Abstract
Liver retransplantation in patients with primary sclerosing cholangitis (PSC) has not been well studied. The aims of this study were to characterize patients with PSC listed for and undergoing retransplantation and to describe the outcomes in these patients. The United Network for Organ Sharing/Organ Procurement and Transplantation Network database was used to identify all primary liver transplantations and subsequent relistings and first retransplantations in adults with PSC between 1987 and 2015. A total of 5080 adults underwent primary transplantation for PSC during this period, and of the 1803 who experienced graft failure (GF), 762 were relisted, and 636 underwent retransplantation. Younger patients and patients with GF due to vascular thrombosis or biliary complications were more likely to be relisted, whereas those with Medicaid insurance or GF due to infection were less likely. Both 5-year graft and patient survival after retransplantation were inferior to primary transplantation (P < 0.001). Five-year survival after retransplantation for disease recurrence (REC), however, was similar to primary transplantation (graft survival, P = 0.45; patient survival, P = 0.09) and superior to other indications for retransplantation (graft and patient survival, P < 0.001). On multivariate analysis, mechanical ventilation, creatinine, bilirubin, albumin, advanced donor age, and a living donor were associated with poorer outcomes after retransplantation. In conclusion, although survival after liver retransplantation in patients with PSC was overall inferior to primary transplantation, outcomes after retransplantation for PSC REC were similar to primary transplantation at 5 years. Retransplantation may therefore represent a treatment option with the potential for excellent outcomes in patients with REC of PSC in the appropriate clinical circumstances.
It is well established that survival after liver retransplantation (re-LT) is inferior to primary liver transplantation (LT).(1–12) Because of the survival differences and the shortage of available organs for LT, re-LT has been a controversial practice. Attempts have therefore been made to identify patient subgroups or risk factors associated with poor outcomes.(10–17) Nearly all prior studies, however, have included all etiologies of liver disease, and few have explored re-LT in a particular disease in more detail outside of hepatitis C virus infection (INF). Although many aspects of re-LT may be similar across indications for the primary LT, there may also be disease-specific features affecting outcomes, particularly when performed for recurrent disease.
Re-LT in patients with primary sclerosing cholangitis (PSC) has not been well characterized. Recurrence (REC) of PSC occurs in up to 20% of LT recipients within 5 years and confers an estimated 4-fold increase in the risk of graft failure (GF) or mortality.(18–23) Therefore, re-LT may be considered in these patients to extend their survival. However, they have been on longterm immunosuppression and are likely to require a more technically complex surgery, both of which may impact their outcomes. In order to better understand re-LT in patients with PSC, we performed this analysis using the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN) database with the following objectives: to characterize the patients with PSC listed for and undergoing re-LT, to describe the outcomes in these patients, and to evaluate the risk factors for GF and mortality after re-LT.
Patients and Methods
STUDY POPULATION AND DATA COLLECTION
The UNOS/OPTN database as of September 25, 2015 was used to identify all primary LTs for adults (18 years or older) with PSC as well as any subsequent wait-list registrations and first re-LTs in these patients between October 1, 1987 and June 30, 2015. Multiorgan transplants and transplant events with missing survival information were excluded.
Variables extracted included age, sex, race/ethnicity, inflammatory bowel disease (IBD) diagnosis, secondary diagnosis of primary liver malignancy, insurance, educational attainment, UNOS region, date of listing, time on the waiting list, reason for wait-list removal, date of LT, location at LT (in the intensive care unit [ICU], hospitalized, or ambulatory), mechanical ventilation status at LT, Model for End-Stage Liver Disease (MELD) score at LT (available after February 27, 2002), total bilirubin at LT, creatinine at LT, albumin at LT, donor age, donor type (living or deceased donor), donation after cardiac death (DCD) donor status, graft type (split/partial or whole), graft status (functioning or failed), graft survival time (time from LT until GF), causes of GF, patient status (alive or deceased), patient survival time (time from LT until death), and causes of death.
GF was reported at the time of re-LT or patient death. Early GF was defined as GF within 30 days. The causes of GF were classified into the following categories: disease REC, biliary complications (BCs), vascular thrombosis (VT), chronic rejection (CR), acute rejection (AR), primary nonfunction (PNF), INF, and other. The etiologies of GF were determined from both coded and free-text responses. This was ascertained for 247/1041 (23.7%) of patients not listed for re-LT and 659/762 (86.5%) of those relisted, including 566/629 (90.0%) of re-LT recipients. Multiple etiologies could be reported, and no primary cause was indicated. Patients were therefore considered to have each reported etiology.
Wait-list mortality was defined as death on the waiting list or removal because of being too sick for LT. Causes of death were grouped into the following categories: INF, hemorrhage, cardiovascular, pulmonary, renal failure, multisystem organ failure, malignancy, GF, and other. Each patient could have up to 3 contributing causes of death, and at least 1 was reported for 1158/1248 (92.8%) primary LT recipients and 219/229 (95.6%) re-LT recipients.
Transplant events were categorized as primary LT or re-LT. The re-LT events were further grouped as early (within 30 days of primary LT) or late (more than 30 days after primary LT). The study population was additionally divided into 2 eras: the first from 1987 to 2004 and the second from 2005 to 2015, each containing approximately half of the total LTs in the study cohort.
STATISTICAL ANALYSIS
Descriptive statistics were calculated for the demographic and clinical characteristics. Comparisons were performed using chi-square tests or Fisher’s exact tests for categorical variables and t tests or Wilcoxon rank sum tests for continuous variables, depending on the normality of the distribution.
Logistic regression was performed to identify factors associated with listing for re-LT in patients with GF. Potential covariates included demographics (age, sex, race/ethnicity), insurance, blood type, IBD diagnosis, UNOS region, living donor for primary LT, DCD donor for primary LT, and early failure of the primary LT graft. Educational attainment was not considered due to a high proportion of missing data. One model was performed among all patients who experienced GF, and a second incorporated the causes of GF and was performed among the subset with a reported etiology.
Graft and patient survival curves were computed using Kaplan-Meier methods and compared using log-rank tests. Survival was compared between re-LT and primary LT events, which included the first LT for re-LT recipients. The early and late re-LT groups were also compared with each other. Recipients retransplanted due to disease REC were compared with re-LTs for other indications and to primary LTs to evaluate their survival relative to these groups.
Cox proportional hazards regression was performed to adjust for donor and recipient factors that may affect graft and patient survival and to identify predictors of GF and mortality. Potential covariates included recipient demographics, IBD diagnosis, UNOS region, primary liver malignancy, ICU status, mechanical ventilation status, and biochemical parameters (total bilirubin, creatinine, and albumin), as well as donor characteristics (living donor, DCD donor, donor age 60 years or older, and a split graft), and the era of LT. In analyses performed among re-LT recipients, the time to re-LT and the causes of GF were also considered. The 10.0% of re-LTs without a reported cause of GF were included and were assigned to their own category.
Predictors with P < 0.05 in univariate analyses were entered and retained in the multivariate models at the same level of significance using a stepwise selection process. UNOS region was considered for inclusion in all multivariate models in order to assess its impact after adjustment for other factors. Collinearity was tested for among included variables.
All analyses were performed using SAS Studio software, version 3.5 (SAS Institute, Cary, NC), and a P value < 0.05 was considered statistically significant. This study was approved by the Duke University Health System institutional review board.
