) and mice (7), respectively. IRGs in mice control T. gondii intracellular proliferation by binding to the parasitophorous vacuolar membrane (PVM) to disrupt its integrity, leading to the release of the parasites into the host cell cytosol, where they are destroyed (8). GBPs also act by binding to the PVM in T. gondii-infected human cells (9).
Members of a relatively recently reported family of small GTPases, called GTPase immunity-associated proteins (GIMAPs), are conserved and expressed prominently in mammalian (including human) immune cells (10, 11). However, their functions and molecular mechanisms in mammalian host innate immunity against intracellular pathogens are yet to be deciphered. Herein, we provide evidence that T. gondii infection in the LEW rat upregulates expression of GIMAPs that mediate restriction of growth of intracellular parasites through induction of lysosomal fusion to the parasite without IFN-γ activation.
RESULTS
T. gondii infection upregulates expression of the GIMAP 4, 5, and 6 genes in LEW rat.
Analysis of the extracted rat RNA samples prior to library generation showed that they were all of high quality for RNA sequencing, with RNA integrity numbers (RIN) of 9.5/10 or above. Due to the nonavailability of a complete LEW rat reference genome, the generated RNA sequences were aligned to the sequence of a mixed-strain genome assembly for female BN/SsNHsdMCW and male SHR rats as the reference genome. Importantly, alignment of the LEW rat sequences to this reference genome depicted very negligible differences between the LEW and BN rat sequences, and as such, strain differences in sequence were ignored overall. By analysis of variance (ANOVA), using a false discovery rate (FDR) of 0.05, the gene expression patterns related to the four experimental groups (T. gondii-infected and uninfected LEW and BN rat groups; n = 4 per group) were generated, with the number of reads that aligned uniquely within 32,662 rat genes and 8,637 T. gondii genes ranging from 13.1 million to 18.3 million per sample. Among these genes, those for GIMAP 4, GIMAP 5, and GIMAP 6 were found to be expressed at significantly higher levels in the T. gondii-infected LEW rats than in the T. gondii-infected BN rats (Table 1). By a four-way comparison analysis (infected LEW rats versus infected BN rats, infected LEW rats versus uninfected LEW rats, infected BN rats versus uninfected BN rats, uninfected LEW rats versus uninfected BN rats), it was evident that T. gondii augmented expression of the GIMAP genes only in the LEW rats (Table 1). Consistently, by real-time PCR analysis of the expression of the GIMAP genes in primary peritoneal cells isolated from the T. gondii-infected LEW and BN rats, GIMAP 4, GIMAP 5, and GIMAP 6 were found to be expressed at 2.6-fold, 7.5-fold, and 3.6-fold higher levels in the infected LEW rats than in the infected BN rats (see Fig. S1 in the supplemental material). On the other hand, there was no significant (P < 0.05) difference in the expression levels of all the GIMAP genes between the uninfected LEW rats and the uninfected BN rats (Fig. S1). Interestingly, real-time PCR analysis of the expression of the GIMAP genes in nonhematopoietic cells (intestinal epithelial cells) did not show any significant differences among the different treatment groups of LEW and BN rats (Fig. S2). This suggested that GIMAP upregulation is restricted to cells of hematopoietic origin.
TABLE 1.
Differentially expressed GIMAP genes in LEW versus BN rats in response to T. gondii infectiona
| Ensembl gene no. | Gene name | Fold change in expression (FDR) |
|||
|---|---|---|---|---|---|
| BNToxo/BNPBS | LEWToxo/LEWPBS | LEWToxo/BNToxo | LEWPBS/BNPBS | ||
| ENSRNOG00000008369 | GIMAP 4 | ND | 4.603 (0.0001) | 2.625 (0.05) | ND |
| ENSRNOG00000008416 | GIMAP 5 | ND | 3.362 (0.01) | 2.500 (0.05) | ND |
| ENSRNOG00000033338 | GIMAP 6 | ND | 2.392 (0.01) | 2.496 (0.05) | ND |
ND, not significantly different (P < 0.05); FDR, false discovery rate; BNToxo and LEWToxo, T. gondii-inoculated BN and LEW rats, respectively; BNPBS and LEWPBS, PBS-inoculated BN and LEW rats, respectively.
Sequence analysis showed that GIMAP 4 coded for a protein with 328 amino acid residues that was 100% identical to the rat GIMAP 4 with GenBank accession number AM285343 reported previously (12). GIMAP 5 coded for two protein variants, herein called GIMAP 5_201 and GIMAP 5_203, with 326 and 308 amino acid residues, respectively. GIMAP 5_201 and GIMAP 5_203 were 100% identical to their respective variant rat proteins (GenBank accession numbers NP_001029085.1 and NP_663713.1, respectively) reported previously (13). The two GIMAP 5 variants were identical, except in the N terminus, where GIMAP 5_201 had an additional 18 amino acid residues, with notable differences in amino acid residues being depicted in the sequence alignment in Fig. 1. GIMAP 6 coded for a 304-amino-acid-long protein that was 100% identical to the rat GIMAP 6 with GenBank accession number ABB03711.1 previously reported. Sequence alignment showed that all the GIMAPs had a conserved AIG1-type G domain containing a typical Walker A motif (G1), a Walker B motif (G3), as well as G2, G4, and G5 motifs (Fig. 1), characteristic of small GTPases (14).
FIG 1.
Alignment of the amino acid sequences encoded by the GIMAP genes cloned from LEW rat cDNA for inducible overexpression in NR8383 cells. The amino acid sequences of GIMAP 4, GIMAP 5_201, GIMAP 5_203, and GIMAP 6 were aligned using the Clustal O (v1.2.4) program. The AIG1-type G domain common to all GIMAPs is highlighted in gray. The motifs G1, G2, G3, G4, and G5 are boxed. Asterisks indicate positions with a single, fully conserved residue.
Overexpression of LEW rat GIMAPs in NR8383 cells restricts parasite growth.
