Abstract
Introduction:
Quality of life (QOL) is increasingly recognized as an important endpoint in cancer therapies. However, few data are available on QOL in patients who have received radiotherapy as adjuvant treatment for cancer stomach.
Methods:
Thirty patients who underwent curative resection were enrolled and received chemoradiotherapy (45 Gy in 25 fractions using three-dimensional conformal radiotherapy technique), together with 5-fluorouracil and leucovorin. The European Organization for Research and Treatment of Cancer QOL questionnaire C30 and STO Q22 was assessed at four time points: pre- and postchemoradiotherapy and at 1-month and 6-month follow-up.
Results:
Mean age of the patients was 54 years. Male:female ratio was 4:1. Stage II and Stage III disease was present in 60% and 30% of patients, respectively. All patients were able to complete the chemoradiotherapy protocol. Our study found out significant impairment in QOL for emotional functioning, fatigue, nausea and vomiting and dyspnea. Results showed that QOL levels decrease postchemoradiotherapy; however, QOL levels returned to baseline at 1-month and 6-month follow-up period.
Conclusion:
Chemoradiotherapy as adjuvant treatment for cancer stomach patients who have undergone resection with curative intent is a safe and well-tolerated regimen with respect to QOL.
Keywords: Chemoradiotherapy, gastric cancer, quality of life
Introduction
Almost one million new cases of stomach cancer were estimated to have occurred in 2012 (952,000 cases, 6.8% of the total), making it the fifth most common malignancy in the world.[1] Five-year overall survival (OS) in the United States is only 27% compared with 69% in Japan, where routine screening for gastric cancer is performed and the majority of patients have localized disease at presentation.[2] The incidence of gastric cancer in India is overall less compared to the worldwide incidence. Gastric cancer is the second most common cause of cancer-related deaths among Indian men and women.[3] Overall 5-year survival rate approximates 20% and has undergone minimal change over the last decade.[2]
The primary curative treatment of gastric carcinoma is surgical resection. However, in view of high incidence of locoregional failure, it is recommended that patients with resected gastric cancer should receive adjuvant treatment.[4] Therefore, it is important to consider postoperative adjuvant therapy in the form of chemotherapy or radiotherapy or combined chemoradiotherapy. The addition of radiotherapy to postoperative chemotherapy experienced a superior disease-free survival, especially in patients with node-positive disease.[5] More importantly, the median OS and 3-year survival were significantly better in patients who were treated with chemoradiation. However, chemoradiotherapy was associated with significantly increased toxicity, particularly hematologic and gastrointestinal. Despite its toxicity, postoperative chemoradiotherapy became the standard of care for high-risk resectable gastric cancer in the United States.[6]
Quality of life (QOL) is increasingly recognized as an important endpoint in cancer treatment. However, few data are available on QOL in patients who have received abdominal radiation therapy.
The aim of the study was to evaluate the impact on QOL in patients receiving chemoradiotherapy as adjuvant treatment after curative resection in patients with gastric cancer.
Methods
The study was conducted in the Department of Radiotherapy over a period of 12 months. Individuals were recruited from patients with a primary diagnosis of gastric cancer who underwent curative resection after taking written and informed consent. Type of the study: observational and analytical and the sample size was 30.
Patient criteria
The inclusion criteria were age ≥18 years, ECOG performance 0–2, histologically proven gastric malignancy (adenocarcinoma), adequate bone marrow function, and no prior chemotherapy or radiotherapy. Patients with metastatic disease, cardiac morbidity, poor nutritional status, significant postsurgical morbidity, single functioning kidney which may be in the radiation field, and already on immunosuppressive drugs were excluded.
Treatment design
Treatment began within 6 weeks of surgery, but treatment was delayed for one more week to permit full recovery with restoration of reasonable nutritional intake. All patients underwent chest radiographs and abdominopelvic computed tomography (CT) to exclude distant metastases. A total of 30 patients who underwent gastric resection with curative intent, irrespective of the type of surgery, and nodal dissection were taken up in the study.
