ABSTRACT
Chagas disease affects 6 to 7 million people worldwide, resulting in significant disease burdens and health care costs in countries of endemicity. Chemotherapeutic treatment is restricted to two parasiticidal drugs, benznidazole and nifurtimox. Both drugs are highly effective during acute disease but are only minimally effective during chronic disease and fraught with significant adverse clinical effects. In experimental models, vaccines can be used to induce parasite-specific balanced TH1/TH2 immune responses that effectively reduce parasite burdens and associated inflammation while minimizing adverse effects. The objective of this study was to determine the feasibility of vaccine-linked chemotherapy for reducing the amount of benznidazole required to significantly reduce blood and tissue parasite burdens. In this study, we were able to achieve a 4-fold reduction in the amount of benznidazole required to significantly reduce blood and tissue parasite burdens by combining the low-dose benznidazole with a recombinant vaccine candidate, Tc24 C4, formulated with a synthetic Toll-like 4 receptor agonist, E6020, in a squalene oil-in-water emulsion. Additionally, vaccination induced a robust parasite-specific balanced TH1/TH2 immune response. We concluded that vaccine-linked chemotherapy is a feasible option for advancement to clinical use for improving the tolerability and efficacy of benznidazole.
KEYWORDS: Chagas disease, Trypanosoma cruzi, E6020 adjuvant, benznidazole, recombinant protein vaccine, CD8+ T cell response
INTRODUCTION
Chagas disease, caused by infection with the intracellular protozoal parasite Trypanosoma cruzi, affects approximately 5.7 to 6.7 million people worldwide, with the vast majority of cases in the Americas (1, 2). Acute infection lasts for approximately the first 2 to 3 months and clinically can present with no symptoms or a nonspecific febrile illness, with readily detectable parasitemia due to rapid replication of parasites within tissues (3). The evolution of a parasite-specific TH1-biased immune response results in reduction of parasitemia to undetectable levels through the actions of parasite-specific lytic antibodies and cytotoxic cellular responses. After the initial acute phase, infected individuals transition to the clinically asymptomatic indeterminate phase (3). The majority of infected individuals remain in this indeterminate phase indefinitely, but approximately 30% of individuals develop chronic Chagasic cardiomyopathy years to decades after initial infection (4). Chronic Chagasic cardiomyopathy is caused by multiple factors, including tissue damage caused by parasite persistence, dysautonomia, and microvascular disturbances (3, 5, 6). These factors initially manifest as conduction disturbances in the cardiovascular system, progressing to cardiac aneurisms, dilated cardiomyopathy, and sudden cardiac death during end-stage disease (4). Chagas disease is the leading cause of nonischemic dilated cardiac disease in Latin America and represents a significant economic burden to health care systems (7–9). The full disease burden of Chagasic cardiomyopathy is being elucidated, but some efforts indicate that it affects more than one million people, with recent studies suggesting that 17 to 18% of Chagasic cardiomyopathy patients will die over the next 5 years (10, 11).
Current treatment for Chagas disease is challenging, being restricted to the two chemotherapeutic agents benznidazole and nifurtimox. Both have prolonged treatment courses, 2 to 3 months in length, and limited efficacy beyond the acute phase of disease (12). Treatment frequently causes significant side effects after the first few weeks of treatment, causing up to 40% of individuals to discontinue treatment (13–15). Thus, novel drugs and reduced dosing treatment regimens have been explored to improve efficacy and tolerability. Reduced-time, combination drug, and novel drug treatment schemes have been tested in preclinical models with some success. Treatment with the combination of benznidazole and posaconazole for 10 days, half of the standard treatment time, has been shown to cure one-half to two-thirds of infected mice (16, 17). When treatment with posaconazole preceded intermittent treatment with benznidazole for 60 days, 100% of mice were cured (16). AmBisome, a liposomal formulation of amphotericin B used to treat visceral leishmaniasis, has been shown to significantly reduce blood and tissue parasite burdens while prolonging survival in both acute and chronic mouse infections with T. cruzi (18). While novel drugs and combination treatments are a promising option, the lack of successful translation from preclinical models to human testing, compounded with the existence of naturally drug-resistant T. cruzi strains, limits the effectiveness of these treatment options (19–21). Additionally, chemotherapy alone does not reverse existing cardiac disease or prevent cardiac death in chronically infected individuals (10, 15) Thus, there is an urgent need for additional therapeutic options for Chagas disease.
Studies of the immune response against natural and experimental T. cruzi infections have elucidated several key parameters that are correlated with the presence or absence of clinical disease. A TH1-directed immune response with antigen-specific gamma interferon (IFN-γ) as well as CD8+ effector cells is essential for controlling tissue parasite burdens (22). T. cruzi is able to modulate the immune system in its favor to survive and secretes several molecules that promote interleukin-10 (IL-10) secretion by dendritic cells (23). However, IL-4 and IL-10 are necessary cytokines to modulate or ameliorate parasite-induced inflammation and tissue pathology (24). Studies in chronic indeterminate human patients without disease and chronic patients with disease have confirmed that antigen-specific IFN-γ balanced by IL-10 correlates with the absence of clinical disease (25). When parasite-specific immune responses are evaluated after benznidazole treatment, antigen-specific IFN-γ is initially increased and is believed to enhance efficacy of the drug (26, 27). Further studies in preclinical models show that benznidazole cure rates are significantly reduced in mice lacking IFN-γ, IL-12, and tumor necrosis factor alpha (TNF-α) (28). However, treatment with suboptimal benznidazole combined with exogenous IL-12 enhanced drug efficacy (29). Taken together, these data suggest that limiting disease progression is dependent upon a balanced TH1/TH2 parasite-specific immune response controlling parasite burdens while limiting tissue pathology and organ dysfunction. Further, efficacy of benznidazole is due to both direct parasiticidal effects and appropriate parasite-specific immune responses of the host.
Capitalizing on the knowledge that immune control of T. cruzi correlates with reduced disease in both naturally infected humans and experimental animal models, numerous vaccine candidates have been designed to induce or boost TH1-directed T. cruzi-specific immune responses. The Tc24 flagellar calcium binding protein of T. cruzi has been studied extensively as a vaccine candidate delivered as a DNA vaccine in mice and dogs and has been shown to reduce parasite burdens and cardiac pathology (22, 30–32). Since DNA vaccines historically have not translated to effective vaccines in humans, further studies have evaluated the Tc24 antigen as a recombinant protein antigen which has more potential for successful translation to a clinical vaccine (33). Initial studies have demonstrated efficacy of preventative Tc24 recombinant protein-based vaccines, which reduced parasite burdens and increased survival through IFN-γ-driven immune control of the parasite (34, 35). Further studies have demonstrated that candidate Tc24 vaccines are partially efficacious when used therapeutically, reducing cardiac parasite burdens and inflammation (36, 37). Thus, vaccines are a very attractive therapeutic option for treating Chagas disease that could be used alone or in combination with specific antiparasitic drug therapy as a way to reduce tissue parasite burdens and enhance host immune control of infection while minimizing side effects. Since the adaptive immune response is too slow in developing a proper specific CD8+ T cell response against T. cruzi in an acute infection (38), we hypothesized that reducing the parasite burden first with a low dose of benznidazole, followed by boosting the parasite-specific immune response with a vaccine would give the immune system enough time to elicit a proper CD8+ T cell response to clear the infection. Here, we report a proof-of-concept study demonstrating that the combined effects of low-dose benznidazole treatment and vaccination significantly reduce blood and cardiac parasite burdens as well as cardiac inflammation in acutely infected mice. The vaccine is comprised of a recombinant form of the Tc24 antigen in which four canonical cysteines were replaced with serines (Tc24 C4) in order to prevent intermolecular disulfide bond formation and aggregation during scale-up production and manufacture (37). The Tc24 C4 antigen is formulated with E6020 (Tc24 C4/E6020), a synthetic Toll-like receptor 4 (TLR4) agonist, in a squalene oil-in-water emulsion to augment specific yet balanced TH1 cellular immune responses. Here, we describe both significant parasite reductions and reduced cardiac inflammation relative to levels in controls following administration of the vaccine linked to benznidazole chemotherapy.
RESULTS
Combination treatment significantly reduces parasite burdens.
