Abstract
Context:
There is a paucity of any significant data on the estrogen receptor (ER) and progesterone receptor (PR) status of breast cancer patients from the eastern part of India.
Aims:
This study aims to document the ER and PR status of breast cancer patients in the eastern Indian population, as catered by two premier tertiary care hospitals in Kolkata.
Subjects and Methods:
All breast cancer patients registered between January 1, 2013 and December 31, 2015, in the Departments of Oncology, of IPGMER and SSKM Hospitals and R. G. Kar Medical College and Hospital, Kolkata, who had at least undergone a core biopsy or surgery, were analyzed retrospectively for documentation of their ER and PR status, using the 2010 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) interpretation guidelines.
Results:
Over a period of 3 years, a total of 927 patients were included for the study. A total of 825 (89%) patients had their ER and PR data available for evaluation. ER and PR positive was seen in 312 (37.82%) patients, ER and PR negative in 399 (48.36%) patients, ER positive and PR negative in 71 (8.6%) patients, and ER negative and PR positive results was found in 43 (5.21%) patients.
Conclusions:
This is the first multi-institutional documentation of ER and PR status from eastern India, having a modest number of patients and one of the earliest documentations using the latest ASCO/CAP interpretation guidelines. These findings resemble the data from the south and also reiterate the fact that majority of the Indian breast cancer patients are still ER and PR negative in spite of the changes in the interpretation guidelines.
Keywords: 2010 American Society of Clinical Oncology/College of American Pathologists guidelines, breast cancer, eastern India, estrogen receptor/progesterone receptor status, Kolkata
Introduction
In view of an unmet need, both in terms of estrogen receptor (ER) and progesterone receptor (PR) status reporting from the eastern part of India and the lack of any significant national data on the ER and PR status based on the 2010 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) interpretation guidelines, this study was envisaged for multi-institutional documentation of the hormone receptor status of breast cancer patients of the eastern region of the country, following the 2010 ASCO/CAP interpretation guidelines.
Subjects and Methods
The study was undertaken in two tertiary care hospitals in Kolkata, IPGMER and SSKM Hospitals and R. G. Kar Medical College and Hospital. A nonfunded and epidemiological nature of the study deemed an ethical committee clearance unnecessary, as per the institutional protocols. The centers were so chosen that its draining patient population was representative of the entire eastern part of India, including the North East, and the neighboring countries, such as Nepal, Bangladesh, and Bhutan. All breast cancer patients registered in the Departments of Radiotherapy (taking care of all domains of oncology) of the respective hospitals, between January 1, 2013 and December 31, 2015, who had at least undergone a core biopsy or surgery for their disease were included in this study. They were analyzed retrospectively for documentation of their ER and PR status, using the 2010 ASCO/CAP interpretation guidelines. The data were prepared on an Excel sheet and analyzed manually for the interpretation of the results.
Results
Over a period of 3 years, 927 breast cancer patients were analyzed. Out of these, 582 (62.78%) patients underwent surgery and 345 (37.21%) patients had a core biopsy. ER and PR reports were not initially available for 287 patients. Out of these, paraffin-embedded tissues of 185 patients could be retrieved and were sent for ER and PR evaluation. Finally, ER and PR data of 825 (89%) patients were available for evaluation. The ER and PR evaluations were done in the Departments of Pathology of the two concerned hospitals by immunohistochemistry. The kits used for assay were the standard approved kits, as available in the respective teaching hospitals over the concerned study period. The results were interpreted as positive when more than or equal to 1% of tumor cells showed positive nuclear staining, as per the ASCO/CAP interpretation guidelines 2010.
The results of the ER and PR status were as shown in Table 1 and Figure 1.
Table 1.
Results of estrogen receptor/progesterone receptor status
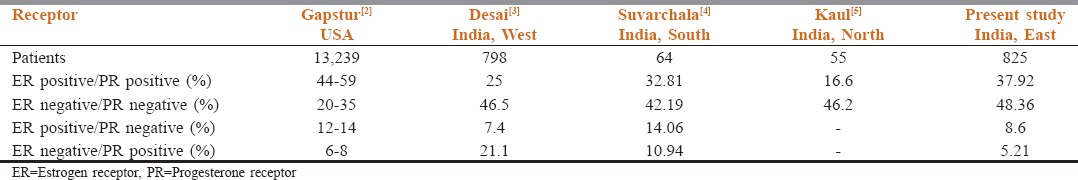
Figure 1.
