Abstract
Purpose: The use of combined estrogen-progestin menopausal hormone therapy (MHT) has been shown to increase the risk of breast cancer, however, recent observational studies have suggested that the association between MHT and breast cancer may be modified by race. The objective of this study was to investigate the association between MHT use and incidence of invasive breast cancer in Black and White women aged ≥40 years at diagnosis after accounting for racial differences in patterns of MHT use and formulation.
Methods: Data from the Carolina Breast Cancer Study, a population-based case–control study of Black and White women in North Carolina conducted between 1993 and 2001, was used to analyze 1474 invasive breast cancer cases and 1339 controls using unconditional logistic regression.
Results: Black women were less likely than White women to use any MHT and were more likely to use an unopposed-estrogen formulation. Combined estrogen-progestin MHT use was associated with a greater odds of breast cancer in White (adjusted odds ratio [OR] 1.48, 95% confidence interval [CI]: 1.03–2.13) and Black (OR 1.43, 95% CI: 0.76–2.70) women, although the estimate in Black women was imprecise. In contrast, use of unopposed-estrogen MHT among women with prior hysterectomy was not associated with breast cancer in women of either race.
Conclusion: The association between MHT and invasive breast cancer appears to be similar in both Black and White women after accounting for differences in formulation and prior hysterectomy. These findings emphasize the importance of accounting for MHT formulation in race-stratified analyses of breast cancer risk.
Keywords: : breast cancer, African American, hormone therapy, menopause, hysterectomy
Introduction
Menopausal hormone therapy (MHT) is a treatment for menopausal symptoms and for health consequences of early onset of menopause, such as osteoporosis and depression.1 However, many observational studies have indicated an increased risk of breast cancer associated with the use of MHT, particularly combined estrogen plus progestin therapy. Results from a large randomized trial of the Women's Health Initiative (WHI) indicated a 24% increase in invasive breast cancer risk among postmenopausal women treated with combined estrogen-progestin after a mean of 5.2 years of follow-up [hazard ratio (HR) 1.24, 95% confidence interval (CI): 1.01–1.54],2 although no significant association with breast cancer was found in the unopposed-estrogen trial among women with prior hysterectomy when the intervention phase was stopped (HR 0.77, 95% CI: 0.59–1.01).3,4 The use of MHT in the United States has declined dramatically since the results of the WHI trial were published in 2003, and subsequent reductions in the incidence of breast cancer in the population have been documented.5–8
Despite overall declines in MHT use, much of what is known about the effect of hormone therapy on breast cancer risk in postmenopausal women has originated from studies conducted in predominantly White study populations. Evidence concerning the effect of MHT among Black women is limited and inconsistent. Results from an analysis of a large number of postmenopausal women in the Breast Cancer Surveillance Consortium, a longitudinal registry of 1.6 million screening mammograms, reported a positive association between any hormone therapy use and breast cancer among White women, but no association among Black women.9 Among women with natural menopause in the Nashville Breast Health Study, ever use of any hormone therapy was positively associated with estrogen receptor positive breast cancer in White women but inversely associated in Black women.10 Results from an early phase of the Carolina Breast Cancer Study also showed an inverse association between combined estrogen-progestin MHT use and breast cancer in Black women.11
In contrast, the original WHI combined estrogen-progestin trial reported no significant modification of the reported association by race, although Black women comprised only 6.8% of the study population.2 A later reanalysis of both WHI trials with a greater number of breast cancer cases showed the same result, indicating no significant modification of reported associations between either estrogen-only or estrogen plus progestin therapy and breast cancer according to race.12 An analysis of 32,559 women aged 40 years and older in the Black Women's Health Study indicated estrogen plus progestin MHT use for ≥5 years was associated with a greater incidence rate of breast cancer compared to never use (incidence rate ratio [IRR] 1.45, 95% CI: 0.94–2.23).13
To elucidate inconsistencies in previous findings of the MHT-breast cancer risk relationship in Black women, we sought to evaluate racial differences in the association between MHT and breast cancer risk after accounting for differences in patterns of MHT use, formulation, and prior hysterectomy. We investigated the association between MHT and the incidence of invasive breast cancer overall and according to tumor subtype and hysterectomy status among Black and White women in Phase 1 and 2 of the Carolina Breast Cancer Study from 1993 to 2001.
Materials and Methods
Study population
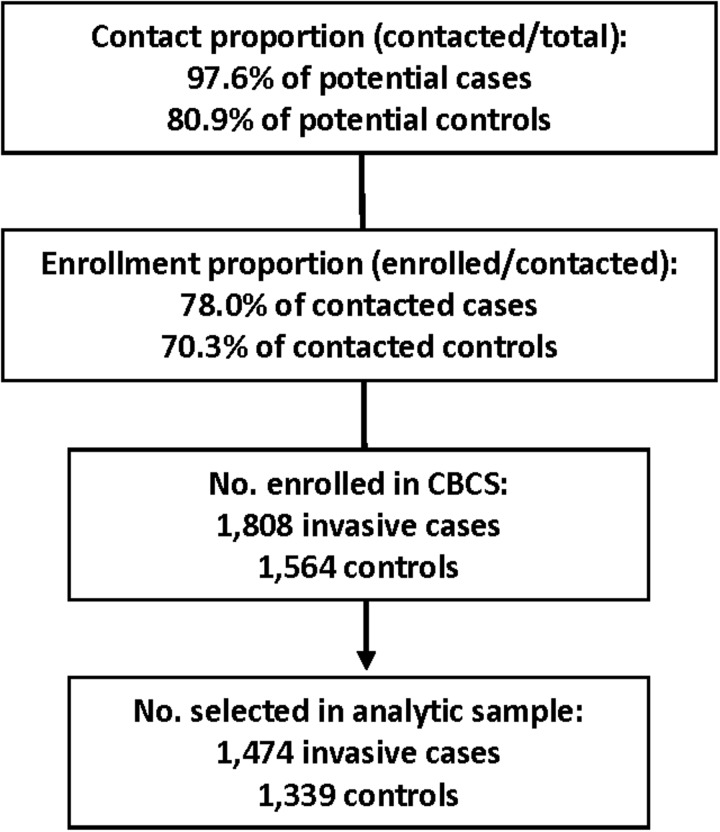
The Carolina Breast Cancer Study (CBCS) is a population-based, case–control study of incident breast cancer among women in 24 counties in central and eastern North Carolina. Cases were identified from the North Carolina Central Cancer registry. Eligible cases were aged 20–74 years and were diagnosed with a first primary breast cancer between 1993 and 2000. Figure 1 presents the contact and enrollment proportions according to case/control status. Younger (<50 years) and Black cases were oversampled to provide sufficient sample sizes for racially stratified analyses. Controls were selected during the same time period and from the same geographic area as cases using North Carolina Driver's License and Medicare beneficiary lists. Controls were frequency-matched to cases using randomized recruitment according to race and 5-year age group. Details on overall study response rates have been published previously.14,15 Participants were interviewed in-person by trained nurses using a standardized questionnaire to obtain information on demographics and potential risk factors for breast cancer. The interview was conducted within 1 year of diagnosis date for 95% of cases. Detailed information on hormone use, family history of cancer, reproductive and menstrual history, socioeconomic status, occupational exposures, and behavioral risk factors for breast cancer was collected. Race was self-reported. Anthropometric measurements were taken by trained nurses at the time of interview to obtain body mass index (BMI).
