ABSTRACT
Trypanosoma cruzi is a protozoan parasite that causes Chagas disease (CD). CD is a persistent, lifelong infection affecting many organs, most notably the heart, where it may result in acute myocarditis and chronic cardiomyopathy. The pathological features include myocardial inflammation and fibrosis. In the Brazil strain-infected CD-1 mouse, which recapitulates many of the features of human infection, we found increased plasma levels of resolvin D1 (RvD1), a specialized proresolving mediator of inflammation, during both the acute and chronic phases of infection (>100 days postinfection) as determined by enzyme-linked immunosorbent assay (ELISA). Additionally, ELISA on lysates of trypomastigotes of both strains Tulahuen and Brazil revealed elevated levels of RvD1 compared with lysates of cultured epimastigotes of T. cruzi, tachyzoites of Toxoplasma gondii, trypomastigotes of Trypanosoma brucei, cultured L6E9 myoblasts, and culture medium containing no cells. Lysates of T. cruzi-infected myoblasts also displayed increased levels of RvD1. Lipid mediator metabolomics confirmed that the trypomastigotes of T. cruzi produced RvD1, RvD5, and RvE2, which have been demonstrated to modulate the host response to bacterial infections. Plasma RvD1 levels may be both host and parasite derived. Since T. cruzi synthesizes specialized proresolving mediators of inflammation, as well as proinflammatory eicosanoids, such as thromboxane A2, one may speculate that by using these lipid mediators to modulate its microenvironment, the parasite is able to survive.
KEYWORDS: Trypanosoma cruzi, Chagas disease, resolvins, resolvin D1, resolvin D5, resolvin E2, inflammation, immune modulation, eicosanoids, resolvin, host-parasite relationship
INTRODUCTION
The parasite Trypanosoma cruzi is the etiologic agent of Chagas disease. It is endemic in Mexico and Central and South America, where millions of persons are infected or at risk (1). In recent years, due to migration of individuals from areas of endemicity, there has been increased recognition of Chagas disease in the United States, Europe, and other areas of nonendemicity (2–4). Additionally, vector transmission of the parasite has been documented in the United States (5). Although any nucleated cell in the body can be infected, the cardiovascular system is among the organs targeted by the parasite, causing acute myocarditis and chronic cardiomyopathy.
Acute infection with T. cruzi results in an intense inflammatory response associated with increased expression of proinflammatory mediators, including cytokines, chemokines, and endothelin-1 (6). We have also previously described the release of the bioactive lipid mediators (LMs) prostaglandin F2α (PGF2α) and thromboxane A2 (TXA2) from T. cruzi, although TXA2 is preferentially synthesized (7). TXA2 is a proinflammatory vasoconstrictor with prothrombotic activity through enhanced platelet activation/aggregation. PGF2α is a proinflammatory vasoconstrictor substance like TXA2 (8); however, it opposes platelet activation by TXA2 and platelet-activating factor (9). Both receptors (TP and FP) couple to Gαq and Gα13, activate the small GTPase Rho, and promote cellular activation; however, FP expression on leukocytes is not well documented, suggesting that the effects of FP on inflammation are likely indirect while the effects of TP are direct (8). A previous report indicated that TP can be activated by PGF2α (10), suggesting a single focal point for the action of proinflammatory prostaglandins in Chagas disease and reinforcing the prominence of TP in the pathogenesis of the disease.
Acute inflammation is a natural host-protective mechanism mounted by the body in response to injury or invading pathogens. When self-limited, it restores homeostasis (11); however, if left uncontrolled, excessive inflammation can lead to chronic tissue damage (12). Resolution of inflammation is an active process orchestrated by a genus of potent molecules known as specialized proresolving mediators (SPM) (13). SPM, which include the resolvins, protectins, and maresins, are bioactive autacoids with both anti-inflammatory and proresolving properties. They are enzymatically produced from essential fatty acids (EFA), with distinct stereochemistries. Resolvin D1 (RvD1) biosynthesis involves sequential oxygenations of docosahexaenoic acid (DHA) by 15-lipoxygenase (15-LO) and 5-LO (14). Using a total organic synthetic approach, the complete stereochemistry of RvD1 has been established as 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (15). RvD1 displays potent leukocyte-directed actions in the picogram-nanogram concentration range in vivo (14). In Escherichia coli-induced peritonitis, RvD1 reduces bacterial titers and hypothermia, increases survival, and enhances microbial containment and killing by phagocytes (16). The aspirin-triggered epimer of RvD1 has been found to regulate the host immune response in Chagas disease patients by decreasing gamma interferon (IFN-γ) and the percentage of necrotic cells in the peripheral blood mononuclear cell (PBMC) pool and reducing the rate of T. cruzi antigen-stimulated PBMC proliferation in cultured cells (17). The source of resolvins and their relevance to T cruzi infection have not been investigated.
The full complement of the T. cruzi lipidome is yet to be determined. We asked whether T. cruzi infection resulted in upregulation of SPM expression and whether there were contributions to the proresolution pathway from both host and parasite. We hypothesized that infection with T. cruzi results in increased expression of resolvins both in vitro and in vivo. Indeed, here, we demonstrate that mice infected with T. cruzi display increased levels of RvD1 and that the trypomastigote form of the parasite contains RvD1, RvD5, and RvE2. Thus, it is of interest that T. cruzi synthesized both a proinflammatory vasoconstrictor (TXA2) and SPM (resolvins) that are essential regulators of the host response and that promote transition to chronic infection. This observation may have new implications for the pathogenesis of Chagas disease.
