Abstract
Background:
In Egypt, there has been a remarkable increase in the proportion of hepatocellular carcinoma (HCC) among chronic liver diseases patients. This rising proportion may be explained by the increasing risk factors as hepatitis C virus (HCV) infection, hepatitis B virus (HBV) infection, improvement of the diagnostic tools of HCC as well as the extended survival among patients with cirrhosis to allow time for some of them to develop HCC. The aim of this study was to study the epidemiology of HCC in Nile delta over the last decade.
Methods:
The study was carried out on patients diagnosed as HCC in liver cancer clinic in Tanta University Hospital, Egypt, from January 2005 to January 2015. This retrospective study reviewed the files of HCC patients with special stress on age, sex, residence, occupation, smoking, and viral markers.
Results:
Over the last decade, 1440 HCC patients were diagnosed or referred to liver cancer clinic in Tropical Medicine Department in Tanta University Hospital from January 2005 to January 2015. The mean age of HCC patients was 56.13 ± 9.53 years. Nearly, half of the patients with HCC were smokers and quarter of HCC patients were diabetics. HBV surface antigen-positive patients were only 3.26%, and the majority of patients were HCV-Ab positive (94.86% of patients).
Conclusions:
In Nile delta, hepatitis C rather than hepatitis B was linked to the development of HCC in our region which may be related to the high prevalence of HCV in this area.
Keywords: Epidemiology, hepatitis C virus, hepatocellular carcinoma, Nile delta
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy, and its prevalence is increasing in developing countries.[1] HCC is the third most common cause of cancer-related deaths complicating liver cirrhosis in most cases.[2]
In Egypt, there was a remarkable increase in the proportion of HCC among patients with chronic liver diseases. This increasing incidence may be explained by the increasing risk factors including the emergence of hepatitis C virus (HCV) over the equal time frame, the contribution of hepatitis B virus (HBV) infection, improvement of the screening programs and diagnostic tools for liver cancer as well as the improved survival rates among patients with cirrhosis to allow time for some of them to develop HCC.[3]
HCC is complex in pathogenesis, which has been contemplated as diverse biologic characteristics in distinct populations.[4]
HBV is considered as one of the major risk factors responsible for the development of liver cirrhosis and HCC. Previous data suggest that the HBx protein may additionally have an important role in HCC pathogenesis through inactivation of tumor suppressor gene (p53) contributing to the development of HCC.[5]
Chronic HCV infection is a major risk factor for the development of HCC. Egypt has shown increasing importance for HCV infection in the etiology of liver cancer and the declining influence of HBV risk factors of HCC and HBV/HCV infection (25% and 15%, respectively).[6]
Some studies linked HCC to diabetes mellitus (DM) and/or obesity, and as the blend of risk factors differs in different parts of the world, we design this retrospective study to clarify the epidemiology of HCC in Nile delta, Egypt and its relation to age, sex, residence, smoking, occupation, and viral hepatitis.
Methods
HCC management unit in Tropical Medicine Department in Tanta University Hospital had their data registered and took consent from patients to use it for scientific research. The patient's files from January 2005 to January 2015 were reviewed.
The diagnosis of HCC was based on detection of hepatic focal lesion by one dynamic imaging techniques (dynamic computed tomography, dynamic magnetic resonance imaging) typical for HCC or alpha-fetoprotein >200 if the hepatic focal lesion above 2 cm in cirrhotic liver or two dynamic imaging techniques typical for HCC if hepatic focal lesion 1–2 cm in cirrhotic liver. Otherwise, diagnosis of HCC depended on detection of hepatic focal lesion and alpha-fetoprotein >400.
This retrospective study included the data of HCC patients who met these criteria, with special stress on their age, sex, residential history, for example, urban or rural type of residence, occupational history, for example, exposure to farming, smoking, presence of previous bilharzial infection (or history of antibilharzial treatment), their clinical and laboratory data including liver function tests and serum alpha-fetoprotein.
The data of radiological investigations, for example, abdominal ultrasonography: with stress on the following data: the liver: regarding the size, texture, border, reflectivity, homogeneity, periportal thickening, and hepatic veins. Focal lesions were reported regarding their number, site, size, shape, echogenicity, halo sign and neovascularization by color Doppler assessment, portal vein regarding its diameter, patency, direction of flow, respiratory variation, and velocity by color Doppler assessment. The spleen also was assessed regarding its size, splenic vein diameter, and the presence of collaterals. The presence of ascites and internal echoes and the presence of any lymph nodes and extrahepatic spread were also documented.