FIG. 1.
Contact and enrollment in the Carolina Breast Cancer Study (Phase 1 and 2) for invasive breast cancer cases and controls.
The present analysis includes invasive cases and controls who were aged ≥40 at the time of selection into the study. This age cut point was chosen to include women who used MHT before menopause onset. Among ever MHT users in our sample, 31% began therapy while premenopausal, indicating a significant number of users were prescribed MHT prophylactically for suspected health benefits or for perimenopausal symptoms occurring gradually before the cessation of ovarian function. Including premenopausal women in our study allowed for the investigation of associations between timing of MHT initiation or cessation and breast cancer.
The study sample was also restricted to women who self-identified as Black/African American or White. Women who had undergone natural menopause (ceased menstruation in the absence of hysterectomy) or bilateral oophorectomy by the time of selection/diagnosis were considered postmenopausal. Age at menopause was equal to the age at surgery for women who underwent bilateral oophorectomy and age at cessation of menstruation for women with no history of gynecologic surgery. Women who underwent hysterectomy alone or in conjunction with unilateral oophorectomy before natural menopause were considered postmenopausal at age 50 years and assigned an age at menopause equal to 50. This imputation method was shown to yield similar results to a more precise method in an analysis of risk factors for breast cancer using data from the Nurse's Health Study.16 Women with chemotherapy or radiation induced menopause were excluded (n = 24). The final analytical sample consisted of 1474 cases and 1339 controls.
Exposure assessment
MHT use was ascertained during the interview with a photo card of commonly prescribed therapies to help participants accurately recall history of use and formulation. Information on type of hormone, dose, duration of use, and age at first/last use was collected. Ever MHT use was defined as treatment with any formulation of hormone therapy for ≥3 months at any point before the time of selection or diagnosis. For analyses examining timing and duration of use, ever users were grouped into multiple categories: recency of initiation (<5 to ≥5 years), recency of last use (Current user, <5 to ≥5 years), and total duration of use (<5 to 5–10, >10 years). Type of therapy was also grouped into three categories of formulation use: unopposed estrogen only, estrogen always with progestin, and estrogen sometimes with progestin. Other combinations of estrogen and progestin use were rare and excluded from analyses of formulation due to insufficient sample sizes. Recency of initiation was analyzed as a joint exposure with MHT formulation when sample size was sufficient.
Outcome assessment
The primary outcome of interest was incident invasive breast cancer. The secondary outcome was incident subtype-specific invasive breast cancer. For subtype-specific analyses, cases were categorized as having either: (1) estrogen receptor (ER) or progesterone receptor (PR) positive (ER+/PR−, ER−/PR+, ER+/PR+) or (2) estrogen and progesterone receptor negative (ER−/PR−) tumors. Controls were the comparison group. Tumor subtypes were abstracted from medical records for 80% of cases and determined using immunohistochemistry assays for others. Cases with missing information for ER or PR status were excluded from subtype-specific analyses (n = 65).
Stratification
Analysis of the relationship between MHT and breast cancer was stratified by race to compare measures of association in Black and White women separately. The subtype-specific analysis was not stratified by race due to insufficient sample size. Analyses were also stratified by hysterectomy status to minimize confounding by indication for this surgery, since hysterectomy is an indication for unopposed estrogen MHT and is independently associated with decreased breast cancer risk.17–19 Women with hysterectomy may not be comparable to women without surgery when attempting to make inferences about hormonal risk factors for breast cancer given differences in timing of menopause, endogenous estrogen exposure, duration and formulation of MHT, and indications for surgery that may confer differences in breast cancer risk independent of MHT. Women were classified as having undergone hysterectomy if they reported having their uterus surgically removed before study selection. Analyses not stratified by hysterectomy status were adjusted for gynecologic surgery status (no surgery, hysterectomy alone, any oophorectomy with or without hysterectomy) using model adjustment for comparison with stratified analyses.
Statistical analysis
Percentages for descriptive characteristics among controls were weighted by the inverse of their sampling probability to obtain prevalence estimates in the source population in central and eastern North Carolina. Unconditional logistic regression was used to estimate odds ratios (OR) and 95% CI as measures of association between MHT use and case status. All tests of association (other than likelihood ratio tests for the evaluation of modification) were evaluated at a significance level of α = 0.05. Never MHT users were the referent group for all analyses. An offset term was incorporated in all models to account for the sampling probabilities used to select cases and controls in the CBCS design.