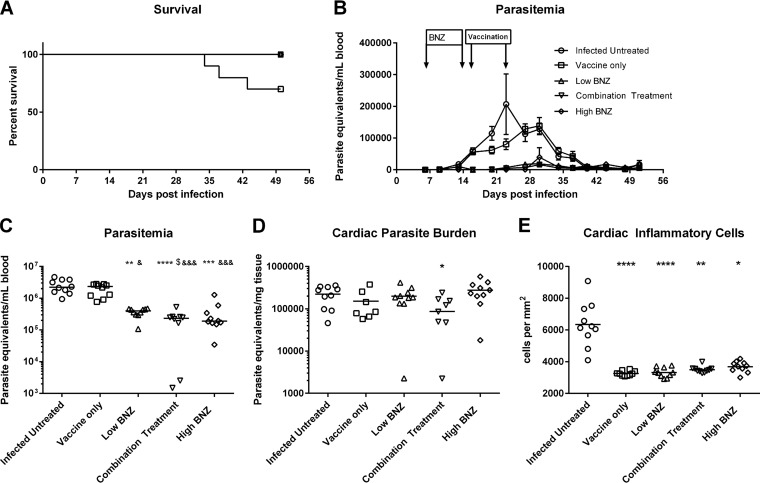
Acutely infected mice were treated therapeutically with a low dose of benznidazole, the Tc24 C4/E6020 squalene emulsion (SE) vaccine, or a combination treatment of benznidazole followed by vaccination. Survival was reduced in mice receiving vaccine alone (70% survival) compared to that of all other groups (100% survival), but this reduction did not reach statistical significance (Fig. 1A). Peak parasitemia in infected untreated mice occurred earlier, at approximately 23 days of infection, than in infected mice receiving treatment, where the peaks occurred at approximately 30 days of infection (Fig. 1B). In a comparison of the areas under the curve between groups, combination treatment reduced overall parasitemia by almost 93% compared to the level for untreated mice (Table 1). This reduction was statistically significant compared to that in untreated mice as well as in mice receiving either vaccine or low-dose benznidazole alone (Fig. 1C). Combination treatment also reduced cardiac parasite burdens by 61% (Table 1), which was the only statistically significant reduction in all the treatment groups (Fig. 1D). Taken together, these data show that combination treatment was the most effective for reducing circulating and tissue parasite burdens.
FIG 1.
Survival, parasite burdens, and cardiac inflammation. Mice were infected with T. cruzi H1 and then treated with benznidazole, vaccine, or a combination treatment. Survival was monitored daily, and parasitemia was measured twice weekly from day 7 until day 53 postinfection. Parasitemia and cardiac parasite burdens were quantified by quantitative real-time PCR. Cardiac inflammation was quantified from representative images of H&E-stained tissue sections using Image J Fiji software. Survival curves were compared to those of the infected untreated control using a Mantel-Cox log rank test. A P value of ≤0.05 was considered statistically significant. For parasitemia, cardiac parasite burden, and cardiac inflammatory infiltrate, groups were compared using Kruskal-Wallis one-way ANOVA and Dunn's multiple-comparison test. Significance is indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001 (comparing results for the treated groups to the those of the infected untreated control); &, P ≤ 0.05; &&&, P ≤ 0.001 (comparing results for the groups to the those of the vaccine-only group); $, P ≤ 0.05 (comparing results for the groups to those of the low-dose benznidazole group). Survival was not significantly different between treatment groups (A). The parasitemia curves are shown in panel B. Combination treatment significantly reduced total parasitemia (C) and cardiac parasite burdens (D). All mice receiving either single treatment or combination treatment had significantly reduced cardiac inflammation (E). BNZ, benznidazole.
TABLE 1.
Compiled data of parasitemia, cardiac parasite burden, and cardiac inflammationa
| Groupb | Parasitemia |
Cardiac parasite burden |
Cardiac inflammation |
|||
|---|---|---|---|---|---|---|
| No. of parasites/ml blood | % reduction | No. of parasites/mg tissue | % reduction | No. of inflammatory cells/mm2 tissue | % reduction | |
| Infected untreated | 2.56 × 106 | NA | 2.22 × 105 | NA | 6,350 | NA |
| Vaccine only | 1.96 × 106 | 23.55 | 1.51 × 105 | 31.86 | 3,263 | 48.61 D |
| Low BNZ | 3.67 × 105 | 85.65 BE | 2.00 × 105 | 10.10 | 3,326 | 47.62 D |
| Combination treatment | 1.92 × 105 | 92.49 DFG | 8.67 × 104 | 60.94 A | 3,499 | 44.90 B |
| High BNZ | 3.33 × 105 | 86.98 CF | 2.75 × 105 | 23.80 | 3,681 | 42.03 A |
Data were analyzed as described in the legend of Fig. 2. Combination treatment resulted in the highest reductions in parasitemia and cardiac parasites, which was greater than reductions with any single treatment. All treatments resulted in at least a 42% reduction in cardiac inflammation. Significance is indicated as follows: A, P ≤ 0.05; B, P ≤ 0.01; C, P ≤ 0.001; D, P ≤ 0.0001 (for comparisons of results for the treated groups to those of the infected untreated control); E, P ≤ 0.05; F, P ≤ 0.000 (for comparisons of results to those of the vaccine-only group); G, P ≤ 0.05 (for comparisons of results to those of the low-dose benznidazole group). NA, not available.
BNZ, benznidazole.
Therapeutic treatment significantly reduces cardiac inflammation.
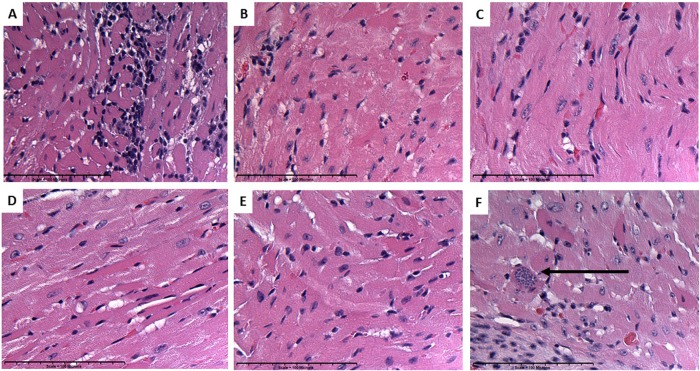
Acute myocarditis, as evidenced by infiltration of cardiac tissue with lymphocytes and other inflammatory cells, is characteristic of acute infection with T. cruzi (30). Inflammatory cells were observed microscopically in the cardiac tissue of acutely infected untreated mice (Fig. 2A), and in some cases T. cruzi amastigote nests were seen (Fig. 2F). Treatment of acutely infected mice therapeutically with the Tc24 C4 vaccine (Fig. 2B), either dose of benznidazole (Fig. 2C and E), or the combination treatment (Fig. 2D) significantly reduced infiltration of cells into cardiac tissue compared to levels in infected untreated controls (Fig. 1E). Treatments reduced inflammatory cells in cardiac tissue by 42 to 48% (Table 1). These data show that benznidazole alone, vaccine alone, and the combination treatment are all effective at reducing cardiac inflammation in acutely infected mice.
FIG 2.
Representative images of H&E-stained sections of cardiac tissue from infected control and treated mice. Inflammatory cells were more prevalent in acutely infected untreated mice (A), and amastigote nests were observed (F) (arrows). Acutely infected mice treated with vaccine only (B), low-doze benznidazole (C), combination treatment (D) or high-dose benznidazole (E) had significantly reduced numbers of inflammatory cells in cardiac tissue.
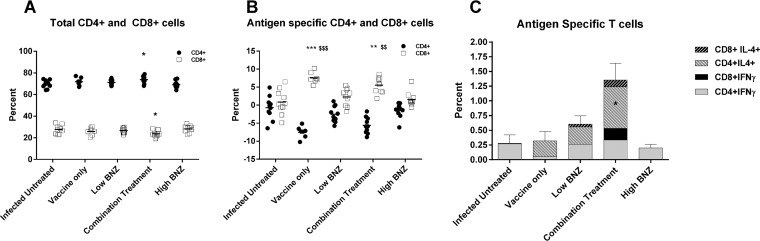
Vaccination increases antigen-specific CD8+ T cells.
In our model, acutely infected mice treated therapeutically with the combination treatment had significantly increased total CD4+ T cell counts compared to level sin infected mice that were left untreated (Fig. 3A). Interestingly, there was a concomitant decrease in total CD8+ T cells (Fig. 3A). Upon in vitro restimulation with recombinant Tc24 C4 protein, mice receiving vaccine alone or combination treatment had significantly decreased percentages of CD4+ cells (Fig. 3B) and concurrently increased percentages of CD8+ T cells (Fig. 3B), indicating that in vivo boosting with the vaccine primes the CD8+ population for expansion when cells are restimulated with antigen. Measurement of cytokine-producing cells indicated that the total percentage of CD4+ IL-4-positive (IL-4+) cells was significantly increased with combination treatment (Fig. 3C), but there were no significant increases in IFN-γ-producing T cells or CD8+ IL-4+ cells (Fig. 3C). These data indicate that therapeutic vaccination boosts the capacity for antigen-specific CD8+ T cell expansion upon restimulation but does not significantly increase the percentage of antigen-specific cytokine-producing T cells.
