ABSTRACT
Staphylococcus aureus nasal carriage is transient in most humans and usually benign, but dissemination of S. aureus to extranasal sites causes the majority of clinical infections, and S. aureus is a major cause of serious infections in the United States. A better understanding of innate nasal decolonization mechanisms is urgently needed, as are relevant models for studying S. aureus clearance. Here, we screened a population of healthy smokers for nasal S. aureus carriage and compared the participants' abilities to clear experimentally applied nasal S. aureus before and after completion of a smoking cessation program. We determined that cigarette smoking increases the mean nasal S. aureus load (2.6 × 104 CFU/swab) compared to the load observed in healthy nonsmokers (1.7 × 103 CFU/swab) and might increase the rate of S. aureus nasal carriage in otherwise-healthy adults: 22 of 99 smokers carried S. aureus at the screening visit, while only 4 of 30 nonsmokers screened positive during the same time period. Only 6 of 19 experimental inoculation studies in active smokers resulted in S. aureus clearance within the month of follow-up, while in the cessation group, 6 of 9 subjects cleared nasal S. aureus and carriage duration averaged 21 ± 4 days. Smoking cessation associated with enhanced expression of S. aureus-associated interleukin-1β (IL-1β) and granulocyte colony-stimulating factor (G-CSF) in nasal fluids. Participants who failed to clear S. aureus exhibited a higher nasal S. aureus load and elevated nasal interleukin-1 receptor antagonist (IL-1RA) expression at the preexperiment study visits. We conclude that smokers exhibit higher S. aureus loads than nonsmokers and that innate immune pathways, including G-CSF expression and signaling through the IL-1 axis, are important mediators of nasal S. aureus clearance.
KEYWORDS: Staphylococcus aureus, experimental colonization, host defense, humans, nasal carriage, smoking cessation
INTRODUCTION
Staphylococcus aureus colonizes healthy humans and various mammals, including cows, pigs, and house pets. Treatment of S. aureus infections and the rise in antibiotic-resistant infections inflict a tremendous burden on health care and agriculture. S. aureus colonizes most often the nose and pharynx of humans, with the nasal vestibule considered the reservoir (1–3). The majority of nasal S. aureus strains classify as methicillin-susceptible S. aureus (MSSA), although methicillin-resistant S. aureus (MRSA) is hosted and disseminated by healthy and hospitalized S. aureus carriers. The overall carriage rate in humans ranges from 20 to 50%, with higher rates reported for children, industrial hog workers, immunocompromised individuals, and patients presenting with clinical extranasal S. aureus infections (4, 5). Considering that most invasive S. aureus isolates are genetically indistinct from corresponding nasal isolates (6, 7), nasal S. aureus carriage represents a major risk factor for S. aureus disease, postoperative complications, and mortality.
The determinants of nasal S. aureus carriage include bacterial, environmental, and host immune factors. S. aureus adhesion to the nasal mucosa is governed by wall teichoic acid, clumping factor B, and numerous additional microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) (8–11). The ability of S. aureus to respond to nutrient limitation also appears to be critical to nasal colonization, although no differences were found between nutrient levels in nasal secretions from carriers and from noncarriers (12). Perhaps most critical to the onset of nasal carriage is the immune status of the host. Atopic dermatitis, HIV, diabetes, and dialysis patients have a higher incidence of S. aureus carriage, as do newborns and nondiabetics with a body mass index (BMI) of >30 (13–15). The nasal fluids of human S. aureus carriers have less intrinsic anti-S. aureus activity than do nasal fluids collected from noncarriers (16, 17). Healthy volunteers who cleared experimentally inoculated nasal S. aureus produced a rapid response marked by elevated inflammatory factors in nasal fluid collected 2 days postinoculation, whereas subjects who failed to clear S. aureus presented a delayed and dysregulated response (18). Deciphering the roles of specific human nasal host defense factors regulating the onset of nasal S. aureus carriage will be pivotal for designing better decolonization strategies.
Despite a decline in recent years, an estimated 16.7% of men and 13.6% of women in the United States (approximately 36.5 million adults) currently smoke cigarettes (19), and smoking-related diseases continue as a leading cause of hospitalizations and death. Whether or not smoking influences nasal carriage of S. aureus remains an important question. Some studies associated smoking and secondhand smoke with increased S. aureus carriage rates (20–23), and others declared no association (24, 25), while the Tromsø (Norway) Staph and Skin Study 2007-08 indicated that smoking might be protective (26). Notably, S. aureus carriage triples the risk for postoperative skin and soft tissue infections (27), and smoking associates with higher infection rates (28). In the current study, we determined the nasal S. aureus carriage rate in healthy smokers who attend the University of Central Florida (UCF). We tested the ability of carriers to clear their experimentally inoculated strain both while smoking and after cessation, and we identified nasal host defense factors associated with nasal S. aureus. We demonstrate that healthy smokers exhibit a higher nasal S. aureus load than nonsmokers and are less likely to clear experimentally inoculated S. aureus than nonsmokers and those who successfully quit smoking.
RESULTS
Elevated nasal S. aureus load in healthy smokers compared to nonsmokers.