All models were adjusted for confounders of age at selection/diagnosis, age at menopause, and educational attainment based on a minimally sufficient adjustment set identified a priori using a directed acyclic graph.20 Estimates in the hysterectomy group were adjusted for history of bilateral oophorectomy, which was identified a priori as a potential confounder. Subtype-specific analyses were also adjusted for race. Additional covariates including BMI, age at menarche, smoking history, first degree family history of breast cancer, parity, and age at first full-term pregnancy were adjusted for in separate models but adjustment for these variables resulted in similar estimates (results not shown). Likelihood ratio tests were used to evaluate the presence of modification by race and obesity status (BMI ≥30) separately. Tests were conducted in nested models with the addition of an interaction term between ever use of any MHT and the variable of interest. All likelihood ratio tests were evaluated at a significance level of α = 0.1.
All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). The study protocol was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
Results
Descriptive characteristics
Table 1 presents distributions of characteristics among cases and controls stratified by ever versus never MHT use, with weighted proportions for controls as estimates of prevalence in the source population. Among controls, the proportion of women who ever used MHT was 35%. These women were more likely to be White, possess some college-level education, have leaner BMI <25, and be postmenopausal. Controls who ever used MHT were also more likely to have undergone any gynecologic surgery (68%) than never users (24%). The most common surgery among never users was hysterectomy without oophorectomy (14%). Among postmenopausal controls, ever users were more likely to have an earlier age at menopause.
Table 1.
Characteristics of Cases and Controls by Menopausal Hormone Therapy use in Phase 1 and 2 of the Carolina Breast Cancer Study, 1993–2001 (n = 2813)
| Ever users (n = 943) | Never users (n = 1870) | |||||
|---|---|---|---|---|---|---|
| Controls | Controls | |||||
| Cases No. | No. | Weighted %a | Cases No. | No. | Weighted %a | |
| Median age (IQR) | 59 (11) | 55 (13) | 53 (20) | 48 (14) | ||
| Race | ||||||
| White | 322 | 304 | (86) | 504 | 422 | (74) |
| Black | 151 | 166 | (14) | 497 | 447 | (26) |
| Education | ||||||
| ≤High school | 194 | 218 | (39) | 514 | 421 | (41) |
| Some college | 147 | 161 | (39) | 233 | 210 | (26) |
| ≥College | 132 | 91 | (21) | 254 | 237 | (34) |
| Missing | 1 | |||||
| Measured BMI | ||||||
| ≤24 | 171 | 150 | (40) | 297 | 240 | (36) |
| 25–29 | 158 | 161 | (33) | 282 | 248 | (29) |
| ≥30 | 138 | 150 | (28) | 395 | 362 | (35) |
| Missing | 6 | 9 | 27 | 19 | ||
| Median BMI (IQR) | 27 (24–32) | 28 (24–34) | ||||
| Parity | ||||||
| 0 | 72 | 54 | (11) | 136 | 78 | (10) |
| 1 | 82 | 78 | (16) | 146 | 152 | (19) |
| 2 | 147 | 147 | (34) | 304 | 271 | (36) |
| ≥3 | 172 | 191 | (38) | 415 | 368 | (36) |
| Age at first birth | ||||||
| Nulliparous | 72 | 54 | (11) | 142 | 81 | (10) |
| <20 | 126 | 154 | (28) | 317 | 283 | (25) |
| 20–24 | 163 | 169 | (38) | 292 | 278 | (34) |
| >24 | 112 | 93 | (23) | 250 | 227 | (31) |
| Age at menopause | ||||||
| Premenopausal | 35 | 31 | (7) | 441 | 386 | (53) |
| ≤39 | 51 | 76 | (16) | 39 | 40 | (4) |
| 40–44 | 54 | 56 | (13) | 54 | 41 | (4) |
| 45–49 | 104 | 80 | (18) | 121 | 94 | (10) |
| 50–54 | 199 | 187 | (43) | 292 | 268 | (28) |
| ≥55 | 23 | 22 | (4) | 41 | 25 | (2) |
| Missing | 7 | 18 | 13 | 15 | ||
| Type of gynecologic surgery | ||||||
| None | 205 | 139 | (32) | 767 | 633 | (76) |
| Hysterectomy + Bilat. oophorectomy | 118 | 145 | (27) | 30 | 40 | (3) |
| Hysterectomy + Unilat. oophorectomy | 31 | 42 | (8) | 45 | 38 | (4) |
| Hysterectomy only | 86 | 103 | (23) | 125 | 134 | (14) |
| Bilateral oophorectomy only | 2 | 3 | (1) | 3 | 0 | (0) |
| Otherb | 31 | 37 | (10) | 31 | 24 | (4) |
| Missing | 1 | |||||
| Age at menarche | ||||||
| <13 | 247 | 215 | (46) | 498 | 395 | (44) |
| ≥13 | 225 | 252 | (54) | 502 | 470 | (56) |
| Missing | 1 | 3 | 1 | 4 | ||
| Oral contraceptive use | ||||||
| Never | 175 | 186 | (35) | 406 | 339 | (30) |
| Ever | 293 | 278 | (65) | 594 | 525 | (70) |
| Missing | 5 | 6 | 1 | 5 | ||
| Hormone therapy formulation | ||||||
| Unopposed estrogen only | 253 | 299 | (58) | |||
| Estrogen always with progestin | 143 | 95 | (24) | |||
| Estrogen sometimes with progestin | 45 | 50 | (12) | |||
| Estrogen and progestin never together | 11 | 6 | (2) | |||
| Progestin only | 21 | 20 | (4) | |||
| Duration of hormone therapy use (years) | ||||||
| <5 | 243 | 241 | (49) | |||
| 5–10 | 104 | 91 | (22) | |||
| >10 | 119 | 135 | (29) | |||
| Missing | 7 | 3 | ||||
Includes White and Black women aged ≥40 years. Excludes women with chemotherapy/radiation induced menopause.
Percentages are weighted by the inverse of the sampling probability and reflect estimated prevalence in the source population in North Carolina.
Women who had gynecologic surgery but specific procedure is unknown.
BMI, body mass index.
