ABSTRACT
Antigenic variation of the immunodominant MgpB and MgpC proteins has been suggested to be a mechanism of immune evasion of the human pathogen Mycoplasma genitalium, a cause of several reproductive tract disease syndromes. Phase variation resulting in the loss of adherence has also been documented, but the molecular mechanisms underlying this process and its role in pathogenesis are still poorly understood. In this study, we isolated and characterized 40 spontaneous, nonadherent phase variants from in vitro-passaged M. genitalium cultures. In all cases, nonadherence was associated with the loss of MgpBC protein expression, attributable to sequence changes in the mgpBC expression site. Phase variants were grouped into seven classes on the basis of the nature of the mutation. Consistent with the established role of RecA in phase variation, 31 (79.5%) variants arose via recombination with MgPa repeat regions that contain mgpBC variable sequences. The remaining mutants arose via nonsense or frameshift mutations. As expected, revertants were obtained for phase variants that were predicted to be reversible but not for those that arose via an irreversible mechanism. Furthermore, phase variants were enriched in M. genitalium cultures exposed to antibodies reacting to the extracellular, conserved C terminus of MgpB but not in cultures exposed to antibodies reacting to an intracellular domain of MgpB or the cytoplasmic HU protein. Genetic characterization of the antibody-selected phase variants confirmed that they arose via reversible and irreversible recombination and point mutations within mgpBC. These phase variants resisted antibody-mediated growth inhibition, suggesting that phase variation promotes immune evasion.
KEYWORDS: Mycoplasma genitalium, homologous recombination, phase variation
INTRODUCTION
Mycoplasma genitalium is a strict human pathogen causing numerous reproductive tract syndromes, including urethritis in men and cervicitis, pelvic inflammatory disease, preterm birth, and spontaneous abortion in women (1). Remarkably, M. genitalium infections persist for months to years (2–7), increasing the risk of serious sequelae and sexual transmission. Exacerbating this problem is the fact that many patients are unaware of their infection, as they are often asymptomatic and therefore are unlikely to seek treatment. Furthermore, treatment of M. genitalium infection is complicated by the inherent resistance of the organism to cell wall synthesis-inhibiting antibiotics, the poor effectiveness of doxycycline, and increasing resistance to azithromycin and moxifloxacin. In fact, recent studies indicate that 20 to 100% of M. genitalium infections worldwide are untreatable with azithromycin (8, 9). Furthermore, M. genitalium strains resistant to both azithromycin and moxifloxacin have been described in several studies (10, 11), further complicating successful treatment.
M. genitalium cells have a flask-shaped morphology attributable to a complex tip organelle required for adherence and gliding motility. The major adhesin, MgpB, and its accessory protein, MgpC, are predominantly located on the surface of the tip organelle and mediate adherence to a variety of cell types and inanimate surfaces (plastic and glass). We recently described the membrane topology and antibody accessibility of defined regions of MgpB and found that the majority of the protein is extracellular and that the protein is anchored by a single transmembrane domain near the C terminus, with a short domain extending into the cytoplasm (12). Using attachment inhibition experiments, we have shown that the adherence domain lies within a 180-amino-acid region just proximal to the transmembrane domain. However, similar studies using MgpC-reactive antibodies have failed to identify an adherence epitope within MgpC (unpublished). The topology of MgpB was corroborated in a recent study in which isolated MgpB/MgpC complexes were characterized by electron microscopy (13); their close association is consistent with the known reciprocal stabilization of these two proteins (14).
While the complete MgpB and MgpC proteins are expressed from a single expression site (mgpBC), multiple copies of the variable regions of mgpB (regions B, EF, and G) and mgpC (region KLM) are present in nine loci, called MgPars, scattered around the genome. MgpB/C proteins are not expressed from the MgPars, as only partial mgpBC sequences are present and adjacent variable regions have different reading frames, which in some cases are separated by AT-rich regions encoding multiple stop codons in all three frames (15, 16). We have shown that segments of the mgpB and mgpC variable regions exchange reciprocally with sequences within the MgPars in vitro and in vivo to express antigenic variants of the MgpB and MgpC proteins (16).
In addition to antigenic variation, M. genitalium undergoes spontaneous phase variation in which MgpB and MgpC are not expressed, manifested as a nonadherent phenotype and a loss of red blood cell binding (hemadsorption [HA]). M. genitalium phase variants were first described by Mernaugh et al. (17) and grouped into class I or II on the basis of the expression of a 140-kDa protein (MgpB). Later, the nonadherent phenotypes of one class I mutant and one class II mutant were characterized and explained by the irreversible deletion of portions of mgpC and mgpBC, respectively. These deletions resulted from single recombination events involving specific variable regions of the expression site and the MgPar5 region, which is located immediately downstream (14). As MgpB and MgpC are reciprocally stabilized, deletion of one gene results in loss of the expression of both proteins (14). In addition to the irreversible class I and II phase variants, reversible phase variants resulting from the reciprocal exchange of two expression site-variable regions with an MgPar were predicted and detected by PCR in heterogeneous populations (14, 18). However, reversible phase variants have not yet been isolated. More recently, we have shown that the accumulation of phase variants requires RecA (19) and RuvAB (20), consistent with the role of the genes encoding these proteins in recombination.
Here we describe the isolation and characterization of a number of phase variants and determine the genetic changes responsible for phase variation. We demonstrate that the majority of these variants arise from recombination events affecting the mgpBC genes and are reversible in many cases. Furthermore, antibodies specific for the extracellular C terminus of MgpB (containing the adherence domain) select for phase variants in vitro, and we demonstrate that these variants resist antibody-dependent growth inhibition. Overall, our study supports a model of high-frequency on/off switching of MgpBC expression that prevents recognition by the immune system.
RESULTS
Isolation of spontaneous phase variants.
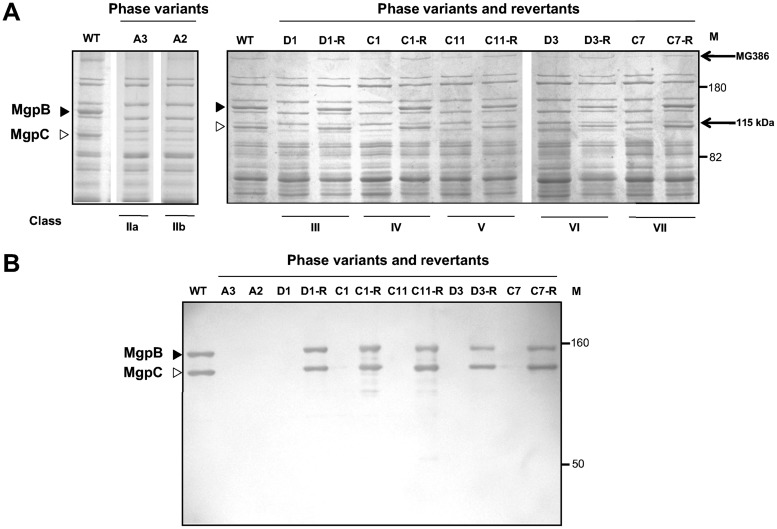
Spontaneous phase variants were isolated from serially passaged cultures of M. genitalium strain G37-C (16), our single-colony cloned isolate that expresses mgpBC sequences identical to those of the fully sequenced type strain. Four independent cultures (cultures A to D) were propagated in SP-4 broth for 10 passages, after which colonies were screened for binding to sheep red blood cells (SRBCs) (hemadsorption [HA]) to identify phase variants. HA-negative [HA(−)] colonies were cultured in 24-well plates to screen for the loss of adherence to plastic and then subjected to a second round of single-colony cloning. Fully or partially adherent clones were eliminated. In this way, between 8 and 13 HA-negative clones were identified in each of the four passaged cultures, with a total of 40 phase variants being obtained. SDS-PAGE analysis (Fig. 1A; see also Fig. S1 in the supplemental material) indicated that each phase variant lacked the MgpB and MgpC proteins, confirmed by immunoblotting (Fig. 1B) and consistent with their nonadherent phenotype and previous results (14, 17). In addition, each phase variant exhibited changes in the expression of other proteins previously noted to coincide with the loss of MgpB and/or MgpC (14), including reduced expression of MG386 and the appearance of an unidentified 115-kDa protein (Fig. 1A and S1).
FIG 1.
(A) Protein profiles of representative phase variants and revertants. Whole-cell lysates (10 μg of protein) of M. genitalium strain G37-C were electrophoresed through 8% SDS-polyacrylamide gels and stained with Coomassie blue. The profiles obtained with lysates for transposon-tagged phase variants D1, C1, C11, D3, and C7 and revertants are shown here and are identical to those obtained with lysates for untagged phase variants (see Fig. S1 in the supplemental material); the A3 and A2 lysates were from untagged phase variants. The MgpB (filled triangles) and MgpC (open triangles) protein bands were found in wild-type (WT) extracts but absent in each phase variant. The reduced expression of MG386 (P200) and the appearance of a 115-kDa unknown protein are indicated on the right. Molecular mass standards (in kilodaltons; lanes M) are marked. The protein profiles of all 40 phase variants are shown in Fig. S1. (B) Immunoblots of M. genitalium whole-cell lysates. MgpB and MgpC were detected by combining rabbit serum specific for each protein. MgpB and MgpC expression was lost from each phase variant and restored in revertants to levels similar to those in wild-type M. genitalium.
Genetic analysis and classification of phase variants.
As each phase variant lacked MgpB and MgpC protein expression, we examined the mgpBC locus by a combination of PCR, sequencing, and Southern blotting. This analysis demonstrated that the nonadherent phenotype could be attributed to a mutation in the mgpBC locus in all 40 phase variants. Alignment with expression site and MgPar sequences identified the precise recombination breakpoints for each phase variant (Table 1). Several phase variants shared identical mutations/recombination breakpoints and were assumed to be siblings arising from a common progenitor in the inoculum or from phase variation during serial passage. For example, five class IIa mutants (A3, A8, B1, B4, and B7) recombined at bp 222250/229527, suggesting that this variant was present at low levels in the inoculum. Taking into account groups of siblings, we identified 22 different mechanisms of phase variation in our collection, adding to the previously described class I and class II mutations (14). On the basis of the mechanism causing phase variation (see below), 39 mutants were grouped into seven classes (detailed in Table 1). One phase variant, A6, could not be classified: although portions of mgpBC were deleted from the expression site in this mutant, the mechanism producing this architecture could not be unambiguously determined (data not shown); this mutant was not analyzed further.