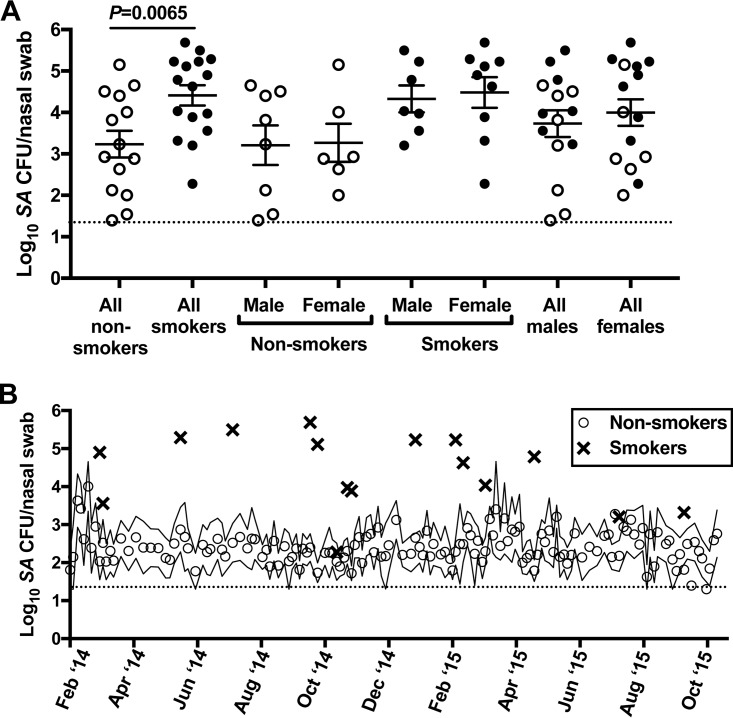
From January 2014 to November 2015, we screened 30 healthy nonsmokers and 99 healthy smokers for nasal S. aureus. The smoker cohort was young (mean, 28 years; median, 25 years; range, 18 to 57 years), 46% female, and racially diverse (75% white, 13% Hispanic, 7% Asian, 5% black). The average smoking history was 8.9 ± 6.0 years (range, 2.5 to 24 years), and most subjects reported smoking 2 to 5 cigarettes per day. Twenty-two of 99 smokers (22.2%) were S. aureus positive in one or both nostrils at the screening appointment, while 4 of 30 nonsmokers (13%) exhibited nasal S. aureus positivity at their initial visit. For the smoker group, 18 S. aureus carriers consented and enrolled in further study visits to test S. aureus clearance rates during active smoking versus after cessation. The 30 nonsmokers submitted nasal swabs every 4 to 7 days for several months (mean, 44 swab visits) to monitor natural S. aureus carriage. This group was similarly matched to the smoker group demographically: mean age, 25 years; median, 22 years; range, 19 to 43 years; 43% female; 60% white, 7% Hispanic, 30% Asian, 3% black. Sixteen nonsmokers were nasal S. aureus negative at every swab visit, while 14 were nasally colonized with S. aureus at least once during their respective evaluation periods (participant information and carriage statistics are shown in Table 1). We compared the nasal S. aureus loads from smokers' screening visits (average of 2 nostril CFU counts for 16 of 18 participants with countable agar plates) with the 14 healthy nonsmokers' average S. aureus loads from S. aureus-positive visits (Fig. 1A). In nonsmokers, the nasal S. aureus load averaged 1,698 CFU/swab and was similar for men and women (Fig. 1A). Smokers' mean S. aureus load (25,704 CFU/swab) was significantly greater than that of nonsmokers, independent of gender (Fig. 1A). To determine whether seasonal variation might contribute to this difference, we also plotted the 16 S. aureus loads recorded for smokers and the average nasal S. aureus loads of 14 nonsmoker carriers over the course of the evaluation period. Figure 1B shows that nonsmokers averaged 102 to 104 S. aureus CFU/swab throughout the observation period, with slight peaks noted in February and March of both years. Smokers who screened positive for S. aureus exhibited 104 to 106 CFU/swab at visits that spanned both summer and winter seasons. Taken together, these data suggest that smokers exhibit higher nasal S. aureus levels than nonsmokers, independent of gender, and even in a young and healthy population and a climate with mild winters.
TABLE 1.
Nonsmoker participant demographics and nasal S. aureus carriage history
| Nonsmoker ID (n = 14) | Gendera | Age (yr)b | Nasal S. aureus carriage (no. of S. aureus-positive visits/total no. of visits) | Duration of monitoring (wk) |
|---|---|---|---|---|
| D501 | M | 31 | 1/44 | 42 |
| D20 | M | 43 | 68/71 | 77 |
| D547 | F | 40 | 105/127 | 87 |
| D832 | F | 20 | 63/63 | 58 |
| D833 | F | 29 | 1/66 | 71 |
| D834 | F | 21 | 50/71 | 63 |
| D836 | F | 19 | 9/59 | 64 |
| D837 | M | 22 | 55/55 | 57 |
| D840 | M | 21 | 36/63 | 70 |
| D845 | F | 23 | 87/95 | 67 |
| D848 | M | 19 | 19/68 | 61 |
| D852 | M | 21 | 11/36 | 37 |
| D853 | M | 24 | 1/16 | 23 |
| D855 | M | 21 | 29/29 | 24 |
M, male (n = 8 [57%]); F, female (n = 6 [43%]).
Mean age, 25.3 years; range, 19 to 43 years.
FIG 1.
Cigarette smoking associates with elevated natural nasal S. aureus load. (A) Nasal S. aureus load (average S. aureus CFU/swab for two nostrils) at the screening visit of healthy smokers (solid black circles) versus the average nasal S. aureus load per S. aureus-positive visit for nonsmokers (open circles) who were monitored longitudinally during the same time period. Error bars represent the means and standard errors of the means (SEM). (B) Seasonal nasal S. aureus load for smoker screening visits (bold X's) versus the average nasal S. aureus load for 14 nonsmoker carriers (open circles). Solid lines enveloping the open circles indicate the SEM for nonsmoker average nasal S. aureus load. The dotted horizontal line in each panel indicates the S. aureus detection limit for nasal swab samples (20 CFU/swab).
Cessation of smoking associates with increased nasal S. aureus clearance following experimental inoculation.
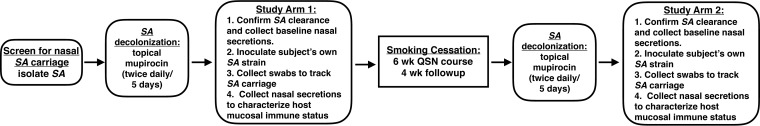
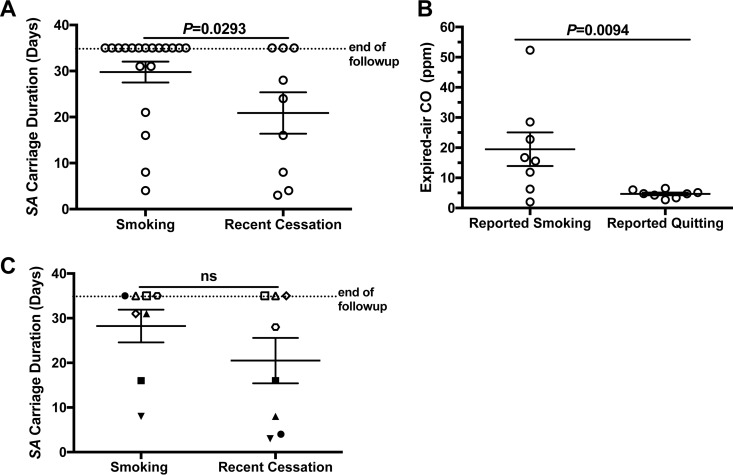
To test whether the host's ability to clear nasal S. aureus improves upon cessation of smoking, participants underwent an experimental nasal inoculation protocol while actively smoking and then again after having successfully quit cigarettes for at least a month (the study design is depicted in Fig. 2). Participants were cleared of their nasal S. aureus using a 5-day/twice-daily treatment with mupirocin ointment. One week after the last application, S. aureus clearance was confirmed and nasal fluids were collected for baseline measurements of mucosal immune status. A week later, nasal swabs were again collected in order to confirm the absence of nasal S. aureus and the restoration of resident non-S. aureus nasal flora to premupirocin levels (18). Each participant then self-applied his or her previously isolated S. aureus strain to each nostril (participant and S. aureus isolate data are shown in Table 2). Nasal swabs were performed the next day, immediately prior to a repeat inoculation, and swab samples were collected every 3 to 4 days thereafter for a month of follow-up. Nasal fluids were collected at days 3, 8, 11, 15 or 16, 23 or 24, and 31 or 32 postinoculation. We previously reported that in healthy nonsmokers, 10 of 15 studies resulted in the clearance of inoculated S. aureus within 1 month (18). For active smokers, only 6 of the 19 studies resulted in clearance of nasal S. aureus within the month, and the average ± standard deviation (SD) carriage duration was at minimum 30 ± 2 days, as follow-up did not extend beyond 34 days (Fig. 3A). In the cessation group, 6 of 9 subjects cleared their S. aureus and carriage duration averaged 21 ± 4 days (Fig. 3A). To aid with confirmation of active smoking versus cessation, expired-air carbon monoxide (CO) was measured at most of the participants' study visits. A previous study demonstrated that an expired-air CO level of ≤4 ppm indicates abstinence from cigarettes for at least 24 h (29). Nonsmokers' readings on our meter ranged from 2 to 5 ppm (data not shown). For the study participants, expired-air CO was 19.50 ± 5.56 ppm for reported smokers and 4.684 ± 0.44 ppm for reported quitters (Fig. 3B). It should be noted that the active-smoker group contained two subjects with average expired-air CO values that were as low as in the cessation group (Fig. 3B). A pairwise comparison of the 8 participants who completed both studies indicated a trend of shorter S. aureus carriage duration for the cessation group (P = 0.0619) (Fig. 3C). Interestingly, these 8 participants partitioned into one group that cleared S. aureus within 16 days after smoking cessation (Fig. 3C, solid symbols) and another group that could not effectively clear nasal S. aureus in one or both study arms (Fig. 3C, open symbols). Considering our previous findings demonstrating that dysregulation of host defense factors associated with longer carriage duration in individuals who underwent the same experimental inoculation procedure as the one utilized here (18), this partitioning suggests that differences in host mucosal immune effectors might render some individuals susceptible to long-term nasal S. aureus carriage regardless of smoking status.