Chemoradiotherapy was administered on an outpatient or inpatient basis. The treatment regimen of fluorouracil and leucovorin as developed by the North Central Cancer Treatment Group was administered before and after radiation. Chemotherapy for 5 days with fluorouracil 425 mg/m2/day and leucovorin 20 mg/m2/day was administered on day 1 and was followed by chemoradiotherapy which began 4 weeks after the start of the initial cycle of chemotherapy. Chemoradiotherapy consisted of 4500 cGy of radiation at 180 cGy/day on 6 MV linear accelerator, 5 days/week for 5 weeks, with fluorouracil (400 mg/m2) and leucovorin (20 mg/m2) on the first 4 days and the last 4 days of radiotherapy. For radiotherapy, all patients underwent CT simulation wherein immobilization cast was made and patients underwent contrast-enhanced CT scan – both oral and intravenous (IV). All patients underwent three-dimensional conformal radiotherapy planning. Four weeks after the completion of chemoradiotherapy, the patients received two more courses of chemotherapy using the same regimen.[6]
Quality of life analysis
The patients were provided with QOL questionnaire pro forma as per European Organization for Research and Treatment of Cancer QOL (EORTC) guidelines. This consisted of 2 questionnaires – EORTC quality of life questionnaire (QLQ)-C30 which pertained to the general symptoms of cancer and STO Q22 which pertained to stomach-specific symptoms. The eortc qlq-C30 is a self-administered, cancer-specific questionnaire. It has five functional scales (each scale consisting of questions reflecting the physical, role, social, emotional, and cognitive functioning of the patient), seven symptom scales (fatigue, pain, nausea and vomiting, dyspnea, insomnia, appetite loss, and diarrhea), and global health status and overall qol scales. The stomach-specific QOL consists of symptom scales (dysphagia, stomach pain, reflux symptoms, eating restrictions, anxiety, dry mouth, taste, body image). This measure has been rigorously developed and has demonstrated reliability, validity, and responsiveness in a variety of cancer populations, both for discriminative and for evaluative purposes. The patients were provided with the QOL pro forma at 4 time periods: once after the surgery (prechemoRT) level, then again after completion of chemoradiotherapy, i.e., postRT level, followed by at 1 month and 6 months of follow-up period.
Analysis
Each QOL domain was scored and reported separately using previously described standard methods. Raw scores were linearly transformed to give values between 1 and 100. Higher scores in the functional domains and in global QOL indicate better functioning; a higher symptom score indicates worst symptom.[7] Statistical analysis was conducted utilizing SPSS version 22 (Corp, Armonk, NY, USA). Interpretation and analysis of obtained results were carried out using following tests: qualitative data were expressed in terms of frequency/percentage. Quantitative data were expressed in terms of mean ± standard deviation repeated measure – ANOVA was used to compare the QOL score at different time intervals.
Results
The baseline characteristics of the study group are shown in Table 1.
Table 1.
Comparing baseline characteristics

The mean and median age was 54.6 and 54.5 years, respectively. The range was between 32 and 79 years. The majority of patients were in the 5th decade of life (11 patients, 36.6%). In sex distribution, majority of patients were males, i.e., 24 (80%) and females were 6 (20%) and male:female ratio was 4:1. As per ECOG scoring criteria, majority of patients were of good performance status, i.e., I and II being 93.33%. Only two patients were of ECOG PS III. In habits, 23 patients were smokers (76.6%) and 15 patients (50%) also consumed alcohol. Subsite analysis showed that majority of patients had disease in antrum and pylorus (15 patients – 50%) followed by growth in the body of stomach and gastroesophageal (GE) junction tumors.
Preoperative workup was done with endoscopy + biopsy, contrast-enhanced CT (CECT) abdomen and pelvis and chest X-ray. CECT accurately predicted the site and extent of the primary tumor in 20 patients (66.6%). For nodal status, CECT showed no evidence of lymphadenopathy in 21 patients (70%) and showed the presence of perigastric lymphadenopathy in nine patients.
