Abstract
Aim:
The aim of the study was to analyze the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status over 7 years in South Indian women with breast cancer. Further analysis of a subgroup was done to study clinically defined subtypes and the role of preanalytical factors in needle core biopsies (NCBs) and excised specimens.
Materials and Methods:
This was a retrospective study from January 2010 to December 2016. Patients diagnosed with invasive breast cancer and available immunohistochemistry (IHC) reports of ER, PR, and HER2 status were analyzed. The cases for the year 2016 were analyzed further to observe the impact of preanalytical factors on the IHC staining patterns and surrogate status.
Results:
A total of 5436 patients were included with a median age of 48 years. Among these, 65% were ≤ 55 years. The overall incidence of hormone receptor (HR)-positive patients was 48%; HER2 positive, 15%; and triple-negative breast cancer (TNBC), 37%. The incidence of HR positive, HER2 positive, and TNBC were 45%, 16%, and 39% and 53%, 13%, and 34% in patients <56 years and over 55 years, respectively (P < 0.001). There was an increase in HR positivity and decrease in TNBCs over time. There was no significant difference in the staining patterns in NCBs and excised specimens.
Conclusion:
With time, there is an increase in hormone-positive tumors which may be attributed to better IHC techniques and tissue handling. There was no statistical difference in the patterns of ER, PR, and HER2 immunostaining in core biopsy and excised specimens.
Keywords: Breast cancer, human epidermal growth factor receptor-2, hormone receptor, immunohistochemistry, triple-negative breast cancer
Introduction
Breast cancer is the second most common cancer worldwide and the most common cancer in women.[1] In Indian women, it is the most common cancer in the urban area and second only to cervical cancer in rural population.[2] Determination of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status in patients with breast cancer is now considered a standard due to their predictive and prognostic implications.[3] When compared to the Western population, Indian women show higher incidence of hormone receptor (HR)-negative breast cancer.[4] Moreover, the incidence of HR-positive tumor increases with age whereas triple-negative breast cancer and HER2-positive tumor decrease. Thus, younger women harbor relatively more aggressive and advanced cancers with poor prognosis than older women.[5,6,7] The present study is an attempt to study the incidence and trend of ER, PR, and HER2 status on immunohistochemistry (IHC) staining over 7 years. In addition, a subgroup of patients was further analyzed to validate the role of preanalytical factors in the reporting of HR and HER2 status by comparing the IHC staining results of core needle biopsies with that of excised specimens.
Materials and Methods
This was a retrospective study from the archives of the department of pathology from January 2010 to December 2016. Patients diagnosed with invasive breast carcinoma and available reports of ER, PR, and HER2 status on IHC were analyzed. These included all histologic subtypes and grades. The histopathological and IHC reporting done was in accordance with the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines. The cold ischemic time was <1 h for all biopsies and most resected specimens. All the specimens were fixed in 10% neutral phosphate-buffered formalin (NBF) for 12–18 h. Excision specimens were cut immediately and one bit for IHC was fixed separately.
For IHC, 5μ sections were cut from the appropriate block on silane-coated slides. These were deparaffinized in xylene, and graded alcohol was used for rehydration. From the year 2010–2013, the IHC procedure was manual and antigen retrieval was done using a pressure cooker and citrate buffer (pH: 6) or EDTA, after blocking with 1% bovine serum albumin. The mouse monoclonal antibodies used were used: ER – 1D5 (dil 1:60), PR – PR88 (dil 1:60), and HER2 – CB11 (dil 1:30) from BioGenex. Primary antibody was applied for the specified period as suggested by the manufacturer in a moist chamber. After incubation with secondary antibody and washing, the sections were counterstained with hematoxylin and dehydrated before mounting with DPX mountant. An external positive control was run with every batch.
From 2014 onward, automated IHC (Ventana) was started and the details of the antibodies used are given in Table 1. ER and PR scoring for all cases was done using Allred scoring.[4] ER and PR were considered positive for cases which scored 3+ or more on Allred score. HER2 scoring was done according to the ASCO/CAP guidelines.[7] Equivocal (2+) results on IHC for HER2 were excluded from the study. Ki67 immunostaining was added to ER, PR, and HER2 from 2015 onward. The cases for the year 2016 formed a subgroup that was analyzed further with respect to grade (Nottingham combined histologic grade), IHC4 as a molecular surrogate, and differences between biopsy and resection specimens in terms of IHC staining patterns.
Table 1.