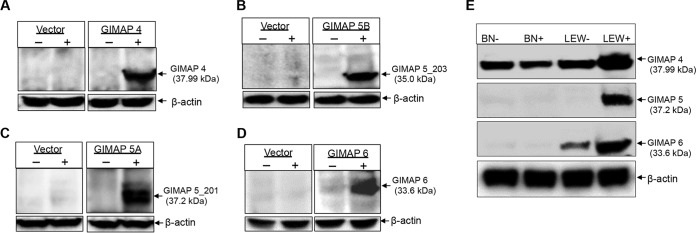
To determine the role of GIMAP 4, 5, and 6, we engineered a macrophage cell line, NR8383 (derived from a T. gondii-susceptible Sprague-Dawley rat strain), for inducible overexpression of GIMAP 4, 5, or 6 individually, as well as the empty expression vector. By Western blotting, we analyzed selected clones of the transgenic NR8383 cells and identified those that were doxycycline inducible for overexpression of the GIMAP transgenes (Fig. 2A to D). By densitometric analysis of the protein bands from the Western blots, GIMAP 4, GIMAP 5, and GIMAP 6 were expressed at levels about 10-fold, 5-fold, and 8-fold higher, respectively, in the induced NR8383 cells than in the uninduced NR8383 cells. Consistently, Western blotting showed that the primary peritoneal cells of T. gondii-infected LEW rats had higher expression levels of endogenous GIMAP 4, 5, and 6 proteins than those of T. gondii-infected BN rats (Fig. 2E). Densitometric analysis of the protein bands indicated that GIMAP 4, GIMAP 5, and GIMAP 6 were expressed at levels about 5-fold, 8-fold, and 10-fold higher, respectively, in the primary peritoneal cells of T. gondii-infected LEW rats than in those of the infected BN rats.
FIG 2.
Western blotting of GIMAPs in rat peritoneal primary cells and in transgenic NR8383 macrophage cell lines. Cloned NR8383 cells engineered for doxycycline-inducible overexpression of LEW rat-derived GIMAP 4 (A), GIMAP 5_203 (B), GIMAP 5_201 (C), and GIMAP 6 (D), along with cells expressing the pLVX-TetOne-Puro empty vector (indicated Vector in each panel), were cultured for 24 h in the absence (−) or presence (+) of 1 μg/ml doxycycline. After 24 h, the cells were harvested and equal amounts of whole-cell protein extracts were analyzed for expression of the respective transgenes. (E) Lewis (LEW) and Brown Norway (BN) rats were intraperitoneally inoculated with PBS (−) or the T. gondii type I RH strain (+), and after 24 h, peritoneal cells were isolated and Western blotting for the expression of GIMAP 4, GIMAP 5, and GIMAP 6 was performed on protein lysates of equal numbers of cells. As a loading control, rat β-actin expression (bottom) was analyzed in equal amounts of the cell lysates.
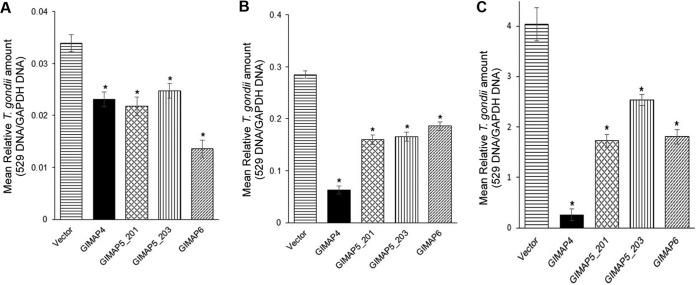
Using selected NR8383 cell clones expressing the GIMAPs, we infected the cells with tachyzoites of the T. gondii type I RH strain, and at different time points of culture, we quantified the growth of the parasites in the cultures by real-time PCR, targeting the amplification of the T. gondii 529 gene. We found that while the parasites proliferated progressively with time in NR8383 cells with the empty expression vector, there were significant (P < 0.05) reductions in the growth of the parasites in cells overexpressing GIMAP 4, 5, or 6 individually (Fig. 3). In comparison to overexpression of the other GIMAPs, overexpression of GIMAP 6 had the most significant inhibitory effect on parasite growth at 24 h (Fig. 3A), while at later time points of 48 h and 72 h, overexpression of GIMAP 4 had the most profound inhibitory effect on T. gondii growth (Fig. 3B and C). Further, overexpression of each of the GIMAP 5 variants exhibited an inhibitory effect on parasite growth, with the GIMAP 5_201 variant being more effective at 72 h than at earlier time points (Fig. 3) postinfection.
FIG 3.
Real-time PCR analysis of the growth rate of T. gondii in NR8383 cells engineered for overexpression of LEW rat-derived GIMAP 4, GIMAP 5_201, GIMAP 5_203, and GIMAP 6 or in NR8383 cells with the pLVX-TetOne-Puro empty plasmid (vector) as a control. The NR8383 cells were cultured in the presence of 1 μg/ml doxycycline for 24 h to induce expression of the respective GIMAP transgene, after which they were infected with T. gondii and maintained in culture with doxycycline. At culture time points of 24 h (A), 48 h (B), and 72 h (C), genomic DNA was extracted from whole-cell cultures and equal amounts of DNA were used as the template for real-time PCR quantification of the amount of T. gondii 529 repetitive and rat GAPDH gene fragments. The relative amount of T. gondii in each culture was derived by dividing the concentration of the T. gondii 529 repetitive gene by the concentration of the rat GAPDH gene. The data shown represent the means from three independent experiments with standard error bars, and levels of statistical significance (relative to the results for cells expressing the empty vector) are depicted by asterisks (*, P < 0.05).
GIMAP 4, 5, and 6 localize to the parasitophorous vacuole membrane.
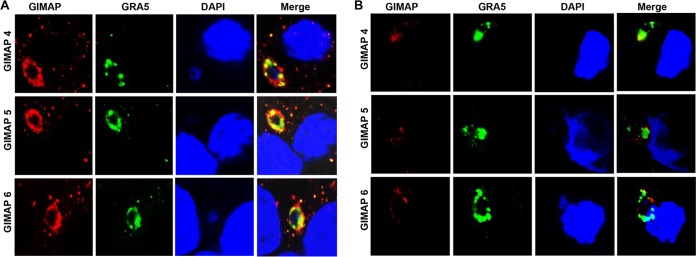
We performed immunofluorescence assays to analyze the localization of overexpressed GIMAPs in T. gondii-infected NR8383 cells after 4 h of infection. We found that individual GIMAP 4, 5, and 6 all abundantly and densely colocalized with the T. gondii GRA5 protein (Fig. 4A), a marker for the parasitophorous vacuole membrane (15). Both variants of GIMAP 5, the GIMAP 5_201 and GIMAP 5_203 variants, were found to colocalize with GRA5 with a similar intensity and pattern. In NR8383 cells expressing the empty vector as a control, low-intensity signals that could be attributed to the endogenous expression of GIMAP 4, 5, and 6 were detected, but they only partially colocalized with the T. gondii GRA5 protein (Fig. 4B).
FIG 4.