FIG 3.
Total and antigen-specific T cell responses. Splenocytes were stimulated in vitro with 100 μg/ml recombinant Tc24 C4 protein or medium alone for 72 h to measure the percentage of antigen-specific or total (unstimulated) T cells, respectively. Cells were stained for viability and surface expression of CD3, CD4, and CD8. To detect cytokine-producing cells, cells were then fixed and permeabilized before staining for intracellular IFN-γ and IL-4. At least 100,000 events were acquired in a live gate on an LSR Fortessa flow cytometer. Data were analyzed using Venturi One software, and groups were compared using Kruskal-Wallis one-way ANOVA and Dunn's multiple-comparison test. Significance is indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (comparing results for the treated groups to the those of the infected untreated control); $$, P ≤ 0.01; $$$, P ≤ 0.001 (comparing results for groups to those of the low-dose benznidazole group). Combination treatment significantly increased total CD4+ cells and decreased CD8+ cells (A). Upon antigen-specific restimulation in vitro, the percentage of antigen-specific CD4+ cells significantly decreased with vaccine alone or combination treatment while antigen-specific CD8+ cells increased significantly (B). Combination treatment also significantly increased CD4+ IL-4+ cells but had no effect on the percentage of CD4+ IFN-γ+, CD8+ IFN-γ+, or CD8+ IL-4+ cells (C).
Vaccination induces a balanced TH1/TH2 response.
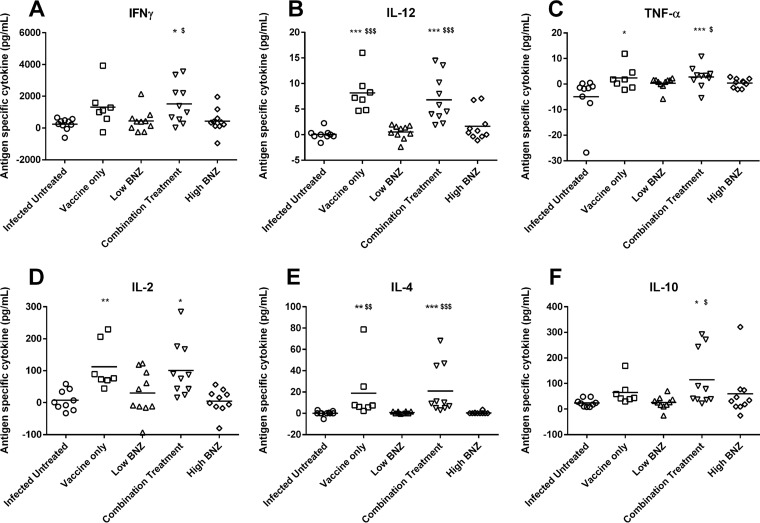
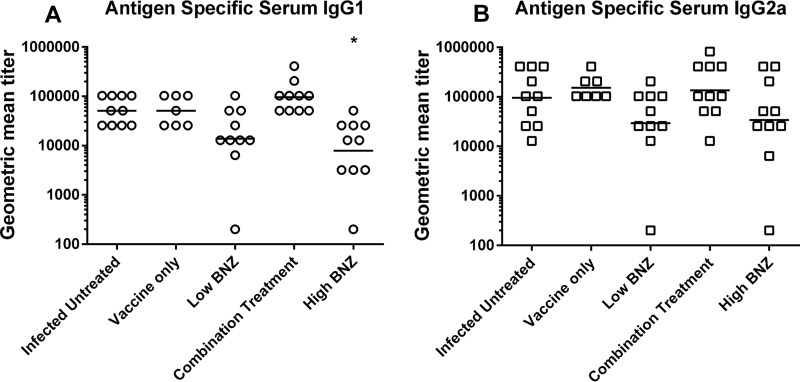
Combination treatment and, to a lesser extent, vaccine alone induced a balanced TH1/TH2 immune response. The cytokine profile elicited upon restimulation with antigen included significant increases in both pro- and anti-inflammatory cytokines. Mice receiving combination treatment had significantly increased secretion of antigen-specific IFN-γ (Fig. 4A). Secretion of IL-12 and TNF-α was increased (Fig. 4B and C), likely due to engagement of the TLR4 receptor on antigen-presenting cells by the E6020 component of the vaccine. IL-2 secretion was also increased (Fig. 4D), which could support the increased percentage of antigen-specific CD8+ cells. Concurrently, cytokines indicative of a healing response, IL-4 and IL-10, were also increased by combination treatment (Fig. 4E and F). IFN-γ, IL-12, TNF-α, IL-4, and IL-10 cytokine responses to combination treatment were significantly greater than those to low-dose benznidazole alone, and benznidazole treatment did not induce significant levels of any cytokine. While vaccine alone induced increased secretion of IL-12, TNF-α, IL-2, and IL-4, maximal responses for all cytokines were achieved by combination treatment. Interestingly, antigen-specific serum antibody titers were not significantly increased by vaccination. However, high-dose benznidazole treatment did significantly decrease antigen-specific IgG1 titers compared to levels in untreated mice (Fig. 5A). These data indicate that combination treatment and, to a lesser extent, vaccine alone induced a balanced TH1/TH2 immune response.
FIG 4.
Antigen-specific cytokine release from splenocytes. Splenocytes were stimulated in vitro with 100 μg/ml recombinant Tc24 C4 protein or medium alone for 96 h. Culture supernatants were centrifuged to remove cells, harvested, and stored at −80°C until quantification of secreted cytokines by Luminex. For each mouse, medium-stimulated cells served as background, and values in these cells were subtracted from the measurement of Tc24 C4-stimulated cells from the same mouse. Groups were compared in GraphPad Prism using Kruskal-Wallis one-way ANOVA and Dunn's multiple-comparison test. Significance is indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (comparing results for the infected groups to the those of the infected untreated control); $, P ≤ 0.05; $$, P ≤ 0.01; $$$, P ≤ 0.001 (comparing results for groups to those of the low-dose benznidazole group). Combination treatment significantly increased secretion of IFN-γ, IL-12, TNF-α, IL-2, IL-4, and IL-10 compared to levels in untreated mice. Additionally, levels of IFN-γ, IL-12, TNF-α, IL-4, and IL-10 were significantly higher than levels in mice treated with low-dose benznidazole alone.
FIG 5.
Antigen-specific serum antibody titers. Serum was isolated from terminal blood samples and frozen at −80°C until use. Antigen-specific IgG1 and IgG2a antibody titers were measured by indirect ELISA. Groups were compared using Kruskal-Wallis one-way ANOVA and Dunn's multiple-comparison test (* P ≤ 0.05, comparing results for the treated groups to those of the infected untreated controls). The level of antigen-specific IgG1 was significantly reduced by high-dose benznidazole treatment (A), but no other treatments had a significant impact on antibody levels.
Antigen-specific IFN-γ and IL-4 release correlates with protection.
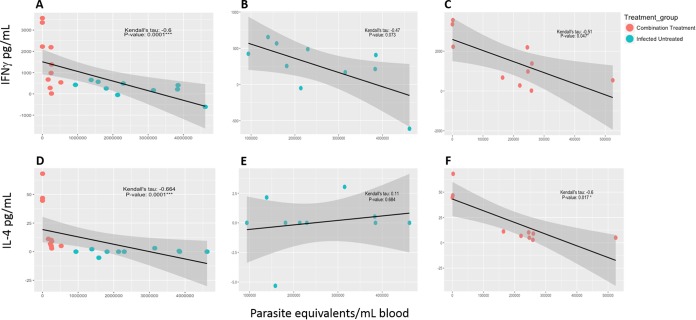
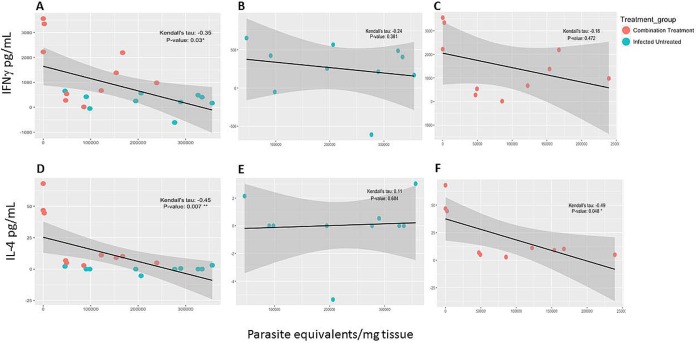
In order to determine if antigen-specific immune responses correlated with reduced parasite burdens and pathology, a correlation analysis was performed to evaluate associations between IFN-γ and IL-4 release and parasite burdens in blood and tissue. Evaluation of cytokine release and parasitemia indicated that the combination treatment group was the only group to show a statistically significant correlation between low parasitemia and higher cytokine levels (Fig. 6A and D). Antigen-specific IFN-γ release was significantly negatively correlated with parasitemia (Fig. 6C), while the level in the infected untreated group did not reach statistical significance (Fig. 6B). Similarly, antigen-specific IL-4 release was significantly negatively correlated with parasitemia in the combination treatment group (Fig. 6E), while there was no significant correlation in the infected untreated group (Fig. 6E). Treatment with vaccine only or benznidazole only also did not show any significant correlation between parasitemia and cytokine levels (see Fig. S1 in the supplemental material). Tissue parasite burdens also tended to decrease when cytokine levels were higher (Fig. 7A and D). Antigen-specific IL-4 release was significantly negatively correlated with cardiac parasite burdens in the combination treatment group (Fig. 7F), while there was no significant correlation in the infected untreated group. The observed negative correlations between IFN-γ release and cardiac parasite burdens were not statistically significant (Fig. 7B and C). Similar to findings for parasitemia, treatment with vaccine only or benznidazole only also did not show any significant correlation between tissue parasite burdens and cytokine levels (Fig. S2). Overall, the statistically significant negative correlations between cytokine release and parasitemia in the combination treatment group suggest that antigen-specific IFN-γ and IL-4 secretion could serve as predictors of treatment efficacy.