All surgeries were done with a curative intent. Majority of the patients underwent a distal gastrectomy (11 patients – 36.6%) followed by proximal and subtotal gastrectomy done in six patients each – 20%. Total gastrectomy was done in five patients – 16.6% and Ivor Lewis surgery along with proximal gastrectomy was done in two patients – 6.6%. All patients were staged according to the AJCC 7th edition tumor, node, metastasis staging classification. T3 and T4 disease were present in 10 (33.3%) and 11 (36.6%), respectively. Nodal status could not be assessed in four patients; nodes were negative in seven patients in which a minimum of 12 nodes were assessed. N1, N2, and N3 nodes were present in 8, 9, and 2 patients, respectively.
Functional symptoms of the QLQ-C30 included emotional, physical functioning, role functioning, cognitive, and social symptoms. The functional scales are summarized in Figure 1. The emotional functioning score was the lowest after completion of chemoradiotherapy which improved 1-month follow-up.
Figure 1.
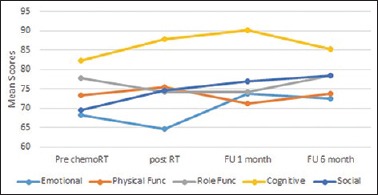
The mean scores of functional scale across all time points as per QLQ-C30
Symptom scale included fatigue, pain, nausea and vomiting, dyspnea, insomnia, appetite loss, and diarrhea. The symptom scale is summarized in Figure 2. Fatigue and nausea and vomiting were the worst postradiotherapy and gradually improved at 1-month and 6-month follow-up period. For dyspnea, the QOL score was worse at 6 months. Insomnia scores worsened gradually; however, they were not significant. Significant results were not obtained when diarrhea, appetite loss, and general pain were assessed.
Figure 2.
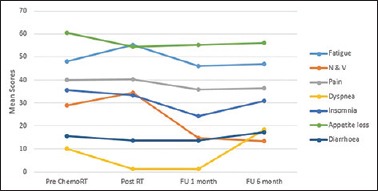
The mean scores of symptom scales across all time points as per QLQ-C30
The stomach-specific QOL consists of various symptom scales (dysphagia, stomach pain, reflux symptoms, eating restrictions, anxiety, dry mouth, taste, body image). Their mean scores are summarized in Figure 3. Dysphagia was worse postradiotherapy. On follow-up of the patient, dysphagia gradually improved with the best scores recorded at 6-month interval. Pain scores worsened at 6 months probably because three patients developed regional failure. Patients were the most anxious at the end of radiotherapy treatment, and least at 1-month follow-up period. For taste, the QOL score was worse postradiotherapy; however, it returned to baseline at 1-month and 6-month follow-up. Significant results were not obtained for eating restrictions, dry mouth, and body image.
Figure 3.
