Abstract
Background:
Outcome in multiple myeloma (MM) has improved substantially over recent years as a result of the availability of multiple novel agents with acceptable safety profile.
Study Design:
Prospective observational study at a tertiary care institute.
Methods:
Twenty-five newly diagnosed patients of MM were treated with bortezomib and dexamethasone induction with monitoring for response and safety, followed by peripheral blood autologous stem cell transplant (PBASCT) in eligible patients or maintenance.
Results:
Out of 25 patients, 32% attained complete response (CR), 56% very good partial response (VGPR), 4% PR, and 8% showed no response. The overall response rate was 92%. In our study, 56% of patients showed hematological side effects, out of which thrombocytopenia was seen in 32%, anemia in 16%, and leukopenia in 8%. Six patients developed bortezomib-induced peripheral neuropathy, out of which four had grade 1 (66.66%), one had grade 2 (16.66%), and 1 (16.66%) had grade 3 toxicity. Sixteen patients were eligible for PBASCT, out of which eight patients received this therapy while as remaining eight patients opted for two more cycles of induction therapy followed by maintenance. After completing 18 months of maintenance, all the eight patients who underwent PBASCT were in CR. Out of the 15 patients who did not receive PBASCT five attained CR, eight VGPR while as two patients relapsed.
Conclusion:
Bortezomib plus dexamethasone is highly effective and well-tolerated regimen for frontline treatment of MM with a higher quality of response in an advanced stage and renal failure patients.
Keywords: Bortezomib and dexamethasone, multiple myeloma, response rate
Introduction
Multiple myeloma (MM) accounts for 1% of malignant tumors and 10%–15% of hematopoietic neoplasms. The survival ranges from few months to more than 10 years with the availability of novel agents such as thalidomide, lenalidomide, and bortezomib over recent years.[1]
Significantly higher response rates approximately 70%–90% have been observed with bortezomib plus dexamethasone combination. No adverse effect on stem cell mobilization has been noted with bortezomib plus dexamethasone.[2] Frequently observed toxicities associated with bortezomib-based therapy in patients with MM include peripheral neuropathy, thrombocytopenia, leukopenia, and gastrointestinal dysfunction.[3,4]
With this background and based on growing body of literature, the present study was conducted at a tertiary care hospital of north India to analyze the efficacy and tolerability of bortezomib plus dexamethasone in newly diagnosed MM patients.
Materials and Methods
This study was conducted in the Departments of Clinical Hematology and Medical Oncology of a tertiary care hospital from north India. The main aim of this study was to assess the efficacy and tolerability of bortezomib plus dexamethasone in newly diagnosed patients of MM. It was an open label single arm prospective observational study. A total of 25 newly diagnosed patients of MM were enrolled after taking an informed consent. The primary endpoint of our study was the response and safety after four cycles of bortezomib plus dexamethasone induction therapy. The study was started after the clearance from ethical committee, an independently functioning body of the hospital.
Newly diagnosed and previously untreated patients whose age was >18 years with symptomatic disease were included in the study irrespective of eligibility for peripheral blood autologous stem cell transplant (PBASCT). Patients with, relapse or refractory disease, associated with another cancer, grade 2–4 peripheral neuropathy before the start of treatment or uncontrolled cardiovascular disease (NYHA class 3–4) were excluded from this study.
Before the start of study, demographic data, detailed medical history, and examination including clinical assessment of neuropathy and all the baseline investigations (complete blood count, ESR, kidney function tests, liver function tests, serum and urine protein electrophoresis, serum and urine immunofixation electrophoresis, free light chain ratio, bone marrow examination, cytogenetics, serum β2 microglobulin, serum lactate dehydrogenase, serum uric acid, random blood glucose, and serum calcium) were documented for each patient. The International Myeloma Working Group (IMWG) criteria were used for diagnosis and International Staging System (ISS) for disease staging.[5] Patients were started on 21 days cycle of bortezomib and dexamethasone regimen.[6] A total of four such cycles were given to each patient and monitored for response and safety. The response was monitored as per IMWG uniform response criteria for MM.[5]
Patients were monitored closely for any adverse effects of the drug combination. After the completion of four cycles, eligible patients were treated with PBASCT followed by maintenance while as noneligible patients were treated with two more cycles of induction chemotherapy followed by maintenance.