Results
LISTING FOR RE-LT
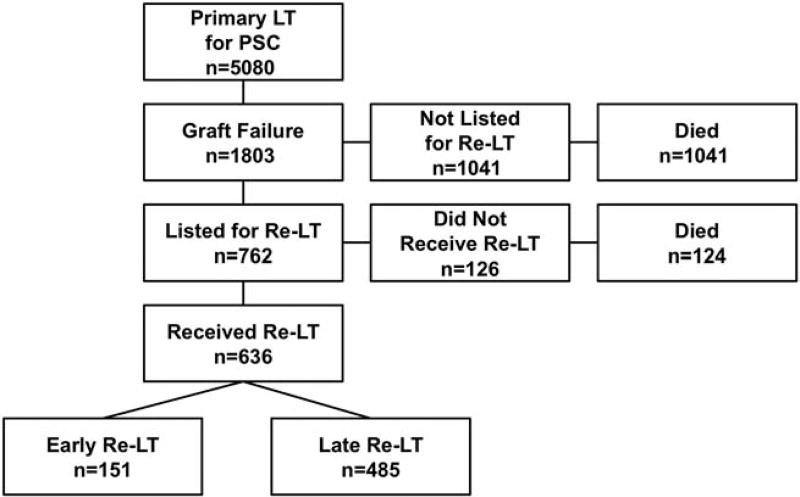
Primary LT for PSC was performed in 5080 adults during the study period. Among this group, 1803 (35.5%) experienced GF, of which 762 (42.3%) were listed for a second LT at a median of 310 days (interquartile range [IQR], 21–2085 days) after primary LT and at a median age of 45 years (IQR, 36–55 years; Fig. 1). The most common indications for relisting were VT and disease REC (Table 1). Some patients had multiple reported causes of GF, and the most frequently co-occurring etiologies were BCs and VT (40/659 with a reported etiology) and BCs and REC (38/659). Relisted patients were more likely to be younger, have private insurance, have a DCD graft, and have experienced early GF (Table 2). Among individuals with a reported etiology of GF, those with VT or BCs were more likely to be relisted, whereas patients with INF and other etiologies were less likely.
FIG. 1.
Flow diagram showing the subsets of patients with PSC included in this analysis.
TABLE 1.
PSC Patients With GF According to Wait-List Status and Re-LT
| Not Relisted (n = 1041) |
Relisted (n = 762) |
P Value | Not Retransplanted (n = 126) |
Retransplanted (n = 636) |
P Value | |
|---|---|---|---|---|---|---|
| Age* | 53 (43–61) | 42 (33–52) | <0.001 | 51 (41–58) | 45 (36–55) | 0.002 |
| Male sex | 685 (65.8) | 550 (72.2) | 0.004 | 86 (68.3) | 464 (73.0) | 0.28 |
| Race/ethnicity | 0.28 | 0.44 | ||||
| White | 845 (81.2) | 620 (81.4) | 98 (77.8) | 525 (82.5) | ||
| Black | 128 (12.3) | 106 (13.9) | 22 (17.5) | 82 (12.9) | ||
| Hispanic | 51 (4.9) | 28 (3.7) | 4 (3.2) | 22 (3.5) | ||
| Asian | 13 (1.2) | 4 (0.5) | 1 (0.8) | 5 (0.8) | ||
| Other | 4 (0.4) | 4 (0.5) | 1 (0.8) | 2 (0.3) | ||
| Blood type | 0.23 | 0.54 | ||||
| A | 434 (41.7) | 312 (40.9) | 55 (43.7) | 256 (40.3) | ||
| B | 110 (10.6) | 103 (13.6) | 16 (12.7) | 88 (13.8) | ||
| AB | 44 (4.2) | 36 (4.7) | 3 (2.4) | 33 (5.2) | ||
| O | 453 (43.5) | 311 (40.8) | 52 (41.3) | 259 (40.7) | ||
| IBD | 731 (70.2) | 548 (71.9) | 0.43 | 87 (69.0) | 461 (72.5) | 0.43 |
| Ulcerative colitis | 495 (47.6) | 370 (48.6) | 0.67 | 62 (49.2) | 308 (48.4) | 0.87 |
| Crohn’s disease | 177 (17.0) | 123 (16.1) | 0.63 | 19 (15.1) | 104 (16.4) | 0.72 |
| Other | 59 (5.7) | 55 (7.2) | 0.18 | 6 (4.8) | 49 (7.7) | 0.24 |
| Insurance* | <0.001 | 0.24 | ||||
| Private | 739 (71.3) | 609 (80.3) | 85 (68.0) | 470 (73.9) | ||
| Medicaid | 71 (6.8) | 46 (6.1) | 6 (4.8) | 38 (6.0) | ||
| Medicare | 189 (18.2) | 72 (9.5) | 29 (23.2) | 100 (15.7) | ||
| Other | 38 (3.7) | 31 (4.1) | 5 (4.0) | 28 (4.4) | ||
| Education*,† | <0.001 | 0.04 | ||||
| High school | 315 (42.8) | 184 (33.0) | 35 (40.7) | 138 (29.6) | ||
| Some college or beyond | 421 (57.2) | 373 (67.0) | 51 (59.3) | 329 (70.4) | ||
| UNOS region* | 0.24 | 0.85 | ||||
| 1 | 42 (4.0) | 29 (3.8) | 6 (4.8) | 21 (3.3) | ||
| 2 | 107 (10.3) | 82 (10.8) | 16 (12.7) | 66 (10.4) | ||
| 3 | 147 (14.1) | 103 (13.5) | 13 (10.3) | 97 (15.3) | ||
| 4 | 86 (8.3) | 42 (5.5) | 7 (5.6) | 36 (5.7) | ||
| 5 | 120 (11.5) | 77 (10.1) | 13 (10.3) | 67 (10.5) | ||
| 6 | 37 (3.6) | 19 (2.5) | 4 (3.2) | 15 (2.4) | ||
| 7 | 128 (12.3) | 110 (14.4) | 19 (15.1) | 81 (12.7) | ||
| 8 | 101 (9.7) | 82 (10.8) | 10 (7.9) | 73 (11.5) | ||
| 9 | 53 (5.1) | 54 (7.1) | 11 (8.7) | 42 (6.6) | ||
| 10 | 110 (10.6) | 87 (11.4) | 15 (11.9) | 76 (11.9) | ||
| 11 | 110 (10.6) | 77 (10.1) | 12 (9.5) | 62 (9.7) | ||
| Living donor recipient | 64 (6.1) | 79 (10.4) | 0.001 | 10 (7.9) | 69 (10.8) | 0.32 |
| DCD recipient | 16 (1.5) | 28 (3.7) | 0.004 | 3 (2.4) | 25 (4.0) | 0.60 |
| Etiologies of GF‡ | ||||||
| VT | 20 (8.1) | 209 (31.7) | <0.001 | 14 (15.7) | 195 (34.2) | <0.001 |
| REC | 80 (32.4) | 207 (31.4) | 0.78 | 26 (29.2) | 181 (31.8) | 0.63 |
| PNF | 52 (21.1) | 144 (21.9) | 0.79 | 15 (16.9) | 129 (22.6) | 0.22 |
| BCs | 32 (13.0) | 136 (20.6) | 0.008 | 14 (15.7) | 122 (21.4) | 0.22 |
| CR | 40 (16.2) | 93 (14.1) | 0.43 | 10 (11.2) | 83 (14.6) | 0.40 |
| AR | 18 (7.3) | 55 (8.3) | 0.60 | 7 (7.9) | 48 (8.4) | 0.86 |
| INF | 61 (24.7) | 64 (9.7) | <0.001 | 22 (24.7) | 42 (7.4) | <0.001 |
| Other | 73 (29.6) | 61 (9.3) | <0.001 | 15 (16.9) | 46 (8.1) | 0.008 |
| Multiple etiologies of GF‡ | 84 (34.0) | 235 (35.7) | 0.64 | 26 (29.2) | 209 (36.7) | 0.17 |
NOTE: Data are given as n (%) or median (IQR).