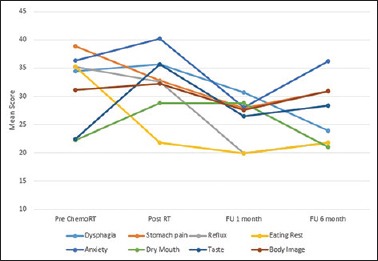
The mead scores of stomach-specific quality of life across all time points as STO Q22
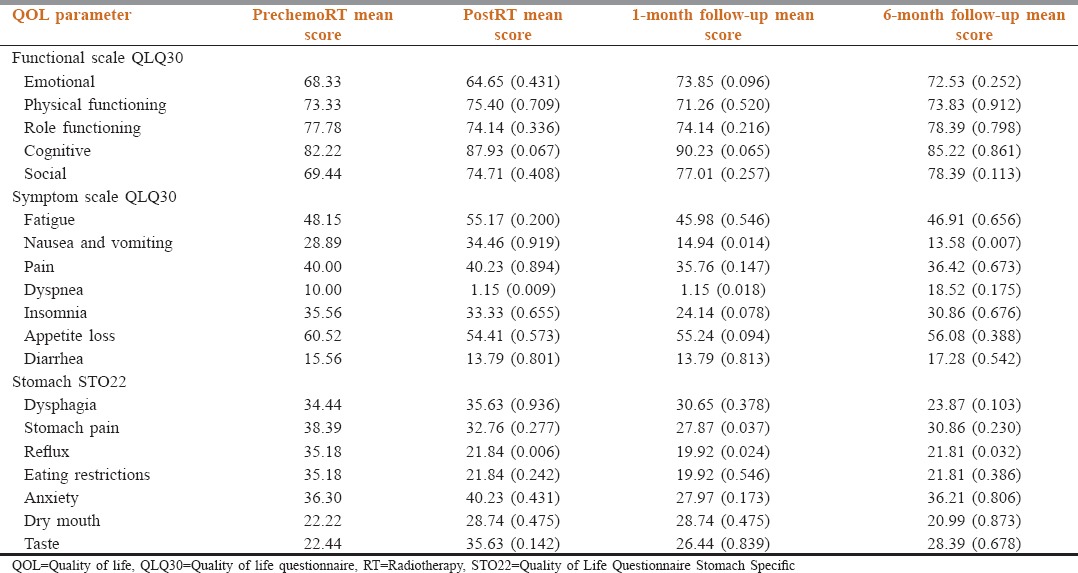
The global QOL is summarized in Figure 4. For Global QoL analysis, it was the maximum and best before the start of chemoradiotherapy. It was the lowest postradiotherapy and gradually improved over 1-month and 6-month follow-up. Table 2 shows the QOL scores at various time points.
Figure 4.
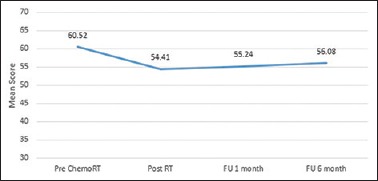
The global quality of life across all time points
Table 2.
Quality of life scores at various time points
Discussion
The treatment of carcinoma stomach has undergone a paradigm shift over the past three decades, with management strategies changing from surgery as single modality in the past combined modality treatment followed by emergence of concurrent chemoradiation protocols. Robust and mature data from various randomized studies and a meta-analysis have shown the superiority of concurrent chemoradiation schedules in locoregional control and OS.
The aim of this study was to study the impact on QOL in patients undergoing adjuvant chemoradiotherapy in resected gastric cancer patients. The principal question was to document the feasibility of chemoradiotherapy regimen in resected gastric cancer patients with curative intent in our patient population. The design and choice treatment in the present study was based on the landmark trial on chemoradiotherapy for cancer stomach that is the intergroup (INT) trial,[6] and QOL analysis was done in concordance with study done by Kassam et al.[8]
All surgeries were done with a curative intent. All surgeries were open surgeries with none being laparoscopic assisted. In pathological T-stage distribution analysis, majority of the patients were locally advanced with only nine patients (30%) having T2 disease on postoperative histopathological examination. However, T3 and T4 disease were present in 10 (33.3%) and 11 (36.6%), respectively, highlighting the fact that patients presented with locally advanced disease which is usually seen in our patient population and is similar to the T-stage classification as per staging in the INT 0116 trial.[6]
Nodal status could not be assessed in four patients and was labeled as Nx. For classification into N0, 1, 2, 3, a minimum of 12 lymph nodes were removed. Nodes were negative in seven patients. N1, N2, and N3 nodes were present in 8, 9, and 3 patients, respectively, again highlighting the fact that majority of patients present with locoregionally advanced disease at the time of presentation. In stage analysis, majority of patients belonged to Stage IIB (12 patients – 40%) followed by Stage IIA and IIIB with six patients each – 20%. This was again similar to the INT trial.[6]
Very few data are available on QOL of patients undergoing adjuvant therapy for gastric and GE adenocarcinoma, and no long-term data are available. Our sample size was small, but the results showed that QOL levels decrease postchemoradiotherapy; however, the treatment was generally well tolerated and the QOL levels returned to baseline at 1-month and 6-month follow-up period. Our study found out statistically significant impairment in QOL for emotional functioning, fatigue, nausea and vomiting and dyspnea.