Results
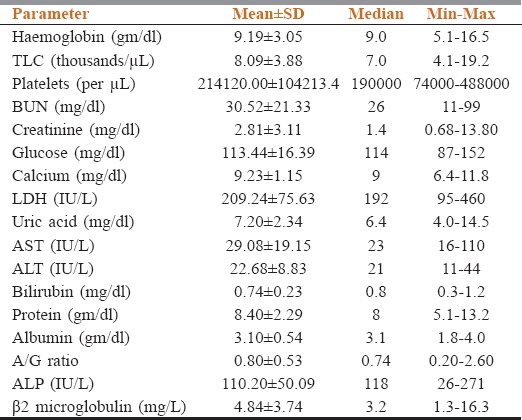
The study population comprised of seventeen male and eight female MM patients (50–80 years); anemia was seen in 80%, renal failure in 44%, spontaneous fractures in 40%, and bone pains in 28% of patients. Hypocalcemia was seen in 16% of patients and hyperuricemia in 40% of patients at presentation [Table 1].
Table 1.
Base line blood profile of 25 newly diagnosed multiple myeloma patients
After two cycles of induction therapy, 64% of patients attained very good partial response (VGPR), 24% of patients attained PR, 4% of patients showed no response, and another 4% of patients showed minimal response. After completing the four cycles of induction, 32% of patients attained complete response (CR), 56% of patients attained VGPR, and 4% of patients attained PR. There were 8% of patients who showed no response after the completion of four cycles of therapy. Overall response rate (ORR) was 92% in these 25 patients. The two nonresponding patients were light chain MM and biclonal MM type [Figure 1].
Figure 1.
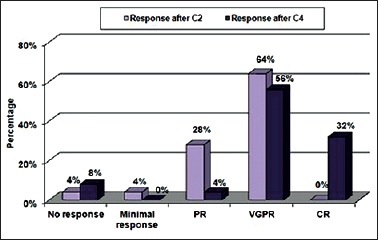
Induction response of bortezomib and dexamethasone in multiple myeloma, where C2: cycle 2 and C4: cycle 4
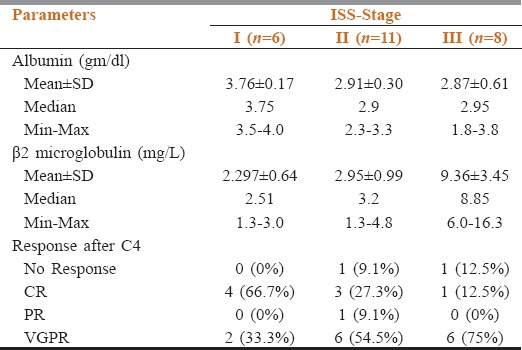
Of 25 patients, 6 patients were in ISS-I, 11 patients in ISS-II, and 8 patients in ISS-III disease stage. Of 6 patients of ISS-I MM, four patients showed CR and two patients showed VGPR after four cycles of therapy (ORR: 100%). Of 11 patients of ISS-II disease, six patients showed VGPR, three patients showed CR, one patient showed PR, and one patient showed no response (ORR: 90%). Out of eight patients of ISS-III disease, six patients showed VGPR (75%), one patient showed CR (12.5), and one patient showed no response (ORR: 87.5%) [Table 2].
Table 2.
Stage wise response of 25 multiple myeloma patients
In the present study, out of 25 patients, 11 had renal failure at presentation. Of these 11 patients, seven patients attained VGPR, three patients attained the PR, and one patient attained minimal response after two cycles of treatment. After the completion of four cycles of treatment, five patients were in VGPR (45.45%), four patients in CR (36.36%), one patient in PR (9%), and one patient showed no response (9%). ORR was 91%. Mean ± standard deviation (SD) of serum creatinine at the start of treatment was 5.05 ± 3.64 (mg/dl) and mean ± SD of serum creatinine after the completion of treatment was 1.31 ± 0.73 (mg/dl). Eight patients (72.72%) showed complete reversal of renal failure and three patients (27.27%) showed partial reversal of renal failure [Figures 2 and 3].