Among both cases and controls, the median duration of total hormone therapy use was 60 months for White and 36 months for Black users, but the median age at first use was similar for both Black and White users (45 and 46 years, respectively). Unopposed estrogen was the most common MHT formulation used, although the proportion of women using estrogen plus progestin was higher among cases than controls. Among ever users, the prevalence of estrogen-only MHT use was greater among Black than White women (75% vs. 50%). Likelihood ratio tests did not indicate the presence of modification by race in the overall sample or by obesity status (BMI ≥30) in Black or White women (p > 0.1). There was also no indication of modification by these variables within strata of hysterectomy status (p > 0.1).
Hormone therapy and breast cancer
Table 2 presents associations between MHT and breast cancer. Estrogen always with progestin MHT use was associated with a greater odds of breast cancer in White women (OR 1.48 95% CI: 1.03–2.13) and appeared similarly associated in Black women (OR 1.43, 95% CI: 0.76–2.70), although the estimate for Black women was imprecise due to a smaller population of Black ever users. Recency of initiation of MHT was not associated with breast cancer in either Black or White women. Estimates for current and longer term (>5 years) MHT use were suggestive of a positive association in White users only. Black women using MHT for 10 or more years exhibited an inverse association with breast cancer (OR 0.67, 95% CI: 0.38–1.17), although the estimate was imprecise.
Table 2.
Odds Ratios and 95% Confidence Interval for the Association Between Hormone Therapy and Invasive Breast Cancer Among Black and White Women in the Carolina Breast Cancer Study, 1993–2001 (n = 2813)
| Black (n = 1261) | White (n = 1552) | |||
|---|---|---|---|---|
| Hormone therapy use | Cases/controls | ORa(95% CI) | Cases/ controls | ORa(95% CI) |
| Never (ref) | 488/438 | 1.00 | 500/415 | 1.00 |
| Ever | 147/164 | 0.82 (0.61–1.10) | 319/288 | 1.19 (0.93–1.52) |
| Formulationb | ||||
| Unopposed estrogen only | 106/130 | 0.75 (0.53–1.05) | 145/169 | 1.02 (0.73–1.44) |
| Progestin always with estrogen | 31/16 | 1.43 (0.76–2.70) | 108/64 | 1.48 (1.03–2.13) |
| Progestin sometimes with estrogen | 5/10 | 0.45 (0.15–1.36) | 39/37 | 1.03 (0.63–1.68) |
| Recency of initiation | ||||
| <5 years | 54/55 | 0.82 (0.54–1.25) | 95/79 | 1.12 (0.79–1.58) |
| ≥5 years | 92/104 | 0.88 (0.62–1.26) | 218/209 | 1.20 (0.90–1.58) |
| Recency of initiation and Formulation | ||||
| <5 years and Unopposed estrogen only | 35/41 | 0.73 (0.44–1.21) | 38/34 | 1.14 (0.66–1.95) |
| <5 years and Progestin always with estrogen | 17/8 | 1.36 (0.56–3.29) | 39/32 | 1.04 (0.62–1.73) |
| Recency of last use | ||||
| Current | 88/94 | 0.86 (0.60–1.23) | 229/203 | 1.24 (0.94–1.62) |
| <5 years | 26/25 | 0.94 (0.52–1.69) | 45/39 | 1.13 (0.71–1.80) |
| ≥5 years | 32/39 | 0.83 (0.50–1.39) | 43/45 | 1.02 (0.63–1.63) |
| Duration | ||||
| <5 years | 90/96 | 0.83 (0.59–1.17) | 151/133 | 1.10 (0.82–1.47) |
| 5 to <10 years | 31/30 | 0.98 (0.57–1.69) | 69/59 | 1.26 (0.84–1.88) |
| ≥10 years | 25/37 | 0.67 (0.38–1.17) | 93/95 | 1.25 (0.86–1.82) |
Odds ratios were adjusted for age at selection/diagnosis, age at menopause, gynecologic surgery, and education. An offset term was incorporated into the model to account for the CBCS sampling design.
Results for women taking progestin only and estrogen in addition to progestin but never simultaneously are not shown due to sparse data, but these women are included in the ever/never, recency of first/last use, and duration models.
CI, confidence interval; OR, odds ratio.
Table 3 presents results for the evaluation of MHT and breast cancer stratified by hysterectomy status. Among women with an intact uterus, current use was positively associated with breast cancer among White women (OR 1.49, 95% CI: 1.03–2.14). There was also a suggestion of a positive association for ever use of progestin always with estrogen (OR 1.40, 95% CI: 0.95–2.05) and initiation of any MHT ≥5 years before selection/diagnosis (OR 1.40, 95% CI: 0.96–2.02). For Black women, estimates were generally null and imprecise as MHT use was less common. Unopposed estrogen-only use appeared inversely associated with breast cancer in Black women (OR 0.48, 95% CI: 0.23–0.97), although this estimate describes a small sample of women with potentially unique clinical characteristics given that this formulation is contraindicated in women with an intact uterus.
Table 3.