Kassam et al. analyzed QOL following chemoradiotherapy as an adjuvant treatment in gastric cancer. Median scores on global QOL and on the social, role, emotional, nausea and vomiting, and fatigue scales showed clinically and statistically significant worsening at completion of radiation. Statistical but not clinical worsening was found for the physical and appetite scales.[8]
Tyrväinen et al.used the SF-36 and D15 to compare QOL in 25 long-term survivors after total gastrectomy for gastric cancer with QOL in a normal population. They found that mental health, physical and social functioning, energy, and vitality were similar in both groups. However, certain dimensions, such as sleeping, eating, and distress, scored worse in cancer survivors.[9] Blazeby et al.reported favorable psychometric properties of the new gastric cancer QOL module, the QLQ-STO22, in 219 gastric cancer patients treated with palliative and radical intent.[10] Goody et al. did a prospective evaluation of QOL during a phase I/II study of adjuvant chemotherapy with image-guided precision radiotherapy for completely resected gastric cancer. They showed that mean scores for global QOL, role and social functioning, fatigue, nausea and vomiting, and appetite declined at completion of radiation; physical functioning showed a statistically significant decline of borderline clinical importance. They concluded that adjuvant gastric chemoradiotherapy incorporating cisplatin worsened global QOL, fatigue, nausea and vomiting and appetite. This regimen is tolerable not only by observer-rated toxicity but also by patient-reported QOL measures.[11]
Functional symptoms included emotional, physical functioning, role functioning, cognitive, and social symptoms. A higher score meant a higher standard of quality and conversely a lower score meant poor health status. In our study, the emotional mean score was the lowest at postradiotherapy level – 64.65. In the study done by Kassam et al. on QOL of chemoradiation in gastric cancer patients, emotional QOL decreased after treatment however statistically improved at follow-up of 2–3 years.[8] It indicates that a patient not only experiences the toxicity of treatment but also suffers emotionally as well. A patient needs adequate counseling and moral support during and after the treatment. We found out that emotional QOL scores improved on follow-up of the patient at 1 and 6 months being approximately equal to 73 and this improvement was statistically significant when compared to postradiotherapy levels (P = 0.014). In other functional scales such as physical and role functioning, cognitive and social scores were not statistically significant in our study.
In symptom scale, a higher score represented a higher level of medical morbidity. We found that our patients experience worsening of fatigue postchemoradiotherapy with the mean score being 55.17. This suggests that patients need adequate diet, nutrition, counseling during and after treatment as a lot of patients practically stop eating due to the side effects of therapy and naturally would experience fatigue. In our study, seven patients (23.33%) required admission after completion of therapy for supportive care which was given in the form of IV fluids or Ryles tube feeding. This was in concordance with the study done by Kassam et al.[8] where fatigue score worsened postradiotherapy where the mean score was in the tune of 65; however, they returned to baseline on follow-up. In our study, statistically significant improvement was seen at 1-month follow-up itself where the mean score was 45.98 with a P = 0.046.
Nausea and vomiting scores also worsened postchemoradiotherapy. This is due to the fact that chemotherapy with leucovorin and 5-fluorouracil are emetogenic and addition of radiotherapy to the stomach bed and drainage sites would lead to increase in reflux. In our study, this proved to be statistically significant with a mean score of 34.46. The symptoms improved tremendously on follow-up with a statistically significant P value as compared to the baseline and postchemoradiotherapy mean scores. This is in tune with the study done by Kassam et al. where nausea and vomiting worsened after treatment but improved on follow-up.[8]
Another symptom which statistically significantly worsened was dyspnea. However, it was not related to morbidity associated with chemoradiotherapy, rather it was due to progression of disease in the form of lung metastasis at 6-month follow-up period.