The data of abdominal triphasic computed tomography were reported with stress on the following data: presence of cirrhosis, splenomegaly, portal vein patency, ascites, focal lesion (number, site, size, shape, arterial enhancement), and portal vein diameter and patency.
Results
One thousand four hundred and forty patients with HCC were diagnosed or referred to liver cancer clinic and liver cancer early detection clinic in Tropical Medicine Department in Tanta University Hospital from January 2005 to January 2015. Their mean age was 56.13 ± 9.53 years. They were 1123 (78%) males and 317 (22%) females. About 85.6% of our patient had their residence in rural areas. Among our 1440 HCC patients, 26.1% were diabetics, 44.86% were smokers, 37.8% of patients were Child's A, 35.3% were Child's B and 26.9% were Child's C. The demographic and clinical data of all studied patients are shown in Table 1 and the laboratory data of the studied patients is shown in Table 2.
Table 1.
Demographic and clinical data of all studied patients (n=1440)
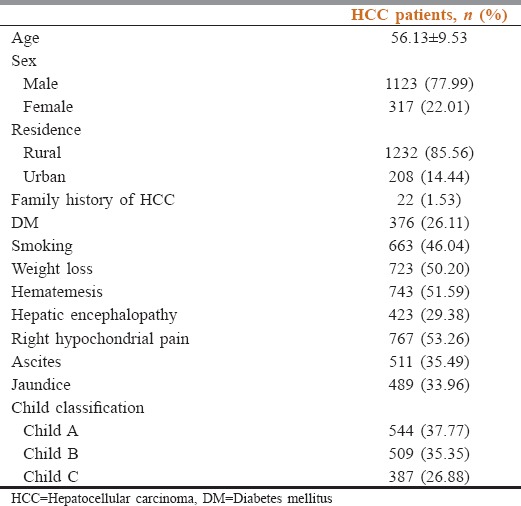
Table 2.
Laboratory data of hepatocellular carcinoma patients

As regards number of the lesions, single lesion was detected in 47.5% of patients versus 52.5% had multiple lesions. While regarding the size of the tumor, 42.29% of patients had tumor size below 3 cm, in 12.77%, the size of tumor was 3–5 cm, while 44.93% of patients presented with tumor size above 5 cm. Nearly, 7.5% of HCC patients had portal vein thrombosis. The characters of focal lesions are shown in Table 3.
Table 3.
Characters of focal lesions

Concerning the etiology of HCC, HBV surface antigen (HBsAg)-positive patients were only 3.26%, while the majority of patients were HCV-Ab positive (94.86% of patients). Therefore, HCV was the predominant cause of HCC.
Discussion
This retrospective study reviewed the files of HCC patients over the last decade. In the current study, HCC was much higher in males. This was in agreement with Di Bisceglie[7] who reported that men are two to three times higher than women in most regions. Higher prevalence of HCC in males may be explained by the higher susceptibility of males to environmental carcinogens and greater exposure to environmental risk factors, for example, pesticides and aflatoxin.[8]
Kew[4] reported male to female ratio of 3.7:1 in HCC patients, especially in population at high risk.
Our HCC patients had a mean age of 56.13 ± 9.53 years. This was much lower than Ryder[8] who reported that the average age of HCC was 66 years and the seventh or eighth decades of life, respectively. However, Kew[4] stated that the incidence of HCC increases progressively with advancing age, but in areas, with high risk, the mean age is definitely younger.
In this study, 85.56% of HCC cases were from rural areas. These results agree with that of el-Zayadi et al.[3] who stated that most of HCC cases (75.2%) resided in rural areas. This may be due to more exposure to environmental carcinogens, for example, pesticides and exposure to aflatoxin in contaminated foods, for example, corn. Aflatoxin may induce the characteristic mutation in p53 preparing the way for HCV to cause liver cancer.[9]
Concerning smoking, our study showed that 46.04% of HCC cases had a history of smoking and about half of them were heavy smokers; a nearly similar result was reported by Mohamed et al.,[10] who reported that 49.6% of HCC cases had a history of smoking. Smoking may cause DNA damage and abnormal cell proliferation.
Nearly, one-quarter of our patients had DM. Davila et al., 2005[11] stated that diabetes may be associated with a 2–3-fold increase in the risk of HCC, regardless of the presence of other major HCC risk factors.