For comparison of relisted and not relisted, variable extracted from primary LT event. After relisting, represents status at wait-list registration. Distribution of data in retransplanted and not retransplanted may therefore vary slightly from total in relisted column.
Available for 736/1041 not relisted, 557/762 relisted, 86/126 not retransplanted, and 467/636 retransplanted.
Available for 247/1041 not relisted, 659/762 relisted, 89/126 not retransplanted, and 570/636 retransplanted. Percentages expressed as a proportion of those with a reported etiology.
TABLE 2.
Predictors of Listing for Re-LT in Patients With PSC
| Multivariate, OR (95% CI) |
P Value | |
|---|---|---|
| All patients with GF | ||
| Age at primary LT* | 0.75 (0.72–0.78) | <0.001 |
| Insurance | <0.001 | |
| Private | Reference | |
| Medicaid | 0.46 (0.30–0.70) | |
| Medicare | 0.66 (0.48–0.92) | |
| Other | 0.94 (0.59–1.60) | |
| DCD recipient | 3.08 (1.57–6.06) | 0.001 |
| Early GF† | 3.56 (2.65–4.78) | <0.001 |
| Patients with a reported etiology of GF‡ | ||
| Age at primary LT* | 0.80 (0.74–0.86) | <0.001 |
| Insurance | 0.03 | |
| Private | Reference | |
| Medicaid | 0.40 (0.21–0.76) | |
| Medicare | 0.69 (0.39–1.24) | |
| Other | 0.87 (0.41–1.84) | |
| Early GF† | 4.66 (2.68–8.08) | <0.001 |
| GF due to VT | 3.90 (2.32–6.57) | <0.001 |
| GF due to BCs | 2.31 (1.43–3.72) | <0.001 |
| GF due to INF | 0.29 (0.19–0.46) | <0.001 |
| GF due to other etiology§ | 0.27 (0.18–0.42) | <0.001 |
For 5-year increments in age.
Defined as GF within 30 days.
Available for 247/1041 not relisted and 659/762 relisted.
Etiology other than disease REC, CR, AR, BCs, INF, VT, and PNF.
Of the 762 patients who were relisted, 636 (83.5%) underwent re-LT after a median of 30 days (IQR, 5–144 days) on the waiting list. Those who were retransplanted were younger at relisting, more likely to have GF due to VT, and less likely to have GF due to INF (Table 1). Ninety-six (12.6%) individuals died on the waiting list after a median interval of 74 days (IQR, 10–384 days). Of the 64 patients listed with GF due to INF, 15 (23.4%) died on the waiting list, the highest proportion of any etiology. Thirty individuals (3.9%) were removed from the waiting list for reasons other than re-LT and death, though all but 2 eventually died. Ultimately, 124/762 (16.3%) patients died after listing without re-LT. These patients died more commonly of GF (21.8% versus 8.7%; P< 0.001), INF (25.0% versus 17.3%; P= 0.04), and multisystem organ failure (16.9% versus 10.9%; P = 0.048), and less frequently of malignancy (5.6% versus 22.5%; P< 0.001) compared with the 1041 individuals who were not relisted.
GF DUE TO DISEASE REC
Of the 287 patients with GF due to disease REC, 207 (72.1%) were listed for re-LT at a median of 2228 days (IQR, 1147–3312 days) after primary LT. The 80 individuals not relisted died most commonly of GF (38/78 with a reported cause of death) and malignancy (20/78), primarily cholangiocarcinoma (CCA; n = 15). Among the 207 relisted, 181 (87.4%) underwent re-LT after a median of 106 days (IQR, 31–323 days). Eighteen (8.7%) died on the waiting list after a median of 178 days (IQR, 87–783 days), of which 10 (55.6%) died within 6 months. The overall most common cause of death was GF (n = 7), 3 of which occurred within 6 months. Other causes of death included multisystem organ failure (n = 3) and INF (n = 2).
RECIPIENTS OF RE-LT
Of the 636 re-LTs, 151 (23.7%) were early (within 30 days of primary LT) and 485 (76.3%) were late (beyond 30 days). Seven re-LT recipients had missing posttransplant information, leaving 629 (151 early and 478 late) for analysis.
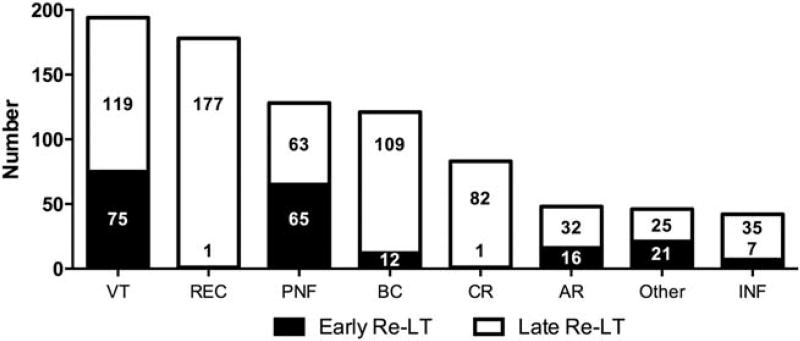
The overall median time between LTs was 397 days (IQR, 33–2362 days), and similar to relisting, the 2 most frequent indications were VT and disease REC (Fig. 2). Early re-LT was typically necessitated by VT or PNF, which together contributed to more than 95% of failed grafts in the early period. The causes of GF leading to late re-LT were more varied, but disease REC was the most common (42.0%). The interval to late re-LT was similarly variable and ranged from 31 to 7064 days.
FIG. 2.
Indications for re-LT in patients with PSC. Bars represent the total number of re-LTs performed for each indication with stratification by early (≤30 days) and late (>30 days) time intervals. An etiology of GF was reported for 566/629 re-LTs, and multiple contributing causes could be reported.
Compared with primary LT, the re-LT recipients were younger, fewer had a primary liver malignancy, a significantly greater proportion were in the ICU, and more were on mechanical ventilation, particularly in early re-LT (Table 3). In addition, re-LT recipients had a greater degree of hepatic and renal dysfunction, as indicated by their biochemical parameters and MELD scores.
TABLE 3.