Immunofluorescence colocalization of T. gondii GRA5 and GIMAPs in NR8383 cells infected with the T. gondii type I RH strain. NR8383 cells engineered for inducible overexpression of GIMAP 4, 5, and 6 (A), as well as NR8383 cells with the empty pLVX-TetOne-Puro expression vector (B), were induced with doxycycline for 24 h and thereafter cultured with T. gondii tachyzoites for 4 h, followed by immunofluorescence analyses using combinations of anti-GIMAP antibodies (red stain, Alexa Fluor 594), anti-GRA5 antibody (green stain, Alexa Fluor 488), and a nucleus stain (DAPI). GIMAP 4, 5_201, and 6 densely colocalized with GRA5 (Merge panels in panel A) in NR8383 cells expressing the respective transgenes, while in cells expressing the empty vector, only very scanty and partial overlap was observed (Merge panels in panel B).
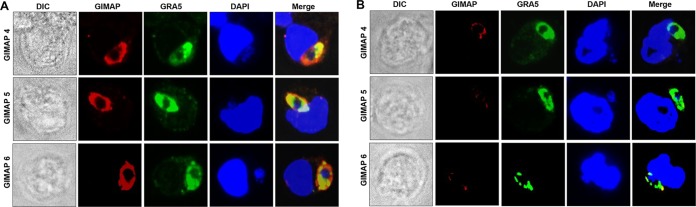
Similarly, in T. gondii-infected LEW rats' primary peritoneal cells, we found that GIMAP 4, 5, or 6 individually abundantly and densely colocalized with the T. gondii GRA5 protein (Fig. 5A). On the other hand, in T. gondii-infected BN rat peritoneal cells, GIMAP 5 and GIMAP 6 were undetectable, while GIMAP 4 was barely detectable (Fig. 5B), consistent with the Western blotting results (Fig. 2E).
FIG 5.
Immunofluorescence colocalization of T. gondii GRA5 and GIMAPs in rat primary peritoneal cells infected with the T. gondii type I RH strain. Primary peritoneal cells freshly isolated from Lewis rat (A) and Brown Norway rat (B) were cultured with T. gondii tachyzoites for 12 h, followed by immunofluorescence analyses using combinations of anti-GIMAP antibodies (red stain, Alexa Fluor 594), anti-GRA5 antibody (green stain, Alexa Fluor 488), and a nucleus stain (DAPI). Images of the cells were also captured by differential interference contrast (DIC). In Lewis rat cells, GIMAP 4, 5, and 6 densely colocalized with GRA5 (Merge panels in panel A). In Brown Norway rat cells, a very low fluorescence signal of GIMAP 4 that partially overlapped the GRA5 signals was detectable, while GIMAP 5 and GIMAP 6 were undetectable (Merge panels in panel B).
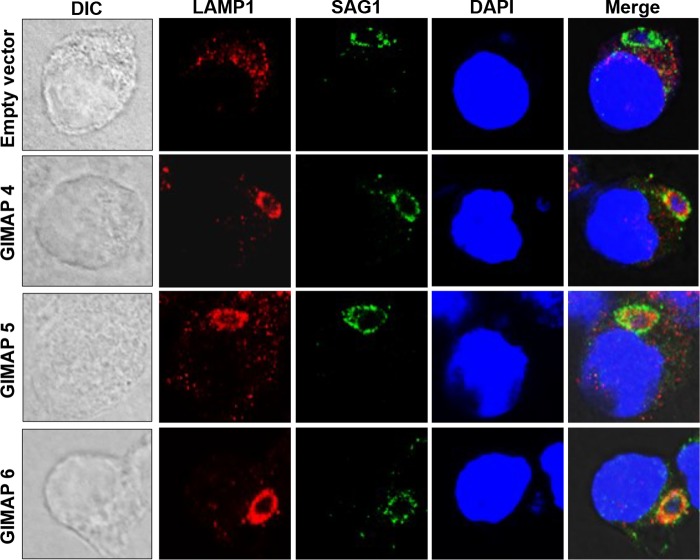
GIMAP 4, 5, and 6 induce lysosomal fusion to the parasite surface membrane.
To determine whether the localization of overexpressed GIMAP 4, 5, or 6 on the PVM would induce the fusion of lysosomes to the parasites, we analyzed the distribution pattern of LAMP1, a lysosome-specific marker protein (16), in T. gondii-infected NR8383 cells expressing the empty vector or the individual GIMAPs. We found that after 4 h of infection, while LAMP1 appeared diffusely distributed in the cytoplasm of the NR8383 cells with the empty vector, LAMP1 densely colocalized with the T. gondii SAG1 protein and extended into the parasites in the cells expressing each of the GIMAPs (Fig. 6). Overexpression of either variant of GIMAP 5 induced the translocation of LAMP1 to the parasite surface. Together, these findings indicate that the dense accumulation of GIMAP 4, 5, or 6 on the PVM induces the fusion of lysosomes to the PVM, with extension of the lysosomal content into the parasite inside the parasitophorous vacuole.
FIG 6.
Immunofluorescence colocalization of T. gondii SAG1 and lysosome marker protein LAMP1 in NR8383 cells infected with the T. gondii type I RH strain. NR8383 cells engineered for inducible overexpression of GIMAP 4, 5_201, and 6, as well as NR8383 cells with the empty pLVX-TetOne-Puro expression vector, were induced with doxycycline for 24 h and thereafter cultured with T. gondii tachyzoites for 4 h, followed by immunofluorescence analyses using combinations of anti-LAMP1 antibody (red stain), anti-SAG1 antibody (green stain), and the DAPI nuclear stain (blue). In GIMAP 4-, 5_201-, and 6-expressing cells, LAMP1 colocalized with SAG1 (Merge panels), while in cells expressing the empty vector, LAMP1 was sparsely distributed in the host cell cytosol and did not appear to overlap SAG1 (Merge panels).
DISCUSSION
The disparity in the LEW and BN rats' responses to T. gondii infection presents an ideal opportunity for elucidating host molecular mechanisms that are important in host resistance to T. gondii infection. The refractoriness of the LEW rat to toxoplasmosis has thus far been associated with a rat genomic locus named Toxo1 on chromosome 10 (5), but the mechanisms are still poorly defined. In other studies, we have identified two genes, called NALP1 (NLRP1) and ALOX12, in the orthologous Toxo1 locus on chromosome 17 of the human genome and demonstrated that they play roles in resistance to toxoplasmosis by mediating infected host cell death and inflammatory cytokine production (17, 18). Cellular invasion by pathogens activates the NLRP1 inflammasome, which leads to initiation of pyroptosis, a caspase-1-dependent highly inflammatory cell death process often observed during infection with cytosolic pathogens (19), including T. gondii (5, 20). Despite these earlier observations, it is quite evident that the LEW rat engages undefined intertwined mechanisms to orchestrate the rapid and early killing of invading T. gondii (5, 20).