RESULTS
Animal studies: parasitology, pathology, and cardiac imaging.
Parasitemia was first detected at 15 days postinfection (dpi), peaked at 4 × 105 to 5 × 105 trypomastigotes/ml at 35 to 40 dpi, and waned by 60 dpi, when parasitemia was undetectable by microscopy (Fig. 1A). Mortality was 50% during acute infection (Fig. 1B). At 100 dpi, cardiac imaging demonstrated an enlarged heart and a significant increase in the right ventricular internal diameter, consistent with previous reports (18) (Fig. 1C and D). Histopathological examination of the heart during the acute phase revealed myonecrosis and parasite pseudocysts (Fig. 1E). During the chronic phase, there were cardiac myocyte hypertrophy, chronic inflammation, and fibrosis, consistent with previous reports (6).
FIG 1.
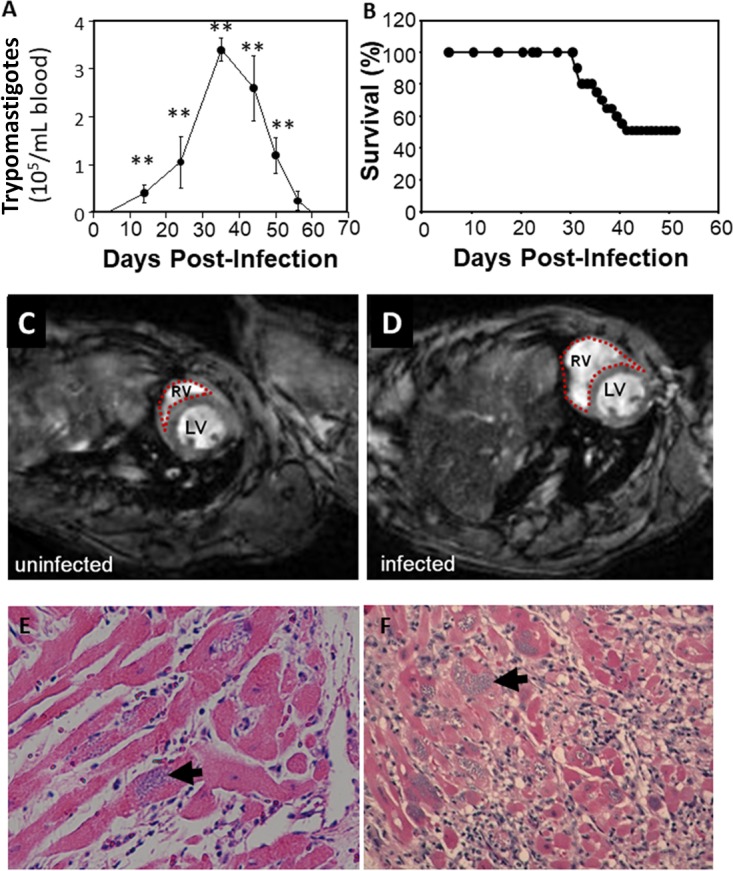
Kinetics of cardiomyopathy in T. cruzi infection. (A and B) Parasitemia (A) and mortality (B) in CD-1 mice infected with 5 × 104 trypomastigotes of T. cruzi strain Brazil. (C and D) Representative cardiac MRIs of control (C) and infected (D) mouse hearts revealing an enlarged right ventricle (RV). (E and F) Representative histopathology of the heart during acute and chronic infection showing pseudocysts of amastigotes (arrows) and inflammation. The images are representative of groups of 5 mice each. **, P ≤ 0.05.
Plasma resolvin D1 increases during acute T. cruzi infection.
There was a rapid rise in RvD1 (in micrograms per milliliter of plasma) in infected mice that was evident at 20 dpi and remained elevated to 140 dpi, during the chronic phase of infection (Fig. 2A). This profile suggests that RvD1 is derived from both the parasite and the host. To assess this hypothesis, we examined RvD1 levels in parasite lysates.
FIG 2.