Odds Ratios and 95% Confidence Interval for the Association Between Hormone Therapy and Invasive Breast Cancer Among Black and White Women According to Hysterectomy Status in the Carolina Breast Cancer Study, 1993–2001 (n = 2813)
| Intact uterus (n = 1844) | Hysterectomy (n = 968) | |||||||
|---|---|---|---|---|---|---|---|---|
| Black | White | Black | White | |||||
| Hormone therapy use | Cases/controls | ORa(95% CI) | Cases/controls | ORa(95% CI) | Cases/controls | ORa(95% CI) | Cases/controls | ORa(95% CI) |
| Never (ref) | 353/297 | 1.00 | 427/337 | 1.00 | 135/141 | 1.00 | 73/78 | 1.00 |
| Ever | 50/42 | 0.77 (0.48–1.24) | 177/117 | 1.23 (0.91–1.68) | 97/122 | 0.88 (0.59–1.32) | 142/171 | 0.98 (0.64–1.51) |
| Formulationb | ||||||||
| Unopposed estrogen only | 17/21 | 0.48 (0.23–0.97) | 25/22 | 1.01 (0.54–1.89) | 89/109 | 0.87 (0.58–1.31) | 120/147 | 0.97 (0.62–1.50) |
| Progestin always with estrogen | 26/14 | 1.18 (0.58–2.37) | 104/62 | 1.40 (0.95–2.05) | 5/2 | 2.32 (0.43–12.45) | 4/2 | 2.85 (0.48–17.07) |
| Progestin sometimes with estrogen | 2/3 | 0.50 (0.08–3.21) | 31/20 | 1.24 (0.67–2.28) | 3/7 | 0.50 (0.12–2.12) | 8/17 | 0.56 (0.21–1.48) |
| Recency of initiation | ||||||||
| <5 years | 25/19 | 0.78 (0.41–1.49) | 62/48 | 0.97 (0.63–1.49) | 29/36 | 0.88 (0.49–1.58) | 33/31 | 1.13 (0.62–2.08) |
| ≥5 years | 25/20 | 0.90 (0.47–1.70) | 111/69 | 1.40 (0.96–2.02) | 67/84 | 0.92 (0.59–1.44) | 107/140 | 0.91 (0.58–1.45) |
| Recency of last use | ||||||||
| Current | 33/20 | 1.04 (0.57–1.92) | 124/68 | 1.49 (1.03–2.14) | 55/74 | 0.84 (0.53–1.34) | 105/135 | 0.93 (0.60–1.45) |
| <5 years | 7/11 | 0.43 (0.16–1.18) | 27/24 | 0.89 (0.49–1.60) | 19/14 | 1.58 (0.72–3.47) | 18/15 | 1.42 (0.65–3.11) |
| ≥5 years | 10/7 | 1.02 (0.37–2.80) | 25/25 | 0.87 (0.48–1.59) | 22/32 | 0.82 (0.44–1.52) | 18/20 | 1.12 (0.51–2.47) |
| Duration | ||||||||
| <5 years | 37/29 | 0.82 (0.48–1.41) | 96/80 | 0.95 (0.67–1.36) | 53/67 | 0.89 (0.56–1.42) | 55/53 | 1.16 (0.69–1.97) |
| 5 to <10 years | 9/9 | 0.65 (0.24–1.72) | 46/18 | 2.18 (1.21–3.95) | 22/21 | 1.22 (0.61–2.42) | 23/41 | 0.65 (0.35–1.22) |
| ≥10 years | 4/4 | 0.68 (0.16–2.87) | 31/19 | 1.52 (0.81–2.84) | 21/33 | 0.71 (0.37–1.33) | 62/76 | 1.05 (0.62–1.76) |
Odds ratios were adjusted for age at selection/diagnosis, age at menopause, education, and bilateral oophorectomy status. An offset term was incorporated into the model to account for the CBCS sampling design.
Results for women taking progestin only and estrogen in addition to progestin but never simultaneously are not shown due to sparse data, but these observations are included in the ever/never, recency, and duration models.
Among women with prior hysterectomy, use of unopposed estrogen-only accounted for 87% of total MHT use, which was expected given this formulation increases the risk of endometrial cancer and is contraindicated in women with an intact uterus.6,21 Unopposed estrogen-only use was not associated with the odds of breast cancer in White (OR 0.97, 95% CI: 0.62–1.50) or Black (OR 0.87, 95% CI: 0.58–1.31) women. Estimates for the combined estrogen and progestin formulation were too imprecise to draw inferences for either race group. Alternative categorizations of gynecologic surgery, such as the inclusion of bilateral or unilateral oophorectomy, were analyzed but resulted in similar estimates (results not shown).
Hormone therapy and breast cancer subtypes
Table 4 presents associations between MHT and hormone receptor status of breast tumors. Among women with an intact uterus, estrogen always with progestin MHT use appeared to be associated with a greater odds of ER+ or PR+ breast cancer (OR 1.36, 95% CI: 0.95–1.94), as did current MHT use (OR 1.39, 95% CI: 1.00–1.93) and use for 5 to <10 years (OR 1.83, 95% CI: 1.09–3.06). There were no associations between MHT use and ER− and PR− breast cancer. Among women with prior hysterectomy, there was no evidence of an association between ever use of unopposed estrogen-only MHT and either ER+ or PR+, or ER− and PR−, tumors. Recent use (<5 years) appeared to be associated with the odds of ER+ or PR+ breast cancer (OR 1.42, 95% CI: 0.74–2.69), although this result was imprecise and inconsistent with null findings for ever use, recency of initiation, and duration of use.
Table 4.
Odds Ratios and 95% Confidence Interval for the Association Between Hormone Therapy and Breast Cancer Subtype in the Carolina Breast Cancer Study, 1993–2001 (n = 2,813)
| Intact uterus (n = 1844) | Hysterectomy (n = 968) | |||||||
|---|---|---|---|---|---|---|---|---|
| ER+ or PR+ | ER− and PR− | ER+ or PR+ | ER− and PR− | |||||
| Hormone therapy use | Cases/Controls | ORa(95% CI) | Cases/Controls | ORa(95% CI) | Cases/Controls | ORa(95% CI) | Cases/Controls | ORa(95% CI) |
| Never (ref) | 489/634 | 1.00 | 239/634 | 1.00 | 120/219 | 1.00 | 69/219 | 1.00 |
| Ever | 161/159 | 1.08 (0.82–1.43) | 53/159 | 0.95 (0.65–1.40) | 151/293 | 0.95 (0.68–1.33) | 71/293 | 1.04 (0.68–1.60) |
| Formulationb | ||||||||
| Unopposed estrogen only | 23/43 | 0.57 (0.33–0.99) | 17/43 | 1.19 (0.63–2.27) | 132/256 | 0.93 (0.66–1.31) | 61/256 | 1.04 (0.67–1.61) |
| Progestin always with estrogen | 96/76 | 1.36 (0.95–1.94) | 24/76 | 0.90 (0.53–1.52) | 6/4 | 2.76 (0.73–9.49) | 3/4 | 2.83 (0.59–13.57) |
| Progestin sometimes with estrogen | 26/23 | 1.20 (0.66–2.18) | 6/23 | 0.79 (0.31–2.03) | 8/24 | 0.68 (0.28–1.67) | 2/24 | 0.41 (0.09–1.92) |
| Recency of initiation | ||||||||
| <5 years | 54/67 | 0.86 (0.58–1.28) | 27/67 | 0.96 (0.58–1.59) | 32/67 | 0.91 (0.55–1.50) | 24/67 | 1.27 (0.70–2.28) |