Antibody details (automated)

Results
A total of 5436 women fulfilled the criteria for this study and were analyzed. The median age of study population was 48 years (range, 18–94 years). Among these, 3534 (65%) were of ≤55 years and 1902 (35%) were over 55 years. The overall incidence of HR-positive patients (either ER or PR or both) was 48%; HER2 overexpressing subtype, 15%; and triple-negative breast cancer (TNBC), 37% [Table 2].
Table 2.
Receptor expression pattern in all patients

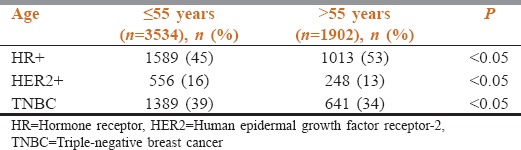
For patients aged ≤55 years, the incidence of HR+, HER2+, and TNBC was 45%, 16%, and 39%, whereas for >55 years, it was 53%, 13%, and 34% respectively. The incidence of HR-positive tumors was higher, whereas that of HER2 positive and TNBC was lower in older patients aged >55 years when compared to young patients aged <55 years, all being statistically significant [Table 3].
Table 3.
Receptor expression pattern in patients ≤55 years and >55 years
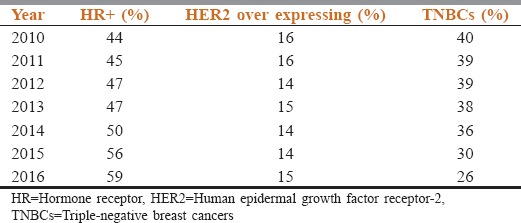
On analysis of HR status over a period of 7 years, there was an increase in the HR positivity and decreased TNBC was noted [Table 4].
Table 4.
Analysis of staining patterns over time
In the year 2016, IHC report of 923 cases of invasive breast cancer was available. In this subgroup, 443 (48%) were Grade 2, 424 (46%) were Grade 3, and rest 56 (6%) patients were Grade 1. Furthermore, analysis was done to see the impact of preanalytical factors that might have influenced IHC staining patterns. Of these cases, 385 were needle core biopsies (NCBs) and 538 were excised specimens. Both were evaluated for receptor expression. The incidence of HR positive, HER2 positive, and TNBC in NCBs and excised specimen were, respectively, 63% and 56% (P = 0.328); 13% and 16% (P = 0.258); and 24% and 28% (P = 0.393). When IHC4 was applied to the cases, 204 (23%) cases were Luminal A-like, 340 (36%) were Luminal B-like, 241 (26%) were basal-like, and 136 (15%) were HER2-like. Of the 340 cases that were Luminal B-like, 288 were HER2 positive and 52 were HER2 negative [Table 5].
Table 5.
Receptor expression and surrogate status in biopsies and excised specimens in 2016

Discussion
The incidence of breast cancer is increasing globally, with an extra surge in Asian countries, especially in premenopausal women.[8] There is an annual rise of 0.5%–2% in the incidence across all regions of India and this rise is even more in younger females less than 45 years.[9] Most Indian studies to date have shown median ages ranging from 48-53 years.[7,8,9,10,11] In our study as well, the median age was 48 years, reinforcing the fact that breast cancer in Indian women occurs at least a decade younger than those in the West.[12] Most western data show a median age around 60 years.[13,14]
In the present era of targeted therapy, IHC and molecular studies are required for diagnosis, prediction, and prognostication of cancers at any site.[15] By the use of complementary DNA microarray profiling, breast cancer has been divided into six molecular subtypes: Luminal A, Luminal B, basal-like, HER2-like, normal epithelial-like, and claudin-low.[16] The IHC surrogates for the molecular subtypes are: Luminal A (ER + or PR + or both, HER2 neu negative), Luminal B (ER + or PR + or both, HER2 neu+) or (ER+, low PR+, HER2 neu−, high Ki67), basal-like (ER−, PR−, HER2 neu±), HER2 neu+ (ER−, PR−, HER2 neu+). Any degree of HR positivity makes the patient suitable for hormone therapy which is safe and administered orally on an outpatient basis.[17]
In our study, a major proportion of the patients were hormone positive (48%) and this expression showed an increase with age, i.e., 45% (<56 years) versus 55% (over 55 years), which is statistically significant. The present study is the largest study from India to date that subcategorizes breast cancers into three groups – HR+, HER2+, and TNBC. There are several studies from the Indian subcontinent regarding either HR positivity alone or HR+[4,8,9,18] and TNBCs[19,20,21,22] with only six studies delineating HER2-positive and HR-negative status as a separate category.[7,10,11,21,22,23,24] Of the latter, only one has a relatively comparable volume of 2001 cases,[7] with 16% of HER2-positive cases which is similar to the 15% found in the present study. It is worthy of note that the percentage of HER2-positive cases in the Indian subcontinent is similar to that described in the Western literature.[16] The reason for this relative uniformity in HER2 status worldwide is unclear. Interestingly, in 2016, Luminal B HER2+ was the largest group among the surrogate molecular subtypes.