In stomach-specific QOL assessment, dysphagia was worse postradiotherapy as compared to preradiotherapy levels due to the immediate effects of radiotherapy; however, dysphagia significantly improved on 1-month and 6-month follow-up. Stomach pain was maximum postsurgery and preradiotherapy. The patients tolerated the radiotherapy treatment in terms of pain, and the best scores were seen at 1-month follow-up which was statistically significant. The patients remained pain free at 6-month follow-up period except for 3 patients who had developed locoregional failure. Patients were most anxious at the end of radiotherapy treatment (mean score: 40.23) and least at 1 month level (mean score: 27.97). This was statistically significant improvement with a P = 0.036. None of the patients experienced hair loss since the chemotherapy regimen used does not lead to alopecia.
The factors associated with poorer QOL outcomes in the setting studied here are not yet clear. The complex relationship between treatment-induced toxicity and QOL has been studied for various cancer sites. Cross-sectional and longitudinal studies have provided important information on the relationship between adverse events and QOL in prostate, head-and-neck, breast, and lung cancers. However, data are lacking on the relationship between long-term toxicity and QOL after radiation treatment for abdominal malignancies. Toxicity and other potential patient and therapy factors associated with poorer QOL outcomes in the short-term and long-term are important areas for future study; these factors may contribute to the tailoring of therapy to the individual patient.
Conclusion
Chemoradiotherapy as adjuvant treatment for cancer stomach patients who have undergone resection with curative intent is a safe and well-tolerated regimen both with respect to QOL. QOL is impaired during the treatment; however, it returns to baseline levels on follow-up at 6 months.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers CD, Parkin D, et al. GLOBOCAN 2012, Cancer Incidence and Mortality Worldwide: IARC Cancer Base 14 No.10. Int J Can. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, et al. Cancer mortality in India: A nationally representative survey. Lancet. 2012;379:1807–16. doi: 10.1016/S0140-6736(12)60358-4. [DOI] [PubMed] [Google Scholar]
- 4.Meyer HJ, Jähne J, Pichlmayr R. Strategies in the surgical treatment of gastric carcinoma. Ann Oncol. 1994;5(Suppl 3):33–6. doi: 10.1093/annonc/5.suppl_3.s33. [DOI] [PubMed] [Google Scholar]
- 5.Costa WL, Jr, Coimbra FJ, Fogaroli RC, Ribeiro HS, Diniz AL, Begnami MD, et al. Adjuvant chemoradiotherapy after D2-lymphadenectomy for gastric cancer: The role of N-ratio in patient selection. Results of a single cancer center: The role of N-ratio in patient selection Results of a single cancer center. Radiat Oncol. 2012;7:169. doi: 10.1186/1748-717X-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 7.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 8.Kassam Z, Mackay H, Buckley CA, Fung S, Pintile M, Kim J, et al. Evaluating the impact on quality of life of chemoradiation in gastric cancer. Curr Oncol. 2010;17:77–84. doi: 10.3747/co.v17i4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyrväinen T, Sand J, Sintonen H, Nordback I. Quality of life in the long-term survivors after total gastrectomy for gastric carcinoma. J Surg Oncol. 2008;97:121–4. doi: 10.1002/jso.20925. [DOI] [PubMed] [Google Scholar]
- 10.Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–8. doi: 10.1016/j.ejca.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Goody RB, Mckay H, Pitcher B, Oza AM, Brierley J, Hyung J, et al. Prospective evaluation of quality of life during a phase I/II study of adjuvant chemotherapy with high precision IGRT for completely resected gastric cancer. J Clin Oncol. 2016;34:164. [Google Scholar]