Recipient and Donor Characteristics at Primary LT and Re-LT in Patients With PSC
| Primary LT (n = 5080) |
Re-LT (n = 629) |
P Value | Early Re-LT (n = 151) |
Late Re-LT (n = 478) |
P Value | |
|---|---|---|---|---|---|---|
| Age at LT, years | 48 (38–57) | 46 (36–55) | <0.001 | 42 (34–55) | 46 (37–55) | 0.10 |
| Male sex | 3453 (68.0) | 458 (72.8) | 0.01 | 104 (68.9) | 354 (74.1) | 0.21 |
| Race/ethnicity | 0.71 | 0.85 | ||||
| White | 4168 (82.0) | 518 (82.4) | 126 (83.4) | 392 (82.0) | ||
| Black | 629 (12.4) | 82 (13.0) | 19 (12.6) | 63 (13.2) | ||
| Hispanic | 187 (3.7) | 22 (3.5) | 6 (4.0) | 16 (3.3) | ||
| Asian | 62 (1.2) | 5 (0.8) | 0 | 5 (1.0) | ||
| Other | 33 (0.6) | 2 (0.3) | 0 | 2 (0.4) | ||
| Primary liver malignancy | 106 (2.1) | 4 (0.6) | 0.01 | 1 (0.7) | 3 (0.6) | >0.99 |
| CCA | 73 (1.4) | 3 (0.5) | 0.048 | 1 (0.7) | 2 (0.4) | 0.56 |
| HCC | 32 (0.6) | 1 (0.2) | 0.26 | 0 (0.0) | 1 (0.2) | >0.99 |
| Other | 1 (<0.1) | 0 (0.0) | >0.99 | 0 (0.0) | 0 (0.0) | |
| Location | <0.001 | <0.001 | ||||
| In ICU | 377 (7.4) | 183 (29.1) | 110 (72.8) | 73 (15.3) | ||
| Hospitalized | 804 (15.8) | 177 (28.1) | 30 (19.9) | 147 (30.8) | ||
| Ambulatory | 3897 (76.7) | 269 (42.8) | 11 (7.3) | 258 (54.0) | ||
| Mechanical ventilation | 100 (2.0) | 102 (16.2) | <0.001 | 66 (43.7) | 36 (7.5) | <0.001 |
| MELD score* | 19 (14–26) | 25 (18–33) | <0.001 | 24 (16–31) | 25 (19–33) | 0.27 |
| Total bilirubin, mg/dL | 6.4 (2.7–15.0) | 10.8 (3.3–24.2) | <0.001 | 8.6 (3.2–18.3) | 11.8 (3.6–26.7) | 0.02 |
| Creatinine, mg/dL | 0.9 (0.7–1.2) | 1.3 (0.9–2.2) | <0.001 | 1.1 (0.8–2.4) | 1.4 (0.9–2.1) | 0.16 |
| Albumin, g/L | 3.0 (2.5–3.5) | 2.8 (2.4–3.3) | <0.001 | 2.7 (2.3–3.2) | 2.9 (2.4–3.3) | 0.13 |
| Living donor | 551 (10.8) | 8 (1.3) | <0.001 | 0 (0.0) | 8 (1.7) | 0.21 |
| DCD donor | 120 (2.4) | 6 (1.0) | 0.02 | 0 (0.0) | 6 (1.3) | 0.34 |
| Donor age ≥ 60 years | 620 (12.2) | 59 (9.4) | 0.04 | 17 (11.3) | 42 (8.8) | 0.36 |
| Split graft | 621 (12.2) | 12 (1.9) | <0.001 | 1 (0.7) | 11 (2.3) | 0.31 |
NOTE: Data are given as n (%) or median (IQR).
Available for transplants performed after February 27, 2002 (n = 3175/5080, 442/629, 81/151, 361/478).
The donor characteristics also differed between the primary LT and re-LT recipients. Primary LT recipients were more likely to receive a split graft as well as a graft from a living donor, DCD donor, and a donor age 60 years or older. These were similar in early and late re-LT.
OUTCOMES OF RE-LT COMPARED WITH PRIMARY TRANSPLANTATION
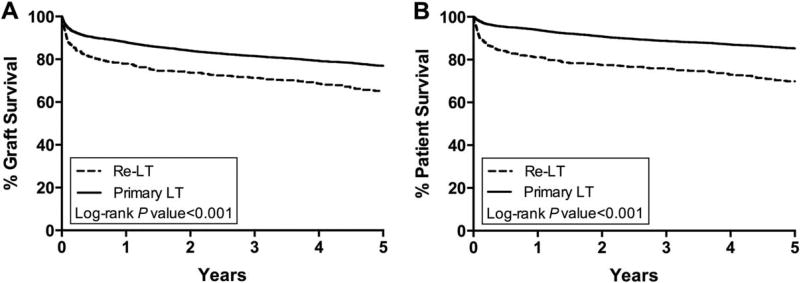
Graft and patient survival after re-LT were inferior compared with primary LT (Fig. 3). Of the 271 re-LT recipients who experienced eventual GF, 49 (18.1%) underwent a third LT. Re-LT recipients were more likely to die from INF and less likely to die of malignancy (Table 4).
FIG. 3.
Kaplan-Meier curves for (A) graft and (B) patient survival of LT recipients with PSC after primary LT and re-LT.
TABLE 4.
Causes of Death After Primary LT and Re-LT in Patients With PSC
| Primary LT (n = 1248) |
All Re-LT (n = 229) |
P Value | Early Re-LT (n = 65) |
Late Re-LT (n = 164) |
P Value | |
|---|---|---|---|---|---|---|
| INF | 210 (18.1) | 68 (31.1) | <0.001 | 19 (30.2) | 49 (31.4) | 0.86 |
| Multisystem organ failure | 134 (11.6) | 33 (15.1) | 0.15 | 9 (14.3) | 24 (15.4) | 0.84 |
| GF | 117 (10.1) | 31 (14.2) | 0.08 | 7 (11.1) | 24 (15.4) | 0.41 |
| Cardiovascular | 118 (10.2) | 25 (11.4) | 0.59 | 6 (9.5) | 19 (12.2) | 0.58 |
| Malignancy | 240 (20.7) | 16 (7.3) | <0.001 | 7 (11.1) | 9 (5.8) | 0.17 |
| Hemorrhage | 54 (4.7) | 13 (5.9) | 0.42 | 4 (6.4) | 9 (5.8) | 0.87 |
| Renal failure | 51 (4.4) | 4 (1.8) | 0.07 | 1 (1.6) | 3 (1.9) | 0.87 |
| Pulmonary | 41 (3.5) | 3 (1.4) | 0.09 | 0 | 3 (1.9) | 0.27 |
| Other | 160 (13.8) | 46 (21.0) | 0.01 | 7 (11.1) | 39 (25.0) | 0.02 |
NOTE: Data are given as n (%). Cause of death was reported for 1158/1248 primary LT mortalities and 219/229 re-LT mortalities, including 63/65 for early re-LT and 156/164 for late re-LT. Up to 3 contributing causes of death could be reported.
Adjustment for recipient and donor characteristics reduced but did not eliminate the increased risk of GF (univariate hazard ratio [HR], 1.76; 95% confidence interval [CI], 1.51–2.04; multivariate HR, 1.47; 95% CI, 1.25–1.72) and mortality (univariate HR, 2.46; 95% CI, 2.09–2.91; multivariate HR, 1.98; 95% CI, 1.65–2.37) at 5 years after re-LT compared with primary LT.
SHORT-TERM GF AND MORTALITY AFTER RE-LT
Of the 271 GFs after re-LT, 75 (27.7%) occurred before 30 days, compared with 14.6% (263/1803) of failed grafts after primary LT. Similarly, 27.1% (62/229) of the overall mortality after re-LT occurred before 30 days, whereas 30-day mortality accounted for only 9.1% (113/1248) of the total deaths after primary LT.
The most common causes of death in re-LT recipients before 30 days were INF (22/62 patients with a reported cause of death) and hemorrhage (9/62). Hemorrhage was a more frequent cause of death compared with after 30 days (14.5% versus 2.6%; P < 0.001). Of the 22 who died of INF, 14 (63.6%) died of generalized sepsis. Compared with primary LT recipients who died before 30 days, early mortalities after re-LT were more likely to be due to INF (35.5% versus 17.7%; P = 0.008) and, specifically, sepsis (22.6% versus 10.6%; P = 0.03).