TABLE 1.
Genotypes of mgpBC phase variants
| Class | Description | Phase varianta | Phase variation mechanismb | Predicted reversibility |
|---|---|---|---|---|
| I | Recombination between regions KLM of mgpC and MgPar5 with deletion of intervening sequences | Class Ic | Deletion of bp 226524 to 230986 between regions KLM of mgpC and MgPar5 | Irreversible |
| IIa | Recombination between regions B of mgpB and MgPar5 with deletion of intervening sequences | A3, A8, B1, B4, B7 | Deletion of bp 222251 to 229526 | Irreversible |
| B9 | Deletion of bp 222586 to 229864 | |||
| C12 | Deletion of bp 222415 to 229681 | |||
| D5 | Deletion of bp 222382 to 229651 | |||
| D6 | Deletion of bp 222305 to 229577 | |||
| D7 | Deletion of bp 222535 to 229813 | |||
| B4a-1d | DNA exchange of 68 bp between regions EF of mgpB and MgPar1 and then deletion of bp 222586 to 229864 | |||
| B4a-3 | DNA exchange of 23 bp between regions EF of mgpB and MgPar5 and then deletion of bp 222251 to 229526 | |||
| IIb | Recombination between regions EF of mgpB and MgPar5 with deletion of intervening sequences | Class IIc | Deletion of bp 224176 to 230169 | Irreversible |
| A2 | Deletion of bp 224133 to 230129 | |||
| A4 | DNA exchange between regions EF of mgpB (bp 223888 to 224133) and MgPar5 (bp 229890 to 230130) and then deletion of bp 224287 to 230280 | |||
| C3, C4, C8 | DNA exchange between regions EF of mgpB (bp 223966 to 224315) and MgPar7 (bp 313348 to 313804) and then deletion of bp 224316 to 230419 | |||
| III | Reciprocal recombination involving two variable regions within mgpBC and one within MgPar | D1 | Reciprocal exchange of DNA extending from mgpB region B to mgpC region KLM (bp 222371 to 226315) with DNA extending from region B to region KLM of MgPar8 (bp 349462 to 350636) | Reversible |
| B4a-4 | Translocation of MgPar1 sequence extending from region B to region EF (bp 85580 to 86325) to mgpB (bp 222175 to 224182) | |||
| IV | Reciprocal recombination involving two variable regions within mgpBC and multiple MgPars | A5 | Reciprocal exchange of DNA extending from KLM of mgpC to KLM of MgPar5 (bp 226540 to 231066) with DNA from KLM of MgPar2 to KLM of MgPar3 (bp 168891 to 175103); in addition, a DNA exchange event replaced MgPar2 KLM sequences at the expression site (bp 169583 to 169738) with MgPar8 sequences (bp 351545 to 351703) | Reversible |
| C1 | Reciprocal exchange of DNA extending from EF to G of mgpB (224067 to 225036) with DNA from EF of MgPar2 to G of MgPar3 (bp 167813 to 175476) | |||
| V | Excision of sequences between variable regions of mgpBC and MgPar5 and subsequent insertion by recombination in another MgPar | A1, B2, B3, B5, B6, B8, B10 | Excision of bp 224326 to 230319 between variable regions EF of mgpB and MgPar5 and reinsertion into region EF of MgPar4, producing the following architecture: 214333/230242 to 230319/224326 to 230173/214260 | Reversible |
| C11 | Excision of bp 222198 to 229473 between regions B of mgpB and MgPar5 and reinsertion into region B of MgPar2, producing the following architecture: 167388/222368 to 229473/222198 to 222367/167389 | |||
| D4 | Excision of bp 222382 to 229651 between regions B of mgpB and MgPar5 and reinsertion into region EF of MgPar7, producing the following architecture: 313735/224360 to 229651/222382 to 224359/313736 | |||
| A7 | DNA exchange between regions KLM of mgpC and MgPar2 and between regions KLM of MgPar2 and MgPar8 and then excision of DNA extending from region B of mgpB to region B of MgPar5 (bp 222382 to 229651) and insertion into region KLM of MgPar2, producing the following architecture: 168925/226576 to 226685/351007 to 351279/226956 to 226575/168926 to 169088/226742 to 226955/351280 to 351544 to 169583 | |||
| C13 | DNA exchange between regions KLM of MgPar5 and MgPar8, followed by excision of DNA spanning KLM of mgpC to KLM of MgPar5, producing the architecture at the expression site of 226940/351265 to 351730/231584 bp, and then subsequent insertion of the excised DNA into region KLM of MgPar9, resulting in 428803/350955 to 351264/226941 to 230968/350802 to 350954/428804 | |||
| VI | Reciprocal recombination of an mgpBC variable region with an MgPar that encodes a frameshift mutation | C6, D3 | Reciprocal DNA translocation between EF of mgpB (bp 224074 to 224228) and MgPar6 (bp 273545 to 273696), which contains a frameshift mutation at bp 273571; in addition, a DNA exchange occurred between regions EF of mgpB (224325 to 224417) and MgPar8 (350141 to 350228); a base change at bp 224937, changing Lys1123 to Arg, could be explained by recombination of region G of mgpB with region G of MgPar3, -5, or -7 | Reversible |
| B4a-6 | Translocation of EF of MgPar6 (bp 273544 to 273638), including the frameshift mutation at bp 273571, to EF of mgpB (bp 224074 to 224176) | |||
| VII | Point mutations in mgpBC resulting in frameshifts (insertions or deletions) or premature translational stops | A9, D2 | Nonsense mutation (TAC to TAA) at bp 222613 of mgpB | Reversible |
| C2, C7, C9, C10 | Frameshift mutation by deletion of 1 bp in a poly(A) tract at bp 222875 of mgpB | |||
| C5 | Frameshift mutation by insertion of TG at bp 224447 of mgpB | |||
| D8 | Frameshift mutation by insertion of CC at bp 222380 of mgpB | |||
| B4a-2 | Frameshift mutation by insertion of AG at bp 224352 of mgpB |
Phase variants shown in bold were further analyzed for reversion.
Genomic coordinates (GenBank accession number NC_000908.2) correspond to the recombination breakpoints (identified by slashes) at the mgpBC expression site and MgPars.
Previously described by Burgos et al. (14).
Phase variants with the underlined notation B4a were obtained after selection with anti-MgpB antibodies. Sequence changes at the expression site are indicated; the corresponding MgPars were not analyzed.
Class I phase variants were previously described (14, 17) and arise from single recombination events involving the KLM region of the mgpC expression site and the homologous KLM region of the adjacent MgPar5, with subsequent deletion of the intervening sequences. Surprisingly, none of the 39 phase variants isolated in our screen were class I.
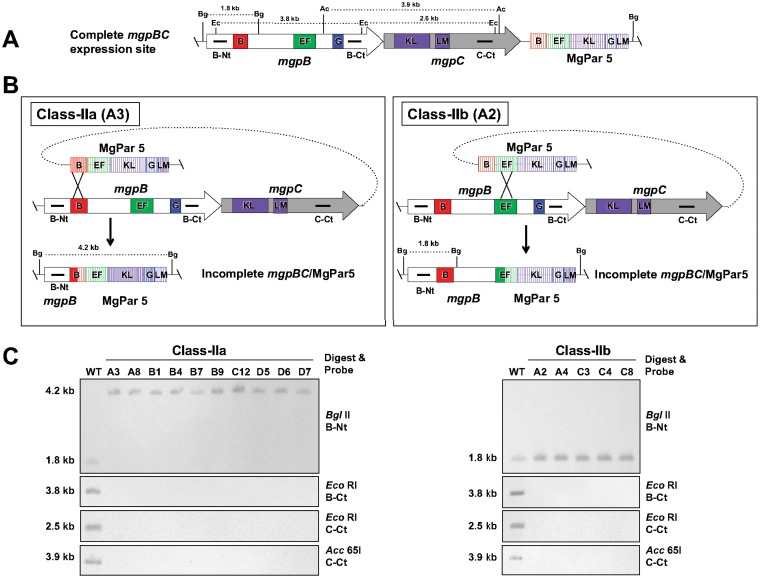
Class II mutants are characterized by the loss of mgpB and mgpC sequences due to single recombination events between variable regions of mgpB and MgPar5. In our screen, these mutants were the most common, representing 15 of the 39 mutants characterized. These mutants were further divided into two groups on the basis of which mgpB variable region recombined with the corresponding region of MgPar5 (Fig. 2): class IIa mutants recombined at region B, and class IIb mutants recombined at region EF, similar to the class II mutant previously described by Burgos et al. (14) and noted in Table 1. Although theoretically possible, no phase variants in which region G of mgpB recombined with MgPar5 were found in our screen. Importantly, Southern blots probed with sequences encoding the N and C termini of mgpB (probes B-Nt and B-Ct, respectively) and the C terminus of mgpC (probe C-Ct) confirmed the architecture of these recombinants. In all cases, the mgpBC sequences between the regions involved in the recombination event were not located elsewhere in the genome (Fig. 2C), predicting that these mutations are irreversible within a clonal population.
FIG 2.
Class IIa and class IIb mutants arise via recombination with MgPar5, resulting in the irreversible deletion of mgpBC sequences. (A) The mgpBC operon and MgPar5, located immediately downstream, are shown, with the positions of repeat regions B, EF, G, and KLM indicated. (B) Class IIa mutants (left) result from a single recombination event between the B regions of mgpB and MgPar5, while class IIb mutants (right) recombine between the EF regions of mgpB and MgPar5. Both class IIa and class IIb mutations irreversibly delete mgpB and mgpC sequences. A representative mutant for each class (A3 and A2) is shown. Horizontal bars mark probes for sequences encoding the N terminus of MgpB (B-Nt), the C terminus of MgpB (B-Ct), and the C terminus of MgpC (C-Ct). The AT-rich sequences between regions EF and KLM of MgPar5, containing stop codons in all three reading frames, are not shown (15). (C) Southern blots using probes B-Nt, B-Ct, and C-Ct for digested genomic DNA of wild-type (WT), class IIa (left), and class IIb (right) phase variants. The fragment sizes resulting from BglII (Bg), EcoRI (Ec), and Acc65I (Ac) digestion are consistent with the proposed architecture in these mutants. Additional BglII and Acc65I sites irrelevant to this analysis are not shown.