FIG 2.
Experimental design and timeline. Healthy smokers were screened for nasal S. aureus (SA) carriage, and S. aureus isolates were genotyped and prepared as frozen stocks for subsequent inoculations. Participants applied topical nasal mupirocin twice daily for 5 days (days −17 to −13, preinoculation) to clear endogenous nasal S. aureus. On day −6 (preinoculation), nasal swabs were collected to confirm S. aureus decolonization, and nasal secretions were collected. On days 0 and 1, participants were swabbed for assessment of S. aureus carriage status and self-applied 2 × 107 CFU of their own S. aureus strain to each nostril. Nasal swabs were collected every 3 to 4 days postinoculation. Nasal secretions were collected at days 3, 8, 11, 15 or 16, 23 or 24, and 31 or 32 postinoculation. Following the month of follow-up after S. aureus inoculation, participants attended a 6-week Quit Smoking Now (QSN) cessation course. Subjects who successfully quit smoking and maintained cessation for an additional 4 weeks repeated the decolonization/recolonization protocol.
TABLE 2.
Smoker participant and S. aureus isolate details
| Smoker ID (n = 18) | Gendera | Age (yr)b | MLST/spa typec | Presence of mecAd | Study arm participatione |
|---|---|---|---|---|---|
| S002 | F | 20 | 59/t216 | − | Smoking |
| S008 | F | 18 | 87/t216 | + | Smoking |
| S012 | M | 22 | 1159/t1685 | − | Smoking |
| S019 | F | 35 | 5/t228 | − | Smoking |
| S036 | M | 24 | 101/t2078 | − | Both |
| S039 | F | 25 | 30/t338 | − | Smoking |
| S042 | F | 18 | 5/t002 | − | Smoking twice (no cessation) |
| S046 | F | 42 | 97/t267 | + | Smoking |
| S051 | M | 38 | 4346/t084 | − | Both |
| S052 | F | 21 | 72/t148 | − | Both |
| S064 | M | 20 | 8/t2866 | − | Both |
| S065 | F | 22 | 8/t2866 | − | Smoking |
| S070 | F | 35 | 59/t1293 | − | Both |
| S072 | M | 28 | 8/t008 | + | Both |
| S076 | M | 27 | 15/t084 | − | Both |
| S080 | M | 26 | 8/t3308 | − | Cessation only |
| S089 | F | 20 | 72/t6759 | − | Both |
| D831 | M | 25 | 22/t852 | + | Smoking twice (no cessation) |
F, female (n = 10 [56%]); M, male (n = 8 [44%]).
Mean age, 25.8 years; range, 18 to 42 years.
MLST, multilocus sequence type; spa, staphylococcal protein A gene.
−, absence; +, presence.
There were 2 study arms: active smoking (19 studies) and cessation (9 studies). Subjects participated in one or both arms or as indicated.
FIG 3.
Cessation from smoking associates with a higher rate of S. aureus clearance during experimental inoculation studies. (A) Comparison of experimental S. aureus carriage duration between all subjects who were actively smoking and those who recently quit smoking. (B) Average expired-air carbon monoxide (CO) values for participants who reported smoking compared to those who reported cessation of cigarette smoking. (C) Pairwise comparison of experimental nasal S. aureus carriage duration for 8 participants (each represented by a unique symbol) who completed both study arms. Open symbols indicate participants who failed to clear S. aureus in one or both study arms. Solid symbols indicate participants who cleared experimentally inoculated S. aureus in one or both study arms. ns, not significant.
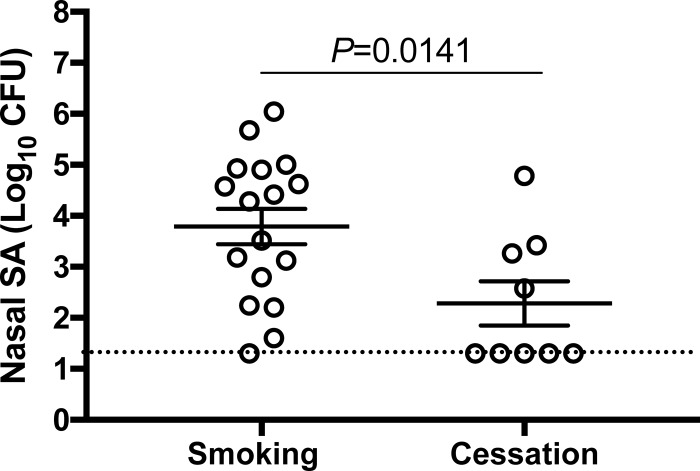
Decreased natural nasal S. aureus load following smoking cessation.
We next compared the natural nasal S. aureus loads of participants who completed a smoking cessation program with those of active smokers. Nasal S. aureus CFU/swab (average of two nostrils) values recorded at the premupirocin (day −17) study visit demonstrate that smoking cessation resulted in nearly a 100-fold decrease in nasal S. aureus load (Fig. 4). Furthermore, all but one of the active smokers' noses maintained S. aureus positivity between the screening visit and the premupirocin visit for study arm 1, while only 4 of the 9 noses of cessation subjects swabbed positive at the premupirocin visit for study arm 2 (Fig. 4). This suggests that after smoking cessation, participants were less likely to be naturally recolonized by S. aureus.
FIG 4.