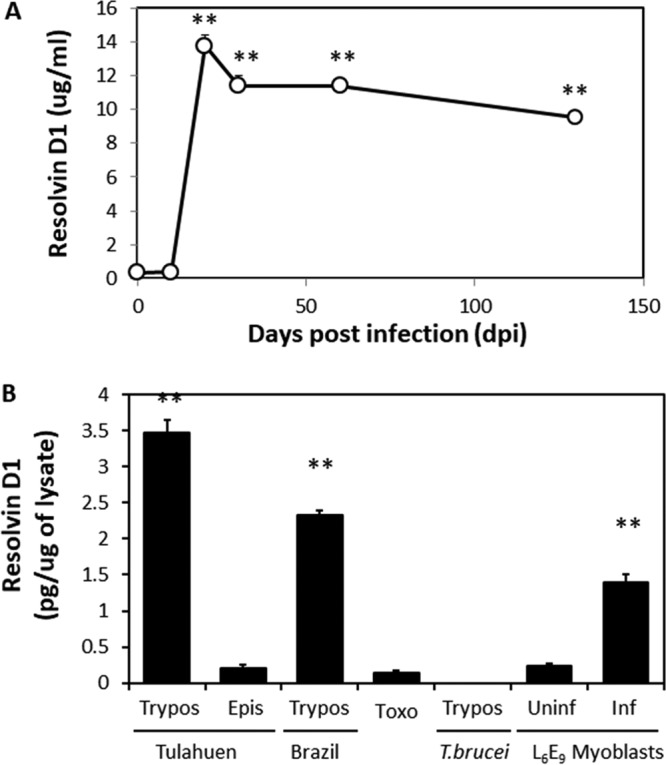
T. cruzi is a source of RvD1 during infection. (A) Serum RvD1 levels in mice inoculated with 5 × 104 strain Brazil trypomastigotes. The data are means and standard deviations (SD) (n = 5). The asterisks indicate significant differences (**, P ≤ 0.05) from uninfected mice. (B) Release of RvD1 from T. cruzi, related protists, and infected L6E9 cells. RvD1 release was measured by ELISA from conditioned medium. The data are means and SD (n = 3). The asterisks indicate significant differences (**, P ≤ 0.05) from epimastigotes and uninfected L6E9 cells. Epis, epimastigote forms of strain Tulahuen; Trypos, trypomastigotes of strains Brazil and Tulahuen; Toxo, lysates of T. gondii strain RH; Uninf and Inf, L6E9 myoblasts uninfected or infected with trypomastigotes of T. cruzi strain Tulahuen, respectively.
Resolvin D1 levels in parasite extracts as determined by enzyme-linked immunosorbent assay (ELISA).
Trypomastigotes of both T. cruzi strains Brazil and Tulahuen had significantly greater amounts of RvD1 than epimastigotes of strains Tulahuen (Fig. 2B) and Brazil (data not shown). RvD1 was not detected at significant levels in trypomastigotes of Trypanosoma brucei, tachyzoites of Toxoplasma gondii, or uninfected L6E9 myoblasts. In contrast, myoblasts infected with T. cruzi strain Tulahuen (which contained intracellular amastigotes) produced significant amounts of RvD1 compared to uninfected myoblasts. Although production was lower than in free trypomastigotes, these data suggest that intracellular amastigotes synthesize RvD1 or that infection stimulates production by myoblasts. To unequivocally determine that RvD1 was being produced, we performed liquid chromatography-tandem mass spectrometry (LC–MS-MS) analysis, the gold standard for identification of bioactive lipid mediators.
LM metabololipidomics.
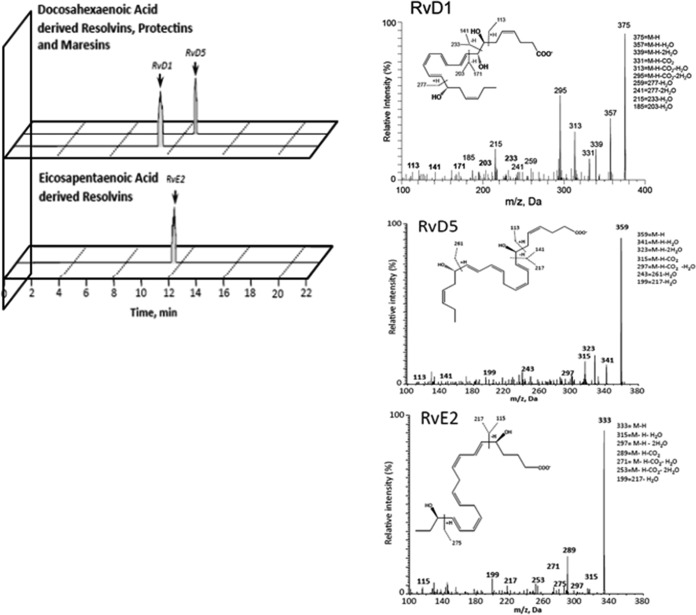
The above-mentioned results clearly indicated that RvD1 is present in trypomastigotes of T. cruzi. Figure 3 shows the characteristic LC–MS-MS spectra used for the identification of RvD1, RvD5, and RvE2 in parasite extracts. The resulting lipidomic profiles are representative of 3 separate parasite lysates from both strains Tulahuen and Brazil. Extracts of uninfected L6E9 myoblasts and liver infusion-tryptose (LIT) medium were negative for lipid species, suggesting that all the identified species were parasite derived (data not shown). Both strains had the precursor lipids DHA, eicosapentaenoic acid (EPA), and arachidonic acid (Table 1), indicating that biosynthesis of all the proinflammatory and proresolving mediators was possible. DHA and its bioactive metabolome were present in significantly larger quantities, indicating a potential preference for biosynthesis of mediators derived from that fatty acid. Consistent with earlier reports from our laboratory and others (8), we identified the arachidonic acid metabolites PGE2, PGD2, and PGF2α in T. cruzi lysates (Table 2). The presence of 5/12/15-HETE (hydroxy-eicosatetraenoic acid) and 5/12/15/18-HEPE (hydroxy-eicosapentaenoic acid) in extracts (Table 3) suggests that active lipoxygenase pathways are present in the parasite, with arachidonic acid and EPA as the respective precursor free fatty acids (FFAs) (19). Potential downstream proinflammatory end products might also include leukotrienes; however, no leukotriene B4 production was identified (Table 3). Thus, the primary class of proinflammatory lipid species produced by T. cruzi is prostaglandins.