Herein, we have performed global transcriptome analysis of the LEW versus BN rat, with or without T. gondii infection, with the goal of unraveling underlying mechanisms for the LEW rat's robust resistance to T. gondii infection. We identified genes of the GTPase immunity-associated protein family, GIMAP 4, 5, and 6, that were significantly upregulated (FDR, 0.05) in the LEW rat (but not in the BN rat) in response to T. gondii infection. GIMAPs are relatively recently reported putative small GTPases that are conserved among mammalian species and higher plants (14, 21, 22). Human and rat genomes each have seven functional GIMAP genes clustered in chromosome 7q36.1 (14) and chromosome 4 (23), respectively. Both human and rat GIMAPs are small proteins of about 34 kDa to 38 kDa (10). All GIMAPs contain an AIG domain (24) and putative coiled-coil domains reminiscent of protein-protein interactions (10). GIMAP 5 and 6 have identical sequences in both LEW and BN rats, while GIMAP 4 in the BN rat has a 21-residue truncation in its carboxyl terminus (12).
We engineered GIMAP 4, 5, and 6 from LEW rat for inducible overexpression in a T. gondii-susceptible macrophage cell line, NR8383. Overexpression of GIMAP 4, 5, or 6 individually in T. gondii-infected NR8383 cells showed that all three GIMAPs colocalized with the T. gondii GRA5 protein. Consistently, the endogenous GIMAP 4, 5, and 6 also colocalized with GRA5 in the T. gondii-infected LEW rats' primary peritoneal cells but were undetectable in the infected BN rats' primary peritoneal cells. GRA5 has been shown to specifically translocate to the PVM in T. gondii-infected host cells (15), and thus, antibodies directed against GRA5 are used in immunostaining assays to delineate the PVM (25). Therefore, our observation that GIMAP 4, 5, and 6 colocalized with GRA5 in T. gondii-infected cells indicated that the GIMAPs accumulated on the PVM. Similarly, IFN-γ-induced GTPases, IRGs in mice and GBPs in humans, have been shown to specifically translocate to the PVM in T. gondii-infected host cells (6, 7). However, unlike GIMAPs, both IRGs and GBPs are larger GTPases, averaging about 47 kDa and 65 kDa, respectively (26).
NK cells and T lymphocytes activated by interleukin-12 produce IFN-γ, which, in turn, induces the generation of IRGs and GBPs (27). Interestingly, in the present study, by analyzing the RNA sequencing data, we observed that, despite having higher expression of GIMAPs, the T. gondii-infected LEW rats had 3.4-fold lower expression of IFN-γ transcripts than the T. gondii-infected BN rats. Additionally, we did not observe significant differential expression of any IRG transcripts between the T. gondii-infected LEW and BN rats. Subsequently, we induced overexpression of GIMAP 4, 5, and 6 in a macrophage cell line (NR8383) in the absence of IFN-γ-producing NK cells or T lymphocytes, but we observed that each of the three GIMAPs translocated to the PVM. Together, these findings suggest that the expression of the GIMAPs and their targeting to the PVM are not driven by IFN-γ.
The mechanism by which PVM-docked IRGs kill intracellular T. gondii is by directly rupturing the PVM membrane, leading to the release of T. gondii tachyzoites into the host cell cytosol (28), where they are degraded (8). On the other hand, GBPs on the PVM restrict parasite growth through recruitment of antimicrobial molecules, including autophagy protein complex, inflammasome, and NADPH oxidase NOX2 (29). In the present study, we observed that each of the overexpressed GIMAP 4, 5, and 6 induced the translocation of the lysosomal marker protein LAMP1 onto the surface membrane of T. gondii tachyzoites in NR8383 cells. LAMP1 was observed not only to delineate the parasite surface but also to extend into the inside of the parasite, indicating lysosomal acidification of the parasite cytosol. A predominantly membrane-bound IRG (IRGM1) on a Mycobacterium bovis phagosome membrane has been shown to interact with SNARE protein complexes, leading to rapid fusion of the degradative lysosomes to the phagosome and destruction of the pathogen (30). In the case of T. gondii, lysosomal fusion to the otherwise nonfusogenic PVM has been demonstrated to kill the parasites in the parasitophorous vacuole (31). Indeed, we found that the growth of the T. gondii type I RH strain in NR8383 cells overexpressing GIMAP 4, 5, or 6 individually was restricted. This implies that the GIMAP-induced lysosome fusion to the PVM was associated with the restriction of intracellular T. gondii growth. While each of the candidate GIMAPs individually inhibited parasite growth, there were notable variations in their effects, with GIMAP 4 having the more profound inhibitory effect. It is likely that endogenous upregulation of GIMAP 4, 5, and 6 together results in additive inhibitory effects against parasite growth in the LEW rat cells.
We have recently reported that, compared to the BN rat, the LEW rat maintains inherently higher levels of reactive oxygen species (ROS) that are associated with restriction of intracellular T. gondii growth (32). ROS in phagocytic cells have been shown to induce the recruitment of autophagy components to phagosomes, which may facilitate lysosomal fusion (33, 34). Together with our present observations, we postulate that T. gondii infection in the LEW rat augments the expression of GIMAPs that translocate to the PVM, where they recruit yet unknown additional components, facilitated by high ROS levels, leading to lysosomal fusion, disruption of the PVM, and killing of the parasites. The NLRP1 and ALOX12 genes, which have been shown to mediate the killing of T. gondii parasites and infected host cells by macrophages (5, 17, 18), activate downstream proinflammatory effector molecules, which leads to restriction of parasite growth, while GIMAPs appear to directly target and destroy the PVM and kill the parasites in the PVM. Taken together, our present and previous findings imply that the LEW rat engages a multifaceted approach, culminating in the rapid killing of both the intracellular T. gondii parasites and the host cell.
MATERIALS AND METHODS
Parasite culture and purification.
Human foreskin fibroblasts (HFFs) were cultured to confluence in Iscove's modified Dulbecco's medium supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 1% (vol/vol) GlutaMAX, and 1% (vol/vol) penicillin-streptomycin-amphotericin B (Fungizone) (Life Technologies) at 37°C with 5% CO2. Tachyzoites of the type I RH strain of T. gondii were maintained in the confluent HFF culture. To extract T. gondii tachyzoites, the infected HFFs were suspended in medium and passed through a 25-gauge needle twice, followed by filtering through a 3-μm-pore-size filter to isolate the parasites from the cell debris. Isolated parasites were washed three times in phosphate-buffered saline (PBS), and their concentration was determined using a hemocytometer.
Rat infection assays, purification of peritoneal cells, and RNA extraction.