Decreased natural nasal S. aureus load in subjects who successfully quit smoking. Nasal S. aureus loads (average S. aureus CFU/swab for both nostrils) at day −17 (preexperiment) of the smoking and cessation study arms. Error bars represent the means and SEM. The dotted horizontal line indicates the limit of detection for nasal S. aureus (20 CFU/swab).
Increased expression of S. aureus-induced G-CSF and IL-1β after smoking cessation.
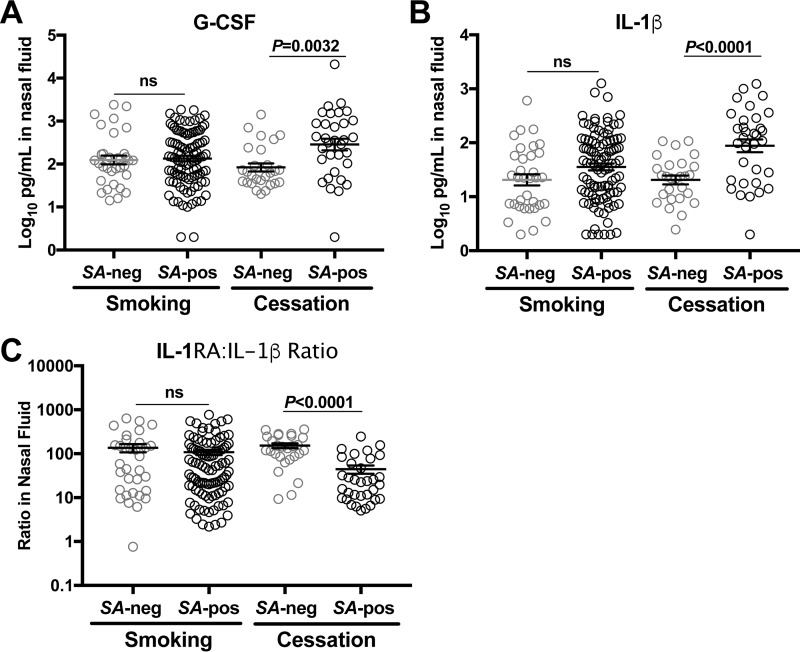
Innate immunity against colonizing S. aureus includes increased neutrophil activity marked by select cytokines and growth factors such as granulocyte colony-stimulating factor (G-CSF), interleukin-1β (IL-1β), and the IL-1 receptor (IL-1R) (18, 30, 31). To assess whether smoking and cessation from smoking associate with different host responses to nasal S. aureus inoculation, nasal fluids that were collected before and after mupirocin treatment and every 5 to 7 days following S. aureus inoculation were analyzed using a multiplex antibody bead array. We compared nasal fluids collected from S. aureus-positive noses with those from S. aureus-negative noses and further stratified the data by smoking status. After experimental nasal S. aureus inoculation, G-CSF and IL-1β were significantly elevated in nasal fluids collected from S. aureus-positive noses compared to S. aureus-negative noses in the cessation group (Fig. 5A and B). The smoking group, in contrast, exhibited no differences in inflammatory marker levels between nasal fluids collected from S. aureus-positive and those from S. aureus-negative noses. The IL-1 receptor antagonist (IL-1RA)-to-IL-1β ratio was significantly reduced in S. aureus-positive versus -negative nasal fluids from cessation subjects, while active smokers exhibited no difference (Fig. 5C). The smoking and cessation groups were comparable in age (means and SD: smoking group, 25.9 ± 7.4 years; cessation group, 26.5 ± 6.4 years), and both contained a mix of males and females (smoking, 8 males and 11 females; cessation, 6 males and 3 females). Elevated S. aureus-associated G-CSF and IL-1 β and a reduced IL-1RA-to-IL-1β ratio in nasal fluids from cessation subjects were observed in both male and female subjects (data not shown). Taken together, these data suggest that the increased rate of S. aureus clearance demonstrated by the cessation group (Fig. 3) was associated with increased neutrophil activity and IL-1 signaling.
FIG 5.
Smoking cessation associates with augmented innate nasal mucosal defense against S. aureus. Participants were cleared of endogenous nasal S. aureus and subsequently reinoculated with their own S. aureus strain as described for Fig. 2. Nasal fluids were collected from each participant at day −17 (prior to mupirocin application for clearance of endogenous S. aureus), day −6 (one week after the last mupirocin application), and at days 3, 8, 11, 15 or 16, 22 or 23, and 31 or 32 postinoculation with autologous S. aureus. Fluids were analyzed using Bio-Rad's 27-plex proinflammatory panel, and results are shown for G-CSF (A), IL-1β (B), and the Il-1RA-to-Il-1β ratio (C), where gray circles represent nasal fluids collected from S. aureus-negative noses (n = 34 for smoking, n = 27 for cessation groups) and black circles represent nasal fluids collected from S. aureus-positive noses (n = 100 for smoking, n = 33 for cessation groups). Error bars represent the means and SEM.
The carriage of experimentally inoculated S. aureus corresponds with an elevated natural nasal S. aureus load and elevated expression of IL-1RA.
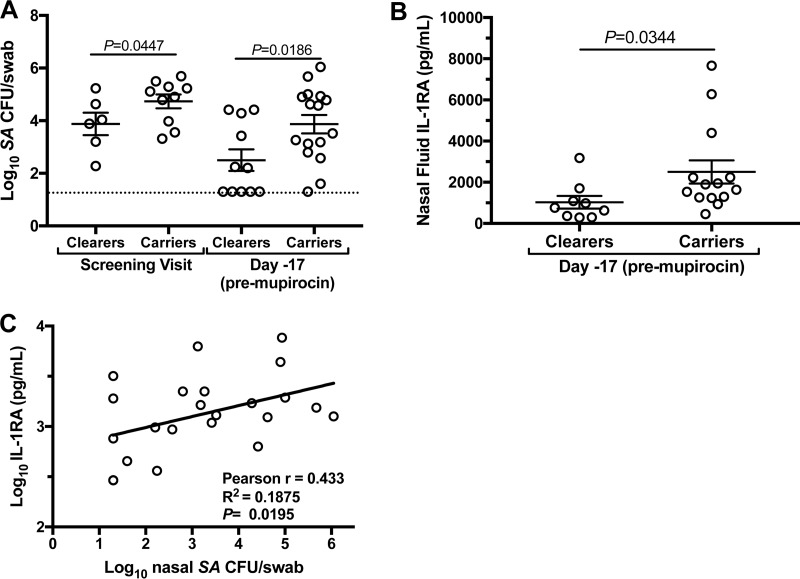
While host mucosal immunity is the most likely determinant of nasal S. aureus carriage status and duration (1, 18), specific host factors associated with nasal S. aureus load or failed S. aureus clearance are not known. Using the current cohort of healthy smokers and smokers who recently quit, we next assessed natural nasal S. aureus loads and secreted nasal immune factors according to whether the subjects cleared experimentally inoculated S. aureus or stayed colonized during the month of follow-up. The “carrier” and “clearer” groups were evenly matched in terms of age and gender (carriers, 24.7 ± 6.1 [mean ± SD] years, 8 males and 9 females; clearers, 27.6 ± 7.4 years, 6 males and 5 females). Consistent with an underlying innate susceptibility for S. aureus, carriers presented elevated nasal S. aureus levels at both the screening and day −17 (premupirocin) visits (Fig. 6A). Carriers also expressed higher levels of IL-1RA at day −17 than did clearers of S. aureus (Fig. 6B), suggesting that carriers' nasal mucosae were naturally “programmed” to antagonize IL-1 signaling. Likewise, nasal S. aureus load showed a modest positive correlation with nasal fluid IL-1RA for all subjects who donated both swab and nasal fluid samples on day −17 (n = 22) (Fig. 6C). Collectively, these data suggest that elevated IL-1RA expression supports nasal S. aureus survival.