FIG 3.
Representative spectra used to identify lipid mediators of the resolving class and LC–MS-MS fragmentation spectra employed for the identification of RvD1, RvD5, and RvE2 in lysates of trypomastigotes of T. cruzi from strains Brazil and Tulahuen.
TABLE 1.
Quantitation of FFA precursors in lysates of trypomastigotes by LC–MS-MS
| FFA precursor speciesa | Mass (m/z) of diagnostic ions at quadrupole position (Q): |
Amt in trypomastigotes of T. cruzi strainb: |
||
|---|---|---|---|---|
| Q1 | Q3 | Tulahuen | Brazil | |
| EPA | 301 | 257 | 1.6–1,194c | 2.9–1,937c |
| DHA | 327 | 283 | 113–15,111c | 62.1–3,841c |
| 4S,14S-diHDHA | 359 | 221 | − | − |
| 17-HDHA | 343 | 245 | 9.7–199.3 | 6.4–242.4 |
| 14-HDHA | 343 | 205 | 15.7–86.8 | 14.9–141.6 |
| 7-HDHA | 343 | 141 | 16.9–262.4 | 11.3–88.2 |
| 4-HDHA | 343 | 101 | 94.5–427.4 | 70.0–471.3 |
| AA | 303 | 259 | 15.5–2,529c | 32–5,766c |
HDHA, hydroxydocosahexaenoic acid; AA, arachidonic acid.
Expression of lipid species quantified from peak height and expressed as picograms per milligram of protein. The data represent the range of samples documented from 3 independent lysates for each strain. −, sample with value below the detection limit.
Amount in nanograms per milligram of protein.
TABLE 2.
Quantitation of prostaglandin species in lysates of trypomastigotes of T. cruzi by LC–MS-MS
| Prostaglandin lipid speciesa | Mass (m/z) of diagnostic ions at quadrupole position (Q): |
Amt in trypomastigotes of T. cruzi strainb: |
||
|---|---|---|---|---|
| Q1 | Q3 | Tulahuen | Brazil | |
| PGD2 | 351 | 189 | 1.4–3.9 | 2.7–23 |
| PGE2 | 351 | 189 | 2.0–21.4 | 6.0–48.6 |
| PGF2α | 353 | 193 | 2.6–38.9 | 2.7–2.6 |
PG, prostaglandin.
Expression of lipid species quantified from peak height and expressed as picograms per milligram of protein. The data represent the range of samples documented from 3 independent lysates for each strain.
TABLE 3.
Quantitation of lipoxygenase-derived lipid species in lysates of T. cruzi trypomastigotes by LC–MS-MS
| Lipoxygenase-derived lipid speciesa | Mass (m/z) of diagnostic ions at quadrupole position (Q): |
Amt in trypomastigotes of T. cruzi strainb: |
||
|---|---|---|---|---|
| Q1 | Q3 | Tulahuen | Brazil | |
| LTB4 | 335 | 195 | − | − |
| 20-OH-LTB4 | 351 | 195 | − | − |
| 20-COOH-LTB4 | 365 | 195 | − | − |
| 5-HETE | 319 | 115 | 54.6–101.6 | 26.3–122.1 |
| 12-HETE | 319 | 179 | 11.1–46.9 | 15.3–143.3 |
| 15-HETE | 319 | 219 | 24.8–166.4 | 39.7–328.1 |
| 5S,15S-diHETE | 335 | 235 | 2.3–5.2 | 5.7–8.0 |
| 5-HEPE | 317 | 115 | 5.4–12.9 | 2.5–31.0 |
| 12-HEPE | 317 | 179 | 0.7–6.1 | 0.6–1.0 |
| 15-HEPE | 317 | 219 | 2.5–3.3 | 2.3–3.4 |
| 18-HEPE | 317 | 259 | 3.8–5.4 | 1.7–2.2 |
| 5S,15S-diHEPE | 333 | 115 | 1.3–27.7 | 1.7–13.6 |
LT, leukotriene.
Expression of lipid species quantified from peak height and expressed as picograms per milligram of protein. The data represent the range of samples documented from 3 independent lysates for each strain. −, sample with value below the detection limit.
An active lipoxygenase pathway would also initiate and promote the biosynthesis of proresolving lipid mediators, such as lipoxins, resolvins, maresins, and protectins. When we examined lysates by LC–MS-MS, no evidence of lipoxins, protectins, or maresins was observed (Table 4). However, their pathway markers were identified, indicating that trypomastigotes of T. cruzi have the enzymatic capability to produce these mediators. Of interest, RvD1 and RvD5 were biosynthesized by both T. cruzi strains Tulahuen and Brazil (Table 4). Moreover, strain Brazil produced significant levels of RvE2 (Table 4), indicating that only strain Brazil utilized EPA to produce proresolving mediators. The level of the precursor of RvE2 (18-HEPE) was lower in strain Brazil, perhaps reflecting increased use of the precursor for RvE2 synthesis. These studies clearly indicate that RvD1 is present in trypomastigotes of T. cruzi and that D and E series resolvins are the likely primary lipid mediators through which T. cruzi dampens the host response to infection.
TABLE 4.