| ≥5 years | 105/89 | 1.28 (0.91–1.79) | 25/89 | 0.95 (0.57–1.59) | 117/224 | 0.97 (0.67–1.40) | 46/224 | 0.96 (0.60–1.55) |
| Recency of last use | ||||||||
| Current | 115/88 | 1.39 (1.00–1.93) | 35/88 | 1.16 (0.73–1.84) | 103/209 | 0.90 (0.62–1.30) | 46/209 | 0.96 (0.60–1.54) |
| <5 years | 24/35 | 0.78 (0.45–1.36) | 8/35 | 0.57 (0.25–1.28) | 21/29 | 1.42 (0.75–2.69) | 14/29 | 2.08 (0.99–4.38) |
| ≥5 years | 21/32 | 0.72 (0.40–1.30) | 10/32 | 1.06 (0.49–2.28) | 26/52 | 1.01 (0.58–1.77) | 10/52 | 0.83 (0.38–1.80) |
| Duration | ||||||||
| <5 years | 85/109 | 0.86 (0.62–1.19) | 40/109 | 0.97 (0.63–1.48) | 63/120 | 0.98 (0.65–1.46) | 34/120 | 1.10 (0.66–1.82) |
| 5 to <10 years | 46/27 | 1.83 (1.09–3.06) | 7/27 | 0.76 (0.31–1.82) | 32/62 | 0.94 (0.56–1.58) | 12/62 | 0.82 (0.40–1.68) |
| ≥10 years | 28/23 | 1.27 (0.71–2.30) | 5/23 | 0.97 (0.34–2.71) | 54/109 | 0.87 (0.55–1.37) | 24/109 | 1.13 (0.63–2.03) |
Cases with missing subtype information were excluded (N = 65).
Odds ratios were adjusted for age at selection/diagnosis, age at menopause, education, and race. An offset term was incorporated into the model to account for the CBCS sampling design.
Results for women taking progestin only and estrogen in addition to progestin but never simultaneously are not shown due to sparse data, but these observations are included in the ever/never, recency, and duration models.
ER, estrogen receptor; PR, progesterone receptor.
Discussion
Results from previous studies have suggested a possible racial difference in the association between combined estrogen and progestin MHT and breast cancer, with estimates in Black women tending to be either null or inversely associated9–11,22,23 and results from the predominantly White WHI trial indicating elevated risk. We found that estrogen always with progestin MHT use was associated with an increased odds of breast cancer in White women and suggestive of a similar association in Black women. The estimate for Black women was imprecise due to a substantially smaller number of Black MHT users in the CBCS compared to White users. Women with prior hysterectomy used unopposed estrogen almost exclusively, and use of this formulation was not associated with breast cancer in either Black or White women. Ours is one of few studies in Black women to examine different formulations of therapy, particularly among those with and without prior hysterectomy.
Previously reported racial differences in the MHT-breast cancer relationship may reflect differences in indications for therapy and in formulations used between Black and White women. Hysterectomy is generally associated with a reduced risk of breast cancer,17–19 and prior hysterectomy is an indication for unopposed estrogen use given that progestin is typically needed only to oppose estrogen's effect on endometrial cancer.3 In our study, women with hysterectomy primarily used unopposed estrogen, which can be considered a wholly different exposure than combined estrogen plus progestin therapy in terms of its association with breast cancer risk. Results from the WHI estrogen-only trial of previously hysterectomized women showed that the risk of breast cancer was not increased with use of estrogen alone.3 There were also proportionally more Black women in the estrogen alone trial (15.1%) than the estrogen plus progestin trial (6.8%), as prior hysterectomy was a criterion for inclusion. Black women in the United States experience a considerably higher incidence of hysterectomy than White women, and rates vary by geographic region with the highest rates occurring in the United States South.24–27 Consistent with this trend, our study showed that the prevalence of hysterectomy was greater among Black (40%) compared to White (30%) women. Black women were also much more likely to receive estrogen-only therapy, as 75% of Black ever users received this formulation compared to just 50% of White ever users. Our results showed no association between unopposed estrogen use and breast cancer among women with prior hysterectomy. Results from previous studies reporting a weak or inverse association between hormone therapy and breast cancer in Black women could be explained by neglecting to adequately account for the disproportionate use of the estrogen-only formulation in this population.
Our results showed that ever use of any MHT was not associated with either hormone receptor positive or ER−/PR− breast cancer. Results from an analysis of White women in the Nashville Breast Health Study indicate that among those who underwent hysterectomy without bilateral oophorectomy, ever use of any hormone therapy was positively associated with both ER+/PR+ and ER−/PR− breast cancer, but inverse associations for both subtypes were observed for women with hysterectomy and bilateral oophorectomy, suggesting a potential role for oophorectomy in modifying the risk of these subtypes compared to hysterectomy alone.28 In our study, we did not investigate whether oophorectomy modified the risk of specific subtypes. Examining results by formulation, our results indicate a positive association between use of estrogen always with progestin and hormone receptor positive tumors. This result has been shown by others in populations of women with and without gynecologic surgery.10,28–32 Among women with hysterectomy, use of estrogen-only was not associated with hormone receptor positive or ER−/PR− tumors, suggesting that, in women with surgical menopause, incidence of these subtypes are not influenced by use of estrogen-only MHT.