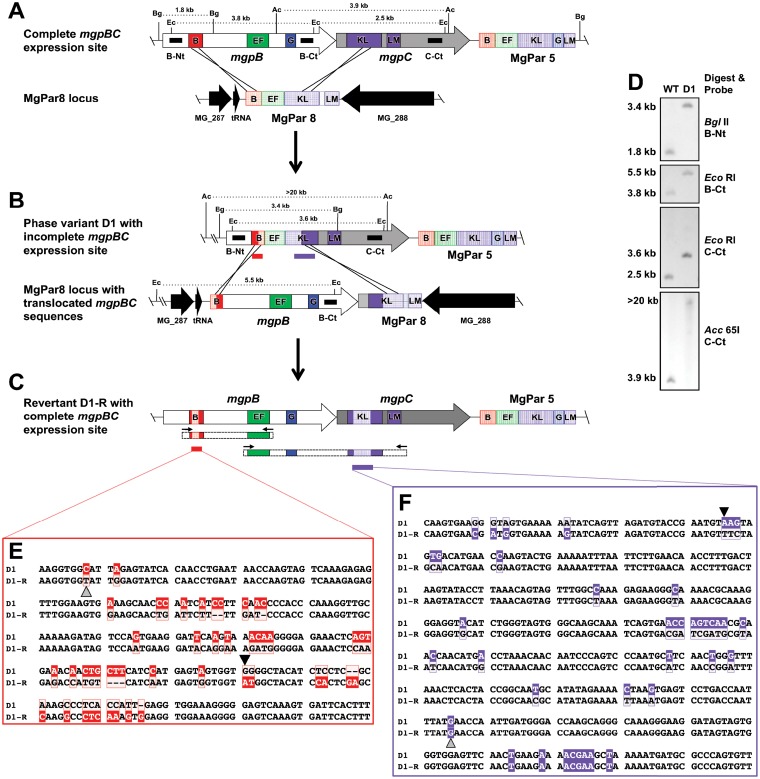
Class III phase variants are generated via double recombination events involving two variable regions of the expression site and the corresponding homologous sequences from a single MgPar. In our study, only one class III phase variant (D1) was isolated (Fig. 3). This particular mutant resulted from the translocation of MgPar8 sequences to the expression site via a double recombination event involving variable regions B and KLM. This resulted in the replacement of mgpB and mgpC sequences from region B to KLM with MgPar8 sequences, including part of region B, all of region EF, and part of region KLM. As shown in Fig. 3B, this DNA rearrangement truncates both the MgpB- and MgpC-coding regions. Interestingly, when the MgPar8 site of phase variant D1 was examined, we found that the conserved and variable sequences of mgpB and mgpC had translocated to the MgPar8 site, indicating that the recombination event was reciprocal in nature (Fig. 3B and D). Therefore, this mutant is predicted to be reversible, as a second double recombination event with the MgPar8 locus could restore the expression site (see below).
FIG 3.
Class III phase variants arise via double recombination involving two variable regions and the corresponding sequences of a single MgPar. The recombination events producing the class III phase variant (D1) and revertant (D1-R) are shown. (A) The complete, wild-type mgpBC expression site (top) and MgPar8 (bottom) with repeat regions B, EF, G, and KLM are indicated. Restriction enzyme sites are shown for BglII (Bg), EcoRI (Ec), and Acc65I (Ac). Black bars mark DNA probes for sequences encoding the N terminus of MgpB (B-Nt), the C terminus of MgpB (B-Ct), and the C terminus of MgpC (C-Ct). Regions B and KLM of the mgpBC expression site recombined (black crossing lines) with homologous regions of MgPar8, resulting in the exchange of most of mgpB and the 5′ end of mgpC with the MgPar8 site, as depicted in panel B. The AT-rich sequences between regions EF and KLM of MgPar5 and MgPar8, containing stop codons in all three reading frames, are not shown (15). Red and purple bars indicate sequences aligned in panels E (region B) and F (region KLM). The expression site of phase variant D1 subsequently exchanged with the MgPar8 locus (B), thereby restoring functional mgpBC in the revertant D1-R shown in panel C. PCR products (dashed rectangles flanked by small arrows) were sequenced to confirm restoration of a functional mgpBC locus. (D) Southern blot of wild-type (WT) and phase variant D1 genomic DNA digested with the restriction enzymes indicated and probed with B-Nt, B-Ct, and C-Ct. The fragment sizes for each digest confirm the schematics shown in panels A and B. (E and F) DNA sequences of PCR products amplified from D1 and D1-R region B (E) and region KLM (F). Nucleotides boxed in solid color are unique to the expression site; nucleotides outlined in color are unique to MgPar8. The recombination breakpoints producing the phase variant are indicated with a small black triangle; the gray triangle marks the crossover point for reversion. Revertant D1-R therefore retains 167 bp (encoding 18 amino acid changes) and 259 bp (encoding 8 amino acid changes) of the MgPar8 sequences in regions B and KLM, respectively.
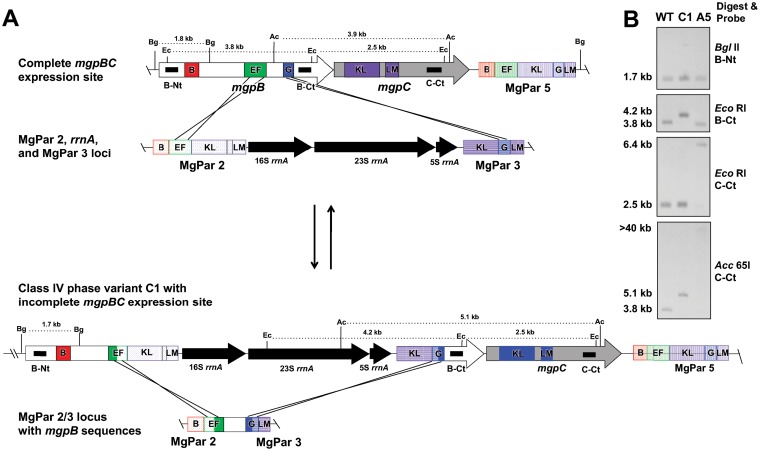
Class IV mutants are also produced via double recombination events affecting the expression site but involving two variable regions and two or three different MgPars. For example, class IV phase variant C1 arose by crossover of regions EF and G of mgpB with region EF of MgPar2 and region G of MgPar3 (Fig. 4). MgPars 2 and 3 lie near each other on the chromosome and are separated by the rRNA operon. This results in the translocation of the rRNA operon to the expression site, with the concomitant movement of the conserved region between EF and G of mgpB to the now fused MgPar2/3 locus. Similarly, a second class IV phase variant (A5) was isolated (Table 1) in which the sequences between KLM of mgpC and KLM of MgPar5 exchanged with KLM of MgPar2 and KLM of MgPar3, respectively. Again, this resulted in the translocation of the rRNA operon to the expression site, interrupting the mgpC gene (not shown). The band sizes shown in Southern blots for both C1 and A5 (Fig. 4B) confirmed the genomic structure deduced from sequencing the recombination breakpoints. Importantly, these phase variants are also predicted to be reversible.
FIG 4.
Class IV phase variants arise by double recombination involving two variable regions of mgpBC and the corresponding sequences of two MgPars, resulting in the reciprocal exchange of intervening sequences. The DNA rearrangements producing class IV phase variant C1 and revertant C1-R are shown. (A) (Top) The mgpBC expression site recombined via regions EF and G with homologous sequences in MgPar2 and MgPar3, respectively, resulting in the translocation of the rrnA operon located between MgPar2 and MgPar3 to the expression site and the reciprocal placement of mgpB conserved sequences in the MgPar2/3 loci (bottom). Reversion of this recombination produced the HA(+) revertant C1-R, restoring mgpBC to the wild type. The AT-rich sequences between regions EF and KLM of MgPar2 and MgPar5, containing stop codons in all three reading frames, are not shown (15). (B) Southern blots of phase variants A5 and C1. In mutant A5, homologous KLM regions of MgPar2 and MgPar3 exchanged with KLM regions of mgpC and MgPar5, thus producing the hybridization patterns shown. The restriction digest sites producing the banding pattern for C1 are indicated in panel A.
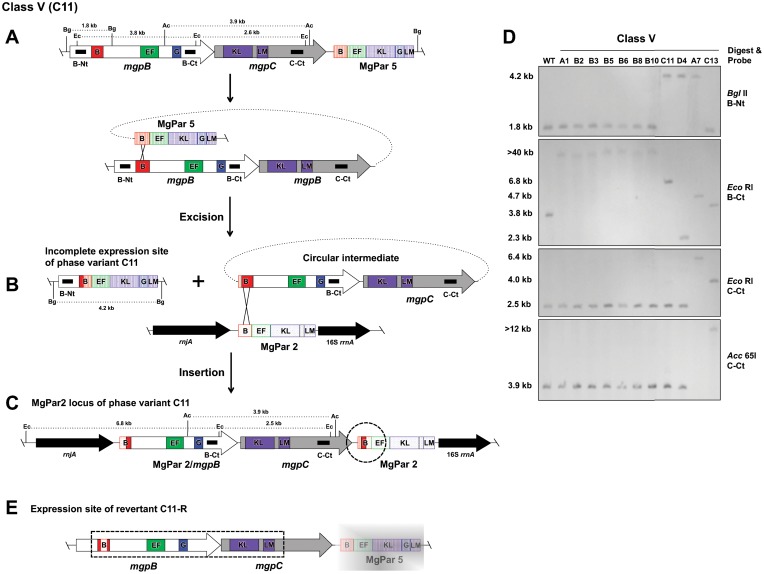
Eleven phase variants resulting from an excision-insertion mechanism were categorized as class V, of which seven (A1, B2, B3, B5, B6, B8, and B10) had identical mutations (Table 1). As an example, the likely recombination events producing class V phase variant C11 are detailed in Fig. 5. Sequencing of the expression site of C11 indicated that most of mgpBC was deleted from the expression site, similar to the findings for class II mutants. In particular, only the 5′ end of mgpB up to region B remained, and then regions B of mgpB and MgPar5 merged. However, unlike the irreversible class II mutants in which mgpBC conserved sequences are deleted, class V expression site sequences are archived elsewhere in the genome, as demonstrated by Southern blotting (Fig. 5D). Further, Southern blot analysis of C11 suggested that the conserved mgpBC sequences had recombined with the MgPar2 locus. Surprisingly, we found that the 3′ end of the MgPar2 locus of phase variant C11 contained 169 bp of expression site region B sequences between MgPar5 and MgPar2 region B sequences (Fig. 5C, dotted circle). This architecture suggests that recombination between regions B of mgpB and MgPar5 first produced a circular intermediate (Fig. 5B), which subsequently inserted via region B into MgPar2 (Fig. 5C). Examination of the other class V mutants similarly suggests the production of a circular intermediate during phase variation.