FIG 6.
Correlation analysis for cytokines and parasitemia. Correlation analysis was performed to evaluate associations between antigen-specific IFN-γ and IL-4 release and parasitemia as a measure of efficacy. Data were loaded into R, version 3.3.2, using the read_tsv function from the readr package, version 1.0.0. Parameters within the data were selected using the dplyr package, version 0.5.0. Subsequently, the PerformanceAnalytics package, version 1.4.3541, was used to visualize correlations between all parameters and to select groups within the data for further analysis. These groups were highlighted with a correlation plot using the GGally package, version 1.3.0. Kendall's tau coefficient and related P value were calculated using the rcor.test function within the ltm package, version 1.0-0, for correlation comparison. Output from this comparison was modified to present the correlation and related P value in tabular format using the dplyr package, version 0.5.0. Negative and positive associations were considered as alternative hypotheses. P values less than or equal to 0.05 were considered significant. There was a statistically significant correlation between IFN-γ (A) or IL-4 (D) secretion and parasitemia. In the combination treatment group there were statistically significant negative correlations between IFN-γ secretion and parasite burdens in the blood (C), as well as between IL-4 secretion and parasite burdens in the blood (F). Infected mice that did not receive treatment did not have statistically significant correlations between IFN-γ secretion and parasite burdens in the blood (B) or between IL-4 secretion and parasite burdens in the blood (E).
FIG 7.
Correlation analysis for cytokines and cardiac parasites. Correlation analysis was performed to evaluate associations between antigen-specific IFN-γ and IL-4 release and cardiac parasites as a measure of efficacy. Data were loaded into R, version 3.3.2, using the read_tsv function from the readr package, version 1.0.0. Parameters within the data were selected using the dplyr package, version 0.5.0. Subsequently, the Performance Analytics package, version 1.4.3541, was used to visualize correlations between all parameters and to select groups within the data for further analysis. These groups were highlighted with correlation plot using the GGally package, version 1.3.0. Kendall's tau coefficient and related P value were calculated using the rcor.test function within the ltm package, version 1.0-0, for correlation comparison. Output from this comparison was modified to present the correlation and related P value in tabular format using the dplyr package, version 0.5.0. Negative and positive were considered as alternative hypotheses. P values less than or equal to 0.05 were considered significant. There was a statistically significant correlation between IFN-γ (A) or IL-4 (D) secretion and cardiac parasite burden. In the combination treatment group there was a statistically significant negative correlation between IL-4 secretion and cardiac parasite burden (F), but there was not a significant correlation between IFN-γ secretion and cardiac parasite burden (C). Infected mice that did not receive treatment did not have statistically significant correlations between IFN-γ secretion and parasite burdens in the blood (B), or between IL-4 secretion and parasite burdens in the blood (E).
DISCUSSION
The Texas Children's Hospital Center for Vaccine Development is developing a therapeutic vaccine against Chagas disease, which would result in both a significant reduction in disease burden and cost savings for health care systems in countries of endemicity (9, 33). This vaccine could be used to improve the efficacy and tolerability of current standard benznidazole therapy. Benznidazole treatment is most effective during acute disease, which is believed to be due in part to the higher levels of parasite-induced proinflammatory cytokines (39, 40) (41–43). Indeed, parasite-specific IFN-γ release is higher in benznidazole-treated cured individuals than in those who do not achieve cure (44). Extensive studies in mouse models have demonstrated that IL-12, IFN-γ, and TNF-α stimulate inducible nitric oxide synthase (iNOS)-induced reactive nitrogen intermediates (RNI) which control parasite burdens (41–43, 45), and the absence of these mediators abrogates benznidazole efficacy (28). Multiple, full-dose courses of benznidazole can cure mice and induce a stable population of antigen-specific CD8+ memory cells (46). We have previously shown that the Tc24 C4/E6020 vaccine induces significant levels of antigen-specific IFN-γ in naive mice (37). Importantly, therapeutic vaccination of acutely affected mice with a Tc24 C4/E6020 vaccine significantly reduces cardiac parasite burdens, while vaccination with E6020 adjuvant alone does not (37). Here, we show that combining the rapid parasiticidal effects of benznidazole with the immune-boosting effects of the candidate Tc24 C4/E6020 SE vaccine results in improved therapeutic treatment efficacy in acutely infected mice. This improved efficacy was achieved by using both a low dose of benznidazole (25 mg/kg daily for 7 days) and a lower vaccine dose than previously used (25 μg Tc24 C4 plus 5 μg of E6020 SE for this study compared to 25 μg Tc24 C4 plus 25 μg of E6020 SE for the prior study). Further, combination treatment induced the largest amounts of IFN-γ and TNF-α, as well as significantly increased IL-12, IL-2, and percentages of antigen-specific CD8+ cells. While this treatment regime did not achieve parasitologic cure, it did result in significantly reduced parasite burdens and cardiac inflammation with a reduced dose of benznidazole. We propose that the improved efficacy is due to the synergism of direct parasiticidal effects of benznidazole, multiple parasite-specific immune control mechanisms, and boosting of benznidazole efficacy by IFN-γ. This model demonstrates the feasibility of combining chemotherapy with vaccination as a multipronged approach to treat Chagas disease.
Chronic Chagasic cardiomyopathy is a chronic inflammatory disease, leading to fibrosis and ultimately resulting in heart failure and death (6, 47). Parasite persistence is a key factor driving inflammation (48). Experimental animal models have proven that IFN-γ is essential for control of parasitism in vivo, but high IFN-γ levels in the absence of IL-4 results in inflammation in the heart (24). Inflammation also causes extracellular matrix deposition in cardiac tissue which can develop into fibrosis (49). Linking parasites to fibrosis highlights the dominant role of parasite persistence in the pathogenesis of Chagasic cardiomyopathy (50). Thus, elimination of parasites and control of inflammation are both key to ameliorating disease progression. In our model, initiating treatment with benznidazole significantly reduced parasite burdens, minimizing the key driver of chronic inflammation. Following benznidazole treatment with vaccine increased IFN-γ production, which enhanced parasite reduction through multiple mechanisms, and was balanced by IL-4 and IL-10, which limited cardiac inflammation and damage. The induction of a balanced parasite-specific immune response would support favorable remodeling of cardiac tissue after control of parasite burdens and less formation of fibrotic tissue. In the mouse model of acute infection, while the reduction in cardiac inflammation subsequent to combination treatment was significant, it was not significantly greater than that with vaccine or low-dose benznidazole alone. It is possible that due to the short term of the studies, the effect of combination treatment could not be fully realized; thus, longer studies evaluating a chronic-infection model of disease are necessary to determine whether combination treatment is superior to drug or vaccine monotherapy for preventing cardiac fibrosis. Additionally, studies evaluating the combination of vaccine with novel chemotherapies, such as posaconazole, would determine whether a combination treatment strategy could rescue drugs that have previously failed to cure Chagas disease in humans (19).