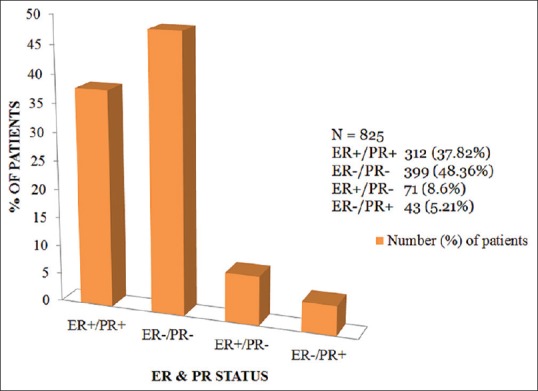
Results of estrogen receptor/progesterone receptor status from Kolkata
The reasons identified for the unavailability of ER and PR reports in 102 (11%) patients were financial constraints, loss of paraffin blocks, and poor embedding of tissue to the available blocks.
Discussion
The relevance of ER and PR status as a predictive marker in breast cancer patients has been established beyond doubt for the last few decades. As per the 2010 ASCO/CAP guidelines, nuclear staining of more than or equal to 1% cells is the current standard to interpret a positive result.[1]
Data from the US suggest that around 44%–59% patients were both ER and PR positive, and only 20%–35% were negative for both ER and PR.[2] The Indian data,[3,4,5,6] on the other hand, suggest a larger number of patients negative for both ER and PR status (42%–48%) [Table 2].
Table 2.
Comparative table showing estrogen receptor/progesterone receptor status reports from the USA and different parts of India (n=825)

This study from Kolkata is one of the first significant reporting of the ER and PR status of breast cancer patients from the east and perhaps the only multi-institutional reporting from India. It is also one of the earliest of its kind to report the ER and PR status based on the 2010 ASCO/CAP interpretation guidelines. The study included a modest number of patients (n = 825), second only to the data from Tata Memorial Hospital, Mumbai, by Dinshaw (n = 1022).[6]
In this study, 37.82% patients were both ER and PR positive and 48.63% patients were both ER and PR negative. Both of these values, as a percentage, are the highest ever reported from of India. These findings also reiterate the fact that the majority of the Indian breast cancer patients are still ER and PR negative in spite of the changes in the interpretation guidelines from the cut off of 10% to 1% nuclear staining. As far as the zonal comparison is concerned, this data from the east look similar to that from the south,[4] though not grossly different from the rest of the country.[3,5,6] We are currently tabulating the Ki67% and HER 2/ neu status of the same set of patients, to enable a complete molecular sub-typing of the breast cancer patients of eastern India in the near future.
Conclusions
This multi-institutional reporting of ER & PR status of 825 breast cancer patients from Kolkata, based on the 2010 ASCO/CAP interpretation guidelines, suggest that the east is not grossly different from the rest of the country, with around half of the patients being negative for both ER & PR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for Imunohistochemical testing of estrogen and progesterone receptors in breast cancer. [Last cited on 2017 Mar 31];JCO. 2010 28:2784–95. doi: 10.1200/JCO.2009.25.6529. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20404251 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gapstur SM, Dupuis J, Gann P, Collila S, Winchester DP. Hormone receptor status of breast tumors in black, Hispanic, and non-Hispanic white women. An analysis of 13,239 cases. [Last cited on 2017 Mar 31];Cancer. 1996 77:1465–71. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1465::AID-CNCR7>3.0.CO;2-B. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8608530 . [DOI] [PubMed] [Google Scholar]
- 3.Desai SB, Moonim MT, Gill AK, Punia RS, Naresh KN, Chinoy RF, et al. Hormone receptor status of breast cancer in India: A study of 798 tumours. [Last cited on 2017 Mar 31];Breast. 2000 9:267–70. doi: 10.1054/brst.2000.0134. Available from: https://www.ncbi.nlm.nih.gov/pubmed/14732176 . [DOI] [PubMed] [Google Scholar]
- 4.Suvarchala SB, Nageswararao R. Carcinoma breast – Histopathological and hormone receptors correlation. [Last cited on 2017 Mar 31];J Biosci Tech. 2011 2:340–8. Available from: http://www.jbstonline.com/documents/vol2issue4/jbst2011020404.pdf . [Google Scholar]
- 5.Kaul R, Sharma J, Minhas SS, Mardi K. Hormone receptor status of breast cancer in the Himalayan region of Northern India. [Last cited on 2017 Mar 31];Indian J Surg. 2011 73:9–12. doi: 10.1007/s12262-010-0121-5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3077188/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinshaw KA, Budrukkar AN, Chinoy RF, Sarin R, Badwe R, Hawaldar R, et al. Profile of prognostic factors in 1022 Indian women with early-stage breast cancer treated with breast-conserving therapy. [Last cited on 2017 Mar 31];Int J Radiat Oncol Biol Phys. 2005 63:1132–41. doi: 10.1016/j.ijrobp.2005.03.071. Available from: http://www.redjournal.org/article/S0360-3016 (05)00712-1/fulltext . [DOI] [PubMed] [Google Scholar]