OUTCOMES OF EARLY AND LATE RE-LT
When compared separately with primary LT, early and late re-LT both experienced inferior graft and patient survival (P < 0.001 for all). Five-year graft survival was similar between the 2 re-LT groups (early 58.6% versus late 65.6%; P = 0.08), but 5-year patient survival was decreased after early re-LT (62.3% versus 70.8%; P = 0.047). When early re-LT was further divided into those that occurred within 7 days (n = 51) and 8–30 days (n = 100), the re-LTs performed within 1 week experienced significantly inferior graft and patient survival compared with the 8–30 day and the late re-LTs, whereas the 8–30 day and late re-LTs were similar (Table 5). After adjustment for recipient illness severity at the time of re-LT and donor factors, however, the interval to re-LT was no longer significantly associated with either GF or mortality. The causes of death after early and late re-LT were similar (Table 4).
TABLE 5.
Risk Factors for GF and Mortality After Re-LT in Patients With PSC
| GF
|
Mortality
|
|||
|---|---|---|---|---|
| Univariate HR (95% CI) |
Multivariate HR (95% CI) |
Univariate HR (95% CI) |
Multivariate HR (95% CI) |
|
| At 30 days | ||||
| Re-LT for REC | 0.47 (0.25–0.87) | 0.36 (0.17–0.77) | ||
| Re-LT for PNF | 2.19 (1.36–3.52) | 2.28 (1.35–3.83) | ||
| Contemporary era* | 0.59 (0.37–0.93) | 0.54 (0.32–0.89) | ||
| Time to re-LT | ||||
| 0–7 days | Reference | Reference | ||
| 8–30 days | 0.45 (0.20–0.98) | 0.41 (0.18–0.95) | ||
| >30 days | 0.40 (0.21–0.73) | 0.35 (0.18–0.66) | ||
| In ICU | 2.36 (1.50–3.71) | 2.72 (1.65–4.48) | ||
| Mechanical ventilation | 3.39 (2.12–5.41) | 2.97 (1.85–4.78) | 4.07 (2.46–6.74) | 3.97 (2.36–6.67) |
| Total bilirubin | 1.02 (1.00–1.03) | 1.02 (1.00–1.04) | ||
| Creatinine | 1.36 (1.20–1.54) | 1.30 (1.14–1.48) | 1.37 (1.19–1.57) | 1.34 (1.15–1.56) |
| Albumin† | 0.68 (0.48–0.98) | 0.60 (0.42–0.87) | ||
| Donor age ≥ 60 years | 2.01 (1.09–3.73) | 2.28 (1.19–4.38) | ||
| At 5 years | ||||
| Re-LT for REC | 0.56 (0.40–0.80) | 0.62 (0.43–0.90) | 0.47 (0.32–0.70) | 0.54 (0.36–0.82) |
| Re-LT for PNF | 1.58 (1.16–2.15) | 1.49 (1.06–2.10) | ||
| Contemporary era* | 0.60 (0.46–0.80) | 0.68 (0.51–0.91) | 0.58 (0.43–0.79) | 0.69 (0.50–0.95) |
| Time to re-LT | ||||
| 0–7 days | Reference | Reference | ||
| 8–30 days | 0.53 (0.32–0.89) | 0.54 (0.31–0.92) | ||
| >30 days | 0.51 (0.34–0.77) | 0.48 (0.31–0.76) | ||
| In ICU | 1.71 (1.29–2.26) | 1.83 (1.36–2.48) | ||
| Mechanical ventilation | 2.07 (1.51–2.84) | 1.64 (1.18–2.28) | 2.12 (1.51–2.99) | 1.60 (1.12–2.28) |
| Total bilirubin | 1.02 (1.01–1.03) | 1.02 (1.01–1.02) | 1.02 (1.01–1.03) | 1.02 (1.01–1.03) |
| Creatinine | 1.22 (1.12–1.33) | 1.18 (1.08–1.30) | 1.21 (1.10–1.33) | 1.15 (1.04–1.28) |
| Living donor‡ | 3.03 (1.34–6.82) | 5.74 (2.49–13.22) | ||
| Donor age ≥ 60 years | 2.00 (1.36–2.94) | 1.94 (1.31–2.87) | 2.12 (1.41–3.20) | 2.01 (1.33–3.04) |
NOTE: Only covariates significant (P < 0.05) in univariate analyses and included in multivariate models are shown. Nonsignificant covariates included the following: age, sex, race/ethnicity, IBD diagnosis, UNOS region, primary liver malignancy, DCD donor, split graft, other indications for re-LT, and number of causes of GF.
Transplants performed from 2005 to 2015 versus 1987 to 2004.
Nonsignificant in univariate analysis of 30-day GF.
Nonsignificant in univariate analysis of 5-year mortality.
OUTCOMES OF RE-LT FOR DISEASE REC
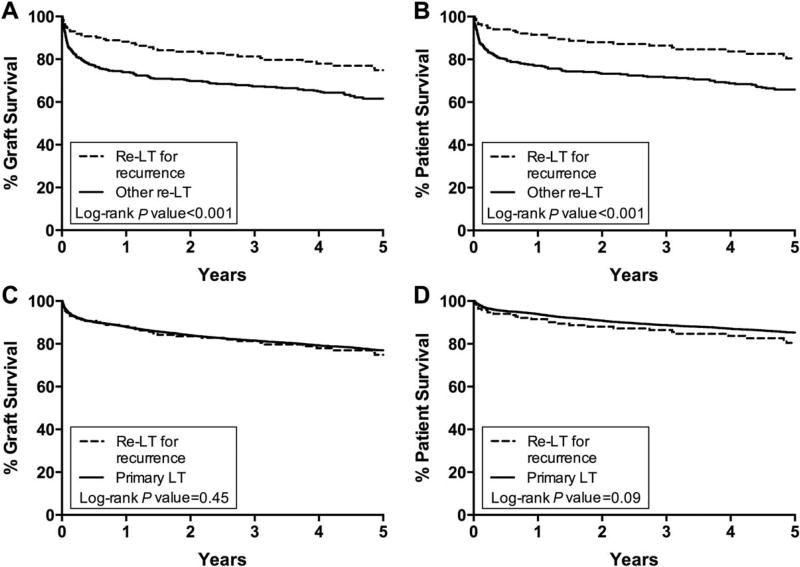
The baseline characteristics at re-LT by etiology of GF are shown in Supporting Table 1, and their outcomes are shown in Table 6. Compared with other re-LT recipients, the individuals with REC were less critically ill at the time of re-LT, with a smaller proportion in the ICU or on mechanical ventilation (Table 7). They also experienced significantly better outcomes (Fig. 4A,B). Adjustment for recipient and donor characteristics attenuated the decreased risk of GF and mortality at 30 days in patients retransplanted for REC, but it did not eliminate the 5-year survival benefit (Table 5).
TABLE 6.