Quantitation of proresolving lipid mediators in lysates of trypomastigotes of T. cruzi by LC–MS-MS
| Proresolving lipid speciesa | Mass (m/z) of diagnostic ions at quadrupole position (Q): |
Amt in trypomastigotes of T .cruzi strainb: |
||
|---|---|---|---|---|
| Q1 | Q3 | Tulahuen | Brazil | |
| RvD1 | 375 | 141 | 1.2–1.4 | 1.8–7.0 |
| RvD2 | 375 | 215 | − | − |
| RvD3 | 375 | 147 | − | − |
| RvD5 | 359 | 199 | 1.4–1.6 | 0.7–1.9 |
| RvD6 | 359 | 159 | − | − |
| RvE1 | 349 | 161 | − | − |
| RvE2 | 333 | 253 | − | 9.5–23.6 |
| RvE3 | 333 | 201 | − | − |
| PD1 | 359 | 153 | − | − |
| 22-OH-PD1 | 375 | 153 | − | − |
| 22-COOH-PD1 | 389 | 153 | − | − |
| MaR1 | 359 | 250 | − | − |
| LXA4 | 351 | 115 | − | − |
| LXB4 | 351 | 115 | − | − |
| LXA5 | 349 | 215 | − | − |
| LXB5 | 349 | 221 | − | − |
RvD, resolvins derived from docosahexenoic acid; RvE, resolvins derived from eicosapentaenoic acid; PD, protectin; MaR, maresin; LX, lipoxin.
Expression of lipid species quantified from peak height and expressed as picograms per milligram of protein. The data represent the range of samples documented from 3 independent lysates for each strain. −, sample with value below the detection limit.
DISCUSSION
The heart is an important focus of infection by T. cruzi, although other tissues and organs are infected as well. Acute T. cruzi infection is characterized by upregulation of proinflammatory cytokines and chemokines and an influx of inflammatory cells. In the setting of Chagas disease, as in other disease states, the acute inflammatory response is a “double-edged” sword, in that it is needed to control the infection but also leads to tissue injury. The chronic phase of Chagas disease is accompanied by the appearance of chronic inflammatory cells and cardiac remodeling (i.e., fibrosis). The present study clearly demonstrates, for the first time, that T. cruzi trypomastigotes and amastigotes produce RvD1, which likely contributes to the resolution of inflammation. This was confirmed by both ELISA and LM metabolomic profiling. In addition, T. cruzi-infected mice displayed elevated circulating RvD1 levels during the acute phase of infection and well into the chronic phase, when peripheral parasitemia had waned and, in the mouse model, cardiomyopathy was present. Lysates of trypomastigotes of strains Brazil and Tulahuen contained RvD5, while trypomastigotes of strain Brazil contained RvE2. We did not identify RvD1 in a variety of other protozoan parasites (T. gondii and T. brucei) and noninfected mammalian cells.
Historically, the resolution of inflammation was considered a passive process resulting from the loss or dilution of proinflammatory mediators from the extracellular milieu. It is now understood, however, that the resolution of inflammation is instead an active and programmed event (13). The mediators of the resolution of inflammation, SPM (including the resolvins, protectins, and maresins), are produced enzymatically from EFA. RvD1 is one of these bioactive mediators, and as noted in E. coli-induced peritonitis, RvD1 reduces bacterial titers and hypothermia, increases survival, and enhances microbial containment and killing by phagocytic cells (16). RvD5 has also been shown to be involved in the setting of experimental E. coli infection by enhancing phagocytosis. A combination of RvD5 with the antibiotic ciprofloxacin was able to accelerate the antibiotic effect, and the same was observed in the setting of experimental staphylococcal infection (16). RvE2, identified by LM metabololipidomics, has potent leukocyte-directed actions that regulate chemotaxis of human neutrophils, enhancing phagocytosis and anti-inflammatory cytokine production. RvE2 rapidly downregulated surface expression of human leukocyte integrins in whole blood and dampened responses to platelet-activating factor. These actions appear to be mediated by leukocyte G protein-coupled receptors. Collectively, these observations indicate that RvE2 can stimulate host-protective actions throughout initiation and resolution in the innate inflammatory responses (20).
The ability of the parasite and host to liberate proresolving mediators is likely to alter the course of disease. The identification of 15- and 5(S),15(S)-HETE in T. cruzi extracts suggests 15-LO activity (which has been previously reported in parasites such as T. gondii) (21); however, this has not been previously documented, and no data are available comparing disease progression in 15-LO−/− mice or with pharmacological antagonists of 15-LO activity. Conversely, a comparison of 5-LO activities has been performed (22). 5-LO activity is essential for synthesis of many proresolving mediators (such as E series resolvins and lipoxins). Direct comparison of 5-LO-null mice (intact parasite RvD1 synthesis) and infected mice treated with nordihydroguaiaretic acid (NDGA) (1.25 mg/mouse/day), a 5-LO inhibitor (inhibiting both mouse and parasite RvD1), indicated that NDGA treatment promoted earlier development of parasitemia and greater lethality than in the 5-LO-null mice (22), although the numbers of cardiac amastigote nests and antioxidant protection were similar. These differences suggest that NDGA may have inhibited parasite- and host-derived 5-LO activity, that parasite 5-LO derivatives may play a significant role in suppressing early parasite growth during acute infection, and that they promote survival of the host through the acute infection. Our data suggest that, of the available mediators, only RvE2 production would be affected by such a difference (as no lipoxin or leukotriene production was observed in the T. cruzi strains tested). These data indicate that the liberation of SPM from the parasite may be important in mediating the transition from acute to chronic disease and promoting host survival during experimental T. cruzi infection.
