Abstract
Objective: The goal of this research was to evaluate c(RGDyK) conjugated to phosphonate-based cross-bridged chelators using Cu-free click chemistry in the 4T1 mouse mammary tumor bone metastasis model in comparison with 64Cu-CB-TE2A-c(RGDyK), which previously showed selective binding to integrin αvβ3 on osteoclasts.
Experimental: Two phosphonate-based cross-bridged chelators (CB-TE1A1P and CB-TE1K1P) were conjugated to c(RGDyK) through bio-orthogonal strain-promoted alkyne–azide cycloaddition. In vitro and in vivo evaluation of the 64Cu-labeled TE1A1P-DBCO-c(RGDyK) (AP-c(RGDyK)), TE1K1P-PEG4-DBCO-c(RGDyK) (KP-c(RGDyK)), and CB-TE2A-c(RGDyK) were compared in the 4T1 mouse model of bone metastasis. The affinities of the unconjugated and chelator-c(RGDyK) analogs for αvβ3 integrin were determined using a competitive-binding assay. For in vivo evaluation, BALB/c mice were injected with 1 × 105 4T1/Luc cells in the left ventricle. Formation of metastases was monitored by bioluminescence imaging (BLI) followed by small-animal PET/CT 2 h postinjection of radiotracers.
Results: The chelator–peptide conjugates showed similar affinity to integrin αvβ3, in the low nM range. PET imaging demonstrated a higher uptake in bones having metastases for all 64Cu-labeled c(RGDyK) analogs compared with bones in nontumor-bearing mice. The correlation between uptake of 64Cu-AP-c(RGDyK) and 64Cu-KP-c(RGDyK) in bones with metastases based on PET/CT imaging, and osteoclast number based on histomorphometry, was improved over the previously investigated 64Cu-CB-TE2A-c(RGDyK).
Conclusion: These data suggest that the phosphonate chelator conjugates of c(RDGyK) peptides are promising PET tracers suitable for imaging tumor-associated osteoclasts in bone metastases.
Keywords: : osteoclast, αvβ3 integrin, click chemistry, copper-64
Introduction
Molecular imaging aims to noninvasively visualize the localization and staging of disease, as well as the response to therapy.1,2 A variety of imaging probes have been developed for different molecular targets. The vitronectin receptor, αvβ3 integrin, is known to play an important role in tumor-induced angiogenesis and tumor metastasis.3 Peptides containing an Arg-Gly-Asp (RGD) sequence are well known to bind preferentially to the αvβ3 integrin receptor.4 A plethora of synthetic peptides containing the RGD sequences have been radiolabeled with 99mTc, 111In, 123I, 68Ga, 64Cu, 18F, and 90Y and evaluated for their ability to target the αvβ3 receptor.5,6 Most of them showed in vitro high affinity for αvβ3 integrin and allowed targeting of receptor-positive tumors in vivo.
Osteoclasts are macrophage-related multinucleated cells that resorb bone and osteoclast bone resorbing activity involves a number of differentiation and activation steps.7 Common cancers (e.g., breast cancer) frequently develop bone metastases, which are often osteolytic in nature due to activation of osteoclast differentiation.8 The αvβ3 integrin receptor is overexpressed in osteoclasts and plays a key role in osteoclast activation and migration, mediating the attachment of osteoclast to bone matrix.9,10 The first report of the role of αvβ3 integrin in bone biology involved a monoclonal antibody (13C2) containing an RGD sequence that was raised against αvβ3 integrin in osteoclasts and was found to inhibit bone resorption in vitro.11,12 Subsequently, numerous studies were performed with ligands containing RGD sequences that inhibited bone resorption, osteoclast formation, attachment, and spreading in vitro.13–16
During the last decade or more, αvβ3 integrin was investigated as a target protein for in vivo osteoclast imaging in osteolytic bone disease. Sprague et al. demonstrated the specific uptake of 64Cu-CB-TE2A-c(RGDyK) in osteoclasts in vitro but not in bone marrow macrophages.17 In the same study, 64Cu-CB-TE2A-c(RGDyK) was shown to be highly effective as an osteoclast PET imaging agent in a pharmacological mouse model of direct administration of parathyroid hormone into the calvarium.17 Wadas et al. imaged osteolytic bone metastases and monitored the physiologic changes in the bone metastatic microenvironment after osteoclast-inhibiting bisphosphonate therapy with 64Cu-CB-TE2A-c(RGDyK) in a transgenic mouse model that spontaneously develops osteolytic tumors throughout the vertebrae and hind limbs.18 Subsequently it was demonstrated that 64Cu-CB-TE2A-c(RGDyK) and PET/CT could monitor increased osteoclast numbers in other osteoclast-related pathologies and their treatments.19
The chelator CB-TE2A for 64Cu has been used with success in a number of preclinical PET imaging studies. Although 64Cu-labeled CB-TE2A bioconjugates show significant improvement over nonbridged macrocycles such as DOTA or TETA with respect to in vivo stability,20 one of the disadvantages of CB-TE2A is the requirement of harsh conditions for radiolabeling with 64Cu, typically 95°C for 1–1.5 h.21,22 In this study, the authors investigated phosphonate-based cross-bridged chelators, CB-TE1A1P and CB-TE1K1P, conjugated to c(RGDyK) via click chemistry using published chemistry from their laboratory.23,24 These 64Cu-labeled c(RGDyK) peptides were compared to the previously reported CB-TE2A conjugate of c(RGDyK) for imaging osteoclasts in the 4T1 mouse mammary tumor model of bone metastases.