Outcomes of Retransplanted Patients With PSC by GF Etiology
| VT (n = 194) |
REC (n = 178) |
PNF (n = 128) |
BC (n = 121) |
CR (n = 83) |
AR (n = 48) |
Other (n = 46) |
INF (n = 42) |
Unknown (n = 63) |
|
|---|---|---|---|---|---|---|---|---|---|
| 30-day graft survival | 86.1 | 93.2 | 76.6 | 93.4 | 89.0 | 87.5 | 87.0 | 88.0 | 88.8 |
| 1-year graft survival | 75.6 | 86.8 | 65.2 | 76.5 | 76.4 | 68.6 | 77.8 | 73.3 | 80.1 |
| 5-year graft survival | 63.5 | 73.6 | 54.5 | 64.6 | 56.6 | 54.6 | 69.4 | 63.2 | 70.7 |
| 30-day patient survival | 88.1 | 95.4 | 82.4 | 94.2 | 90.2 | 87.5 | 87.0 | 88.0 | 90.4 |
| 1-year patient survival | 79.0 | 90.5 | 71.4 | 78.8 | 77.3 | 68.6 | 77.8 | 75.5 | 81.6 |
| 5-year patient survival | 67.5 | 79.5 | 61.4 | 69.1 | 62.8 | 56.7 | 69.4 | 65.1 | 72.0 |
NOTE: Data are given as a percentage. Kaplan-Meier survival probability at 30-day, 1-year, and 5-year intervals.
TABLE 7.
Recipient and Donor Characteristics at Re-LT for Disease REC in Patients With PSC
| Re-LT for REC (n = 178) |
Other Re-LT (n = 451) |
P Value* | Primary LT (n = 5080) |
P Value† | |
|---|---|---|---|---|---|
| Age at LT, years | 47 (38–56) | 45 (36–55) | 0.08 | 48 (38–57) | 0.44 |
| Male sex | 134 (75.3) | 324 (71.8) | 0.38 | 3453 (68.0) | 0.04 |
| Race/ethnicity | 0.43 | 0.60 | |||
| White | 140 (78.7) | 378 (83.8) | 4168 (82.0) | ||
| Black | 28 (15.7) | 54 (12.0) | 629 (12.4) | ||
| Hispanic | 8 (4.5) | 14 (3.1) | 187 (3.7) | ||
| Asian | 1 (0.6) | 4 (0.9) | 62 (1.2) | ||
| Other | 1 (0.6) | 1 (0.2) | 33 (0.6) | ||
| Location | <0.001 | <0.001 | |||
| In ICU | 21 (11.8) | 162 (35.9) | 377 (7.4) | ||
| Hospitalized | 44 (24.7) | 133 (29.5) | 804 (15.8) | ||
| Ambulatory | 113 (63.5) | 156 (34.6) | 3897 (76.7) | ||
| Mechanical ventilation | 10 (5.6) | 92 (20.4) | <0.001 | 100 (2.0) | 0.004 |
| MELD score‡ | 25 (20–34) | 24 (17–32) | 0.12 | 19 (14–26) | <0.001 |
| Total bilirubin | 13.3 (5.4–26.5) | 9.4 (3.1–22.6) | 0.01 | 6.4 (2.7–15.0) | <0.001 |
| Creatinine | 1.4 (0.9–2.5) | 1.3 (0.9–2.1) | 0.11 | 0.9 (0.7–1.2) | <0.001 |
| Albumin | 2.8 (2.3–3.4) | 2.8 (2.4–3.3) | 0.49 | 3.0 (2.5–3.5) | 0.08 |
| Living donor | 4 (2.2) | 4 (0.9) | 0.23 | 551 (10.8) | <0.001 |
| DCD donor | 2 (1.1) | 4 (0.9) | 0.68 | 120 (2.4) | 0.44 |
| Donor age ≥ 60 years | 14 (7.9) | 45 (10.0) | 0.41 | 620 (12.2) | 0.08 |
| Split graft | 4 (2.2) | 8 (1.8) | 0.75 | 621 (12.2) | <0.001 |
NOTE: Data are given as n (%) or median (IQR).
Comparison between re-LT for disease REC versus other indications.
Comparison between re-LT for disease REC versus primary LT.
Available for transplants performed after February 27, 2002 (n = 164/178, 278/451, and 3175/5080).
FIG. 4.
Kaplan-Meier curves for graft and patient survival following re-LT for PSC REC compared (A and B) with other indications for re-LT and (C and D) with primary LT.
When compared with primary LT, the recipients of re-LT for disease REC were more likely to be in the ICU or on mechanical ventilation at LT, and they also had a greater degree of hepatic and renal dysfunction (Table 7). However, their outcomes were similar at 5 years (Fig. 4C,D). The graft and patient survival remained similar after adjusting for recipient and donor factors and the time period in which the transplant was performed (Supporting Table 2). There were also no significant differences in outcomes when limited to the more recent era (re-LT for REC versus primary LT: graft survival at 5 years, 75.7% versus 78.7%, P = 0.35; patient survival at 5 years, 82.0% versus 85.8%, P = 0.25).
PREDICTORS OF GF AND MORTALITY AFTER RE-LT
The risk factors for GF and mortality at 30 days and 5 years after re-LT are shown in Table 5. Recipient factors associated with greater severity of illness (on mechanical ventilation and more abnormal biochemical parameters) were associated with an increased risk of GF and mortality. Of the donor characteristics, a donor age of 60 years or older was independently associated with increased risk of GF and mortality at 5 years, and receipt of a graft from a living donor conferred an increased risk of GF at 5 years. UNOS region was not a significant factor affecting outcomes and neither was recipient age, sex, race/ethnicity, primary liver malignancy, number of causes of GF, a DCD donor, or a split graft.
Among re-LT recipients with disease REC, the only significant predictor of 30-day outcomes was mechanical ventilation (Supporting Table 3). Factors associated with 5-year survival were similar to those for all re-LT recipients and included mechanical ventilation, bilirubin, creatinine, a living donor, and a donor age of 60 years or older.
Discussion
Retransplantation has been a controversial practice due to its historically inferior survival rates. With the scarcity of organs available for LT, understanding the factors and groups of patients associated with improved survival after re-LT is important in order to limit futility. In this study, we characterized the patients with PSC listed for and receiving re-LT and their outcomes in order to better understand re-LT in this population. We used a robust data set that allowed for analysis of more re-LT recipients with PSC than any previous study. It also permitted a meaningful comparison between the survival after primary LT and re-LT for PSC REC. To the best of our knowledge, this study represents the first specific comparison of these outcomes.
A variety of factors influenced whether a patient was listed for re-LT, including GF etiology, a DCD graft, and socioeconomic status. Patients with GF due to VT were more likely to be relisted as well as more likely to receive re-LT. This may have been due to many having early hepatic artery thrombosis, a complication that typically requires re-LT and for which MELD exception points may be granted. Individuals with GF due to INF were both less likely to be relisted and retransplanted, and they were the most likely to die on the waiting list. Without more information about these INFs, however, it is difficult to draw many conclusions from this finding.
DCD recipients were also more likely to be relisted, consistent with previous studies.(24) DCD grafts are known to have inferior survival compared with donation after brain death grafts due to an increased risk of ischemic cholangiopathy, and DCD recipients have previously been found to have an increased rate of relisting as a result.(24–26) Similarly, among the DCD recipients in this analysis, the most common cause of GF was BCs, which likely contributed to the increased likelihood of relisting in these patients.
In addition, relisted patients were significantly more likely to be college-educated, and Medicaid insurance was an independent predictor of not being relisted. Disparities in access to and outcomes of primary LT have previously been shown for patients with Medicaid, though decreased access to re-LT has not been demonstrated before.(27–29) This finding warrants further study into disparities in listing for re-LT.