Previously, our groups reported that T. cruzi synthesizes the lipid mediators TXA2 and PGF2α (7). TXA2 is both a potent vasoconstrictor and a proinflammatory mediator synthesized by both trypomastigotes and amastigotes (7, 19). The majority of TXA2 in the sera of infected mice is derived from the parasite (7). In the present report, we have demonstrated that trypomastigotes and amastigotes of both T. cruzi strains Brazil and Tulahuen contain RvD1, RvD5, and RvE2, important proresolving mediators. Plasma RvD1 levels are elevated in T. cruzi (strain Brazil)-infected mice, and it is possible that the source of the RvD1 is, in part, the parasite itself. The synthesis of proinflammatory and proresolving lipid mediators suggests that the parasite is able to modulate its microenvironment through these metabolites. If the net result is a damping down of the inflammatory response, this may be important in the perpetuation of the infection into the chronic phase. There are other anti-inflammatory factors present during T. cruzi infection, such as interleukin-10. In that regard, it was recently demonstrated that there is an interaction between RvD1 and interleukin-10 in the resolution of inflammation in adipose tissue in the experimental obese state (23). These observations suggest that the addition of such an antiparasitic regimen may ameliorate some of the consequences of the infection. Resolvins dampen the inflammatory response and promote resolution of infections, which prevents fibrosis and enables parasite persistence and the perpetuation of the parasite life cycle (24).
The biosynthesis of lipid mediators by T. cruzi is increasingly complex but may provide advantages for longevity of infection. The parasite genome encodes terminal synthases for some eicosanoid species, and the ability of T. cruzi to elevate the host plasma lipid mediator levels in mice with the respective terminal synthase knocked out (such as TXA2 synthase-null mice) suggests that these pathways are active during disease (7). While T. cruzi proteins, such as PGF2α synthase, are highly homologous to other prokaryotic enzymes (such as Saccharomyces cerevisiae old yellow enzyme [T. cruzi old yellow enzyme {TcOYE}] and T. brucei synthase [T. brucei PGF2α synthase {TbPGFS}]) (25–27), their primary sequences have significant differences from mammalian terminal enzymes (26, 27). Moreover, the T. cruzi enzymes are resistant to pharmacological inhibitors of mammalian terminal synthases, indicating that the active sites are also topographically or structurally different (25). Thus, parasite-derived eicosanoids manipulate host responses during infection but are intractable to current therapies.
Most interestingly, the terminal synthase for the primary T. cruzi prostanoid, TXA2, has resisted genomic efforts (such as cDNA library screening and homology-based cloning) to identify it, prompting suggestions that the parasite may coopt host synthetic pathways by the process of trogocytosis (28). Similarly, the 15-LO and 5-LO activities needed for D and E series resolvin biosynthesis are not predicted from the T. cruzi genome. It therefore remains to be determined whether the biosynthetic pathways exist in T. cruzi or are serendipitously stolen from infected host cells. Only the examination of chronically infected null mice, once parasite numbers have declined, is likely to yield these answers.
Previously, we reported that T. cruzi produces the proinflammatory and prothrombotic lipid TXA2. Here, we demonstrate that the parasite also produces several proresolving lipids. Taken together, the data show that the parasite may be able to modulate its microenvironment and enhance persistence. On the other hand, the administration of RvD1 may ameliorate inflammation and fibrosis in the heart. Experiments addressing this are under way.
MATERIALS AND METHODS
Animal ethics statement.
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
Parasitology and pathology.
T. cruzi strains Brazil and Tulahuen were maintained by serial passage in C3H mice (Jackson Laboratories, Bar Harbor, ME). For studies with strain Brazil, 8-week-old male CD-1 mice were infected with 5 × 104 parasites. Parasitemia was determined by counting in a hemocytometer chamber as previously described (29). Trypomastigotes of both T. cruzi strains Brazil and Tulahuen were propagated in a myoblast line (L6E9) as previously described (30). T. gondii strain RH was maintained in cultured human foreskin fibroblasts. T. brucei brucei strain Lister 427 was provided to us by George Cross (Rockefeller University, New York, NY). Hearts were fixed in 10% (vol/vol) neutral buffered formalin, and sections (5 μm) were stained with hematoxylin and eosin (H&E).
Cardiac MRI.
For magnetic resonance imaging (MRI), mice were anesthetized by isoflurane inhalation (2% [vol/vol] in medical air administered via a nose cone). MRI-compatible electrocardiogram (ECG) electrodes were inserted in the left front and right rear paws, and the ECG signal was used as a trigger signal with a Small Animal Instruments (Stony Brook, NY) physiological monitoring system. Mice were positioned in a 35-mm (inside diameter [i.d.]) hydrogen (1H) volume coil (Molecules2Man Imaging Co., Cleveland, OH) (18). Body temperature was maintained at 34 to 35°C using warm air, with feedback from a body surface thermocouple, and a small respiratory sensor balloon, taped onto the abdomen, provided respiratory gating. Images were acquired using a 9.4-T Varian Direct Drive animal magnetic resonance imaging and spectroscopic system (Agilent Technologies, Inc., Santa Clara, CA). One-millimeter-thick slices were acquired in short-axis orientation using an ECG-triggered and respiratory-gated multiframe tagged cine sequence. The imaging parameters used were a field of view (FOV) of 40 by 40 mm, a matrix size of 256 by 256, an echo time (TE) of 2.6 ms, a repetition time (TR) of 5.5 ms, a flip angle of 25°, and a number of averages of 2.