FIG 6.
Clearance of experimentally inoculated nasal S. aureus correlated with nasal S. aureus load at screening and elevated IL-1RA in nasal secretions. (A) Nasal S. aureus levels at the screening and day −17 (premupirocin) visits of participants who eventually cleared (“Clearers”) or carried (“Carriers”) experimentally inoculated S. aureus. The dotted horizontal line indicates the limit of detection. (B) Participants who carried experimentally inoculated S. aureus (“Carriers”) exhibited elevated natural (day −17/premupirocin) expression of IL-1RA compared to participants who cleared (“Clearers”). In panels A and B, error bars represent the means and SEM. (C) Positive correlation between natural nasal S. aureus load (x axis) and IL-1RA expression in nasal fluid (y axis) collected from subjects at day −17.
DISCUSSION
Nasally carried S. aureus strains cause the majority of clinical infections, but the events leading to the establishment of human nasal S. aureus carriage are not well defined. Thus, methods for studying S. aureus interaction with human nasal mucosa are urgently needed in order to identify novel decolonization strategies. In the presented studies, we utilized natural human nasal carriers of S. aureus to study early colonization events following participants' self-inoculation with previously isolated autologous isolates.
We postulated that nasal inflammatory dysregulation might render cigarette smokers more susceptible to S. aureus nasal carriage than nonsmokers based on the paradigm that smoking causes both inflammatory and immunosuppressive effects and smokers are at increased risk for infections (32, 33). Several reports have described the effect of cigarette smoke extract (CSE) on innate immunity: CSE-exposed neutrophils exhibit spontaneous superoxide production but attenuated bacterially induced superoxide production and reduced phagocytic function (34, 35). Smokers' macrophages express lower cell surface levels of Toll-like receptor 2 (TLR2) and scavenger receptor, which might be important for recognizing and responding to S. aureus and resolving acute inflammation (34, 36). Smoke-exposed S. aureus strain USA300 showed reduced susceptibility to killing by macrophages and the antimicrobial peptide LL-37 (37). We observed a 22% nasal S. aureus carriage rate for 99 smokers who attended a single screening visit, while only 4 of 30 (13%) healthy nonsmokers were nasal S. aureus positive at their first swab appointment during the same period. Therefore, age- and climate-matched healthy smokers might have been more susceptible to nasal S. aureus carriage than their nonsmoker counterparts. A limitation of our study design was that while consenting smokers were enrolled in the decolonization/inoculation protocol following S. aureus positivity at screening, those who screened negative were not reswabbed at later dates. As S. aureus nasal carriage is often a transient state, especially in otherwise-healthy adults (38, 39), a thorough assessment of nasal S. aureus carriage rate in smokers, or any population, would require repeated measurements. Notably, we determined that the smoker-carriers' nasal S. aureus load was greater than the nonsmokers' load independent of age, gender, or climate (Fig. 1). This might be more meaningful than carriage rate comparisons when considering how smoking and nasal S. aureus carriage impact health care, especially in light of the known link between persistent S. aureus carriers (who often exhibit nasal S. aureus loads of 106 CFU/swab, like the ones that we observed in smokers) and nosocomial S. aureus infections (40).
During experimental nasal S. aureus inoculation, active smokers were at a disadvantage compared to individuals who recently quit smoking and healthy nonsmokers with regard to successful S. aureus clearance during the month of follow-up (Fig. 3). Smokers also had an elevated premupirocin nasal S. aureus load compared to the cessation group (Fig. 4). These findings correspond with smokers' enhanced susceptibility to clinical S. aureus infections and postoperative S. aureus-mediated complications (28, 33). Combined with the observation that month-long carriers of experimentally inoculated S. aureus also presented higher natural nasal S. aureus loads (Fig. 6A), this study suggests that the employed autologous inoculation model is physiologically relevant and effective for measuring human mucosal immune activity against colonizing S. aureus strains.
Production of IL-1β and chemokine-mediated neutrophil chemotaxis are demonstrated hallmarks of the acute anti-S. aureus response in a mouse cutaneous infection model (30). We and others have shown that IL-1β attenuates S. aureus adhesion and growth on nasal epithelial cells (41) and induces S. aureus killing by neutrophils (42). The present study demonstrates that augmented S. aureus-induced IL-1β and G-CSF and a decreased IL-1RA-to-IL-1β ratio in S. aureus-positive noses associate with cessation from smoking (Fig. 5) and improved S. aureus clearance (Fig. 3). This corroborates our previous investigation of experimentally inoculated healthy nonsmokers that showed the importance of early IL-1β and G-CSF signaling in mediating S. aureus clearance (18). This is also the first human study to show that elevated natural nasal IL-1RA levels correspond with elevated nasal S. aureus loads (Fig. 6B and C). Levels of nasal IL-1RA ranged from hundreds of picograms per ml to nearly 10 ng/ml over time and between donors, which likely contributed the statistically modest positive correlation (Pearson's r = 0.433; R2 = 0.18) (Fig. 6C), but the significant nonzero slope (P = 0.0195) (Fig. 6C) and 3-fold-increased nasal IL-1RA in carriers versus clearers of experimentally inoculated S. aureus (Fig. 6B) suggest a role for IL-1RA in supporting nasal S. aureus survival. Interestingly, elevated IL-1RA levels are associated with obesity and diabetes (43), two conditions that predict higher rates of S. aureus carriage, skin infections, and postoperative complications related to S. aureus infection. Little is known currently about IL-1R signaling specific to human nasal mucosa, but IL-1R-deficient mice and MyD88-deficient mice had a higher S. aureus burden and a decreased neutrophil recruitment in a cutaneous infection model than did TLR-deficient mice or wild-type mice (31). The authors also discovered that IL-1R/MyD88 signaling in resident but not newly recruited bone marrow-derived skin cells promoted neutrophil-mediated host defense against S. aureus. Taking these past and present studies in aggregate, it is tempting to speculate that classified “noncarriers” and “intermittent carriers” of nasal S. aureus possess innate IL-1β/IL-1R signaling cascades that mediate the rapid eradication of diverse S. aureus strains. Persistent carriers of nasal S. aureus, in contrast, elaborate a delayed but sustained cytokine response to S. aureus (18) leading to enhanced antimicrobial peptide expression (17, 44), which likely serves to keep nasal carriage asymptomatic. Further studies are needed to test the role of IL-1RA levels across diverse populations of nasal S. aureus carriers, especially when taking into account the known role for elevated IL-1RA in predicting the onset of type 2 diabetes (45) and the link between IL-1R polymorphisms and S. aureus osteomyelitis (46).