The protocol approved by the University of Illinois at Urbana—Champaign Institutional Animal Care and Use Committee was followed in performing experimental procedures involving rats. Male Lewis (LEW) and Brown Norway (BN) rats, aged 4 weeks, were obtained from Charles River. After 10 days of acclimatization, the rats were divided into two groups (4 animals in each group) per rat strain. For each rat strain, the treatment group animals were each intraperitoneally inoculated with 3.5 × 106 freshly isolated T. gondii RH strain tachyzoites constitutively expressing cytosolic yellow fluorescent protein (20), while the control group animals were inoculated with an equal volume of sterile PBS. After 24 h, the rats were sacrificed, and immediately, peritoneal lavage was performed to isolate peritoneal cells in RPMI medium. About 1 × 107 of the freshly isolated rat peritoneal cells were used for total RNA extraction using an RNeasy minikit (Qiagen). A Qubit (v3.0) fluorometer RNA BR kit (Life technologies) and an Agilent 2100 bioanalyzer (Agilent Technologies) were used to determine the quantity and quality of the RNA samples, respectively. The RNA samples were stored at −80°C until use.
Generation of RNA libraries and RNA sequencing.
RNA library production and sequencing were done essentially as we have previously reported (32). Briefly, freshly extracted RNA samples were used to prepare RNA libraries at the Roy J. Carver Biotechnology Center's High Throughput Sequencing and Genotyping Unit of the University of Illinois at Urbana—Champaign, using Illumina's TruSeq Stranded mRNAseq sample preparation kit (Illumina). The libraries were quantitated by fluorometry (Qubit), and their quality was determined using a bioanalyzer. The libraries were then diluted to 10 nM and quantified by quantitative PCR. Sequencing of the RNA libraries was done using a HiSeq SBS sequencing kit (v4) and a HiSeq2500 system (Illumina). Residual adapter content and low-quality bases on the generated sequences were trimmed using Trimmomatic (version 0.33), while the bcl2fastq (v2.17.1.14) conversion software (Illumina) was used to generate and demultiplex Fastq files from the sequence data. An ASCII offset of 33, known as Sanger scores, was used to determine the quality scores line in Fastq files. Feature Counts in the Sub-read (v1.5.0) package were used to derive gene counts, while gene annotations, including gene ontology terms, were downloaded from Ensembl (release 84) for Rnor_6.0 and ToxoDB.org (release 28) for T. gondii GT1. The SAMtools (v1.3) idxstats tool was used to determine the number of reads mapping to each rat chromosome and to the T. gondii genome.
Real-time PCR and Western blotting analyses of expression of candidate genes.
To confirm differential expression of candidate genes by real-time PCR and Western blotting, the LEW and BN rat infection assays described above were repeated, and after 24 h, the rats were sacrificed and peritoneal cells were extracted in PBS. Additionally, for each rat, 2 cm of the ileum was resected, washed in PBS to remove the intestinal content, and then homogenized in the TRIzol reagent (Invitrogen). The homogenate was spun down, and the supernatant was used for RNA extraction following the TRIzol reagent homogenization procedure. About 1 × 107 freshly isolated peritoneal cells were also used for total RNA extraction with the TRIzol reagent. For each sample, 2 μg of RNA was used for the synthesis of first-strand cDNA using a SuperScript II reverse transcriptase kit (Invitrogen). The primer pairs used were RT-GIMAP 4-F and RT-GIMAP 4-R for the GIMAP 4 gene, RT-GIMAP 5-F and RT-GIMAP 5-R for the GIMAP5 gene, RT-GIMAP 6-F and RT-GIMAP 6-R for the GIMAP 6 gene, and GAPDH-F and GAPDH-R for the rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (Table 2). The primer pairs generated DNA fragments of 104 bp, 130 bp, 87 bp, and 300 bp for the GIMAP 4, GIMAP 5, GIMAP 6, and GAPDH genes, respectively. PCR products for each gene were fractionated on an agarose gel, and the DNA bands were extracted using a QIAquick gel extraction kit (Qiagen). The concentration of the purified DNA fragments was measured by use of a NanoDrop spectrophotometer (Fisher). Tenfold serial dilutions of the extracted DNA fragments were made and used as quantification standards for real-time PCR. Each real-time PCR mixture contained 1 μl of cDNA template, 1 μl of primer mix (500 nM [each] primer), and 10 μl of SsoFast EvaGreen supermix (Bio-Rad), with the final volume made up to 50 μl with nuclease-free water. The cycling conditions included an initial denaturation for 10 min at 98°C, 40 cycles at 98°C for 15 s and 60°C for 1 min, and a final melting curve step. Cycling was performed using a 7500 real-time PCR system (Applied Biosystems). Transcript quantities were derived by the system software using the generated standard curves. The relative amount of the GIMAP gene transcripts was derived by dividing the concentration of the respective GIMAP gene by the concentration of the rat GAPDH gene from each cDNA sample.
TABLE 2.
Primers used in this study
| Primera | Sequence (5′–3′) |
|---|---|
| GIMAP 4-F | CCCTCGTAAAGAATTCATGGAAGCCCAGTACAGT |
| GIMAP 4-R | GAGGTGGTCTGGATCCCTAGTCTCTCATAAACTGGTTGA |
| GIMAP 5_201-F | CCCTCGTAAAGAATTCATGGAGGACCATGGCTT |
| GIMAP 5_201-R | GAGGTGGTCTGGATCCTCATTTCCACCTGCCAAT |
| GIMAP 5_203-F | CCCTCGTAAAGAATTCATGGAAGGCCTTCAGAAGA |
| GIMAP 5_203-R | GAGGTGGTCTGGATCCTCATTTCCACCTGCCAAT |
| GIMAP 6-F | CCCTCGTAAAGAATTCATGAATTGGCTTTACAGTAAAAC |
| GIMAP 6-R | GAGGTGGTCTGGATCCTTAAAGGGTTTTGCTGGAGA |
| pLVX-F | ATGTAAACCAGGGCGCCTAT |
| pLVX-R | ACCCGTCTTTGGATTAGGCA |
| 529-F | AGCTGCGTCTGTCGGGATGAGA |
| 529-R | ACCCTCGCCTTCATCTACAGT |
| GAPDH-F | TTCATTGACCTCAACTACATGGTTTACA |
| GAPDH-R | TCTGCAGCTGAAGTAGAAGACATGCT |
| RT-GIMAP4-F | CACTCGCTGTGTTGCTCTGA |
| RT-GIMAP4-R | GAAGTTTCCGTGTGGCCTTG |
| RT-GIMAP5-F | GCTTCCTAGTGGTGGACACG |
| RT-GIMAP5-R | AGTTGGGTCACCAGCAACAA |
| RT-GIMAP6-F | CAAAATCAGCGCTCGACCAG |
| RT-GIMAP6-R | GGGGTGTCGATCACCTCAAG |
F, forward; R, reverse.