General considerations regarding ELISA and LC–MS-MS assay.
T. cruzi epimastigotes were cultured in LIT medium, which we assayed (by both ELISA and LC–MS-MS) and which contained no resolvins. They were washed 3 times in phosphate-buffered saline (PBS) (containing no resolvins), and lysates were made from them. Trypomastigotes of T. cruzi were obtained from infected myoblast cultures, which we confirmed (by both ELISA and LC–MS-MS) contained no resolvins. Lysates of T. brucei trypomastigotes and tachyzoites of T. gondii strain RH prepared in similar fashion contained no resolvins, indicating that the resolvin content of T. cruzi was not an artifact of the culture conditions. Positive controls for LC–MS-MS were the known-authentic standards for the resolvins. Negative controls were the L6E9 myoblasts, which we found to have no detectable amounts of resolvins. The limit of detection of this LC–MS-MS system was ∼10 to 15 pmol. RvD1 was measured by ELISA in lysates of 1 × 106 trypomastigotes of both T. cruzi strains Brazil and Tulahuen and trypomastigotes of T. brucei brucei according to the manufacturer's specifications (Cayman Chemical Company, Ann Arbor, MI, USA). Blood was collected from infected CD-1 mice by retro-orbital bleeding, centrifuged, and frozen at −80°C until it was used. RvD1 levels for all experiments were determined according to absorbance measured between 405 and 420 nm using a microplate reader.
LM metabololipidomics.
LM metabololipidomics was performed as described previously (31). After hypotonic lysis, parasites were further sonicated, snap-frozen, and stored at −80°C until they were analyzed. Briefly, parasite lysates were placed in ice-cold methanol (containing a deuterium-labeled internal standard [d5-RvD2, d4-PGE2, d5-LXA4, or d4-LTB4; 500 pg each]) to facilitate quantification and recovery. Samples were held at −20°C for 45 min to allow protein precipitation and centrifuged. The supernatants were collected and placed onto an automated extraction system (Biotage) using a C18 column. Bioactive LMs were collected and injected into an LC system (Shimadzu) coupled to a Qtrap 5500 (AB Sciex). Identification was conducted using published criteria, including matching the retention time to synthetic standards and a minimum of 6 diagnostic ions in the MS-MS mode, as reported previously (31).
Statistical analysis.
All other data are expressed as means and standard errors of the mean (SEM) and were analyzed using GraphPad Prism statistical software (GraphPad Software Inc., San Diego, CA). For analysis of differences between groups, the Student t test was performed. A level of significance of 5% was chosen to indicate differences between means.
ACKNOWLEDGMENTS
This work was supported by NIH grants PO1 GM095467 (C.N.S.) and AI-214000 (H.B.T.).
REFERENCES
- 1.Nunes MC, Dones W, Morillo CA, Encina JJ, Ribeiro AL, Council on Chagas Disease of the Interamerican Society of Cardiology. 2013. Chagas disease: an overview of clinical and epidemiological aspects. J Am Coll Cardiol 62:767–776. doi: 10.1016/j.jacc.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 2.Tanowitz HB, Weiss LM, Montgomery SP. 2011. Chagas disease has now gone global. PLoS Negl Trop Dis 5:e1136. doi: 10.1371/journal.pntd.0001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Requena-Mendez A, Moore DA, Subira C, Munoz J. 2016. Addressing the neglect: Chagas disease in London, UK. Lancet Glob Health 4:e231-3. doi: 10.1016/S2214-109X(16)00047-4. [DOI] [PubMed] [Google Scholar]
- 4.Traina MI, Sanchez DR, Hernandez S, Bradfield JS, Labedi MR, Ngab TA, Steurer F, Montgomery SP, Meymandi SK. 2015. Prevalence and impact of Chagas disease among Latin American immigrants with nonischemic cardiomyopathy in Los Angeles, California. Circ Heart Fail 8:938–943. doi: 10.1161/CIRCHEARTFAILURE.115.002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, Woc-Colburn L, Hotez PJ, Murray KO. 2015. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg 92:325–330. doi: 10.4269/ajtmh.14-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanowitz HB, Machado FS, Spray DC, Friedman JM, Weiss OS, Lora JN, Nagajyothi J, Moraes DN, Garg NJ, Nunes MC, Ribeiro AL. 2015. Developments in the management of Chagas cardiomyopathy. Expert Rev Cardiovasc Ther 13:1393–1409. doi: 10.1586/14779072.2015.1103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashton AW, Mukherjee S, Nagajyothi FN, Huang H, Braunstein VL, Desruisseaux MS, Factor SM, Lopez L, Berman JW, Wittner M, Scherer PE, Capra V, Coffman TM, Serhan CN, Gotlinger K, Wu KK, Weiss LM, Tanowitz HB. 2007. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exp Med 204:929–940. doi: 10.1084/jem.20062432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hata AN, Breyer RM. 2004. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther 103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong RA, Jones RL, Wilson NH. 1985. Mechanism of the inhibition of platelet aggregation produced by prostaglandin F2 alpha. Prostaglandins 29:601–610. doi: 10.1016/0090-6980(85)90083-8. [DOI] [PubMed] [Google Scholar]