Given the increased complexity of re-LT, the decision of whether to pursue re-LT and to relist a patient may be highly variable. Yet, there was no difference in the proportion relisted and undergoing re-LT between UNOS regions, and the outcomes after re-LT also did not differ. A center-level analysis, however, may be more revealing of the variation in re-LT practices and outcomes.
Consistent with studies of re-LT in general, the outcomes of re-LT in patients with PSC were overall inferior to primary LT, though survival has improved more recently.(1–17,30–35) The survival differences were primarily due to increased short-term GF and mortality, with twice as many of the overall failed grafts and deaths occurring before 30 days after re-LT compared with primary LT. The most common cause of death among these early mortalities was INF, largely sepsis, which has also been true of re-LT recipients in a series of other etiologies.(2–4,12,13,33–35)
In our analysis, mechanical ventilation and increased hepatic and renal dysfunction were independently associated with GF and mortality in re-LT recipients, highlighting the impact of the preoperative status on postoperative outcomes. These same predictors have been identified in prior studies of re-LT, as has advanced donor age, which was also a risk factor in these patients.(4,10,11,13–16,34) Unlike other studies, recipient age was not associated with GF or mortality.(3,10,15,32,34,36) Also, many previous analyses have identified the time to re-LT as a predictor of outcomes, but after adjusting for other factors, there was not an association between the time to re-LT and survival in these patients.(1,2,10–12,14,16,17) Interestingly, use of a living donor in the setting of re-LT was a significant predictor of GF at 5 years. Given the relatively few recipients who underwent living donor LT (n = 8) though, it is difficult to draw any definitive conclusions from this finding, and further research is needed to evaluate the role of living donor LT in re-LT. The finding that having a primary liver malignancy at re-LT did not significantly affect outcomes is also hard to interpret with such a small number of these patients (n = 4).
Although overall re-LT recipients with PSC experienced inferior survival compared with primary LTs, this was not true of every indication for re-LT. A novel finding of this study is that the outcomes of re-LT for PSC REC were not only superior to other indications for re-LT, but they were similar to primary LT. REC of PSC occurs in up to 20% of LT recipients at 5 years and increases the risk of GF as well as mortality.(18–23) Re-LT may therefore be considered in these patients in order to prolong their survival. Given the traditionally poor outcomes after re-LT, similarly inferior survival might have been expected in recipients of re-LT for PSC REC, and some may have been hesitant to offer re-LT to these patients. Our analysis, however, demonstrates that the outcomes in these re-LT recipients were similar to primary LT for PSC at 5 years after LT. Furthermore, although the longterm survival after re-LT was excellent, the majority of wait-list mortalities occurred within 6 months, highlighting the risk of not receiving re-LT. Together, the favorable post-re-LT outcomes and the high proportion of waitlist mortalities occurring soon after relisting support the consideration of re-LT in patients with REC of PSC.
Several limitations of this analysis and important qualifiers to the previous statement should be noted though. First, because 897/1803 patients did not have a reported etiology of GF, some individuals with GF due to REC may not have been reported, and therefore would not have been included in this study. There is also likely a significant selection bias among the individuals listed for re-LT. REC is a slow developing, late cause of GF, allowing for re-LT to occur under more elective circumstances. By virtue of being relisted, these individuals had already been deemed fit to undergo re-LT and may have been predisposed to better outcomes. Therefore, these findings are likely not applicable to every patient with recurrent PSC. Future research should be performed to identify the individuals who may benefit from re-LT like the cohort in this study. It appears, however, that clinician assessments of these patients were largely accurate.
Also, though the use of the UNOS/OPTN database allowed for the analysis of more re-LT recipients with PSC than any prior study, there are inherent limitations with retrospective review of a large administrative database, including missing, incomplete, or potentially inaccurate data. We were particularly interested in the cause of failure in the primary graft as a factor affecting post-re-LT outcomes, especially re-LT in the setting of PSC REC. We cannot know definitively, however, that these were truly cases of REC and not CR or other diagnoses, which is perhaps highlighted by the 1 case of REC that was reported to occur within 30 days of LT. Given our relatively large sample size though, it is unlikely that a minority being reported incorrectly could produce the results we observed. Unless there was a more systematic problem, misclassification of other diagnoses as REC or vice versa would likely only increase the similarity between the groups, not further distinguish REC from the others.
In this study, we reported the characteristics and outcomes of a large number of re-LT recipients with PSC, and we identified the novel finding that PSC patients retransplanted for disease REC appear to have similar survival to primary LT recipients at 5 years after LT. In addition, a majority of the patients who died on the waiting list did so within 6 months of relisting. We therefore believe that the transplant community should consider re-LT in patients with REC of PSC. An important caveat to this statement is that the patients included in this analysis were likely highly selected to undergo re-LT for their favorable pre-LT characteristics. So, although our finding of good outcomes after re-LT is encouraging, these patients should continue to be evaluated carefully, taking into account the predictors of poor outcomes that we and others have identified, particularly mechanical ventilation and donor factors. Under the right circumstances, however, re-LT in individuals with recurrent PSC may represent a treatment option with the potential for excellent outcomes. Further research to better characterize the patients with PSC REC who may benefit from re-LT is warranted.
Supplementary Material
Acknowledgments
This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Abbreviations
- AR
acute rejection
- BC
biliary complication
- CCA
cholangiocarcinoma
- CI
confidence interval
- CR
chronic rejection
- DCD
donation after cardiac death
- GF
graft failure
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- IBD
inflammatory bowel disease
- ICU
intensive care unit
- INF
infection
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- OR
odds ratio
- PNF
primary nonfunction
- PSC
primary sclerosing cholangitis
- REC
recurrence
- re-LT
liver retransplantation
- UNOS
United Network for Organ Sharing
- VT
vascular thrombosis
Footnotes
Additional supporting information may be found in the online version of this article.
Potential conflict of interest: Nothing to report.