Figure 2.
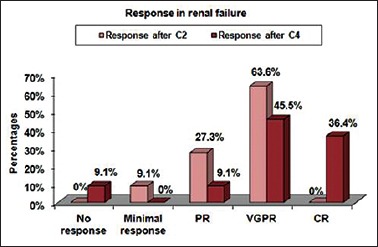
Response in renal failure patients, where C2: cycle 2 and C4: cycle 4
Figure 3.
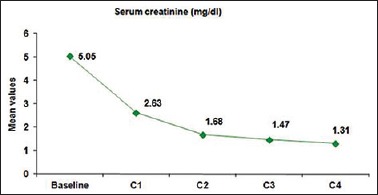
Line diagram showing improvement of serum creatinine with each cycle of treatment
In our study, 56% of patients showed hematological side effects, out of which thrombocytopenia was seen in 32% (all grade 1), anemia in 16% (8% grade 2 and 8% grade 3), and leukopenia in 8% (all grade 1). The hematological side effects were graded as per national cancer institute toxicity criteria. In the present study, bortezomib-induced peripheral neuropathy (BIPN) was the most important nonhematological toxicity seen with the combination of bortezomib plus dexamethasone. Six out of 25 patients developed BIPN, out of which four had grade 1 (66.66%), one had grade 2 (16.66%), and one (16.66%) had grade 3 toxicity. No patient developed grade 4 toxicity. Other common side effects seen in our study were mild nausea (52%) and vomiting (20%). Out of these side effects, none was of grade 3/4 intensity. Transient rise in temperature was seen in three patients. Two patients had mild infection in the upper respiratory tract during the treatment which subsided with the use of short course of antibiotic therapy. Other less common side effects were diarrhea (8%) and constipation (4%) [Figure 4].
Figure 4.
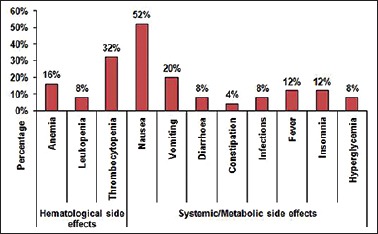
Nonneuropathic side effect profile in multiple myeloma
Of 25 patients, sixteen patients were eligible for autologous stem cell transplant, seven patients were ineligible, and remaining two nonresponding patients were started on different protocol. Of 16 eligible patients, eight patients finally underwent high-dose therapy and autologous stem cell transplant while as remaining eight patients opted for two more cycles of induction therapy followed by maintenance. After 18 months of maintenance, all the eight patients who underwent peripheral blood autologous transplant were in CR. Of 15 patients who did not receive autologous transplant, five patients were in CR, eight patients in VGPR while as two patients relapsed. Hence, after 18 months of completion of induction therapy, 13 (52%) patients were in CR, and 8 (32%) patients were in VGPR.
Discussion
The major objective for the frontline treatment of MM is the achievement of CR or at least VGPR; treatment of choice remains high-dose melphalan with PBASCT after induction therapy.[2]
Compared to published studies, the present study has shown higher ORR (92%), CR (32%), and VGPR (56%) at the end of four cycles of bortezomib plus dexamethasone; with improved quality of response through all the ISS risk stages as compared to phase II PETHEMA trial and famous IFM phase III study.[7,8,9] Post 18 months of induction therapy 13 patients (52%) were in CR and 8 patients (32%) were in VGPR.
Approximately 30% of patients with diagnosed MM present with baseline renal dysfunction.[10] A recent subanalyses of patients with impaired renal function from two phase 2 studies showed that renal dysfunction did not appear to have a negative impact on response rates or toxicity.[11] In the present study, 72.72% of patients showed complete reversal of renal failure and 27.27% of patients showed partial reversal of renal failure as defined by Burnette et al.[12] In comparison to previous studies, the response as per CR is comparable but with higher ORR. In addition, the present study showed higher rates of renal response (CR renal and PR renal).