FIG 5.
Class V phase variants arise via an excision-reinsertion mechanism in which a variable region within mgpBC recombines with MgPar5, producing a circular intermediate that subsequently reinserts in a second MgPar. The DNA rearrangements producing the class V phase variant C11 and revertant C11-R are shown. (A) The mgpBC operon and MgPar5 located immediately downstream are shown, with the positions of the variable regions (B, EF, G, and KLM) indicated. A single recombination involving variable regions B of the expression site and MgPar5 results in the excision of mgpBC sequences and the production of a circular intermediate, shown in panel B. Subsequent insertion of the circular intermediate via recombination with region B of MgPar2, flanked by rnjA and the 16S rrnA gene, is shown in panel C. The dashed circle indicates where mgpB-specific region B sequences were found between MgPar5 and MgPar2 region B sequences, evidence for the creation of a circular intermediate to produce phase variants of this class. The AT-rich sequences between regions EF and KLM of MgPar2 and MgPar5 containing stop codons in all three reading frames are not shown (15). (D) Southern blot analysis using probes B-Nt, B-Ct, and C-Ct for digested genomic DNAs of the wild type and class V mutants. The fragment sizes resulting from BglII (Bg), EcoRI (Ec), and Acc65I (Ac) digestions are also indicated. The various band sizes for the different class V mutants depend on the variable region involved in the excision and the MgPar in which the recombination intermediate inserted. (E) Expression site of revertant C11-R, with the region confirmed by PCR and sequencing being marked by the dashed rectangle. C11-R mgpB region B contains 224 bp of MgPar2 region B; not shown is the MgPar7-specific sequence change of 3 bp in region EF. The sequence of MgPar5 of revertant C11-R was not determined, as indicated by the faded gray rectangle; similarly, the MgPar2 locus of C11-R was not analyzed (not shown).
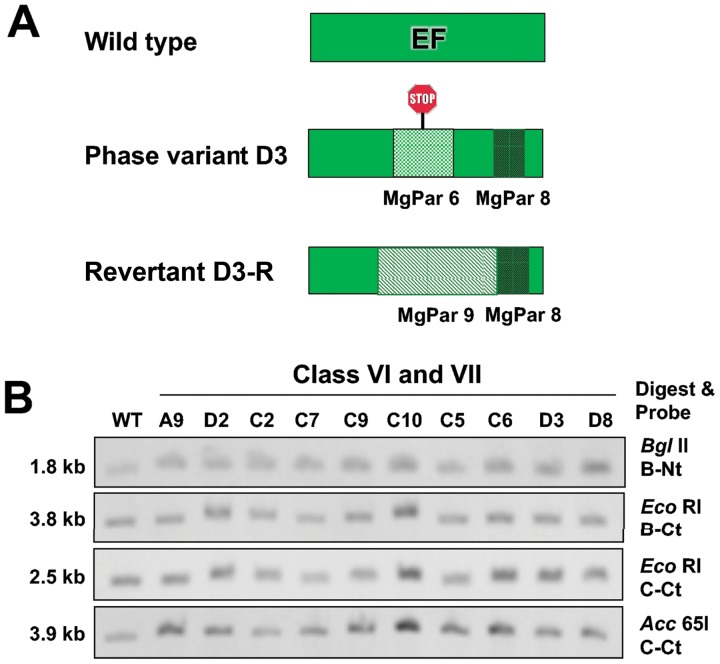
Ten of the phase variants had small mutations in mgpB and were categorized as class VI or class VII (Table 1). The two class VI mutants, C6 and D3, arose via reciprocal exchange of expression site region EF with MgPar6 (Fig. 6). MgPar6 of M. genitalium strain G37 is unusual in that it contains only a variable region (EF) and it has a single-base-pair deletion, thus introducing a frameshift in mgpB after recombination into the expression site. In addition, MgPar8-specific EF sequences were found in mgpB of phase variants C6 and D3, indicating that a second reciprocal recombination event occurred in these mutants. Figure 6B shows Southern blots for these mutants, confirming that the sizes of the hybridizing bands are unchanged, as predicted.
FIG 6.
Class VI and class VII phase variants are produced by small sequence changes within mgpBC resulting from recombination-dependent and -independent mechanisms, respectively. (A) Schematic showing that class VI phase variant D3 arose via recombination with MgPar6 EF (thereby introducing a frameshift and premature stop, indicated with a stop sign). This mutant also recombined with MgPar8, replacing expression site EF with 87 bp of MgPar8 EF. The expression site region EF of phase variant D3-R subsequently recombined with 297 bp of MgPar9 (replacing MgPar6, expression site, and MgPar8 sequences), thus restoring wild-type MgpB expression. (B) Southern blot showing that the band sizes are unchanged relative to those for the wild type (WT) for class VI mutants (C6 and D3) and the class VII phase variants (A9, D2, C2, C7, C9, C10, C5, and D8), consistent with the small sequence changes in the expression site of these mutants (described in Table 1).
Like the class VI mutants, the eight class VII mutants isolated in this study contain small sequence changes that leave the structure of the expression site unchanged. In class VII mutants, expression of MgpB is prevented by nonsense mutations or 1- to 2-bp indels in mgpB (Table 1). The class VII mutants differed from the other phase variants, in that they did not arise via a recombination-dependent mechanism.
Isolation of revertants.
The possibility that phase variants are reversible was explored by using representatives of each class of phase variant: A3 (class IIa), A2 (class IIb), D1 (class III), C1 (class IV), C11 (class V), D3 (class VI), and C7 (class VII). To rule out the possibility that wild-type HA-positive [HA(+)] cells were carried through during single-colony cloning of phase variants, each mutant was marked with a minitransposon (MTnTetM438 [21]) conferring tetracycline resistance by electroporation. Tetracycline-resistant colonies arising after electroporation were cultured, were confirmed to be nonadherent, and had protein profiles identical to those of untagged phase variants (Fig. 1A and S1). Cultures of the transposon-tagged phase variants were enriched for HA(+) revertants by binding to washed sheep red blood cells (see Materials and Methods). HA(+) revertants were obtained for phase variants of class III (D1-R), class IV (C1-R), class V (C11-R), class VI (D3-R), and class VII (C7-R), consistent with their predicted reversibility. In contrast, no HA(+) revertants were obtained for class IIa or IIb phase variants, consistent with the predicted irreversibility due to the loss of mgpBC sequences from the genome. SDS-PAGE and immunoblot analyses confirmed that MgpB and MgpC expression was restored in each revertant (Fig. 1A and B).
The mgpBC expression site of each revertant was analyzed by PCR and sequencing to determine the mechanism restoring adherence. Analysis of the class III revertant D1-R revealed that a second double recombination event that restored expression of mgpBC had occurred. As expected, the mgpBC locus had exchanged with MgPar8 (which, in phase variant D1, contains most of mgpB and the 5′ end of mgpC), thereby translocating these sequences back to the expression site (Fig. 3B). However, the crossover points producing the revertant (extending from bp 222196 in region B to bp 226575 in region KLM) differed from those of the phase variant, such that MgPar8-specific sequences were retained in mgpBC (Fig. 3C, E, and F). As a result, the D1-R revertant is predicted to express antigenic variants of MgpB and MgpC that differ from the wild-type sequence in 18 and 8 amino acids, respectively, supporting a role for phase variation and reversion as a means to avoid antibody-mediated killing.
Examination of a revertant (C1-R) of the class IV mutant C1 indicated that the mgpBC sequence of the revertant was identical to that of wild-type mgpBC (Fig. 4 and data not shown). This suggests that the same recombination crossover points were utilized for phase variation and for reversion.
A revertant of class V phase variant C11 was isolated, and analysis of the C11-R expression site by PCR and sequencing confirmed that the complete mgpBC genes were restored (Fig. 5D), suggesting that reversion occurred via recombination between the truncated expression site and the MgPar2 locus of phase variant C11. Alignment of mgpB region B of C11-R showed that a 224-bp segment of MgPar2 was present in the expression site, predicting the expression of an antigenic variant of MgpB region B differing from the wild-type sequence in 11 amino acids. In addition a small, 3-bp exchange with MgPar7 predicts a 1-amino-acid change in region EF (not shown). Although we did not sequence the MgPar5 region of C11-R, a number of possible reciprocal recombination events could have restored MgpBC expression. For example, exchange of DNA spanning mgpB region B to region EF of MgPar2 with regions B to EF of MgPar5 or the reversal of the original recombination event, including the production of a circular intermediate, would have reconstituted the mgpBC expression site.
Class VI mutants, which are nonadherent by virtue of the frameshift mutation encoded by region EF of MgPar6, are readily reversible, as exchange with any of the other eight MgPars which contain region EF could restore MgpB expression. Consequently, after enrichment with SRBCs, we observed significantly more revertants in class VI than in the other classes. When we examined one such revertant (D3-R), we found that the frameshifted MgPar6 EF sequences within the expression site (plus additional flanking DNA) were replaced by homologous sequences from MgPar9, thus restoring expression of MgpB and predicting 18 amino acid changes compared to the sequence of wild-type MgpB (Fig. 6).