References
- 1.Powelson JA, Cosimi AB, Lewis WD, Rohrer RJ, Freeman RB, Vacanti JP, et al. Hepatic retransplantation in New England–a regional experience and survival model. Transplantation. 1993;55:802–806. doi: 10.1097/00007890-199304000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Markmann JF, Markowitz JS, Yersiz H, Morrisey M, Farmer DG, Farmer D, et al. Long-term survival after retransplantation of the liver. Ann Surg. 1997;226:408–418. doi: 10.1097/00000658-199710000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong T, Devlin J, Rolando N, Heaton N, Williams R. Clinical characteristics affecting the outcome of liver retransplantation. Transplantation. 1997;64:878–882. doi: 10.1097/00007890-199709270-00015. [DOI] [PubMed] [Google Scholar]
- 4.Watt KD, Lyden ER, McCashland TM. Poor survival after liver retransplantation: is hepatitis C to blame? Liver Transpl. 2003;9:1019–1024. doi: 10.1053/jlts.2003.50206. [DOI] [PubMed] [Google Scholar]
- 5.Ghobrial RM, Gornbein J, Steadman R, Danino N, Markmann JF, Holt C, et al. Pretransplant model to predict posttransplant survival in liver transplant patients. Ann Surg. 2002;236:315–322. doi: 10.1097/00000658-200209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo HY, Maheshwari A, Thuluvath PJ. Retransplantation of liver: primary graft nonfunction and hepatitis C virus are associated with worse outcome. Liver Transpl. 2003;9:897–904. doi: 10.1053/jlts.2003.50176. [DOI] [PubMed] [Google Scholar]
- 7.Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905–918. doi: 10.1097/01.sla.0000164077.77912.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, et al. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg. 2013;258:409–421. doi: 10.1097/SLA.0b013e3182a15db4. [DOI] [PubMed] [Google Scholar]
- 9.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. Liver. Am J Transplant. 2016;16(S2):69–98. doi: 10.1111/ajt.13668. [DOI] [PubMed] [Google Scholar]
- 10.Doyle HR, Morelli F, McMichael J, Doria C, Aldrighetti L, Starzl TE, Marino IR. Hepatic retransplantation-an analysis of risk factors associated with outcome. Transplantation. 1996;61:1499–1505. doi: 10.1097/00007890-199605270-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markmann JF, Gornbein J, Markowitz JS, Levy MF, Klintmalm GB, Yersiz H, et al. A simple model to estimate survival after retransplantation of the liver. Transplantation. 1999;67:422–430. doi: 10.1097/00007890-199902150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay D, Linhares MM, Huguet E, Delvart V, Castaing D, Adam R, et al. Decision for retransplantation of the liver: an experience- and cost-based analysis. Ann Surg. 2002;236:713–721. doi: 10.1097/01.SLA.0000036264.66247.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong JC, Kaldas FM, Kositamongkol P, Petrowsky H, Farmer DG, Markovic D, et al. Predictive index for long-term survival after retransplantation of the liver in adult recipients: analysis of a 26-year experience in a single center. Ann Surg. 2011;254:444–448. doi: 10.1097/SLA.0b013e31822c5878. [DOI] [PubMed] [Google Scholar]
- 14.Linhares MM, Azoulay D, Matos D, Castelo-Filho A, Triviño T, Goldenberg A, et al. Liver retransplantation: a model for determining long-term survival. Transplantation. 2006;81:1016–1021. doi: 10.1097/01.tp.0000203798.96491.2f. [DOI] [PubMed] [Google Scholar]
- 15.Rosen HR, Madden JP, Martin P. A model to predict survival following liver retransplantation. Hepatology. 1999;29:365–370. doi: 10.1002/hep.510290221. [DOI] [PubMed] [Google Scholar]
- 16.Rosen HR, Prieto M, Casanovas-Taltavull T, Cuervas-Mons V, Guckelberger O, Muiesan P, et al. Validation and refinement of survival models for liver retransplantation. Hepatology. 2003;38:460–469. doi: 10.1053/jhep.2003.50328. [DOI] [PubMed] [Google Scholar]
- 17.Kim WR, Wiesner RH, Poterucha JJ, Therneau TM, Malinchoc M, Benson JT, et al. Hepatic retransplantation in cholestatic liver disease: impact of the interval to retransplantation on survival and resource utilization. Hepatology. 1999;30:395–400. doi: 10.1002/hep.510300210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, Neuberger J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–340. doi: 10.1002/lt.21679. [DOI] [PubMed] [Google Scholar]
- 19.Alexander J, Lord JD, Yeh MM, Cuevas C, Bakthavatsalam R, Kowdley KV. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:245–251. doi: 10.1002/lt.21394. [DOI] [PubMed] [Google Scholar]
- 20.Campsen J, Zimmerman MA, Trotter JF, Wachs M, Bak T, Steinberg T, Kam I. Clinically recurrent primary sclerosing cholangitis following liver transplantation: a time course. Liver Transpl. 2008;14:181–185. doi: 10.1002/lt.21313. [DOI] [PubMed] [Google Scholar]
- 21.Cholongitas E, Shusang V, Papatheodoridis GV, Marelli L, Manousou P, Rolando N, et al. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:138–143. doi: 10.1002/lt.21260. [DOI] [PubMed] [Google Scholar]
- 22.Graziadei IW, Wiesner RH, Batts KP, Marotta PJ, LaRusso NF, Porayko MK, et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050–1056. doi: 10.1002/hep.510290427. [DOI] [PubMed] [Google Scholar]
- 23.Ravikumar R, Tsochatzis E, Jose S, Allison M, Athale A, Creamer F, et al. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J Hepatol. 2015;63:1139–1146. doi: 10.1016/j.jhep.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Selck FW, Grossman EB, Ratner LE, Renz JF. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased-donor pool. Ann Surg. 2008;248:599–607. doi: 10.1097/SLA.0b013e31818a080e. [DOI] [PubMed] [Google Scholar]
- 25.Abt PL, Desai NM, Crawford MD, Forman LM, Markmann JW, Olthoff KM, Markmann JF. Survival following liver transplantation from non-heart-beating donors. Ann Surg. 2004;239:87–92. doi: 10.1097/01.sla.0000103063.82181.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renz JF, Kin C, Kinkhabwala M, Jan D, Varadarajan R, Goldstein M, et al. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Ann Surg. 2005;242:556–563. doi: 10.1097/01.sla.0000183973.49899.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryce CL, Angus DC, Arnold RM, Chang CC, Farrell MH, Manzarbeitia C, et al. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9:2092–2101. doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemmer N, Zacharias V, Kaiser TE, Neff GW. Access to liver transplantation in the MELD era: role of ethnicity and insurance. Dig Dis Sci. 2009;54:1794–1797. doi: 10.1007/s10620-008-0567-5. [DOI] [PubMed] [Google Scholar]
- 29.Yoo HY, Thuluvath PJ. Outcome of liver transplantation in adult recipients: influence of neighborhood income, education, and insurance. Liver Transpl. 2004;10:235–243. doi: 10.1002/lt.20069. [DOI] [PubMed] [Google Scholar]
- 30.Yao FY, Saab S, Bass NM, Hirose R, Ly D, Terrault N, et al. Prediction of survival after liver retransplantation for late graft failure based on preoperative prognostic scores. Hepatology. 2004;39:230–238. doi: 10.1002/hep.20005. [DOI] [PubMed] [Google Scholar]
- 31.Ghabril M, Dickson R, Wiesner R. Improving outcomes of liver retransplantation: an analysis of trends and the impact of hepatitis C infection. Am J Transplant. 2008;8:404–411. doi: 10.1111/j.1600-6143.2007.02082.x. [DOI] [PubMed] [Google Scholar]
- 32.Maggi U, Andorno E, Rossi G, De Carlis L, Cillo U, Bresadola F, et al. Liver retransplantation in adults: the largest multicenter Italian study. PLoS One. 2012;7:e46643. doi: 10.1371/journal.pone.0046643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onaca N, Levy MF, Ueno T, Martin AP, Sanchez EQ, Chinnakotla S, et al. An outcome comparison between primary liver transplantation and retransplantation based on the pretransplant MELD score. Transpl Int. 2006;19:282–287. doi: 10.1111/j.1432-2277.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 34.Facciuto M, Heidt D, Guarrera J, Bodian CA, Miller CM, Emre S, et al. Retransplantation for late liver graft failure: predictors of mortality. Liver Transpl. 2000;6:174–179. doi: 10.1002/lt.500060222. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Lee KW, Yi NJ, Lee HW, Choi Y, Suh SW, et al. Outcome and technical aspects of liver retransplantation: analysis of 25-year experience in a single major center. Transplant Proc. 2015;47:727–729. doi: 10.1016/j.transproceed.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt TM, Kumer SC, Pruett TL, Argo CK, Northup PG. Advanced recipient age (>60 years) alone should not be a contraindication to liver retransplantation. Transpl Int. 2009;22:601–605. doi: 10.1111/j.1432-2277.2009.00845.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.