The analysis of staining patterns over 7 years that showed increasing HR positivity and decrease in TNBCs over time probably reflects the shift from manual to automated immunostaining methods and better preanalytical handling of both in-house and referral samples.
TNBCs comprise 10%–20% of all breast cancers in the Western literature and are the most aggressive subtype with poor prognosis.[25] Indian data show high rates of TNBCs as compared to the Western literature, and this was also observed in our study (39%). TNBCs showed an inverse relation with age. Out of 2030 patients with this subtype, 68% of patients were <55 years of age and 22% were >55 years of age, which was statistically significant. The higher TNBC numbers have been attributed to tumor biology and younger age of patients.[18] The finding of increased TNBC in Asian Indian women outside India also suggests that intrinsic genetic susceptibility/ethnicity may play a role.[26] This mitigates the attribution of high TNBC numbers to poor preanalytical handling of tissue.
However, to test the latter hypothesis (that preanalytical factors, primarily prolonged cold ischemic time, contribute to the relatively low numbers of hormone-positive cancers and high numbers of TNBCs), we compared the IHC staining patterns of NCBs and big specimens in 1 year. The presumption was that all NCBs would be processed ideally, with cold ischemic times of <1 h. Excision specimens were invariably cut immediately on receipt in the laboratory, but one could not always be sure how long the specimen took to arrive in the laboratory after excision. Both types of specimens were, however, fixed in adequate volumes of NBF for 6–72 h. Big specimens that were excised and processed elsewhere (with no details of cold ischemic time, fixative used, and duration of fixation) also contributed to the cases. Interestingly, there was no statistically significant difference between the two groups, implying that our findings were indeed “true.” One of the analytical factors that is known to cause decreased immunostaining is the use of sections that are cut more than 6 weeks before staining. This is avoided in our laboratory by cutting sections for immunostaining on the day prior and not earlier.
Conclusion
With time, there is an increase in hormone-positive tumors which may be attributed to better IHC techniques and tissue handling. Moreover, there seems to be no difference in IHC staining of core biopsy or large specimens, corroborating that preanalytical factors did not account for negative HR staining.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Consolidated Report of Population Based Cancer Registries 2001-2004, Incidence and Distribution of Cancer. Bangalore: Coordinating Unit, National Cancer Registry Programme (ICMR); 2006. National Cancer Registry Programme, Indian Council of Medical Research. Leading sites of cancer; pp. 8–30. [Google Scholar]
- 3.Rastelli F, Crispino S. Factors predictive of response to hormone therapy in breast cancer. Tumori. 2008;94:370–83. doi: 10.1177/030089160809400314. [DOI] [PubMed] [Google Scholar]
- 4.Shet T, Agrawal A, Nadkarni M, Palkar M, Havaldar R, Parmar V, et al. Hormone receptors over the last 8 years in a cancer referral center in India: What was and what is? Indian J Pathol Microbiol. 2009;52:171–4. doi: 10.4103/0377-4929.48909. [DOI] [PubMed] [Google Scholar]
- 5.Bonnier P, Romain S, Charpin C, Lejeune C, Tubiana N, Martin PM, et al. Age as a prognostic factor in breast cancer: Relationship to pathologic and biologic features. Int J Cancer. 1995;62:138–44. doi: 10.1002/ijc.2910620205. [DOI] [PubMed] [Google Scholar]
- 6.Gajdos C, Tartter PI, Bleiweiss IJ, Bodian C, Brower ST. Stage 0 to stage III breast cancer in young women. J Am Coll Surg. 2000;190:523–9. doi: 10.1016/s1072-7515(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh J, Gupta S, Desai S, Shet T, Radhakrishnan S, Suryavanshi P, et al. Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer. 2011;48:391–6. doi: 10.4103/0019-509X.92245. [DOI] [PubMed] [Google Scholar]