As Nile delta has the highest prevalence of HCV in Egypt, according to demographic study performed in 2008, it may be among the areas of higher risk to HCC. In our study, 94.9% of cases were HCV positive; this was higher than that reported in Egyptian study by Mohamed et al.[10] who reported 64.4% of HCC cases were positive and near to results by el-Zayadi et al.[3] who reported 87.9% of HCC cases were positive. Our results go in agreement with Michielsen et al.[12] who stated that in areas with an intermediate rate of liver tumors such as Southern Europe, Egypt, and Japan, HCV is the predominant cause of HCC. HCC was mostly discovered in patients with long-standing cirrhosis due to HCV.
Although HBV is considered worldwide as a major risk factor for liver cirrhosis and HCC, only 3.26% of HCC patients in our study had HBs-antigen seropositivity. Hence, in Nile delta, hepatitis C rather than hepatitis B was linked to the development of HCC in our region which may be related to the high prevalence of HCV in this area. This decrease of HBsAg positivity may be partially attributed to successful control measures of blood transfusion and vaccination and partially to the development of mutant or occult HBV infection, which requires costly assays for diagnosis.[3,13,14]
Conclusions
As in many developing countries, Egypt is undergoing an epidemiologic transition. With increasing environmental risk factors, increasing smoking rates, in addition to the significantly high HCV epidemiology, it is likely that HCC will continue to rise within the next few years. This study could help policymakers to create efficient prevention programs. In the era of new anti-HCV drugs, we hope that this will stop the rising trend of HCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: Current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19–37. doi: 10.2147/HMER.S16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: An epidemiologic view. J Clin Gastroenterol. 2002;35(5 Suppl 2):S72–8. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 3.el-Zayadi AR, Badran HM, Barakat EM, Attia Mel-D, Shawky S, Mohamed MK, et al. Hepatocellular carcinoma in Egypt: A single center study over a decade. World J Gastroenterol. 2005;11:5193–8. doi: 10.3748/wjg.v11.i33.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kew MC. Hepatic tumors and cysts. In: Feldman M, Friedman LS, Sleisenger MH, editors. Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. Philadelphia, London, New York, St. Louis, Sydney: Saunders; 2002. pp. 1577–602. [Google Scholar]
- 5.Szabó E, Páska C, Kaposi Novák P, Schaff Z, Kiss A. Similarities and differences in hepatitis B and C virus induced hepatocarcinogenesis. Pathol Oncol Res. 2004;10:5–11. doi: 10.1007/BF02893401. [DOI] [PubMed] [Google Scholar]
- 6.Lehman EM, Soliman AS, Ismail K, Hablas A, Seifeldin IA, Ramadan M, et al. Patterns of hepatocellular carcinoma incidence in Egypt from a population-based cancer registry. Hepatol Res. 2008;38:465–73. doi: 10.1111/j.1872-034X.2007.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Bisceglie AM. Epidemiology and clinical presentation of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13(9 Pt 2):S169–71. doi: 10.1016/s1051-0443(07)61783-7. [DOI] [PubMed] [Google Scholar]
- 8.Ryder SD British Society of Gastroenterology. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52(Suppl 3):iii1–8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharaf-Eldin M, Salah R, Soliman H, Abdou SH, Abd-Elsalam S, Elkhalawany W, et al. Aflatoxin as an environmental risk factor attributable to liver cancer in Nile delta. Indian J Med Res Pharm Sci. 2016;3:19–26. [Google Scholar]
- 10.Mohamed NH, El Zawahry HM, Mokbtar NM, Faisal SS, Gad El-Mawla N. Review of epidemiologic and clinicopathologic features of 403 hepatocellular carcinoma (HCC) patients. J Egypt Natl Cancer Inst. 2000;12:87–93. [Google Scholar]
- 11.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: A population based case control study. Gut. 2005;54:533–9. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michielsen PP, Francque SM, van Dongen JL. Viral hepatitis and hepatocellular carcinoma. World J Surg Oncol. 2005;3:27. doi: 10.1186/1477-7819-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziada DH, El Sadany S, Soliman H, Abd-Elsalam S, Salama M, Hawash N, et al. Prevalence of hepatocellular carcinoma in chronic hepatitis C patients in Mid Delta, Egypt: A single center study. J Egypt Natl Canc Inst. 2016;28:257–62. doi: 10.1016/j.jnci.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Sheta E, El-Kalla F, El-Gharib M, Kobtan A, Elhendawy M, Abd-Elsalam S, et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: A randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28:1198–203. doi: 10.1097/MEG.0000000000000688. [DOI] [PubMed] [Google Scholar]