Chronic viral and protozoal infections can lead to immune exhaustion, resulting in expression of inhibitory receptors, reduced T cell proliferation, and loss of antigen-specific effector function (51–53). Chronic Chagas disease patients with severe disease do not have detectable levels of T. cruzi antigen-specific CD8+ cells, whereas patients without disease do have detectable levels of parasite-specific CD8+ cells and significant numbers of IFN-γ-producing cells (25). Seropositive children have a higher percentage of polyfunctional IFN-γ+/IL-2+ T cell responses than adults, who predominantly had monofunctional IFN-γ+-only T cells (54). Polyfunctional T cells, particularly IFN-γ, IL-2, and TNF-α triple producers, secrete higher levels of IFN-γ and are more efficient effector cells in protozoal infections (55, 56). These data support the assumption that loss of adequate immune control of parasites leads to parasite-induced tissue damage and disease progression. Therapeutic treatments that preserve robust parasite-specific immune responses would counteract the parasite-mediated immune suppression, controlling tissue damage and ultimately preventing or delaying disease progression. In our model, vaccination alone or following low-dose benznidazole treatment boosted antigen-specific CD8+ cell percentages and increased secretion of IFN-γ, as well as that of other pro- and anti-inflammatory cytokines. Interestingly, while increased secretion of IFN-γ was measured in vaccinated mice, concomitant increases in the percentages of CD4+ IFN-γ or CD8+ IFN-γ cells were not seen. Other cell types not identified in this study, such as NK cells, might be responsible for the observed increase in IFN-γ secretion. It is also possible that the CD4+ IFN-γ+ cells that were detected also produced IL-2 and TNF-α, but further analysis is necessary to characterize vaccine-induced polyfunctional T cells. Increased antigen-specific IFN-γ significantly correlated with reduced parasitemia, and increased antigen-specific IL-4 correlated with reduced parasitemia and cardiac parasite burdens. Absence of parasitemia and parasite-specific immune responses have been used as indicators of drug efficacy in humans (19, 26), and our data show that these parameters serve as informative correlates of protection in our combination treatment model as well.
Limitations of this model were observed. Survival and parasite burdens in mouse models of Chagas disease give clear signals of vaccine efficacy (30, 31, 57–60). However, low-virulence T. cruzi infection models, in which overall survival is high, can elucidate key aspects of disease pathogenesis (61, 62). The model used for the study described here showed high survival overall, but reduced survival was observed in the vaccine-only group (70%) although this observation was not statistically different from the results for the infected untreated control group. It is possible that in the animals that succumbed, the vaccine failed to sufficiently reduce parasite burdens. The mean parasitemia for the infected untreated control group was 2.56 × 106 parasites per ml of blood, while the mean for the vaccine-alone group was 1.96 × 106 parasites per ml of blood, a reduction of 24% that was not statistically significant. The three vaccinated animals that succumbed had 2.74 × 106, 2.47 × 106, and 1.28 × 106 parasites per ml of blood, respectively (data not shown). The two animals with the highest parasitemias may have died due to significant systemic inflammation and organ failure due to high parasite burdens. For the third animal, it appears that the vaccine did have some effect at controlling parasitemia, but it was still not sufficient for survival. It has been shown that delaying therapeutic vaccination during the acute phase from 5 days of infection to 10 days of infection results in progressively increasing parasitemia and increased cardiac inflammation compared to results with earlier administration (30). In our model, vaccination was not initiated until 17 days of infection; thus, delayed vaccination may have had reduced efficacy. Despite the increased parasitemia in the deceased animals, there was no evidence of increased cardiac inflammation. The mean number of cardiac inflammatory cells for the infected untreated control group was 6,350 cells per mm2 of tissue, and for the vaccine-only group it was 3,263 cells per mm2 of tissue. The animals that died had mean numbers of cardiac inflammatory cells of 3,119, 3,122, and 3,529 cells per mm2 of tissue, respectively, indicating that the vaccine did not induce excess inflammation. In prior studies evaluating immunogenicity of the vaccine in naive mice, there was no mortality observed; hence, the possibility of inherent vaccine toxicity is low (37). Despite the deaths in the vaccine group, several pieces of evidence support the assertion that the vaccine alone provides partial protection against acute Chagas disease with minimal toxicity.
Effective treatment for Chagas disease remains a challenge due to the paucity of available antiparasitic drugs, the limited efficacy of the drugs that are available, and the high risk for adverse effects caused by licensed drugs. Research and development efforts have focused not only on identifying new chemotherapeutic options (18, 19), but also on evaluating combination chemotherapy and reduced dosing regimens (16, 17). Concomitantly, over 100 years of research has been dedicated to developing vaccines against Chagas disease, and numerous single- and multiple-antigen candidate vaccines have demonstrated efficacy in preclinical models (63). To our knowledge, this is the first report demonstrating the synergistic effect of vaccine-linked chemotherapy on reducing parasite burdens and cardiac inflammation in a mouse model of acute Chagas disease. This would be a promising therapeutic option to bridge the efficacy and tolerability gaps of traditional chemotherapy with benznidazole.
MATERIALS AND METHODS
Parasites and mice.
Female BALB/c mice (BALB/cAnNTac) were obtained at 5 to 6 weeks of age from Taconic (Taconic Biosciences, Inc.) and allowed to acclimate for 1 week prior to studies. Mice were housed in groups of 5 in small microisolator caging, with ad libitum food and water and a 12-h light/dark cycle. T. cruzi H1 parasites, originally isolated from a human case in Yucatan, Mexico (30), were maintained by serial passage in female BALB/c mice every 25 to 28 days.
Vaccine candidate.
The recombinant Tc24 C4 antigen was expressed and purified in-house according to previously published protocols (37). The endotoxin level of the final purified protein was measured by the Endosafe PTS method (37) and was found to be <0.38 endotoxin units (EU)/mg protein. E6020 dissolved in a stable squalene emulsion (SE) was acquired through Eisai, Inc. Vaccine formulations comprising 25 μg of recombinant Tc24 C4 protein and 5 μg of E6020 in 100 μl of a 2% squalene emulsion in 1× phosphate-buffered saline (PBS), pH 7.4 (E6020 SE) (Eisai Inc.), were freshly prepared and mixed just before injection.
Infection and therapeutic treatment.
A total of 50 mice were each infected with 500 blood-form trypomastigotes of T. cruzi H1 by intraperitoneal injection. At 7 days of infection, mice were randomly divided into groups of 10 and assigned to treatment groups (Table 1). Ten mice served as infected untreated controls. Beginning 7 days after infection, blood was collected twice weekly throughout the study by tail vein microsampling to monitor parasitemia by quantitative PCR. Mice were monitored daily for mortality, and survival curves were plotted using GraphPad Prism software. Benznidazole powder (Laborotorio ELEA) was resuspended in 5% dimethyl sulfoxide (DMSO)–95% HPMC (0.5% hydroxypropyl methylcellulose, 0.4% Tween 80, 0.5% benzyl alcohol in deionized water) to a final concentration of 10 mg/ml. Mice were given 25 mg/kg or 100 mg/kg benznidazole by oral gavage daily from day 7 of infection until day 14 of infection. On days 17 and 24 of infection, mice were vaccinated subcutaneously with 25 μg of recombinant Tc24 C4 protein (37) combined with 5 μg of E6020 SE. Approximately 4 weeks after the boost vaccination, all mice were humanely euthanized, and hearts, whole blood, serum, and spleens were collected for further analysis.
Evaluation of parasite burdens.
To measure parasite burdens, total DNA was isolated from blood and cardiac tissue using a DNeasy blood and tissue kit (Qiagen), and 4 ng of DNA from blood or 50 ng of DNA from cardiac tissue was used in quantitative real-time PCR using TaqMan Fast Advanced master mix (Life Technologies) and oligonucleotides specific for the satellite region of T. cruzi nuclear DNA (primers 5′-ASTCGGCTGATCGTTTTCGA-3′ and 5′-AATTCCTCCAAGCAGCGGATA-3′ and probe 5′-6-FAM-CACACACTGGACACCAA-MGB-3′, where FAM is 6-carboxyfluorescein and MGB is minor groove binder [Life Technologies]) (64, 65). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (primers 5′-CAATGTGTCCGTCGTGGATCT-3′ and 5′-GTCCTCAGTGTAGCCCAAGATG-3′ and probe 5′-6-FAM-CGTGCCGCCTGGAGAAACCTGCC-MGB-3′ [Life Technologies]) (66), and parasite equivalents were calculated based on a standard curve (18, 67). GraphPad Prism software was used to plot parasite equivalents per milliliter of blood over time, and the area under the curve (AUC) was calculated for each animal to determine overall parasitemia. Cardiac parasite burdens were calculated based on a standard curve and expressed as the number of parasites per milligram of tissue.
Quantification of cardiac inflammation.