Participant attendance at frequent sampling visits, repeated over weeks or months, is a challenge, particularly when subjects are otherwise healthy and thus must volunteer their time and compliance. A limitation of the presented studies is sample size, begetting the question of whether valid conclusions may be drawn from 19 smoker and 9 cessation studies. The field is in need of an animal model of S. aureus nasal carriage that exhibits physiological S. aureus load and mucosal host defense, including neutrophil-mediated clearance and antimicrobial peptide production. Until a relevant animal model is validated, it is our view that human studies of S. aureus nasal carriage are valuable regardless of sample size. Mice and rats do not naturally harbor nasal S. aureus, and rodent noses are different structurally and immunologically from human noses (47, 48). Their continued use might complicate the progression of new drug and vaccine candidates from the laboratory to human clinical trials. For now, the utilized cohort of young healthy adults demonstrated that high nasal S. aureus burden associates with cigarette smoking. Participants who successfully quit smoking expressed elevated levels of S. aureus-associated nasal IL-1β and G-CSF and cleared S. aureus faster than active smokers. Participants who did not clear experimental S. aureus presented with higher natural nasal S. aureus load at screening and preexperiment visits, and their nasal S. aureus load was positively correlated with mucosal IL-1RA levels. These findings support the concept that nasal S. aureus carriage depends largely on host immune status rather than bacterial genotype. Furthermore, the enhanced nasal S. aureus load measured in active smokers might warrant the broadened use of healthy smokers in mucosal host defense and nasal microbiome studies.
MATERIALS AND METHODS
Ethics statement.
A protocol for recruitment of healthy participants, collection of nasal swabs and nasal fluid, self-application of topical mupirocin, and self-inoculation of autologous S. aureus was approved by the Institutional Review Board of the University of Central Florida. Participants provided consent at each study visit, and no adverse effects were reported.
Subject screening, sample collection, and inoculation protocol.
In collaboration with UCF Student Health Services, we recruited active smokers who considered themselves healthy, were not taking any medications or oral or topical antibiotics for at least 1 month, and were interested in smoking cessation to be screened for nasal S. aureus carriage. Participants sampled each nostril by rotating a sterile polyester-tipped swab around the anterior vestibule 10 times. Swabs were dipped and swirled into 2 ml Bacto tryptic soy broth (TSB; Becton Dickinson [BD], Franklin Lakes, NJ) to extract and culture the microbes. From each 2-ml mixture of microbes and broth, 0.5 ml was added to 0.5 ml TSB–30% glycerol and frozen immediately at −80°C as a noncultured “early” glycerol stock, 0.1-ml and 10-fold serial dilutions were spread onto BD CHROMagar Staph aureus (Fisher Scientific catalog number 14-432-41) or Remel mannitol salt agar (Fisher Scientific catalog number R01580) for identification of S. aureus, and 0.1-ml and serial 10-fold dilutions were spread onto TSAII agar containing 5% sheep blood (Fisher Scientific catalog number B21261X) for enumeration of S. aureus and non-S. aureus CFU. The remaining broth-microbe mixture was placed in culture overnight (total, 4 ml TSB; 250 rpm, 37°C) for preparation of a cultured “late” glycerol stock the next day. Agar plates were incubated 18 h in a 37°C bacterial oven. S. aureus colonies on TSAII–5% sheep blood agar were confirmed using the BD Staphyloslide Latex Test kit (Fisher Scientific catalog number B4340953) and subcultured overnight to make S. aureus colony glycerol stocks and aliquots of glycerol-free stocks to be used on experiment days. Participants' nasal fluids were collected by suction catheter as described previously (17, 18) and stored at −80°C immediately.
In the first arm of the study, smokers positive for nasal S. aureus at the screening visit were sampled (day −17 nasal swab and nasal fluid collection) as described above and decolonized via self-application of mupirocin nasal ointment (Bactroban; GlaxoSmithKline, Philadelphia, PA) twice daily for 5 days. One week after the last mupirocin application (day −6), participants were sampled to confirm clearance of nasal S. aureus and recovery of commensal nasal microflora and to collect “preinoculation” nasal fluid. At day zero, participants inoculated their nasal mucosa with their own S. aureus isolate, which had been cultured in TSB for 2.5 h (250 rpm, 37°C), washed three times with sterile Hanks' buffered salt solution (HBSS; Invitrogen/ThermoFisher catalog number 21-023-CV), and resuspended such that each nostril received 2 × 107 CFU. Nasal swabs were performed the next day, immediately prior to a repeat inoculation. Swab collections occurred every 3 to 4 days thereafter for a month of follow-up. Nasal fluids were collected at days 3, 8, 11, 15 or 16, 23 or 24, and 31 or 32 postinoculation. Expired-air CO measurements, for confirming smoking status, were obtained at most visits using the Smokerlyzer (coVita LLC, Santa Barbara, CA) according to the manufacturer's instructions. In total, 19 experimental inoculation studies were completed with active smokers.
Smokers who successfully completed the UCF Student Health Services' “Quit Smoking Now” (QSN) cessation course and/or remained smoke free for at least 1 month and consented to a second study underwent a repeat mupirocin decolonization protocol followed by inoculation with autologous S. aureus and month-long sampling as described above. The QSN program consists of six 1-h weekly sessions that provide participants with the knowledge, techniques, and support necessary to quit using tobacco. There is an option for participants to receive free nicotine replacement therapy (NRT) in the form of patches or lozenge or gum. Expired-air CO was measured at each visit to confirm smoking status. Eight participants completed both study arms, while a ninth subject successfully quit smoking before the screening visit (and thus participated only as a member of the cessation group).
Bacterial genotyping and assessment of mupirocin sensitivity.