Finally, a revertant of the class VII phase variant C7 was also analyzed. Sequencing of the expression site of revertant C7-R indicated that the A deleted at bp 222875 in the C7 phase variant (Table 1) had been restored, thus correcting the reading frame and allowing MgpB expression.
Antibody-mediated selection of phase variants.
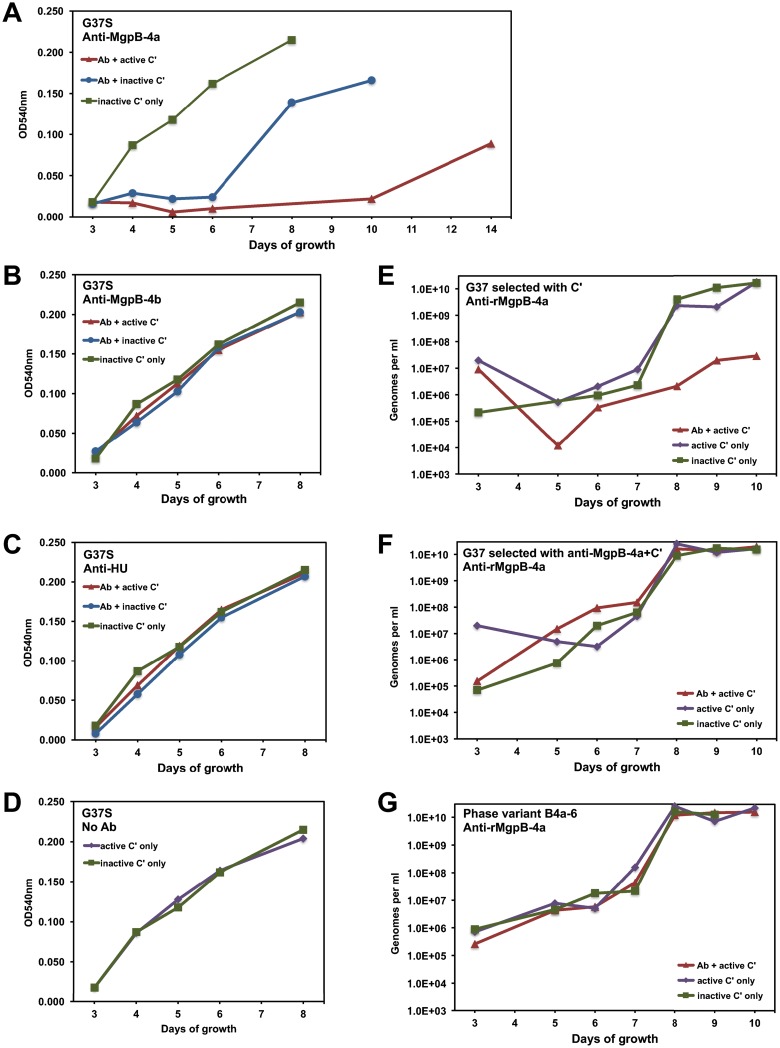
As MgpB and MgpC are immunodominant proteins, we hypothesized that phase variants might avoid antibody-mediated killing of M. genitalium. We therefore measured the effect of anti-MgpB antibodies on the growth and the resulting proportion of phase variants using M. genitalium strain G37-S, the nonclonal stock of G37 maintained in Seattle, WA (15). We reasoned that more phase variants that could avoid antibody-mediated killing would be present in this culture than in the highly purified G37-C stock. As shown in Fig. 7A, the growth of G37-S cultures treated with heat-inactivated complement and anti-recombinant MgpB-4a (anti-rMgpB-4a) antibodies, which bind the extracellular C terminus containing the adherence domain (12), was delayed compared to that of controls treated with only heat-inactivated complement. Growth inhibition was even more pronounced when active complement was included. In contrast, M. genitalium treated with anti-recombinant MgpB-4b (anti-rMgpB-4b), which binds the intracellular domain of MgpB, or with antibodies against recombinant HU (rHU; HU is an intracellular protein) grew similarly to control cultures (Fig. 7B and C). In addition, cultures grew similarly after treatment with active versus inactive complement (Fig. 7D), indicating that growth inhibition could be attributed entirely to antibodies reacting with the C-terminal extracellular domain of MgpB.
FIG 7.
Antibody (Ab)-dependent growth inhibition of wild-type and phase variant M. genitalium. (A to D) Wild-type M. genitalium strain G37-S was incubated for 1 h with antibody (or PBS) and active or heat-inactivated guinea pig complement (C′) and then cultured in SP-4 medium using color change as a measure of growth. The proportion of phase variants during growth is shown in Table 2. OD540nm, optical density at 540 nm. (E to G) The growth of the phase variants is not inhibited by MgpB-specific antibody. The indicated M. genitalium cultures were exposed to MgpB-specific antibody and complement for 1 h and then cultured in SP-4 medium, with growth being measured by qPCR. (E) M. genitalium previously selected by complement alone is susceptible to inhibition by anti-MgpB-4a antibody; (F) M. genitalium previously selected with anti-MgpB-4a and complement is not inhibited under these conditions; (G) a pure culture of antibody-selected phase variant B4a-6 is not inhibited by anti-MgpB-4a and complement.
To determine if treatment with anti-rMgpB-4a antibodies selected for phase variants, we screened for HA(−) colonies arising during the growth of antibody-selected cultures compared to control cultures. Phase variants were more abundant in cultures selected with anti-rMgpB-4a antibodies, especially when active complement was present, reaching 76% of colonies after 10 days of growth (Table 2). Interestingly, the proportion of phase variants in cultures treated with anti-rMgpB-4a and inactive complement increased slightly to 9% after 4 and 6 days of growth and then later decreased to 2%, similar to the findings for cultures treated with inactive complement only. We hypothesize that the growth of adherent, MgpB-positive M. genitalium is inhibited by antibody but that increasing cell density over time effectively reduces the antibody dosage, thus allowing the uninhibited growth of MgpB-positive cells.
TABLE 2.
Proportion of phase variants during growth with antibody and complement
| Antibody target | Complement | % HA(−) coloniesa |
||||
|---|---|---|---|---|---|---|
| Day 4 | Day 6 | Day 8 | Day 10 | Day 14 | ||
| MgpB-4a | Active | 16.18 | 45.64 | 54.77 | 76.05 | 62.71 |
| MgpB-4a | Inactive | 5.77 | 9.09 | 4.41 | 2.17 | — |
| MgpB-4b | Active | 0.79 | 4.32 | — | — | — |
| MgpB-4b | Inactive | 0.90 | 2.34 | — | — | — |
| HU | Active | 0.96 | 2.24 | — | — | — |
| HU | Inactive | 0.95 | 2.91 | — | — | — |
| None | Active | 0.11 | 3.89 | — | — | — |
| None | Inactive | 0.92 | 1.79 | — | — | — |
Aliquots of antibody-selected cultures were plated to agar plates on the day indicated. The percentage of the resulting colonies that were HA(−) is indicated. —, no colonies were obtained, as these late-stationary-phase cultures were no longer viable.
Genetic characterization of antibody-selected phase variants.
In order to compare the mechanism of phase variation of antibody-selected mutants to that of the spontaneous phase variants described above, we cloned single colonies of five nonadherent mutants from cultures selected with anti-rMgpB-4a and complement. Total DNA was isolated from phase variants B4a-1, B4a-2, B4a-3, B4a-4, and B4a-6, and the changes in the mgpBC expression site were determined by PCR and sequencing (Table 1).
Two antibody-selected mutants, B4a-1 and B4a-3, were determined to be class IIa phase variants. Phase variant B4a-1 had breakpoints identical to those of the spontaneous phase variant B9 (Table 1). B4a-1 additionally exchanged 68 bp of MgPar5 region EF with region EF of MgPar1. Similarly, two recombination events occurred to produce antibody-selected strain B4a-3: first, 23 bp of mgpB region EF translocated to MgPar5 region EF, and then a single crossover between mgpB region B and MgPar5 region B occurred, with the breakpoints being identical to those in the spontaneous phase variant A3 described above (Table 1 and Fig. 2B). Variant B4a-2 was found to be a class VII mutant (Table 1), as it contained a 2-bp insertion of AG at bp 224352 (within expression site EF), resulting in a frameshift and premature termination of MgpB. Antibody-selected phase variant B4a-4 arose via a class III mechanism, in which expression site sequences spanning from region B of mgpB to region KLM of mgpC exchanged with region B through region KLM of MgPar1 (Table 1). Finally, antibody-selected variant B4a-6 was categorized as class VI, as it contained an MgPar6-encoded frameshift mutation preventing expression of MgpB (Table 1). These experiments indicate that the antibody-selected mutants were generated by the same mechanisms as the spontaneously occurring mutants.
Growth of phase variants is not inhibited by MgpB-specific antibody and complement.
We reasoned that if antibodies and complement select against MgpB-positive M. genitalium, then phase variants should be resistant to these treatments. To test this hypothesis, we repeated the antibody selection experiments, comparing the growth of three inocula: (i) G37-S previously selected with active complement alone, (ii) G37-S previously selected with anti-rMgpB-4a antibodies and active complement, and (iii) a pure culture of antibody-selected phase variant B4a-6. Each culture was treated with anti-rMgpB-4a plus active complement, active complement alone, or heat-inactivated complement, and growth was monitored by quantitative PCR (qPCR). As shown in Fig. 7, the growth of G37-S previously selected with complement alone was inhibited by anti-rMgpB-4a plus complement but not by complement alone or heat-inactivated complement (Fig. 7E). In contrast, the growth of G37-S previously selected with anti-rMgpB-4a and complement was not subsequently inhibited by anti-rMgpB-4a and complement (Fig. 7F), consistent with the high percentage of phase variants in the inoculum. Finally, the growth of the cloned antibody-selected phase variant B4a-6 was unaffected by anti-rMgpB-4a plus complement (Fig. 7G), thus supporting phase variation as a mechanism to avoid killing by antibodies targeting the adherence domain of MgpB.