- 8.Manjunath S, Prabhu JS, Kaluve R, Correa M, Sridhar TS. Estrogen receptor negative breast cancer in India: Do we really have higher burden of this subtype? Indian J Surg Oncol. 2011;2:122–5. doi: 10.1007/s13193-011-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajan G, Culas TB, Jayalakshmy PS. Estrogen and progesterone receptor status in breast cancer: A cross-sectional study of 450 women in Kerala, South India. World J Surg Oncol. 2014;12:120. doi: 10.1186/1477-7819-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R, Gupta S, Pawar SB, Pawar RS, Gandham SV, Prabhudesai S. Receptor expression in patients in semi urban India. J Cancer Res Ther. 2014;10:26–28. doi: 10.4103/0973-1482.131348. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee G, Lakshmaiah KC, Vijayakumar M, Prabhu JS, Telikicherla D, Sridhar TS, et al. Analysis of clinico-pathological characteristics of Indian breast cancers shows conservation of speci c features in the hormone receptor sub-types. J Integr Oncol. 2016;5:159. [Google Scholar]
- 12.Bustreo S, Osella-Abate S, Cassoni P, Donadio M, Airoldi M, Pedani F, et al. Optimal ki67 cut-off for luminal breast cancer prognostic evaluation: A large case series study with a long-term follow-up. Breast Cancer Res Treat. 2016;157:363–71. doi: 10.1007/s10549-016-3817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson WF, Pfeiffer RM, Dores GM, Sherman ME. Comparison of age distribution patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1899–905. doi: 10.1158/1055-9965.EPI-06-0191. [DOI] [PubMed] [Google Scholar]
- 14.Anderson WF, Reiner AS, Matsuno RK, Pfeiffer RM. Shifting breast cancer trends in the United States. J Clin Oncol. 2007;25:3923–9. doi: 10.1200/JCO.2007.11.6079. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamurthy S, Poornima R, Challa VR, Goud YG. Triple negative breast cancer-our experience and review. Indian J Surg Oncol. 2012;3:12–6. doi: 10.1007/s13193-012-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 18.Desai SB, Moonim MT, Gill AK, Punia RS, Naresh KN, Chinoy RF, et al. Hormone receptor status of breast cancer in India: A study of 798 tumours. Breast. 2000;9:267–70. doi: 10.1054/brst.2000.0134. [DOI] [PubMed] [Google Scholar]
- 19.Nabi MG, Ahangar A, Wahid MA, Kuchay S. Clinicopathological comparison of triple negative breast cancers with non-triple negative breast cancers in a hospital in North India. Niger J Clin Pract. 2015;18:381–6. doi: 10.4103/1119-3077.153248. [DOI] [PubMed] [Google Scholar]
- 20.Sharma D, Singh G. An institutional analysis of clinicopathological features of triple negative breast cancer. Indian J Cancer. 2016;53:566–8. doi: 10.4103/ijc.IJC_534_16. [DOI] [PubMed] [Google Scholar]
- 21.Ajay A, Radhakrishnan P. Clinical pathological and epidemiological study of triple negative breast cancer. Int J Res Med Sci. 2017;5:2657–61. [Google Scholar]
- 22.Sharma M, Sharma JD, Sarma A, Ahmed S, Kataki AC, Saxena R, et al. Triple negative breast cancer in people of North East India: Critical insights gained at a regional cancer centre. Asian Pac J Cancer Prev. 2014;15:4507–11. doi: 10.7314/apjcp.2014.15.11.4507. [DOI] [PubMed] [Google Scholar]
- 23.Patnayak R, Jena A, Rukmangadha N, Chowhan AK, Sambasivaiah K, Phaneendra BV, et al. Hormone receptor status (estrogen receptor, progesterone receptor), human epidermal growth factor-2 and p53 in South Indian breast cancer patients: A tertiary care center experience. Indian J Med Paediatr Oncol. 2015;36:117–22. doi: 10.4103/0971-5851.158844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adusumilli P, Konatam ML, Gundeti S, Bala S, Maddali L. Treatment challenges of Her2-positive breast cancer. Indian J Med Paediatr Oncol. 2017;338:22–7. doi: 10.4103/0971-5851.203511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Jain J, Kumar A, Kumar S, Wadhwa N. Triple negative breast cancer – An overview and review of literature. Asian J Med Sci. 2012;3:16–20. [Google Scholar]
- 26.Kakarala M, Rozek L, Cote M, Liyanage S, Brenner DE. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S. – A SEER analysis. BMC Cancer. 2010;10:191. doi: 10.1186/1471-2407-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]