Donor S. aureus strains were genotyped by multilocus sequence typing (MLST) and spa typing as described previously (49). Isolates were screened for the mecA gene by performing PCR as described originally by Tokue et al. (50) using the primers MR1, 5′-AGACGATCCTTCGGTGAGC-3′, and MR2, 5′-GCTTTTGCAATGTCATTTACTG-3′. The PCR conditions were as follows: 2 μl (∼200 ng) bacterial DNA template was added to 400 μM deoxynucleoside triphosphate (dNTP) mix (Invitrogen catalog number18427088), 200 nM primers, 2 mM MgSO4, 2% dimethyl sulfoxide (DMSO), 1× buffer, and 1 U Platinum Taq (Platinum Taq DNA Polymerase High Fidelity kit; Invitrogen catalog number 11304029). Thermocyler (Bio-Rad T100) settings were 94°C for 5 min, followed by 40 cycles of 94°C for 1 min, 60°C for 1.5 min, and 72°C for 2 min, and a final extension step of 72°C for 10 min. A 1,339-bp PCR product or no amplicon indicated the presence or absence, respectively, of mecA, which encodes the penicillin-binding protein (PBP2A) that confers resistance to β-lactam antibiotics. Donor S. aureus strains were also tested for functional mupirocin resistance by performing turbidity (growth) assays (51). S. aureus isolates were grown to log phase in TSB, diluted to 104 CFU/90 μl in Mueller-Hinton broth (Sigma-Aldrich, St. Louis, MO) containing 5% sucrose, and loaded to a 96-well culture plate. Ten microliters of 2-fold serial dilutions of either mupirocin (catalog number M-7694; Sigma-Aldrich) or vehicle (volume-matched DMSO) was added to the diluted S. aureus culture so that the final concentration of mupirocin ranged from 1 to 16 μg/ml. The culture plate was covered with ThermoSeal A film (catalog number TSA-100; Excel Scientific, Inc.) and placed into a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA) programmed for a 16-h kinetic assay at 37°C. Turbidity measurements at optical density at 550 nm (OD550) were taken every 5 min following 15 s of agitation. Growth curves (OD550 plotted against time) were generated for all wells, and both the input and 16-h incubation cultures were plated on TSAII–5% sheep blood agar for enumeration. All strains grew to 107 to 108 CFU/0.1 ml in antibiotic-free medium. All strains were killed in the presence of ≤1 μg/ml mupirocin.
Measurement of cytokines, chemokines, and growth factors.
Nasal fluids were thawed, processed, and analyzed for cytokine, chemokine, and growth factor levels as described in reference 18. All nasal fluids were assayed with Bio-Rad's Bio-Plex Pro Human Cytokine group I 27-plex panel (Bio-Rad catalog number M50-0KAF0Y). All analytes were detectable in all or some participants' nasal fluids, except IL-2, IL-4, IL-15, and GM-CSF. The detection limits of the analytes in the utilized kits typically ranged between 1 and 8 pg/ml.
Statistical analysis.
Data were analyzed using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA). In most cases, S. aureus counts (CFU/nasal swab) were log transformed. Nasal fluid analytes were analyzed and are presented as raw picograms/milliliter or log10 picograms/milliliter. All participants produced similar volumes of fluid (0.4 to 0.8 ml). Groups (smokers versus recent cessation, carriers versus clearers, males versus females) were compared using unpaired t tests (two tailed). For cytokine analyses, we performed planned comparisons of S. aureus-associated and S. aureus-independent G-CSF, IL-1β, and IL-1RA levels based on previous findings (18). Correlations (Pearson's r, R2, P value) between nasal S. aureus counts and cytokine levels were considered significant at P levels of <0.05 and if linear regression lines were significant for a nonzero slope.
ACKNOWLEDGMENTS
A.M.C. was supported by the Florida Department of Health, James and Esther King Biomedical Research Program, and internal funds from UCF. We declare that we have no conflicts of interest. We are grateful to the participants who donated swab samples and nasal fluids and who participated in the self-inoculation studies.
Author contributions: the recruitment of human subjects and design of in vivo studies were the work of A.M.C., A.L.C., M.S.-O., J.S., and M.G.D. Technical work was performed by A.C.B., C.F.C., and A.L.C. Statistical analysis was performed under the guidance of P.M.T. The manuscript was written by A.L.C.
REFERENCES
- 1.Mulcahy ME, McLoughlin RM. 2016. Host-bacterial crosstalk determines Staphylococcus aureus nasal colonization. Trends Microbiol 24:872–886. doi: 10.1016/j.tim.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Mermel LA, Cartony JM, Covington P, Maxey G, Morse D. 2011. Methicillin-resistant Staphylococcus aureus colonization at different body sites: a prospective, quantitative analysis. J Clin Microbiol 49:1119–1121. doi: 10.1128/JCM.02601-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaspar U, Kriegeskorte A, Schubert T, Peters G, Rudack C, Pieper DH, Wos-Oxley M, Becker K. 2016. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ Microbiol 18:2130–2142. doi: 10.1111/1462-2920.12891. [DOI] [PubMed] [Google Scholar]
- 4.Miller RR, Walker AS, Godwin H, Fung R, Votintseva A, Bowden R, Mant D, Peto TE, Crook DW, Knox K. 2014. Dynamics of acquisition and loss of carriage of Staphylococcus aureus strains in the community: the effect of clonal complex. J Infect 68:426–439. doi: 10.1016/j.jinf.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kock R, Werner P, Friedrich AW, Fegeler C, Becker K, Prevalence of Multiresistant Microorganisms Study Group. 2016. Persistence of nasal colonization with human pathogenic bacteria and associated antimicrobial resistance in the German general population. New Microbes New Infect 9:24–34. doi: 10.1016/j.nmni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abad CL, Pulia MS, Safdar N. 2013. Does the nose know? An update on MRSA decolonization strategies. Curr Infect Dis Rep 15:455–464. doi: 10.1007/s11908-013-0364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 8.Burian M, Rautenberg M, Kohler T, Fritz M, Krismer B, Unger C, Hoffmann WH, Peschel A, Wolz C, Goerke C. 2010. Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis 201:1414–1421. doi: 10.1086/651619. [DOI] [PubMed] [Google Scholar]
- 9.Mulcahy ME, Geoghegan JA, Monk IR, O'Keeffe KM, Walsh EJ, Foster TJ, McLoughlin RM. 2012. Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin. PLoS Pathog 8:e1003092. doi: 10.1371/journal.ppat.1003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannessen M, Sollid JE, Hanssen AM. 2012. Host- and microbe determinants that may influence the success of S. aureus colonization. Front Cell Infect Microbiol 2:56. doi: 10.3389/fcimb.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baur S, Rautenberg M, Faulstich M, Grau T, Severin Y, Unger C, Hoffmann WH, Rudel T, Autenrieth IB, Weidenmaier C. 2014. A nasal epithelial receptor for Staphylococcus aureus WTA governs adhesion to epithelial cells and modulates nasal colonization. PLoS Pathog 10:e1004089. doi: 10.1371/journal.ppat.1004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Unger C, Weidenmaier C, Lalk M, Peschel A. 2014. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10:e1003862. doi: 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen K, Danielsen K, Wilsgaard T, Sangvik M, Sollid JU, Thune I, Eggen AE, Simonsen GS, Furberg AS. 2013. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS One 8:e63716. doi: 10.1371/journal.pone.0063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peacock SJ, de Silva I, Lowy FD. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol 9:605–610. doi: 10.1016/S0966-842X(01)02254-5. [DOI] [PubMed] [Google Scholar]
- 15.Breuer K, Haussler S, Kapp A, Werfel T. 2002. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol 147:55–61. [DOI] [PubMed] [Google Scholar]