- 10.Arakawa H, Lotvall J, Kawikova I, Lofdahl CG, Skoogh BE. 1993. Leukotriene D4- and prostaglandin F2 alpha-induced airflow obstruction and airway plasma exudation in guinea-pig: role of thromboxane and its receptor. Br J Pharmacol 110:127–132. doi: 10.1111/j.1476-5381.1993.tb13781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V, Abbas AK, Aster JC. 2015. Robbins and Cotran pathologic basis of disease, 9th ed, p 69–112. Elsevier-Saunders, Philadelphia, PA. [Google Scholar]
- 12.Tabas I, Glass CK. 2013. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 278:14677–14687. [DOI] [PubMed] [Google Scholar]
- 15.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. 2007. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem 282:9323–9334. [DOI] [PubMed] [Google Scholar]
- 16.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. 2012. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogata H, Teixeira MM, Sousa RC, Silva MV, Correia D, Rodrigues V Jr, Levy BD, Rogerio Ade P. 2016. Effects of aspirin-triggered resolvin D1 on peripheral blood mononuclear cells from patients with Chagas' heart disease. Eur J Pharmacol 777:26–32. doi: 10.1016/j.ejphar.2016.02.058. [DOI] [PubMed] [Google Scholar]
- 18.Prado CM, Fine EJ, Koba W, Zhao D, Rossi MA, Tanowitz HB, Jelicks LA. 2009. Micro-positron emission tomography in the evaluation of Trypanosoma cruzi-induced heart disease: Comparison with other modalities. Am J Trop Med Hyg 81:900–905. doi: 10.4269/ajtmh.2009.09-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado FS, Mukherjee S, Weiss LM, Tanowitz HB, Ashton AW. 2011. Bioactive lipids in Trypanosoma cruzi infection. Adv Parasitol 76:1–31. doi: 10.1016/B978-0-12-385895-5.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. 2012. Resolvin E2 formation and impact in inflammation resolution. J Immunol 188:4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bannenberg GL, Aliberti J, Hong S, Sher A, Serhan C. 2004. Exogenous pathogen and plant 15-lipoxygenase initiate endogenous lipoxin A4 biosynthesis. J Exp Med 199:515–523. doi: 10.1084/jem.20031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges CL, Cecchini R, Tatakihara VL, Malvezi AD, Yamada-Ogatta SF, Rizzo LV, Pinge-Filho P. 2009. 5-Lipoxygenase plays a role in the control of parasite burden and contributes to oxidative damage of erythrocytes in murine Chagas' disease. Immunol Lett 123:38–45. doi: 10.1016/j.imlet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Titos E, Rius B, Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Lopategi A, Dalli J, Lozano JJ, Arroyo V, Delgado S, Serhan CN, Claria J. 2016. Signaling and immunoresolving actions of resolvin D1 in inflamed human visceral adipose tissue. J Immunol 197:3360–3370. doi: 10.4049/jimmunol.1502522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basil MC, Levy BD. 2016. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabututu Z, Martin SK, Nozaki T, Kawazu S, Okada T, Munday CJ, Duszenko M, Lazarus M, Thuita LW, Urade Y, Kubata BK. 2003. Prostaglandin production from arachidonic acid and evidence for a 9,11-endoperoxide prostaglandin H2 reductase in Leishmania. Int J Parasitol 33:221–228. doi: 10.1016/S0020-7519(02)00254-0. [DOI] [PubMed] [Google Scholar]
- 26.Kubata BK, Duszenko M, Kabututu Z, Rawer M, Szallies A, Fujimori K, Inui T, Nozaki T, Yamashita K, Horii T, Urade Y, Hayaishi O. 2000. Identification of a novel prostaglandin f(2alpha) synthase in Trypanosoma brucei. J Exp Med 192:1327–1338. doi: 10.1084/jem.192.9.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okano Y, Inoue T, Kubata BK, Kabututu Z, Urade Y, Matsumura H, Kai Y. 2002. Crystallization and preliminary X-ray crystallographic studies of Trypanosoma brucei prostaglandin F(2 alpha) synthase. J Biochem 132:859–861. doi: 10.1093/oxfordjournals.jbchem.a003298. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S, Mukhopadhyay A, Andriani G, Machado FS, Ashton AW, Huang H, Weiss LM, Tanowitz HB. 2015. Trypanosoma cruzi invasion is associated with trogocytosis. Microbes Infect 17:62–70. doi: 10.1016/j.micinf.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trischmann T, Tanowitz H, Wittner M, Bloom B. 1978. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp Parasitol 45:160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]
- 30.Rowin KS, Tanowitz HB, Wittner M, Nguyen HT, Nadal-Ginard B. 1983. Inhibition of muscle differentiation by Trypanosoma cruzi. Proc Natl Acad Sci U S A 80:6390–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. 2014. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol 307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]