Heart samples were fixed in 10% neutral buffered formalin prior to routine processing for paraffin embedding and sectioning. Sections were stained with hematoxylin and eosin (H&E) to measure influx of inflammatory cells into tissue. Images of three to five representative sections from each mouse were captured at ×100 magnification using a Micromaster microscope (Fisher Scientific) and Micron software by a reviewer blinded to the treatment groups. Images were analyzed using ImageJ Fiji software (National Institutes of Health) to quantify the number of nuclei per millimeter of tissue.
Splenocyte preparation restimulation for measurement of cytokine release.
Spleens were mechanically dissociated by being pressed through a 70-μm-pore-size cell strainer. Splenocytes were rinsed through the screen with RPMI medium supplemented with 10% fetal bovine serum FBS, 1× penicillin-streptomycin (Pen-Strep), and l-glutamine (cRPMI medium) and then pelleted by centrifugation for 5 min at 300 × g at room temperature. The supernatant was decanted, and the splenocyte pellet was resuspended in 1 ml of ammonium-chloride-potassium (ACK) lysis solution for 5 min at room temperature to lyse red blood cells. The lysis solution was diluted 5-fold with cRPMI medium, and then splenocytes were pelleted by centrifugation for 5 min at 300 × g. Splenocytes were resuspended in 3 to 5 ml of cRPMI medium and counted using acridine orange-propidium iodide (AOPI) live/dead dye and a Cellometer Auto 2000 automated cell counter. Then, for each sample, 1 × 106 live splenocytes were incubated in a 96-well non-tissue culture plate with either 100 μg/ml recombinant Tc24 C4 protein, 20 ng/ml phorbol 12-myristate 13-acetate (PMA)–1 mg/ml ionomycin, or medium only for 96 h at 37°C in 5% CO2. To measure IFN-γ and IL-4 in the supernatants, a sandwich enzyme-linked immunosorbent assay (ELISA) method was employed, using mouse IFN-γ and IL-4 ELISA kits (eBioscience) per the manufacturer's instructions. To measure secreted levels of IL-2, IL-10, IL-12, and TNF-α, a Luminex-based assay was used, as previously described, that utilizes a Luminex kit from Bio-Rad and a wall-less 96-well plate from Curiox (68). Cytokine concentrations in the supernatant were calculated based on a standard curve, and for each sample duplicate wells were averaged. The limits of detection were 500 pg/ml to 15.6 pg/ml for IFN-γ and 125 pg/ml to 3.9 pg/ml for IL-4 by ELISA. The lower limits of detection were 0.4 pg/ml for IL-12, 0.6 pg/ml for IL-2, 1.0 pg/ml for IL-10, and 1.4 pg/ml for TNF-α by Luminex. Each measurement below the lower limit of detection was assigned a value of 0. The results from the medium-stimulated cells served as background and were then subtracted from the measurement from the Tc24 C4-stimulated cells from the same mouse to obtain the antigen-specific cytokine values. Antigen-specific cytokine values were plotted in GraphPad Prism software, with negative values representing a decreased response after Tc24 C4 stimulation compared to that with medium only.
Flow cytometry.
To measure CD4- and CD8-specific responses, restimulated splenocytes were collected, washed with PBS, and stained with Live/Dead fixable blue viability dye, anti-CD3e fluorescein isothiocyanate (FITC), anti-CD4 Alexa Fluor 700, and anti-CD8a peridinin chlorophyll protein (PerCP)-Cy5.5. To evaluate intracellular cytokine production, 4.1 μg/ml brefeldin A was added to splenocytes for the last 6 h of incubation. Splenocytes were stained for surface markers as described above, fixed with BD Cytofix/Cytoperm, and permeabilized according to the manufacturer's instructions. Permeabilized splenocytes were stained with anti-IFN-γ allophycocyanin (APC) and anti-IL-4 phycoerythrin (PE)-Cy7. Samples were acquired on an LSR Fortessa instrument, and at least 100,000 total events in a live gate were analyzed using Venturi One, version 6, software. To evaluate antigen-specific responses, the percentage of medium-stimulated cells was subtracted from the percentage of antigen-stimulated cells for each mouse. Data were plotted using GraphPad Prism software.
Serum antibody ELISA.
To measure serum antibodies specific to Tc24 C4, 96-well Nunc ELISA plates were coated with 1.25 μg/ml Tc24 C4 recombinant protein diluted in 1× coating buffer. After overnight incubation at 4°C, the coating solution was removed, and plates were blocked overnight with 0.1% bovine serum albumin (BSA) in PBS-Tween 20 (PBST) at 4°C; then the blocking solution was removed, and plates were sealed and frozen at −80°C until use. Plates were thawed at room temperature and washed twice with PBST, and serially diluted serum samples in 0.1% BSA in PBST were added in duplicate. Bound antibody was quantified by horseradish peroxidase (HRP)-conjugated anti-IgG1 and -IgG2a and TMB (3,3′,5,5′-tetramethylbenzidine) substrate, and the reaction was stopped with 1 M HCl. Absorbance was measured at 450 nm using a Biotek Epoch spectrophotometer. The background optical density at 450 nm (OD450) from wells without serum was subtracted from the average OD450 for each individual well. The average OD450 for the replicate wells for each sample was calculated. The positive cutoff was calculated as the average OD450 plus 3 standard deviations of the naive serum sample at a dilution of 1:400. For each sample, the titer was determined as the lowest dilution with an average OD450 above the positive cutoff (69). Geometric mean titers and standard deviations for each group were calculated using GraphPad Prism software.
Statistical analysis.
Survival curves were compared using a Mantel-Cox log rank test. For parasite burdens, cardiac inflammatory infiltrate, and immune responses, groups were compared using Kruskal-Wallis one-way analysis of variance (ANOVA) and Dunn's multiple-comparison test. To evaluate correlations between individual parameters, data were loaded into R, version 3.3.2, using the read_tsv function from the readr package, version 1.0.0 (70, 71). Parameters within the data were selected using the dplyr package, version 0.5.0 (71, 72). Subsequently, the PerformanceAnalytics package, version 1.4.3541, was used to visualize correlations between all parameters and to select groups within the data for further analysis (73). These groups were highlighted with a correlation plot using the GGally package, version 1.3.0 (74). Kendall's tau coefficient and related P value were calculated using the rcor.test function within the ltm package, version 1.0-0 (75), for correlation comparison. Output from this comparison was modified to present the correlation and related P value in tabular format using the dplyr package, version 0.5.0 (72). Negative and positive associations were considered as alternative hypotheses. P values of ≤0.05 were considered significant.
Animal study approval.
All studies were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine (protocol AN-5973) and were performed in strict compliance with The Guide for the Care and Use of Laboratory Animals (76).
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the Global Health Innovation Technology Fund (G2014-111) and the Carlos Slim Foundation. This project was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (NIAID P30AI036211, NCI P30CA125123, and NCRR S10RR024574) and the assistance of Joel M. Sederstrom and Brandon Saxton.
We are grateful for the generous donation of benznidazole powder from Laborotorio ELEA (Buenos Aires, Argentina), with the assistance of Silvia Gold.
We are involved in the development of a vaccine against Chagas disease.
K.J., L.V., B.K., J.P., P.J.H., and M.E.B. conceived and designed the study. K.J., L.V., B.K., and A.K. conducted the experiments. K.J., L.V., B.K., A.K., and J.V.C.-C. collected data. K.J., L.V., A.D., and J.V.C.-C. analyzed the data. J.P. and F.G. provided reagents. K.J., L.V., J.P., P.J.H., and M.E.B. wrote the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00876-17.