For Western blotting, part of the extracted equal amounts of the rat peritoneal cells was lysed in Laemmli sample buffer. Equal amounts of the cell lysates were fractionated by SDS-PAGE and transferred to nitrocellulose membranes. The primary antibodies used for blotting were goat anti-GIMAP 4 (sc-103519; Santa Cruz Biotechnology), goat anti-GIMAP5 (sc-164476; Santa Cruz Biotechnology), and goat anti-GIMAP 6 (sc-247002; Santa Cruz Biotechnology) for GIMAP 4, 5, and 6, respectively. The secondary antibodies used were a donkey anti-goat IgG (H+L)-horseradish peroxidase (HRP) conjugate (A16005; Invitrogen). Signal generation was performed using Clarity Western enhanced chemiluminescence (ECL) substrate (Bio-Rad), and imaging was done using a FluoroChem R imager (Protein Simple).
Generation of lentiviral particles for inducible expression of LEW rat GIMAP genes.
About 1 μg of total RNA extracted from the LEW rat peritoneal cells using the RNeasy minikit was treated with DNase I (Invitrogen) to remove residual genomic DNA and used for cDNA synthesis with the SuperScript II reverse transcriptase kit (Invitrogen). Full coding sequences of the GIMAP 4, 5, and 6 genes were amplified from the LEW rat cDNA with a CloneAmp HiFi PCR premix kit (Clontech) using the respective primer sets (Table 2), and the amplicons were cloned into the pLVX-TetOne-Puro expression vector using an In-Fusion HD cloning system (Clontech) following the manufacturer's instructions. The recombinant pLVX-TetOne-Puro expression vectors carrying the coding sequences were transformed in Stellar competent Escherichia coli cells (Clontech) and purified using a NucleoBond Xtra Midi Plus kit (Clontech). After sequencing to confirm gene insert identity, the purified pLVX-TetOne-Puro expression plasmids were used with the Lenti-X Packaging Single Shots system (Clontech) to generate lentivirus particles in Lenti-X 293T cells (Clontech). Briefly, Lenti-X 293T cells were cultured to about 80% confluence in petri dishes (10-cm diameter) with 8 ml of Dulbecco modified Eagle medium supplemented with 4.5 mg/ml glucose, 4 mM l-glutamine, 1 mM sodium pyruvate, 1.5 mg/ml sodium bicarbonate, 10% (vol/vol) heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. For each transfection, 7.0 μg of recombinant pLVX-TetOne-Puro expression plasmid in 600 μl of nuclease-free sterile double-distilled water was added to a Lenti-X Packaging Single Shots system, the components were mixed, and the mixture was incubated at room temperature for 10 min. The whole mixture was added dropwise to one petri dish of a Lenti-X 293T cell culture and mixed by gentle rocking. The cells were incubated at 37°C with 5% CO2 for 8 h, after which the medium was changed, the cells were incubated for a further 48 h, and the culture medium was collected. The presence of lentivirus particles in the medium was determined using the Lenti-X GoStix reagent (Clontech) following the manufacturer's protocol. The lentiviral particles in the medium were concentrated using the Lenti-X concentrator reagent (Clontech) following the manufacturer's instructions. The concentrated viral titer was estimated using the Lenti-X GoStix reagent. The viral particles were aliquoted and stored at −80°C until use.
Transduction of NR8383 cells with lentiviral particles.
Lentiviral particles expressing the GIMAP transgenes or the empty pLVX-TetOne-Puro expression vector (control) were used to transduce NR8383 cells (CRL2192; ATCC). Briefly, NR8383 cells were cultured to 70% confluence in petri dishes (10-cm diameter) containing Ham's F-12K medium supplemented with 2 mM l-glutamine, 1.5 g/liter sodium bicarbonate, and 10% (vol/vol) certified tetracycline-free fetal bovine serum (Clontech). Polybrene solution was added to the cell culture to a final concentration of 4 μg/ml and mixed by rocking the cells gently. About 200 μl of thawed lentiviral aliquot (at a titer of about 105 inclusion-forming units) was added to the cells dropwise, and the cells and virus were mixed by rocking gently. The transduced cells were cultured overnight, after which the cells were selected with 12 μg/ml of puromycin (Clontech) for 3 weeks. Puromycin-resistant cells were cloned by limiting dilution. Selected clones were cultured in the absence or presence of 1 μg/ml doxycycline and then analyzed by Western blotting to determine the clones that induced the expression of the transgenes over a 24-h period. The primary antibodies used were goat anti-GIMAP 4 (sc-103519; Santa Cruz Biotechnology), goat anti-GIMAP5 (sc-164476; Santa Cruz Biotechnology), and goat anti-GIMAP 6 (sc-247002; Santa Cruz Biotechnology) for GIMAP 4, 5, and 6, respectively. The secondary antibodies used were a donkey anti-goat IgG (H+L)-HRP conjugate (A16005; Invitrogen). Signal generation was performed using the Clarity Western ECL substrate (Bio-Rad), and imaging was done using the FluoroChem R imager (Protein Simple).
Immunofluorescence assays.
NR8383 cells overexpressing the GIMAP transgenes or the pLVX-TetOne-Puro empty vector were grown on coverslips in 24-well plates in supplemented Ham's F-12K medium and infected with T. gondii type I RH strain tachyzoites at a multiplicity of infection (MOI) of 1:10 (parasites/cells). Similarly, 107 freshly isolated primary peritoneal cells from uninfected LEW and BN rats were seeded on coverslips in 24-well plates in supplemented Ham's F-12K medium and infected with T. gondii type I RH strain tachyzoites at an MOI of 1:10. For immunofluorescence analysis, the cultures were fixed with 3% (wt/vol) formaldehyde in PBS for 30 min at room temperature. Permeabilization of the cells was done with 0.2% Triton X-100 in PBS at room temperature for 10 min, followed by washing with PBS and incubation at 4°C overnight with blocking buffer (0.2% Triton X-100 and 3% bovine serum albumin in PBS) containing primary antibodies against the respective GIMAPs. The primary antibodies used were goat anti-GIMAP 4 (sc-103519; Santa Cruz Biotechnology), goat anti-GIMAP 5 (164476; Santa Cruz Biotechnology), and goat anti-GIMAP 6 (sc-247002; Santa Cruz Biotechnology) for GIMAP 4, 5, and 6, respectively, or rabbit anti-LAMP1 (ab24170; Abcam) and mouse monoclonal antibody against T. gondii GRA5 (BIO.018.6; Biotem) or SAG1 (C65620M; Meridian Life Science). The secondary antibodies used were Alexa Fluor 488-conjugated donkey anti-mouse immunoglobulin antibody (A11001; Invitrogen), Alexa Fluor 594-conjugated donkey anti-goat immunoglobulin antibody (A11058; Invitrogen), and Alexa Fluor 594-conjugated goat anti-rabbit antibody (A-11037; Invitrogen). After washing the cells in blocking buffer to remove unbound primary antibodies, secondary antibodies diluted in blocking buffer at 1:300 were added and the mixture was incubated at room temperature for 1 h. The cells were then washed three times in PBS and air dried, and each coverslip was placed on a glass slide to overlay a drop of ProLong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole; Life Technologies) and sealed with nail polish. The slides were analyzed using a Nikon A1R confocal laser microscope system.