- 16.Cole AM, Dewan P, Ganz T. 1999. Innate antimicrobial activity of nasal secretions. Infect Immun 67:3267–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole AM, Tahk S, Oren A, Yoshioka D, Kim YH, Park A, Ganz T. 2001. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol 8:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole AL, Muthukrishnan G, Chong C, Beavis A, Eade CR, Wood MP, Deichen MG, Cole AM. 2016. Host innate inflammatory factors and staphylococcal protein A influence the duration of human Staphylococcus aureus nasal carriage. Mucosal Immunol 9:1537–1548. doi: 10.1038/mi.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. 2016. Current cigarette smoking among adults—United States, 2005-2015. MMWR Morb Mortal Wkly Rep 65:1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 20.Durmaz R, Tekerekoglu MS, Kalcioglu T, Ozturan O. 2001. Nasal carriage of methicillin-resistant Staphylococcus aureus among smokers and cigarette factory workers. New Microbiol 24:143–147. [PubMed] [Google Scholar]
- 21.Soltani B, Taghavi Ardakani A, Moravveji A, Erami M, Haji Rezaei M, Moniri R, Namazi M. 2014. Risk factors for methicillin resistant Staphylococcus aureus nasal colonization of healthy children. Jundishapur J Microbiol 7:e20025. doi: 10.5812/jjm.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi CS, Yin CS, Bakar AA, Sakewi Z, Naing NN, Jamal F, Othman N. 2006. Nasal carriage of Staphylococcus aureus among healthy adults. J Microbiol Immunol Infect 39:458–464. [PubMed] [Google Scholar]
- 23.Wardyn SE, Forshey BM, Smith TC. 2012. High prevalence of Panton-Valentine leukocidin among methicillin-sensitive Staphylococcus aureus colonization isolates in rural Iowa. Microb Drug Resist 18:427–433. doi: 10.1089/mdr.2011.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito S, Terranova L, Zampiero A, Ierardi V, Rios WP, Pelucchi C, Principi N. 2014. Oropharyngeal and nasal Staphylococcus aureus carriage by healthy children. BMC Infect Dis 14:723. doi: 10.1186/s12879-014-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitazawa K, Sotozono C, Sakamoto M, Sasaki M, Hieda O, Yamasaki T, Kinoshita S. 2016. Nasal and conjunctival screening prior to refractive surgery: an observational and cross-sectional study. BMJ Open 6:e010733. doi: 10.1136/bmjopen-2015-010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen K, Falch BM, Danielsen K, Johannessen M, Ericson Sollid JU, Thune I, Grimnes G, Jorde R, Simonsen GS, Furberg AS. 2012. Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromso Staph and Skin Study. Eur J Clin Microbiol Infect Dis 31:465–473. doi: 10.1007/s10096-011-1331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos N, Stachel A, Phillips M, Vigdorchik J, Slover J, Bosco JA. 2016. Prior Staphylococcus aureus nasal colonization: a risk factor for surgical site infections following decolonization. J Am Acad Orthop Surg 24:880–885. doi: 10.5435/JAAOS-D-16-00165. [DOI] [PubMed] [Google Scholar]
- 28.Claessen FM, Braun Y, van Leeuwen WF, Dyer GS, van den Bekerom MP, Ring D. 2016. What factors are associated with a surgical site infection after operative treatment of an elbow fracture? Clin Orthop Relat Res 474:562–570. doi: 10.1007/s11999-015-4523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins KA, Karelitz JL, Jao NC. 2013. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob Res 15:978–982. doi: 10.1093/ntr/nts205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. 2012. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog 8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. 2006. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Jaspers I. 2014. Cigarette smoke effects on innate immune mechanisms in the nasal mucosa. Potential effects on the microbiome. Ann Am Thorac Soc 11(Suppl 1):S38–S42. doi: 10.1513/AnnalsATS.201306-154MG. [DOI] [PubMed] [Google Scholar]
- 33.Stampfli MR, Anderson GP. 2009. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 34.Guzik K, Skret J, Smagur J, Bzowska M, Gajkowska B, Scott DA, Potempa JS. 2011. Cigarette smoke-exposed neutrophils die unconventionally but are rapidly phagocytosed by macrophages. Cell Death Dis 2:e131. doi: 10.1038/cddis.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews JB, Chen FM, Milward MR, Ling MR, Chapple IL. 2012. Neutrophil superoxide production in the presence of cigarette smoke extract, nicotine and cotinine. J Clin Periodontol 39:626–634. doi: 10.1111/j.1600-051X.2012.01894.x. [DOI] [PubMed] [Google Scholar]
- 36.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. 2005. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res 6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEachern EK, Hwang JH, Sladewski KM, Nicatia S, Dewitz C, Mathew DP, Nizet V, Crotty Alexander LE. 2015. Analysis of the effects of cigarette smoke on staphylococcal virulence phenotypes. Infect Immun 83:2443–2452. doi: 10.1128/IAI.00303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, Verbrugh HA, Wertheim HF. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 199:1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 39.Muthukrishnan G, Lamers RP, Ellis A, Paramanandam V, Persaud AB, Tafur S, Parkinson CL, Cole AM. 2013. Longitudinal genetic analyses of Staphylococcus aureus nasal carriage dynamics in a diverse population. BMC Infect Dis 13:221. doi: 10.1186/1471-2334-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 41.Quinn GA, Tarwater PM, Cole AM. 2009. Subversion of interleukin-1-mediated host defence by a nasal carrier strain of Staphylococcus aureus. Immunology 128:e222–e229. doi: 10.1111/j.1365-2567.2008.02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M, Nizet V, Eckmann L. 2009. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci U S A 106:12873–12878. doi: 10.1073/pnas.0904958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrier S, Darakhshan F, Hajduch E. 2006. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett 580:6289–6294. doi: 10.1016/j.febslet.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 44.van Belkum A, Emonts M, Wertheim H, de Jongh C, Nouwen J, Bartels H, Cole A, Cole A, Hermans P, Boelens H, Toom NL, Snijders S, Verbrugh H, van Leeuwen W. 2007. The role of human innate immune factors in nasal colonization by Staphylococcus aureus. Microbes Infect 9:1471–1477. doi: 10.1016/j.micinf.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabak AG, Schloot NC, Witte DR. 2009. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care 32:421–423. doi: 10.2337/dc08-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alves De Souza C, Queiroz Alves De Souza A, Queiroz Alves De Souza MDS, Dias Leite JA, Silva De Morais M, Barem Rabenhorst SH. 2017. A link between osteomyelitis and IL1RN and IL1B polymorphisms-a study in patients from Northeast Brazil. Acta Orthop 88:556–561. doi: 10.1080/17453674.2017.1348439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mestas J, Hughes CC. 2004. Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 48.Lehrer RI, Lichtenstein AK, Ganz T. 1993. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol 11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 49.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokue Y, Shoji S, Satoh K, Watanabe A, Motomiya M. 1992. Comparison of a polymerase chain reaction assay and a conventional microbiologic method for detection of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 36:6–9. doi: 10.1128/AAC.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamers RP, Eade CR, Waring AJ, Cole AL, Cole AM. 2011. Characterization of the retrocyclin analogue RC-101 as a preventative of Staphylococcus aureus nasal colonization. Antimicrob Agents Chemother 55:5338–5346. doi: 10.1128/AAC.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]