REFERENCES
- 1.GBD Disease and Injury Incidence and Prevalence Collaborators. 2016. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–43. [PubMed] [Google Scholar]
- 3.Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. 2012. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol 34:753–770. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro AL, Nunes MP, Teixeira MM, Rocha MO. 2012. Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol 9:576–589. doi: 10.1038/nrcardio.2012.109. [DOI] [PubMed] [Google Scholar]
- 5.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. 2007. Pathogenesis of chronic Chagas heart disease. Circulation 115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi Mde L, Benvenuti LA, Martins Reis M, Metzger M. 2003. Pathophysiology of the heart in Chagas' disease: current status and new developments. Cardiovasc Res 60:96–107. doi: 10.1016/S0008-6363(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 7.Bocchi EA. 2013. Heart failure in South America. Curr Cardiol Rev 9:147–156. doi: 10.2174/1573403X11309020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BY, Bacon KM, Connor DL, Willig AM, Bailey RR. 2010. The potential economic value of a Trypanosoma cruzi (Chagas disease) vaccine in Latin America. PLoS Negl Trop Dis 4:e916. doi: 10.1371/journal.pntd.0000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BY, Bacon KM, Wateska AR, Bottazzi ME, Dumonteil E, Hotez PJ. 2012. Modeling the economic value of a Chagas' disease therapeutic vaccine. Hum Vaccin Immunother 8:1293–1301. doi: 10.4161/hv.20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S, BENEFIT Investigators . 2015. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 11.Pecoul B, Batista C, Stobbaerts E, Ribeiro I, Vilasanjuan R, Gascon J, Pinazo MJ, Moriana S, Gold S, Pereiro A, Navarro M, Torrico F, Bottazzi ME, Hotez PJ. 2016. The BENEFIT Trial: where do we go from here? PLoS Negl Trop Dis 10:e0004343. doi: 10.1371/journal.pntd.0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A. 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7:157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 13.Urbina JA. 2010. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Jackson Y, Alirol E, Getaz L, Wolff H, Combescure C, Chappuis F. 2010. Tolerance and safety of nifurtimox in patients with chronic Chagas disease. Clin Infect Dis 51:e69–e75. doi: 10.1086/656917. [DOI] [PubMed] [Google Scholar]
- 15.Molina I, Salvador F, Sanchez-Montalva A, Trevino B, Serre N, Sao Aviles A, Almirante B. 2015. Toxic Profile of benznidazole in patients with chronic Chagas disease: risk factors and comparison of the product from two different manufacturers. Antimicrob Agents Chemother 59:6125–6131. doi: 10.1128/AAC.04660-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bustamante JM, Craft JM, Crowe BD, Ketchie SA, Tarleton RL. 2014. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J Infect Dis 209:150–162. doi: 10.1093/infdis/jit420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cencig S, Coltel N, Truyens C, Carlier Y. 2012. Evaluation of benznidazole treatment combined with nifurtimox, posaconazole or AmBisome in mice infected with Trypanosoma cruzi strains. Int J Antimicrob Agents 40:527–532. doi: 10.1016/j.ijantimicag.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Cencig S, Coltel N, Truyens C, Carlier Y. 2011. Parasitic loads in tissues of mice infected with Trypanosoma cruzi and treated with AmBisome. PLoS Negl Trop Dis 5:e1216. doi: 10.1371/journal.pntd.0001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina I, Gomez i Prat J, Salvador F, Trevino B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sanchez-Montalva A, Vidal X, Pahissa A. 2014. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N Engl J Med 370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 20.Filardi LS, Brener Z. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg 81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 21.Neal RA, van Bueren J. 1988. Comparative studies of drug susceptibility of five strains of Trypanosoma cruzi in vivo and in vitro. Trans R Soc Trop Med Hyg 82:709–714. doi: 10.1016/0035-9203(88)90208-8. [DOI] [PubMed] [Google Scholar]
- 22.Limon-Flores AY, Cervera-Cetina R, Tzec-Arjona JL, Ek-Macias L, Sanchez-Burgos G, Ramirez-Sierra MJ, Cruz-Chan JV, VanWynsberghe NR, Dumonteil E. 2010. Effect of a combination DNA vaccine for the prevention and therapy of Trypanosoma cruzi infection in mice: role of CD4+ and CD8+ T cells. Vaccine 28:7414–7419. doi: 10.1016/j.vaccine.2010.08.104. [DOI] [PubMed] [Google Scholar]
- 23.Poncini CV, Alba Soto CD, Batalla E, Solana ME, Gonzalez Cappa SM. 2008. Trypanosoma cruzi induces regulatory dendritic cells in vitro. Infect Immun 76:2633–2641. doi: 10.1128/IAI.01298-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares MB, Silva-Mota KN, Lima RS, Bellintani MC, Pontes-de-Carvalho L, Ribeiro-dos-Santos R. 2001. Modulation of chagasic cardiomyopathy by interleukin-4: dissociation between inflammation and tissue parasitism. Am J Pathol 159:703–709. doi: 10.1016/S0002-9440(10)61741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laucella SA, Postan M, Martin D, Hubby Fralish B, Albareda MC, Alvarez MG, Lococo B, Barbieri G, Viotti RJ, Tarleton RL. 2004. Frequency of interferon-gamma-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infect Dis 189:909–918. doi: 10.1086/381682. [DOI] [PubMed] [Google Scholar]
- 26.Albareda MC, Laucella SA. 2015. Modulation of Trypanosoma cruzi-specific T-cell responses after chemotherapy for chronic Chagas disease. Mem Inst Oswaldo Cruz 110:414–421. doi: 10.1590/0074-02760140386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laucella SA, Mazliah DP, Bertocchi G, Alvarez MG, Cooley G, Viotti R, Albareda MC, Lococo B, Postan M, Armenti A, Tarleton RL. 2009. Changes in Trypanosoma cruzi-specific immune responses after treatment: surrogate markers of treatment efficacy. Clin Infect Dis 49:1675–1684. doi: 10.1086/648072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanha AJ, Alves RO, Murta SM, Silva JS, Ropert C, Gazzinelli RT. 2002. Experimental chemotherapy against Trypanosoma cruzi infection: essential role of endogenous interferon-gamma in mediating parasitologic cure. J Infect Dis 186:823–828. doi: 10.1086/342415. [DOI] [PubMed] [Google Scholar]
- 29.Michailowsky V, Murta SM, Carvalho-Oliveira L, Pereira ME, Ferreira LR, Brener Z, Romanha AJ, Gazzinelli RT. 1998. Interleukin-12 enhances in vivo parasiticidal effect of benznidazole during acute experimental infection with a naturally drug-resistant strain of Trypanosoma cruzi. Antimicrob Agents Chemother 42:2549–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumonteil E, Escobedo-Ortegon J, Reyes-Rodriguez N, Arjona-Torres A, Ramirez-Sierra MJ. 2004. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice. Infect Immun 72:46–53. doi: 10.1128/IAI.72.1.46-53.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Burgos G, Mezquita-Vega RG, Escobedo-Ortegon J, Ramirez-Sierra MJ, Arjona-Torres A, Ouaissi A, Rodrigues MM, Dumonteil E. 2007. Comparative evaluation of therapeutic DNA vaccines against Trypanosoma cruzi in mice. FEMS Immunol Med Microbiol 50:333–341. doi: 10.1111/j.1574-695X.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- 32.Quijano-Hernandez IA, Bolio-Gonzalez ME, Rodriguez-Buenfil JC, Ramirez-Sierra MJ, Dumonteil E. 2008. Therapeutic DNA vaccine against Trypanosoma cruzi infection in dogs. Ann N Y Acad Sci 1149:343–346. doi: 10.1196/annals.1428.098. [DOI] [PubMed] [Google Scholar]
- 33.Dumonteil E, Bottazzi ME, Zhan B, Heffernan MJ, Jones K, Valenzuela JG, Kamhawi S, Ortega J, Rosales SP, Lee BY, Bacon KM, Fleischer B, Slingsby BT, Cravioto MB, Tapia-Conyer R, Hotez PJ. 2012. Accelerating the development of a therapeutic vaccine for human Chagas disease: rationale and prospects. Expert Rev Vaccines 11:1043–1055. doi: 10.1586/erv.12.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Campos V, Martinez-Vega P, Ramirez-Sierra MJ, Rosado-Vallado M, Seid CA, Hudspeth EM, Wei J, Liu Z, Kwityn C, Hammond M, Ortega-Lopez J, Zhan B, Hotez PJ, Bottazzi ME, Dumonteil E. 2015. Expression, purification, immunogenicity, and protective efficacy of a recombinant Tc24 antigen as a vaccine against Trypanosoma cruzi infection in mice. Vaccine 33:4505–4512. doi: 10.1016/j.vaccine.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Taibi A, Plumas-Marty B, Guevara-Espinoza A, Schoneck R, Pessoa H, Loyens M, Piras R, Aguirre T, Gras-Masse H, Bossus M. 1993. Trypanosoma cruzi: immunity-induced in mice and rats by trypomastigote excretory-secretory antigens and identification of a peptide sequence containing a T cell epitope with protective activity. J Immunol 151:2676–2689. [PubMed] [Google Scholar]