Determination of effect of GIMAPs on T. gondii growth in NR8383 cells.
NR8383 cells engineered for inducible overexpression of individual GIMAP transgenes or the pLVX-TetOne-Puro empty vector were grown to confluence in supplemented Ham's F-12K medium in 12-well plates. One day prior to infection, the medium was replaced with fresh medium containing 1 μg/ml doxycycline. After 24 h of culture in the presence of doxycycline, fresh medium with 1 μg/ml doxycycline was added, followed immediately by addition of 4 × 104 freshly extracted T. gondii type I RH strain tachyzoites to each well. At time intervals of culture of 24 h, 48 h, and 72 h postinfection, genomic DNA was extracted from the whole culture of each well using PureLink genomic DNA kits (Invitrogen). The concentration of the extracted genomic DNA from each culture well was measured with a NanoDrop spectrophotometer (Fisher) and adjusted by dilution to make the concentrations uniform in all the samples. Quantification of the amount of the T. gondii 529 repetitive gene (GenBank accession number AF146527) and the rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (GenBank accession number BC029618) was performed on equal amounts of DNA template using real-time PCR. The primer pairs used were 529-F and 529-R for the 529 repetitive gene and GAPDH-F and GAPDH-R for the GAPDH gene (Table 2). The primer pairs generated DNA fragments of 174 bp and 300 bp for the 529 repetitive and GAPDH genes, respectively. PCR products for each gene were fractionated on an agarose gel, and the DNA bands were extracted using a QIAquick gel extraction kit (Qiagen). The concentration of the purified DNA fragments was measured with a NanoDrop spectrophotometer (Fisher). Tenfold serial dilutions of the extracted DNA fragments were made and used as quantification standards for real-time PCR. Each real-time PCR mixture contained 1 μl (containing a uniform concentration of DNA across all samples analyzed) of DNA template, 1 μl of primer mix (500 nM [each] primer), and 10 μl of SsoFast EvaGreen supermix (Bio-Rad), with the final volume made up to 50 μl with nuclease-free water. The cycling conditions included an initial denaturation for 10 min at 98°C, 40 cycles at 98°C for 15 s and 60°C for 1 min, and a final melting curve step. Cycling was performed using a 7500 real-time PCR system (Applied Biosystems). DNA quantities were derived by the system software using the generated standard curves. The relative amount of T. gondii was derived by dividing the concentration of T. gondii 529 repetitive gene by the concentration of the rat GAPDH gene from each DNA sample. Three independent experiments of this assay were performed, with each experiment being done in triplicate.
Data analysis.
The R (v3.3.0) and Bioconductor packages (35, 36) were used to preprocess the raw RNA sequencing data, while analysis was performed with EdgeR's (v3.14.0) negative binomial generalized linear model with likelihood ratio tests and tag-wise dispersion estimates (37, 38, 39). One-way ANOVA was used to compare the data across and between groups, while the false discovery rate method (40) was used to perform multiple-hypothesis-test correction. A weighted gene correlation network analysis (WGCNA; v1.51) using the trimmed mean for M values (TMM) normalized log2 count per million (CPM) values was performed to compare gene expression patterns across treatment groups (41, 42). Heat maps were generated separately for rat and Toxoplasma genes with the ANOVA FDR (P < 0.05) using TMM-normalized log2 CPM values that were scaled to have a mean of 0 and a standard deviation of 1. All the sequencing data were analyzed using log2 scale values and then converted from the log2 fold change (FC) to the regular FC. All other experimental data analyses were performed using a two-tailed Student's t test. P values of 0.05 or less were considered significant.
Accession number(s).
The entire series of RNA sequencing data reported in this study is publicly accessible in the Gene Expression Omnibus (GEO) repository with accession number GSE100203.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded in part by the University of Illinois at Urbana—Champaign to W.H.W.
We are grateful to Alvaro G. Hernandez, Jenny Drnevich Zadeh, and Jessica (Kirkpatrick) Holmes of the Roy J. Carver Biotechnology Center's High Throughput Sequencing and Genotyping Unit of the University of Illinois at Urbana—Champaign for the help in RNA sequencing and analysis of the results.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00582-17.