Besides the high response rate of upfront bortezomib plus dexamethasone in MM patients, there are certain concerns about its safety in these patients including the hematological side effects and neurotoxicity. Out of hematological adverse effects, thrombocytopenia is a common toxicity associated with bortezomib therapy. In comparison to APEX trial, SUMMIT trial, IFM III trial, and phase II PETHEMA trial, the hematological side effects were less severe.[7,8,13,14] In our study, 56% of patients showed hematological side effects, and they were graded as per national cancer institute toxicity criteria. None of the patients showed neutropenia. These side effects were transient and did not require therapy interruption or any other medical intervention.
Peripheral neuropathy is a significant toxicity of bortezomib, requiring dose modification, and potential changes in the treatment plan when it occurs. The results of the SUMMIT and CREST phase 2 trials accurately provide data about the incidence, severity, and risk factors of BIPN.[15]
In the present study, BIPN was the most important nonhematological toxicity. Although the study has shown comparable results (as per the incidence) with previous data the severity of BIPN in this study was less and no patient required discontinuation of therapy. In our study, none of the patients faced the problem of reactivation of herpes zoster, reason being the use of prophylactic acyclovir.
Conclusion
This study adds to the existing data that bortezomib plus dexamethasone is highly effective and well-tolerated regimen for frontline treatment of MM. Further, it highlights the improved quality of response in advanced stage myeloma and renal failure patients. The present study also signifies the improved event-free survival but has a limitation of smaller sample size.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ludwig H, Beksac M, Bladé J, Boccadoro M, Cavenagh J, Cavo M, et al. Current multiple myeloma treatment strategies with novel agents: A European perspective. Oncologist. 2010;15:6–25. doi: 10.1634/theoncologist.2009-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Sonneveld P. Front-line treatment in younger patients with multiple myeloma. Semin Hematol. 2009;46:118–26. doi: 10.1053/j.seminhematol.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 4.Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin Cancer Res. 2004;10:3839–52. doi: 10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–8. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 7.Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: Results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621–9. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 8.Rosiñol L, Oriol A, Mateos MV, Sureda A, García-Sánchez P, Gutiérrez N, et al. Phase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: Efficacy and clinical implications of tumor response kinetics. J Clin Oncol. 2007;25:4452–8. doi: 10.1200/JCO.2007.12.3323. [DOI] [PubMed] [Google Scholar]
- 9.Nakorn TN, Watanaboonyongcharoen P, Niparuck P, Chancharunee S, Intragumtornchai T. Bortezomib plus dexamethasone as the induction therapy in newly diagnosed multiple myeloma matients: A phase II study in Thai patients. J Hematol Transf Med. 2008;18:119–27. [Google Scholar]
- 10.Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002 – Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–26. doi: 10.1200/JCO.2005.03.2086. [DOI] [PubMed] [Google Scholar]
- 11.Jagannath S, Barlogie B, Berenson JR, Singhal S, Alexanian R, Srkalovic G, et al. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impared renal function. Cancer. 2005;103:1195–200. doi: 10.1002/cncr.20888. [DOI] [PubMed] [Google Scholar]
- 12.Burnette BL, Leung N, Rajkumar SV. Renal improvement in myeloma with bortezomib plus plasma exchange. N Engl J Med. 2011;364:2365–6. doi: 10.1056/NEJMc1101834. [DOI] [PubMed] [Google Scholar]
- 13.Lonial S, Richardson PG, San Miguel J, Sonneveld P, Schuster MW, Bladé J, et al. Characterisation of haematological profiles and low risk of thromboembolic events with bortezomib in patients with relapsed multiple myeloma. Br J Haematol. 2008;143:222–9. doi: 10.1111/j.1365-2141.2008.07321.x. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. Aphase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 15.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–20. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]