- 36.Barry MA, Wang Q, Jones KM, Heffernan MJ, Buhaya MH, Beaumier CM, Keegan BP, Zhan B, Dumonteil E, Bottazzi ME, Hotez PJ. 2016. A therapeutic nanoparticle vaccine against Trypanosoma cruzi in a BALB/c mouse model of Chagas disease. Hum Vaccin Immunother 12:976–987. doi: 10.1080/21645515.2015.1119346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seid CA, Jones KM, Pollet J, Keegan B, Hudspeth E, Hammond M, Wei J, McAtee CP, Versteeg L, Gutierrez A, Liu Z, Zhan B, Respress JL, Strych U, Bottazzi ME, Hotez PJ. 2017. Cysteine mutagenesis improves the production without abrogating antigenicity of a recombinant protein vaccine candidate for human Chagas disease. Hum Vaccin Immunother 13:621–633. doi: 10.1080/21645515.2016.1242540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma YW, Louis M, Huang H. 2015. Strategy for the development of vaccines against Chagas disease. J Vaccines Res Vaccin 1:1–8. [Google Scholar]
- 39.Urbina JA. 1999. Chemotherapy of Chagas' disease: the how and the why. J Mol Med (Berl) 77:332–338. doi: 10.1007/s001090050359. [DOI] [PubMed] [Google Scholar]
- 40.Khaw M, Panosian CB. 1995. Human antiprotozoal therapy: past, present, and future. Clin Microbiol Rev 8:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vespa GN, Cunha FQ, Silva JS. 1994. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun 62:5177–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva JS, Vespa GN, Cardoso MA, Aliberti JC, Cunha FQ. 1995. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun 63:4862–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aliberti JC, Cardoso MA, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS. 1996. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun 64:1961–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahia-Oliveira LM, Gomes JA, Cancado JR, Ferrari TC, Lemos EM, Luz ZM, Moreira MC, Gazzinelli G, Correa-Oliveira R. 2000. Immunological and clinical evaluation of chagasic patients subjected to chemotherapy during the acute phase of Trypanosoma cruzi infection 14–30 years ago. J Infect Dis 182:634–638. doi: 10.1086/315743. [DOI] [PubMed] [Google Scholar]
- 45.Gazzinelli RT, Oswald IP, Hieny S, James SL, Sher A. 1992. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol 22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 46.Bustamante JM, Bixby LM, Tarleton RL. 2008. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med 14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higuchi ML, De Morais CF, Pereira Barreto AC, Lopes EA, Stolf N, Bellotti G, Pileggi F. 1987. The role of active myocarditis in the development of heart failure in chronic Chagas' disease: a study based on endomyocardial biopsies. Clin Cardiol 10:665–670. doi: 10.1002/clc.4960101113. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Tarleton RL. 1999. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. J Infect Dis 180:480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- 49.Andrade SG, Stocker-Guerret S, Pimentel AS, Grimaud JA. 1991. Reversibility of cardiac fibrosis in mice chronically infected with Trypanosoma cruzi, under specific chemotherapy. Mem Inst Oswaldo Cruz 86:187–200. doi: 10.1590/S0074-02761991000200008. [DOI] [PubMed] [Google Scholar]
- 50.Machado FS, Tyler KM, Brant F, Esper L, Teixeira MM, Tanowitz HB. 2012. Pathogenesis of Chagas disease: time to move on. Front Biosci (Elite ed) 4:1743–1758. doi: 10.2741/e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gigley JP, Bhadra R, Moretto MM, Khan IA. 2012. T cell exhaustion in protozoan disease. Trends Parasitol 28:377–384. doi: 10.1016/j.pt.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virgin HW, Wherry EJ, Ahmed R. 2009. Redefining chronic viral infection. Cell 138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 53.Odorizzi PM, Wherry EJ. 2012. Inhibitory receptors on lymphocytes: insights from infections. J Immunol 188:2957–2965. doi: 10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albareda MC, De Rissio AM, Tomas G, Serjan A, Alvarez MG, Viotti R, Fichera LE, Esteva MI, Potente D, Armenti A, Tarleton RL, Laucella SA. 2013. Polyfunctional T cell responses in children in early stages of chronic Trypanosoma cruzi infection contrast with monofunctional responses of long-term infected adults. PLoS Negl Trop Dis 7:e2575. doi: 10.1371/journal.pntd.0002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 56.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 57.Matos MN, Cazorla SI, Bivona AE, Morales C, Guzman CA, Malchiodi EL. 2014. Tc52 amino-terminal-domain DNA carried by attenuated Salmonella enterica serovar Typhimurium induces protection against a Trypanosoma cruzi lethal challenge. Infect Immun 82:4265–4275. doi: 10.1128/IAI.02190-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira IR, Vilar-Pereira G, Marques V, da Silva AA, Caetano B, Moreira OC, Machado AV, Bruna-Romero O, Rodrigues MM, Gazzinelli RT, Lannes-Vieira J. 2015. A human type 5 adenovirus-based Trypanosoma cruzi therapeutic vaccine re-programs immune response and reverses chronic cardiomyopathy. PLoS Pathog 11:e1004594. doi: 10.1371/journal.ppat.1004594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta S, Smith C, Auclair S, Delgadillo ADJ, Garg NJ. 2015. Therapeutic efficacy of a subunit vaccine in controlling chronic trypanosoma cruzi infection and Chagas disease is enhanced by glutathione peroxidase over-expression. PLoS One 10:e0130562. doi: 10.1371/journal.pone.0130562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta S, Garg NJ. 2010. Prophylactic efficacy of TcVac2 against Trypanosoma cruzi in mice. PLoS Negl Trop Dis 4:e797. doi: 10.1371/journal.pntd.0000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marinho CR, Nunez-Apaza LN, Bortoluci KR, Bombeiro AL, Bucci DZ, Grisotto MG, Sardinha LR, Jorquera CE, Lira S, Lima MR, Alvarez JM. 2009. Infection by the Sylvio X10/4 clone of Trypanosoma cruzi: relevance of a low-virulence model of Chagas' disease. Microbes Infect 11:1037–1045. doi: 10.1016/j.micinf.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Lewis MD, Francisco AF, Taylor MC, Jayawardhana S, Kelly JM. 2016. Host and parasite genetics shape a link between Trypanosoma cruzi infection dynamics and chronic cardiomyopathy. Cell Microbiol 18:1429–1443. doi: 10.1111/cmi.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beaumier CM, Gillespie PM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. 2016. Status of vaccine research and development of vaccines for Chagas disease. Vaccine 34:2996–3000. doi: 10.1016/j.vaccine.2016.03.074. [DOI] [PubMed] [Google Scholar]
- 64.Melo MF, Moreira OC, Tenorio P, Lorena V, Lorena-Rezende I, Junior WO, Gomes Y, Britto C. 2015. Usefulness of real time PCR to quantify parasite load in serum samples from chronic Chagas disease patients. Parasit Vectors 8:154. doi: 10.1186/s13071-015-0770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piron M, Fisa R, Casamitjana N, Lopez-Chejade P, Puig L, Verges M, Gascon J, Gomez i Prat J, Portus M, Sauleda S. 2007. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop 103:195–200. doi: 10.1016/j.actatropica.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 66.Gangisetty O, Reddy DS. 2009. The optimization of TaqMan real-time RT-PCR assay for transcriptional profiling of GABA-A receptor subunit plasticity. J Neurosci Methods 181:58–66. doi: 10.1016/j.jneumeth.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caldas S, Caldas IS, Diniz LDF, Lima WG, Oliveira RDP, Cecilio AB, Ribeiro I, Talvani A, Bahia MT. 2012. Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta Trop 123:170–177. doi: 10.1016/j.actatropica.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Versteeg L, Le Guezennec X, Zhan B, Liu Z, Angagaw M, Woodhouse JD, Biswas S, Beaumier CM. 2017. Transferring Luminex(R) cytokine assays to a wall-less plate technology: Validation and comparison study with plasma and cell culture supernatants. J Immunol Methods 440:74–82. doi: 10.1016/j.jim.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Crowther JR. 1995. ELISA. Theory and practice. Methods Mol Biol 42:1–218. [DOI] [PubMed] [Google Scholar]
- 70.R Core Development Team. 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 71.Wickham H, Hester J, Francois R. 2016. readr: read tabular data. R Foundation for Statistical Computing, Vienna, Austria: https://cran.r-project.org/package=readr. [Google Scholar]
- 72.Wickham H, Francois R. 2016. dplyr: a grammar of data manipulation. R Foundation for Statistical Computing, Vienna, Austria: https://cran.r-project.org/package=dplyr. [Google Scholar]
- 73.Peterson BG, Carl P. 2014. PerformanceAnalytics: econometric tools for performance and risk analysis. R Foundation for Statistical Computing, Vienna, Austria: https://cran.r-project.org/web/packages/PerformanceAnalytics/index.html. [Google Scholar]
- 74.Schloerke B, Crowley J, Cook D, Briatte F, Marbach M, Thoen E, Elberg A, Larmarange J. 2016. GGally: extension to “ggplot2.” R Foundation for Statistical Computing, Vienna, Austria: https://cran.r-project.org/web/packages/GGally/index.html. [Google Scholar]
- 75.Rizopoulos D. 2006. ltm: an R package for latent variable modelling and item response theory analyses. J Stat Softw 17:1–25. doi: 10.1360/jos170001. [DOI] [Google Scholar]
- 76.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academy Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.