DISCUSSION
Many pathogenic bacteria are able to enhance their survival in vivo by altering the antigenic and functional properties of their surface components by a variety of mechanisms, most notably, by antigenic and phase variation (22). Antigenic variants express surface molecules with altered characteristics, whereas phase variants switch between the on and off expression states of a given molecule. Both mechanisms of variation diversify the phenotypes within a population, potentially facilitating immune avoidance or affording a fitness advantage in varied environments (23).
M. genitalium is a sexually transmitted human pathogen that has a dedicated system of recombination enzymes (19, 20, 24) and repetitive elements (MgPars) to modify by segmental reciprocal recombination the antigenic properties of its major antigens, MgpB and MgpC (15, 16), proteins required for adherence and motility. Burgos et al. proposed that, in addition to antigenic variation, the particular architecture of the MgPars may also generate phase variants that no longer express MgpB/C, thereby modulating adherence of M. genitalium (14). We hypothesize that phase variation may also allow M. genitalium to avoid clearance by antibodies targeting conserved regions of these immunodominant proteins. In the current study, we isolated 40 nonadherent, hemadsorption-negative variants that arose spontaneously during serial passage and determined that all lacked MgpB/C protein expression attributable to changes in the mgpBC locus. Characterization of the resulting mgpBC and MgPar sites revealed that these variants were generated by a variety of genetic mechanisms. Twenty-two (55%) of these phase variants contained unique mutations, which were grouped into seven classes on the basis of the nature of the genetic change. Most phase variants arose via recombination-dependent mechanisms, consistent with previous observations that phase variants are infrequent in cultures of recA and ruvAB recombination-deficient mutants (19, 20). Importantly, the DNA rearrangements in the expression site and MgPars in the isolated variants predicted that most mutations are reversible, a hypothesis that we confirmed experimentally. Finally, we showed that antibodies specific to the conserved C-terminal adhesin domain of MgpB (12) select for phase variants that resist growth inhibition by these antibodies, consistent with a potential role for phase variation in avoiding antibody-mediated killing in vivo.
The finding that all of our isolated phase variants had defects in mgpBC suggests that alteration of these genes is the primary mechanism by which nonadherent phase variants arise in M. genitalium. This is consistent with a previous description of 30 HA(−) variants, which were differentiated into two classes (class I and class II) depending on whether full-length MgpB was expressed at reduced levels or was completely absent, respectively (17). Burgos et al. (14) later determined that deletion of large segments of mgpC (class I) or mgpBC (class II) in these variants was responsible for the nonadherent phenotype. It is important to note that of these 30 mutants, the mgpBC loci of only 1 class I mutant and 1 class II mutant have been described (14) and the remaining 28 phase variants may in fact belong to one of the new phase variant classes defined herein. Building on this nomenclature, we grouped our phase variants into seven different classes based upon the mechanism that altered the mgpBC expression site.
The previously identified class I and class II phase variants arose via recombination between variable regions of mgpC (class I) or mgpB (class II) with homologous sequences of MgPar5 located just downstream, causing the irreversible deletion of the intervening sequences (14, 17). Surprisingly, in our screen we did not isolate any class I mutants, even though the close genetic linkage between mgpC KLM and MgPar5 KLM would predict that these genetic variants would be the most common. Perhaps steric hindrance or other factors reduce the frequency of this recombination event. In contrast, class II irreversible phase variants represented a substantial portion (38%) of the isolates in our study. Irreversible phase variation would presumably be disadvantageous in vivo, as variants would be unable to adhere to host cells. One possible explanation for the high proportion of these variants in our study is that our strategy to clone single colonies and isolate pure HA(−) colonies would necessarily enrich for stable and irreversible variants. In other words, phase variants that revert frequently would be discarded, as colonies would appear as mixed cultures of HA(+) and HA(−) cells. This hypothesis is supported by the report by Mernaugh et al. (17), in which more than 90% of the 500 HA(−) colonies isolated displayed an unstable phenotype. This apparent instability may be a consequence of the propensity of M. genitalium to clump, thereby producing aggregates of adherent and nonadherent cells, or, alternatively, may suggest that most phase variants rapidly revert. Regardless, if irreversible phase variants occur in vivo, it is also possible that they could revert by acquisition of exogenous DNA from other M. genitalium cells via either conjugation, membrane fusion, or uptake of free DNA from lysed bacteria. Evidence for these possibilities is lacking in M. genitalium (25), yet horizontal gene transfer events have been described for other mycoplasma species (26, 27).
The existence of reversible phase variants was initially suggested by Burgos et al. (14) and detected by PCR within M. genitalium cultures (14, 18). In our study, reversible variants constituted the majority (62%) of the mutants isolated and arose by diverse mechanisms. Class III and class IV phase variants were produced by reciprocal recombination of two variable regions of the mgpBC expression site with either a single MgPar locus or multiple MgPar loci, respectively. Class IV DNA rearrangements are especially interesting, in that the single rRNA operon of M. genitalium is translocated to the mgpBC expression site as a consequence of its position between the closely linked MgPar2 and MgPar3 loci (28). Apart from causing a loss of adherence, this genome reorganization might affect rRNA transcription, possibly altering cell growth by affecting ribosome abundance (29–31). Given that MgPars can exchange with each other (16), the rRNA operon could also theoretically be translocated to other MgPar sites distributed around the genome without involving the mgpBC locus.
Class V reversible variants are similar to class I and class II variants in that they originated from single crossovers with MgPar5. However, the DNA rearrangements in the class V variants suggest a model in which the excised circular intermediate produced after the crossover is subsequently integrated into a different MgPar through recombination. As a result, mgpBC sequences are maintained within the genome, thereby enabling phenotype reversion. The high number of class V variants in our screen suggests that circular intermediates are stable in M. genitalium cells and, together with the class II mutants obtained, reinforces the notion that recombination with the adjacent MgPar5 is the most favorable event.
Class VI mutants arose by recombination between regions EF of mgpB and MgPar6, which encode a frameshift mutation. These mutants should revert more frequently than other variants because the frameshift mutation in EF of MgPar6 can be replaced by recombination with any of the EF regions in other MgPars. In fact, class VI revertants were obtained at a higher frequency than other reversible phase variants after red blood cell enrichment. Interestingly, MgPar3 region KLM in M. genitalium strain G37 also encodes a frameshift, providing another way to generate class VI variants, although no MgPar3-derived class VI phase variants were isolated in this study. Notably, of the fully sequenced M. genitalium strains (28, 32), only G37 and M6320 contain frameshifts in MgPar6. However, MgPar sequences of only a few clinical strains are available (33); thus, we cannot predict how common this mechanism of phase variation is among M. genitalium strains.
Although less common, we also identified recombination-independent mechanisms to generate phase variants, which were designated class VII. Of the 10 class VII mutants isolated, 8 originated by frameshift mutations within the mgpBC coding sequence, including 4 mutants with a 1-base deletion in a poly(A) tract in mgpB. Similarly a 1-base insertion in a poly(A) tract of the homologous P1 protein of M. pneumoniae results in nonadherence (34). Such mutations may result from slipped-strand mispairing during DNA replication (35, 36), a common mechanism that generates phase variants in other bacteria (22, 37, 38) and that is perhaps facilitated by the lack of mismatch repair systems in mycoplasmas (39). Consistent with this idea, Escherichia coli and Saccharomyces cerevisiae yeast strains defective in mismatch correction display increased mutation rates in homopolymeric tracts (40, 41).
What might be the role of phase variation in vivo? As M. genitalium forms intimate attachments with host cells (17), the loss of MgpBC expression and disassembly of the attachment organelle (14) may facilitate shedding and transmission to new hosts or to different sites in the same host. Restoration of MgpBC expression would then be necessary to adhere and establish infection. Alternatively, M. genitalium may occupy an intracellular space (42, 43), where an attachment organelle would presumably be an unnecessary and energetically costly structure to maintain. Phase and antigenic variations are also classically presumed to be mechanisms of immune evasion, thereby promoting recurrent and persistent infection. In this regard, in a primate model of M. genitalium infection, the appearance of antibodies to specific sequences of MgpB correlates with the clearance of those sequences from the genital tract, consistent with a model of selection and antigenic variation (44, 45). Although antigenic variants may avoid antibodies recognizing MgpBC variable regions, the C-terminal conserved domain of MgpB is the most immunogenic region (46–48). Herein, we demonstrate that complement and antibodies targeting the conserved adherence domain of MgpB inhibit growth and select for phase variants that ultimately resist killing by antibodies and complement. These results suggest that phase variation may provide an additional immune evasion mechanism by avoiding clearance by antibodies directed to conserved regions of MgpB and/or MgpC. Future studies focused on clinical specimens to detect phase variants in vivo are necessary to establish a role for phase variation in the pathogenesis of M. genitalium.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. genitalium strains G37-S (15) and G37-C (16) were grown in SP-4 broth or on SP-4 agar plates containing 0.8% agar (49). Cultures were grown in 25-cm2 tissue culture flasks containing 5 ml SP-4 medium at 37°C in 5% CO2 until the medium turned orange or yellow (4 to 5 days), indicating fermentation of glucose and growth of M. genitalium.
Isolation and purification of phase variants.
Four separate cultures (cultures A to D) of M. genitalium were serially passaged in SP-4 broth: 25-cm2 tissue culture flasks containing 5 ml of SP-4 broth were inoculated with 25 μl of a frozen stock of G37-C and incubated until the color changed to orange (approximately 5 days). Adherent bacteria were scraped into the supernatant, and 25 μl of this suspension was used to inoculate a new flask. This process was repeated for a total of 10 passages, at which point cultures were plated to SP-4 agar and hemadsorption assays were performed on isolated colonies (see below). HA-negative colonies were cultured in SP-4 broth in 24-well plates, and nonadherent cultures were again plated to SP-4 agar. Random, well-isolated colonies were picked from these plates (without assaying for HA) and screened again for nonadherence in 24-well plates. Subsequent HA assays on single colonies of these cloned phase variants confirmed that all colonies were HA(−) for each phase variant.