Results from several large observational studies have shown current or recent (1–4 years) estrogen plus progestin MHT use to be associated with an increased breast cancer risk among postmenopausal women.33–35 Analyses from the Million Women's Study indicated estrogen plus progestin MHT use initiated <5 versus ≥5 years after menopause was associated with a greater risk of breast cancer (risk ratio [RR] 2.04, 95% CI: 1.95–2.14 vs. RR 1.53, 95% CI: 1.38–1.70).34 Results from the WHI observational cohort study also indicate the association between estrogen plus progestin MHT and breast cancer to be highest for women initiating therapy at the time of menopause onset (HR 1.68, 95% CI: 1.52–1.86), with the association decreasing linearly with increasing time between menopause and MHT initiation.35 Similarly, results from the WHI estrogen plus progestin randomized trial among postmenopausal women indicated an elevated breast cancer risk in women who initiated therapy <5 versus ≥5 years after menopause (HR 1.41, 95% CI: 1.14–1.74 vs. HR 1.15, 95% CI: 0.96–1.37).36 In our study, current use and initiation of MHT ≥5 years before study enrollment was associated with a greater odds of breast cancer only in White women without prior hysterectomy. Results from the two Sisters study of MHT in young women showed that adjustment for recency of last use, age at first use, and menopausal status at first use did not modify observed associations, suggesting that timing of MHT initiation is most critical for breast cancer risk after menopause.37
With respect to unopposed estrogen MHT use among women with hysterectomy, previous observational and experimental studies have yielded inconsistent results for its association with breast cancer risk.3,33,38–40 Current hypotheses on the risk of unopposed estrogen use pertain to the timing of therapy initiation after menopause onset, whereby women initiating therapy within 5 years of menopause tend to exhibit a greater breast cancer risk compared to those who initiate after this period.34,41,42 Conversely, an antitumor effect of unopposed estrogen therapy in estrogen-deprived postmenopausal women has been proposed as a biological mechanism for explaining reductions in breast cancer risk.43 In our study, use of unopposed estrogen therapy was not associated with an increased breast cancer risk among women who underwent hysterectomy. Although the timing of MHT initiation relative to menopause onset among women with hysterectomy was not examined in this study, the median age at first use for Black and White women in this group was the same (44 years), suggesting that timing did not play a substantial role in comparing results by race.
The Carolina Breast Cancer Study is a population-based sample representative of Black and White women in the United States South where the majority of Black Americans reside. Detailed information on menopausal history and gynecologic surgery reduced the potential for misclassification of menopausal status and type of menopause. We used a similar method for imputing age at menopause in our sample as that shown in the Nurse's Health Study. Our imputation method is less biased than assigning age at menopause equal to age at hysterectomy.16,44 Race-specific changes in the patterns and prevalence of hormone therapy after the publication of the WHI results could bias subsequent observational studies of racial differences in the association between MHT and breast cancer risk. In particular, White, educated women may have benefitted more from the change in MHT treatment guidelines relative to nonWhite women.7 This study avoids such bias given the pre-2003 data collection period. However, our results may not reflect risk for women using MHT according to current clinical guidelines that emphasize low-dose, short-term treatment, rather than long-term treatment for prevention of chronic diseases that was more common during the CBCS study period.
Recall bias may have led to misclassification of MHT use, particularly if use was intermittent or if formulation varied frequently. Older participants may also have had difficulty recalling usage history correctly. However, the use of a photo card with commonly prescribed hormone therapy products during the interview likely enhanced recall of MHT formulation and duration. MHT users may have better access to or be more likely to utilize healthcare resources compared to nonusers, which may have introduced detection bias.45 This potential bias may have led to elevated estimates for associations between MHT and breast cancer. It also may affect interpretations of racial differences in associations if Black women experience greater barriers to medical care. While we adjusted all models for educational attainment to account for potential bias related to access to care, the availability of additional information pertaining to socioeconomic status and uptake of medical services may have improved the validity of our estimates. Our study was limited by a small sample size of Black compared to White ever MHT users, which affected the precision of estimates of association in Black women. Despite this, our representative sample revealed important differences in the uptake of MHT, formulation used, and hysterectomy prevalence in Black versus White women that should inform future work on this topic.
The association between estrogen plus progestin therapy and invasive breast cancer is biologically plausible given the established links between endogenous sex hormone exposure and breast cancer risk at critical periods over a woman's lifetime.46 The increased risk associated with estrogen plus progestin therapy has been attributed to progestin's role in stimulating a higher mitotic rate and cell proliferation in breast tissue compared to estrogen alone.47 Although progestin is added to estrogen-only formulations to oppose estrogen's effect on endometrial cancer risk, explorations into use of lower doses and nonsynthetic sources of progesterone could improve the safety profile of conjugated hormone therapy while conferring the same benefits for managing menopausal symptoms.6
In summary, our results emphasize that use of estrogen always with progestin appears to be similarly associated with greater risk of breast cancer in White and Black women. Unopposed estrogen therapy was not associated with breast cancer risk in either race group. Our findings could help explain conflicting results from previous studies of Black women that did not account for racial differences in MHT formulation and surgical indications for use.