REFERENCES
- 1.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Aliberti J. 2005. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat Rev Immunol 5:162–170. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- 3.Phan L, Kasza K, Jalbrzikowski J, Noble AG, Latkany P, Kuo A, Mieler W, Meyers S, Rabiah P, Boyer K, Swisher C, Mets M, Roizen N, Cezar S, Remington J, Meier P, McLeod R, Toxoplasmosis Study Group. 2008. Longitudinal study of new eye lesions in treated congenital toxoplasmosis. Ophthalmology 115:553–559.e8. [DOI] [PubMed] [Google Scholar]
- 4.Dubey JP, Ferreira LR, Alsaad M, Verma SK, Alves DA, Holland GN, McConkey GA. 2016. Experimental toxoplasmosis in rats induced orally with eleven strains of Toxoplasma gondii of seven genotypes: tissue tropism, tissue cyst size, neural lesions, tissue cyst rupture without reactivation, and ocular lesions. PLoS One 11:e0156255. doi: 10.1371/journal.pone.0156255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavailles P, Flori P, Papapietro O, Bisanz C, Lagrange D, Pilloux L, Massera C, Cristinelli S, Jublot D, Bastien O, Loeuillet C, Aldebert D, Touquet B, Fournié GJ, Cesbron-Delauw MF. 2014. A highly conserved Toxo1 haplotype directs resistance to toxoplasmosis and its associated caspase-1 dependent killing of parasite and host macrophage. PLoS Pathog 10:e1004005. doi: 10.1371/journal.ppat.1004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng YS, Colonno RJ, Yin FH. 1983. Interferon induction of fibroblast proteins with guanylate binding activity. J Biol Chem 258:7746–7750. [PubMed] [Google Scholar]
- 7.Boehm U, Guethlein L, Klamp T, Ozbek K, Schaub A, Futterer A, Pfeffer K, Howard JC. 1998. Two families of GTPases dominate the complex cellular response to IFN-gamma. J Immunol 161:6715–6723. [PubMed] [Google Scholar]
- 8.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. 2006. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med 203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virreira Winter S, Niedelman W, Jensen KD, Rosowski EE, Julien L, Spooner E, Caradonna K, Burleigh BA, Saeij JP, Ploegh HL, Frickel EM. 2011. Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PLoS One 6:e24434. doi: 10.1371/journal.pone.0024434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filén S, Lahesmaa R. 2010. GIMAP proteins in T-lymphocytes. J Signal Transduct 2010:268589. doi: 10.1155/2010/268589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascall JC, Rotondo S, Mukadam AS, Oxley D, Webster J, Walker SA, Piron J, Carter C, Ktistakis NT, Butcher GW. 2013. The immune system GTPase GIMAP6 interacts with the Atg8 homologue GABARAPL2 and is recruited to autophagosomes. PLoS One 8:e77782. doi: 10.1371/journal.pone.0077782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter C, Dion C, Schnell S, Coadwell WJ, Graham M, Hepburn L, Morgan G, Hutchings A, Pascall JC, Jacobs H, Miller JR, Butcher GW. 2007. A natural hypomorphic variant of the apoptosis regulator Gimap4/IAN1. J Immunol 179:1784–1795. doi: 10.4049/jimmunol.179.3.1784. [DOI] [PubMed] [Google Scholar]
- 13.Hornum L, Romer J, Markholst H. 2002. The diabetes-prone BB rat carries a frameshift mutation in Ian4, a positional candidate of Iddm1. Diabetes 51:1972–1979. doi: 10.2337/diabetes.51.6.1972. [DOI] [PubMed] [Google Scholar]
- 14.Krucken J, Schroetel RM, Muller IU, Saidani N, Marinovski P, Benten WP, Stamm O, Wunderlich F. 2004. Comparative analysis of the human gimap gene cluster encoding a novel GTPase family. Gene 341:291–304. doi: 10.1016/j.gene.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Lecordier L, Mercier C, Sibley LD, Cesbron-Delauw MF. 1999. Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Mol Biol Cell 10:1277–1287. doi: 10.1091/mbc.10.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clough B, Wright JD, Pereira PM, Hirst EM, Johnston AC, Henriques R, Frickel EM. 2016. K63-linked ubiquitination targets Toxoplasma gondii for endo-lysosomal destruction in IFNγ-stimulated human cells. PLoS Pathog 12:e1006027. doi: 10.1371/journal.ppat.1006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witola WH, Mui E, Hargrave A, Liu S, Hypolite M, Montpetit A, Cavailles P, Bisanz C, Cesbron-Delauw MF, Fournie GJ, McLeod R. 2011. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun 79:756–766. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witola WH, Liu SR, Montpetit A, Welti R, Hypolite M, Roth M, Zhou Y, Mui E, Cesbron-Delauw MF, Fournie GJ, Cavailles P, Bisanz C, Boyer K, Withers S, Noble AG, Swisher CN, Heydemann PT, Rabiah P, Muench SP, McLeod R. 2014. ALOX12 in human toxoplasmosis. Infect Immun 82:2670–2679. doi: 10.1128/IAI.01505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschopp J, Martinon F, Burns K. 2003. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol 4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 20.Cirelli KM, Gorfu G, Hassan MA, Printz M, Crown D, Leppla SH, Grigg ME, Saeij JP, Moayeri M. 2014. Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog 10:e1003927. doi: 10.1371/journal.ppat.1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitta T, Takahama Y. 2007. The lymphocyte guard-IANs: regulation of lymphocyte survival by IAN/GIMAP family proteins. Trends Immunol 28:58–65. doi: 10.1016/j.it.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Wang T, Zhang W, Li X. 2008. Computational identification and analysis of immune-associated nucleotide gene family in Arabidopsis thaliana. J Plant Physiol 165:777–787. doi: 10.1016/j.jplph.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Dion C, Carter C, Hepburn L, Coadwell WJ, Morgan G, Graham M, Pugh N, Anderson G, Butcher GW, Miller JR. 2005. Expression of the Ian family of putative GTPases during T cell development and description of an Ian with three sets of GTP/GDP-binding motifs. Int Immunol 17:1257–1268. doi: 10.1093/intimm/dxh302. [DOI] [PubMed] [Google Scholar]
- 24.Reuber TL, Ausubel FM. 1996. Isolation of Arabidopsis genes that differentiate between resistance responses mediated by the RPS2 and RPM1 disease resistance genes. Plant Cell 8:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson DJ. 2004. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int J Parasitol 9:347–360. doi: 10.1016/j.ijpara.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Kim BH, Shenoy AR, Kumar P, Bradfield CJ, MacMicking JD. 2012. IFN-inducible GTPases in host cell defense. Cell Host Microbe 12:432–444. doi: 10.1016/j.chom.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazzinelli RT, Denkers EY. 2006. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol 6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 28.Howard JC, Hunn JP, Steinfeldt T. 2011. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr Opin Microbiol 14:414–421. doi: 10.1016/j.mib.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 29.MacMicking JD. 2012. Interferon-inducible effector mechanisms in cell autonomous immunity. Nat Rev Immunol 12:367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari S, Choi HP, Matsuzawa T, Pypaert M, MacMicking JD. 2009. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P(2) and PtdIns(3,4,5)P(3) promotes immunity to mycobacteria. Nat Immunol 10:907–917. doi: 10.1038/ni.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. 2006. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest 116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witola WH, Kim CY, Zhang X. 2017. Inherent oxidative stress in the Lewis rat is associated with resistance to toxoplasmosis. Infect Immun 85:e00289-17. doi: 10.1128/IAI.00289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. 2007. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, Glogauer M, Grinstein S, Brumell JH. 2009. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci U S A 106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- 36.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. 2004. Bioconductor: open software development for computational biology and bioinformatics R. Genome Biol 5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson MD, McCarthy DJ, Smyth GK. 2010. EdgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy DJ, Chen Y, Smyth GK. 2012. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Lun ATL, Smyth GK. 2014. Differential expression analysis of complex RNA-seq experiments using edgeR. In Datta S, Nettleton DS (ed), Statistical analysis of next generation sequence data. Springer, New York, NY. [Google Scholar]
- 40.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 57:289–300. [Google Scholar]
- 41.Zhang B, Horvath S. 2005. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 4:Article17. [DOI] [PubMed] [Google Scholar]
- 42.Langfelder P, Horvath S. 2008. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.