Hemadsorption assays.
Hemadsorption assays were performed as previously described (50). Briefly, M. genitalium colonies were exposed to washed sheep red blood cells (SRBCs) diluted 1:50 in phosphate-buffered saline (PBS) for 30 to 60 min, unbound SRBCs were removed by gentle washing with PBS, and then the colonies were visualized by light microscopy. The proportion of HA-deficient colonies was calculated by dividing the number of colonies that failed to adsorb SRBCs by the total number of colonies analyzed.
Analysis of protein profiles.
SDS-PAGE analysis of total M. genitalium proteins was performed according to standard procedures: 8% polyacrylamide gels were loaded with whole-cell lysates (10 μg of total protein per lane), electrophoresed, and then stained with Coomassie blue. Immunoblot assays were performed as described previously (45) using antibodies (diluted 1:1,000) specific to amino acids 1123 to 1136 of MgpB combined with antibodies (diluted 1:5,000) specific to amino acids 36 to 308 of MgpC (44), followed by peroxidase-conjugated goat anti-rabbit IgG (whole molecule; Sigma-Aldrich) and colorimetric detection.
Southern blot analysis.
Southern blot analysis was performed according to standard techniques using M. genitalium genomic DNA isolated from 20-ml cultures with a MasterPure Complete DNA isolation kit (Epicentre). Probes were PCR amplified using the following primers (the primer sequence and genomic binding sites are indicated): probe B-Nt (666 bp) and primers 5′SP140 (GATGAAGGACAAGCTAAGGC, which binds at bp 222797) and 3′SP140 (CATTGTCCTGGATATCAGGG, which binds at bp 223463), probe B-Ct (499 bp) and primers 5′PmgpB_Ct (ATAAGAGTGAAATAGGGATAGGT, which binds at bp 225189) and 3′PmgpB_Ct (TAATCCCAAAGTAACACTAAGGA, which binds at bp 225688), and probe C-Ct (499 bp) and primers 5′PmgpC_Ct (CAATTGCCCCTGATGGTTTTG, which binds at bp 227743) and 3′PmgpC_Ct (TCCAGTTAGGGTTTGGGTGG, which binds at bp 228242). PCR products were gel purified and labeled by random priming according to the instructions accompanying the Roche DIG DNA labeling and detection kit (Sigma-Aldrich).
Transposon tagging of phase variants.
To rule out the possibility that phase variant cultures might have been contaminated with low numbers of wild-type cells (which would appear to be revertants), we marked representative phase variants from each class with a tetracycline-resistant minitransposon by electroporation with plasmid pMTnTetM438 (21) as previously described (19). Well-isolated colonies were cultured and maintained under tetracycline selection (2 μg/ml) to isolate revertants, as described below.
Isolation of revertants.
To isolate adherent revertants, cultures of individual transposon-marked phase variants were combined with washed SRBCs in SP-4 medium and incubated at 37°C in 5% CO2 for 90 min with rotation. SRBCs with attached M. genitalium were centrifuged (2 min at 200 × g), washed three times in SP-4, and then inoculated into 2 ml SP-4 in a 24-well plate. Cultures were incubated until the medium turned orange, at which point the supernatant was discarded, adherent bacteria were washed with PBS, and fresh SP-4 broth was added. This process was repeated until adherent M. genitalium cells were visible in the well (approximately 2 to 3 weeks). Adherent bacteria were scraped from the well and then filtered (pore size, 0.45 μm) and plated on duplicate SP4 agar plates. After 2 to 3 weeks of incubation, one plate was used for HA assays to determine the proportion of HA(+) colonies within the culture. Random colonies were picked from the untreated plate (not screened by the HA assay) and placed into SP-4 broth in T25 flasks. Cultures found to contain only adherent bacteria were frozen and again screened by the HA plate assay to confirm that all colonies were HA(+). One revertant per phase variant was analyzed for restoration of a functional mgpBC expression site by PCR and sequencing.
Antibodies.
Rabbit antibodies specific for rMgpB-4a and rMgpB-4b have been described previously (12). Antibodies to the M. genitalium HU protein were produced by immunizing rabbits (Pacific Immunology, San Diego, CA) with His-tagged recombinant HU as previously described (12). Rabbit IgG antibodies were purified using a Nab protein A/G antibody purification kit (Thermo Fisher). Anti-HU and anti-MgpB-4b antibodies were concentrated using iCON concentrators with a 9-kDa-molecular-mass cutoff (Thermo Fisher) to increase their titer to that of anti-rMgpB-4a antibodies. The titers of purified antibodies against M. genitalium whole-cell lysates were determined using spot blots, and the concentrations were adjusted so that total reactivity was similar between the different antibodies.
Antibody selection of phase variants.
M. genitalium strain G37-S (15), the stock culture of the G-37 type strain maintained and cultured in Seattle, was used for antibody-mediated selection of phase variants. We reasoned that because this culture is not clonal and expresses a variety of MgpB and MgpC sequence variants (15, 16), spontaneous phase variants would be more abundant, thus facilitating the isolation of multiple variants that escape antibody/complement selection. M. genitalium strain G37-S was grown to log phase in tissue culture flasks, and adherent bacteria were scraped into the culture supernatant. After shearing through a 25-gauge needle to disrupt cell aggregates, 25 μl of inoculum was combined with 100 μl of purified rabbit antibody, 2 μl of guinea pig complement (active or heat inactivated), and 73 μl of PBS supplemented with 0.15 mM CaCl2 and 0.5 mM MgCl2. This mixture was incubated at 37°C in 5% CO2 for 1 h, diluted with 5 ml SP-4 medium, and incubated for an additional 7 to 14 days. Every few days the flasks were scraped to dislodge adherent bacteria, and aliquots were removed to measure the color change (A540), for frozen stocks, and for filter plating to determine the proportion of hemadsorption-negative colonies.
Isolation and analysis of antibody-selected phase variants.
Phase variants selected with anti-rMgpB-4a and complement were isolated from a culture that had been exposed to antibodies and complement, incubated for 8 days, and then filter plated to duplicate SP-4 agar plates. HA assays on one set of plates indicated that the proportion of phase variants within this culture was 55%. Fourteen random colonies were picked from untreated plates, cultured in T25 flasks, and then examined for adherence after a color change was observed. The resulting six nonadherent cultures were analyzed further by PCR and sequencing of the mgpBC expression site.
Growth of the wild type and phase variants in the presence of specific antibody and complement.
Genome quantitation, rather than color change, was used to precisely determine the effect of anti-rMgpB-4a antibodies and complement on the growth of the wild type and phase variants. Cultures were treated with antibody and complement as described above and then cultured in SP-4 broth. Every 1 to 2 days, adherent bacteria were scraped into the culture supernatant and an aliquot was removed for genome quantitation by qPCR targeting the strain-typing region of mgpB, as previously described (45).
Supplementary Material
ACKNOWLEDGMENTS
The research reported in this publication was supported by NIAID of the National Institutes of Health under award numbers R21AI109332 and R21AI097702.
We certify that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00866-17.
REFERENCES
- 1.Lis R, Rowhani-Rahbar A, Manhart LE. 2015. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 61:418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 2.Horner PJ, Taylor-Robinson D. 2011. Association of Mycoplasma genitalium with balanoposthitis in men with non-gonococcal urethritis. Sex Transm Infect 87:38–40. doi: 10.1136/sti.2010.044487. [DOI] [PubMed] [Google Scholar]
- 3.Oakeshott P, Hay P, Taylor-Robinson D, Hay S, Dohn B, Kerry S, Jensen JS. 2004. Prevalence of Mycoplasma genitalium in early pregnancy and relationship between its presence and pregnancy outcome. BJOG 111:1464–1467. doi: 10.1111/j.1471-0528.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- 4.Sena AC, Lensing S, Rompalo A, Taylor SN, Martin DH, Lopez LM, Lee JY, Schwebke JR. 2012. Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis infections in men with nongonococcal urethritis: predictors and persistence after therapy. J Infect Dis 206:357–365. doi: 10.1093/infdis/jis356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor-Robinson D, Gilroy CB, Thomas BJ, Hay PE. 2004. Mycoplasma genitalium in chronic non-gonococcal urethritis. Int J STD AIDS 15:21–25. doi: 10.1258/095646204322637209. [DOI] [PubMed] [Google Scholar]
- 6.Taylor-Robinson D, Horner PJ. 2001. The role of Mycoplasma genitalium in non-gonococcal urethritis. Sex Transm Infect 77:229–231. doi: 10.1136/sti.77.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandepitte J, Weiss HA, Kyakuwa N, Nakubulwa S, Muller E, Buve A, Van der Stuyft P, Hayes R, Grosskurth H. 2013. Natural history of Mycoplasma genitalium infection in a cohort of female sex workers in Kampala, Uganda. Sex Transm Dis 40:422–427. doi: 10.1097/OLQ.0b013e31828bfccf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau A, Bradshaw CS, Lewis D, Fairley CK, Chen MY, Kong FY, Hocking JS. 2015. The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis 61:1389–1399. doi: 10.1093/cid/civ644. [DOI] [PubMed] [Google Scholar]
- 9.Unemo M, Jensen JS. 2017. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol 14:139–152. doi: 10.1038/nrurol.2016.268. [DOI] [PubMed] [Google Scholar]
- 10.Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. 2013. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS 24:822–828. doi: 10.1177/0956462413502008. [DOI] [PubMed] [Google Scholar]