Acknowledgments
This research was funded in part by the University Cancer Research Fund of North Carolina and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA58223). Dr. Robinson was supported by the National Cancer Institute (K01-CA172717). We are grateful to the Carolina Population Center (R24 HD050924) for general support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Goodman NF, Cobin RH, Ginzburg SB, Katz IA, Woode DE. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause. Endocr Pract 2011;17 Suppl 6:1–25 [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast, cancer and mammography in healthy postmenopausal women: The women's health initiative randomized trial. JAMA 2003;289:3243–3253 [DOI] [PubMed] [Google Scholar]
- 3.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated, equine estrogen in postmenopausal women with hysterectomy: The women's health initiative randomized controlled trial. JAMA 2004;291:1701–1712 [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: Analyses of data from 2 women's health initiative randomized clinical trials. JAMA Oncol 2015;1:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674 [DOI] [PubMed] [Google Scholar]
- 6.Ghazal S, Pal L. Perspective on hormone therapy 10 years after the WHI. Maturitas 2013;76:208–212 [DOI] [PubMed] [Google Scholar]
- 7.Krieger N, Chen JT, Waterman PD. Decline in US breast cancer rates after the women's health initiative: Socioeconomic and racial/ethnic differentials. Am J Public Health 2010;100:S132–S139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks E, Canfell K. Recent declines in breast cancer incidence: Mounting evidence that reduced use of menopausal hormones is largely responsible. Breast Cancer Res 2010;12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou NQ, Hong SS, Wang WL, Olopade OI, Dignam JJ, Huo DZ. Hormone replacement therapy and breas cancer: Heterogeneous risks by race, weight, and breastdensity. J Natl Cancer Inst 2013;105:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui Y, Deming-Halverson SL, Shrubsole MJ, et al. Associations of hormone-related factors with breast cancer risk according to hormone receptor status among white and african american women. Clin Breast Cancer 2014;14:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moorman PG, Kuwabara H, Millikan RC, Newman B. Menopausal hormones and breast cancer in a biracial population. Am J Public Health 2000;90:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Anderson GL, Aragaki AK, Prentice R. Breast cancer and menopausal hormone therapy by Race/Ethnicity and Body Mass Index. J rNatl Cancer Inst 2016;108:pii; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg L, Palmer JR, Wise LA, Adams-Campbell LL. A prospective study of female hormone use and breast cancer among black women. Arch Intern Med 2006;166:760–765 [DOI] [PubMed] [Google Scholar]
- 14.Moorman PG, Newman B, Millikan RC, Tse CK, Sandler DP. Participation rates in a case-control study: The impact of age, race, and race of interviewer. Ann Epidemiol 1999;9:188–195 [DOI] [PubMed] [Google Scholar]
- 15.Millikan R, Eaton A, Worley K, et al. HER2 codon 655 polymorphism and risk of breast cancer in African Americans and whites. Breast Cancer Res Treat 2003;79:355–364 [DOI] [PubMed] [Google Scholar]
- 16.Rosner B, Colditz GA. Age at Menopause: Imputing age at menopause for women with a hysterectomy with application to risk of Postmenopausal Breast Cancer. Annf Epidemiol 2011;21:450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Press DJ, Sullivan-Halley J, Ursin G, et al. Breast cancer risk and ovariectomy, hysterectomy, and tubal sterilization in the women's contraceptive and reproductive experiences Study. Am J Epidemiol 2011;173:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudet MM, Gapstur SM, Sun J, Teras LR, Campbell PT, Patel AV. Oophorectomy and hysterectomy and cancer incidence in the Cancer Prevention Study-II Nutrition Cohort. Obstet Gynecol 2014;123:1247–1255 [DOI] [PubMed] [Google Scholar]
- 19.Irwin KL, Lee NC, Peterson HB, Rubin GL, Wingo PA, Mandel MG. Hysterectomy, tubal-sterilization, and the risk of breast-cancer. Am J Epidemiol 1988;127:1192–1201 [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48 [PubMed] [Google Scholar]
- 21.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet Gynecol 1995;85:304–313 [DOI] [PubMed] [Google Scholar]
- 22.Hall IJ, Moorman PG, Millikan RC, Newman B. Comparative analysis of breast cancer risk factors among African-American women and White women. Am J Epidemiol 2005;161:40–51 [DOI] [PubMed] [Google Scholar]
- 23.Terry MB, Tehranifar P. Hormone replacement therapy and breast cancer risk: More evidence for risk stratification? J Natl Cancer Inst 2013;105:1342–1343 [DOI] [PubMed] [Google Scholar]
- 24.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: The CARDIA study. Am J Public Health 2009;99:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol 2010;202:514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit 2008;14:Cr24–Cr31 [PubMed] [Google Scholar]
- 27.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol 2007;110:1091–1095 [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, Deming-Halverson S, Beeghly-Fadiel A, et al. Interactions of Hormone Replacement Therapy, bodyweight, and bilateral oophorectomy in breast cancer risk: Clin Cancer Res 2014;20:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritte R, Lukanova A, Berrino F, et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: A large prospective cohort study. Breast Cancer Res 2012;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst 2004;96:218–228 [DOI] [PubMed] [Google Scholar]
- 31.Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: The multiethnic cohort study. Am J Epidemiol 2009;169:1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg L, Bethea TN, Viscidi E, et al. Postmenopausal female hormone use and estrogen receptor-oositive and -negative breast cancer in African American women. J Natl Cancer Inst 2015;108:pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beral V, Bull D, Doll R, Key T, Peto R, Reeves G. Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52, 705 women with breast cancer and 108, 411 women without breast cancer. Lancet 1997;350:1047–1059 [PubMed] [Google Scholar]
- 34.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst 2011;103:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the women's health initiative observational study. J Natl Cancer Inst 2013;105:526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chlebowski RT, Anderson GL. Changing concepts: Menopausal hormone therapy and breast cancer. J Natl Cancer Inst 2012;104:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien KM, Fei C, Sandler DP, Nichols HB, DeRoo LA, Weinberg CR. Hormone therapy and young-onset breast cancer. Am J Epidemiol 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks E, Beral V, Bull D, et al. Breast cancer and hormone-replacement therapy in the million women study. Lancet 2003;362:419–427 [DOI] [PubMed] [Google Scholar]
- 39.Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol 2010;28:3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li CI, Malone KE, Porter PL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA 2003;289:3254–3263 [DOI] [PubMed] [Google Scholar]
- 41.Nichols HB, Trentham-Dietz A, Newcomb PA, et al. Postoophorectomy estrogen use and breast cancer risk. Obstet Gynecol 2012;120:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol 2009;170:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan VC, Ford LG. Paradoxical clinical effect of estrogen on breast cancer risk: A “new” biology of estrogen-induced apoptosis. Cancer Prev Res (Phila) 2011;4:633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pike MC, Ross RK, Spicer DV. Problems involved in including women with simple hysterectomy in epidemiologic studies measuring the effects of hormone replacement therapy on breast cancer risk. Am J Epidemiol 1998;147:718–721 [DOI] [PubMed] [Google Scholar]
- 45.Barrett-Connor E. Postmenopausal estrogen and prevention bias. Ann Intern Med 1991;115:455–456 [DOI] [PubMed] [Google Scholar]
- 46.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst 2002;94:606–616 [DOI] [PubMed] [Google Scholar]
- 47.Key TJA, Pike MC. The role of estrogens and progestagens in the epidemiology and prevention of breast-cancer. Eur J Cancer Clin Oncol 1988;24:29–43 [DOI] [PubMed] [Google Scholar]