- 11.Murray GL, Bradshaw CS, Bissessor M, Danielewski J, Garland SM, Jensen JS, Fairley CK, Tabrizi SN. 2017. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis 23:809–812. doi: 10.3201/eid2305.161745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iverson-Cabral SL, Wood GE, Totten PA. 2015. Analysis of the Mycoplasma genitalium MgpB adhesin to predict membrane topology, investigate antibody accessibility, characterize amino acid diversity, and identify functional and immunogenic epitopes. PLoS One 10:e0138244. doi: 10.1371/journal.pone.0138244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheffer MP, Gonzalez-Gonzalez L, Seybert A, Ratera M, Kunz M, Valpuesta JM, Fita I, Querol E, Pinol J, Martin-Benito J, Frangakis AS. 2017. Structural characterization of the NAP; the major adhesion complex of the human pathogen Mycoplasma genitalium. Mol Microbiol 105:869–879. doi: 10.1111/mmi.13743. [DOI] [PubMed] [Google Scholar]
- 14.Burgos R, Pich OQ, Ferrer-Navarro M, Baseman JB, Querol E, Pinol J. 2006. Mycoplasma genitalium P140 and P110 cytadhesins are reciprocally stabilized and required for cell adhesion and terminal-organelle development. J Bacteriol 188:8627–8637. doi: 10.1128/JB.00978-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iverson-Cabral SL, Astete SG, Cohen CR, Rocha EP, Totten PA. 2006. Intrastrain heterogeneity of the mgpB gene in Mycoplasma genitalium is extensive in vitro and in vivo and suggests that variation is generated via recombination with repetitive chromosomal sequences. Infect Immun 74:3715–3726. doi: 10.1128/IAI.00239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iverson-Cabral SL, Astete SG, Cohen CR, Totten PA. 2007. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol Microbiol 66:55–73. doi: 10.1111/j.1365-2958.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- 17.Mernaugh GR, Dallo SF, Holt SC, Baseman JB. 1993. Properties of adhering and nonadhering populations of Mycoplasma genitalium. Clin Infect Dis 17(Suppl 1):S69–S78. doi: 10.1093/clinids/17.Supplement_1.S69. [DOI] [PubMed] [Google Scholar]
- 18.Lluch-Senar M, Querol E, Pinol J. 2010. Cell division in a minimal bacterium in the absence of ftsZ. Mol Microbiol 78:278–289. doi: 10.1111/j.1365-2958.2010.07306.x. [DOI] [PubMed] [Google Scholar]
- 19.Burgos R, Wood GE, Young L, Glass JI, Totten PA. 2012. RecA mediates MgpB and MgpC phase and antigenic variation in Mycoplasma genitalium, but plays a minor role in DNA repair. Mol Microbiol 85:669–683. doi: 10.1111/j.1365-2958.2012.08130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgos R, Totten PA. 2014. Characterization of the operon encoding the Holliday junction helicase RuvAB from Mycoplasma genitalium and its role in mgpB and mgpC gene variation. J Bacteriol 196:1608–1618. doi: 10.1128/JB.01385-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pich OQ, Burgos R, Planell R, Querol E, Pinol J. 2006. Comparative analysis of antibiotic resistance gene markers in Mycoplasma genitalium: application to studies of the minimal gene complement. Microbiology 152:519–527. doi: 10.1099/mic.0.28287-0. [DOI] [PubMed] [Google Scholar]
- 22.van der Woude MW, Baumler AJ. 2004. Phase and antigenic variation in bacteria. Clin Microbiol Rev 17:581–611. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deitsch KW, Lukehart SA, Stringer JR. 2009. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol 7:493–503. doi: 10.1038/nrmicro2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgos R, Totten PA. 2014. MG428 is a novel positive regulator of recombination that triggers mgpB and mgpC gene variation in Mycoplasma genitalium. Mol Microbiol 94:290–306. doi: 10.1111/mmi.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereyre S, Sirand-Pugnet P, Beven L, Charron A, Renaudin H, Barre A, Avenaud P, Jacob D, Couloux A, Barbe V, de Daruvar A, Blanchard A, Bebear C. 2009. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet 5:e1000677. doi: 10.1371/journal.pgen.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dordet-Frisoni E, Sagne E, Baranowski E, Breton M, Nouvel LX, Blanchard A, Marenda MS, Tardy F, Sirand-Pugnet P, Citti C. 2014. Chromosomal transfers in mycoplasmas: when minimal genomes go mobile. mBio 5:e01958-14. doi: 10.1128/mBio.01958-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teachman AM, French CT, Yu H, Simmons WL, Dybvig K. 2002. Gene transfer in Mycoplasma pulmonis. J Bacteriol 184:947–951. doi: 10.1128/jb.184.4.947-951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, Fleischmann RD, Bult CJ, Kerlavage AR, Sutton G, Kelley JM, Fritchman RD, Weidman JF, Small KV, Sandusky M, Fuhrmann J, Nguyen D, Utterback TR, Saudek DM, Phillips CA, Merrick JM, Tomb JF, Dougherty BA, Bott KF, Hu PC, Lucier TS, Peterson SN, Smith HO, Hutchison CA III, Venter JC. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 29.Gyorfy Z, Draskovits G, Vernyik V, Blattner FF, Gaal T, Posfai G. 2015. Engineered ribosomal RNA operon copy-number variants of E. coli reveal the evolutionary trade-offs shaping rRNA operon number. Nucleic Acids Res 43:1783–1794. doi: 10.1093/nar/gkv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin DJ, Cagliero C, Zhou YN. 2012. Growth rate regulation in Escherichia coli. FEMS Microbiol Rev 36:269–287. doi: 10.1111/j.1574-6976.2011.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura M, Yates JL, Dean D, Post LE. 1980. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein mRNA. Proc Natl Acad Sci U S A 77:7084–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGowin CL, Ma L, Jensen JS, Mancuso MM, Hamasuna R, Adegboye D, Martin DH. 2012. Draft genome sequences of four axenic Mycoplasma genitalium strains isolated from Denmark, Japan, and Australia. J Bacteriol 194:6010–6011. doi: 10.1128/JB.01478-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, Jensen JS, Mancuso M, Hamasuna R, Jia Q, McGowin CL, Martin DH. 2010. Genetic variation in the complete MgPa operon and its repetitive chromosomal elements in clinical strains of Mycoplasma genitalium. PLoS One 5:e15660. doi: 10.1371/journal.pone.0015660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su CJ, Chavoya A, Baseman JB. 1989. Spontaneous mutation results in loss of the cytadhesin (P1) of Mycoplasma pneumoniae. Infect Immun 57:3237–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levinson G, Gutman GA. 1987. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol 4:203–221. [DOI] [PubMed] [Google Scholar]
- 36.Streisinger G, Okada Y, Emrich J, Newton J, Tsugita A, Terzaghi E, Inouye M. 1966. Frameshift mutations and the genetic code. Cold Spring Harbor Symp Quant Biol 31:77–84. doi: 10.1101/SQB.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Giacani L, Brandt SL, Ke W, Reid TB, Molini BJ, Iverson-Cabral S, Ciccarese G, Drago F, Lukehart SA, Centurion-Lara A. 2015. Transcription of TP0126, Treponema pallidum putative OmpW homolog, is regulated by the length of a homopolymeric guanosine repeat. Infect Immun 83:2275–2289. doi: 10.1128/IAI.00360-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simmons WL, Bolland JR, Daubenspeck JM, Dybvig K. 2007. A stochastic mechanism for biofilm formation by Mycoplasma pulmonis. J Bacteriol 189:1905–1913. doi: 10.1128/JB.01512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho FM, Fonseca MM, Batistuzzo De Medeiros S, Scortecci KC, Blaha CA, Agnez-Lima LF. 2005. DNA repair in reduced genome: the Mycoplasma model. Gene 360:111–119. doi: 10.1016/j.gene.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Greene CN, Jinks-Robertson S. 1997. Frameshift intermediates in homopolymer runs are removed efficiently by yeast mismatch repair proteins. Mol Cell Biol 17:2844–2850. doi: 10.1128/MCB.17.5.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaaper RM, Dunn RL. 1987. Spectra of spontaneous mutations in Escherichia coli strains defective in mismatch correction: the nature of in vivo DNA replication errors. Proc Natl Acad Sci U S A 84:6220–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGowin CL, Popov VL, Pyles RB. 2009. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol 9:139. doi: 10.1186/1471-2180-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueno PM, Timenetsky J, Centonze VE, Wewer JJ, Cagle M, Stein MA, Krishnan M, Baseman JB. 2008. Interaction of Mycoplasma genitalium with host cells: evidence for nuclear localization. Microbiology 154:3033–3041. doi: 10.1099/mic.0.2008/020735-0. [DOI] [PubMed] [Google Scholar]
- 44.Wood GE, Iverson-Cabral SL, Patton DL, Cummings PK, Cosgrove Sweeney YT, Totten PA. 2013. Persistence, immune response, and antigenic variation of Mycoplasma genitalium in an experimentally infected pig-tailed macaque (Macaca nemestrina). Infect Immun 81:2938–2951. doi: 10.1128/IAI.01322-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood GE, Patton DL, Cummings PK, Iverson-Cabral SL, Totten PA. 2017. Experimental infection of pig-tailed macaques (Macaca nemestrina) with Mycoplasma genitalium. Infect Immun 85:e00738–16. doi: 10.1128/IAI.00738-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clausen HF, Fedder J, Drasbek M, Nielsen PK, Toft B, Ingerslev HJ, Birkelund S, Christiansen G. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum Reprod 16:1866–1874. doi: 10.1093/humrep/16.9.1866. [DOI] [PubMed] [Google Scholar]
- 47.Iverson-Cabral SL, Manhart LE, Totten PA. 2011. Detection of Mycoplasma genitalium-reactive cervicovaginal antibodies among infected women. Clin Vaccine Immunol 18:1783–1786. doi: 10.1128/CVI.05174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svenstrup HF, Jensen JS, Gevaert K, Birkelund S, Christiansen G. 2006. Identification and characterization of immunogenic proteins of Mycoplasma genitalium. Clin Vaccine Immunol 13:913–922. doi: 10.1128/CVI.00048-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tully JG, Rose DL, Whitcomb RF, Wenzel RP. 1979. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified culture medium. J Infect Dis 139:478–482. doi: 10.1093/infdis/139.4.478. [DOI] [PubMed] [Google Scholar]
- 50.Pich OQ, Burgos R, Ferrer-Navarro M, Querol E, Pinol J. 2006. Mycoplasma genitalium mg200 and mg386 genes are involved in gliding motility but not in cytadherence. Mol Microbiol 60:1509–1519. doi: 10.1111/j.1365-2958.2006.05187.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.