Materials and Methods
Copper-64 (T1/2 = 12.7 h, 17.8% β+) was obtained from Washington University (St. Louis, MO) and University of Wisconsin (Madison, WI). All chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO), unless otherwise specified. Aqueous solutions were prepared using ultrapure water (resistivity, 18 MΩ). Tenta Gel S PHB (O-[4(hydroxymethyl) phenyl] glycol resin) and 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids were purchased from Chem-Impex International, Inc. (Wood Dale, IL). All reagents used in cell culture were purchased from Gibco (Invitrogen, Waltham, MA).
Electrospray ionization mass spectrometry (ESI-MS) was performed on a Waters LCT Premier XE LC-MS station (Milford, MA). Analytical and semipreparative reversed-phase high-performance liquid chromatography (HPLC) was performed using a Waters 1525 Binary HPLC pump with a Waters 2489 UV/visible detector and a model 105-S-1 radioactivity detector (Carroll & Ramsey Associates, Berkeley, CA). Nonradioactive HPLC samples were analyzed on an analytical C18 column (Phenomenex Jupiter C18; 300 Å, 150 × 4.60 mm, 5 μ) and purified on a semipreparative C18 column (Phenomenex Jupiter C18; 300 Å, 250 × 10 mm, 5 μ). Radiochemistry reaction progress and purity were monitored on a rocket C18 column (Alltima, Deerfield, IL). The mobile phase was H2O (0.1% TFA; solvent A) and acetonitrile (0.1% TFA; solvent B). Radioactive samples were counted on an automated well-type gamma counter (Wizard2, 2480 automatic gamma counter; Perkin-Elmer, Waltham, MA). PET/CT data were acquired on an Inveon PET/CT scanner (Siemens Molecular Imaging, Knoxville, TN).
Synthesis of DBCO-CB-TE1A1P and DBCO-PEG4-CB1K1P conjugates of c(RGDyK) [CB-TE1A1P-c(RGDyK); CB-TE1K1P-c(RGDyK)]
CB-TE2A-c(RGDyK), DBCO-CB-TE1A1P, and DBCO-PEG4-CB1K1P were synthesized as previously described.17,23–25 c(RGDyK) was prepared by Fmoc-based solid-phase peptide synthesis. Lysine functionalization and cyclization reactions were performed on resin as described.17 After successful cyclization, the (4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl (Dde) protecting group from the amino side chain of lysine was removed by hydrazine monohydrate-DMF (4:96) for 3 × 10 min. Then, azide-PEG4-NHS was coupled to the resin for 4 h at room temperature (3 eq N3-PEG4-NHS: 6 eq TEA in 1 mL DMF). Cleavage from the resin as well as side chain deprotection was performed in one step using a mixture of TFA/TIPS/H2O (90:5:5). The cleaved peptides were precipitated with ice-cold diethyl ether and washed twice with diethyl ether, followed by purification by semipreparative HPLC using a Phenomenex Jupiter 5 μ C18 300 Å semiprep column (250 × 10 mm) under the following conditions: 0–3 min 90% A, 3–23 min 40% A, 23–27 min 40% A, 27–30 min 90% A, 30–35 min 90% A; Solvent A, 0.1% TFA in water, Solvent B, 0.1% TFA in ACN. ESI-MS[M+H]+: observed 892.9; calculated 892.4.
N3-PEG4-c(RGDyK) was mixed with CB-TE1A1P-DBCO (1:1) in 0.5 mL of 0.1 M ammonium acetate buffer, pH 6.8, and the mixture was incubated at 37°C for 2 h, diluted with water, and purified by reversed-phase semipreparative HPLC using a Phenomenex Jupiter 5 μ C18 300 Å semiprep column (250 × 10 mm), 2 mL/min flow rate under the following gradients: 0–3 m 80% A, 3–30 min 60% A, 30–31 min 10% A, 31–41 min 10% A, 41–45 min 80% A. ESI-MS[M + 2H]2+/2: observed 765.3; calculated 764.9.
N3-PEG4-c(RGDyK) was mixed with CB-TE1K1P-PEG5-DBCO (1:1.5) in 0.5 mL of 0.1 M ammonium acetate buffer, pH 6.8, and the mixture was incubated at 37°C for 2 h, diluted with water, and purified by reversed-phase semipreparative HPLC using a Phenomenex Jupiter 5 μ C18 300 Å semiprep column (250 × 10 mm), 3 mL/min flow rate using the following gradients: 0–3 min 90% A, 3–23 min 40% A, 23–27 min 40% A, 27–30 min 90% A, 30–35 min 90% A. Solvent A, 0.1% TFA in water; Solvent B, 0.1% TFA in ACN. ESI-MS[M + 2H]2+/2: observed 960.9; calculated 960.5.
Integrin-binding assay
The affinity of each peptide for αvβ3 integrin was estimated as previously described.26 Biotinylation of vitronectin was achieved as described.17 Three micrograms of integrin/mL in coating buffer (20-mM Tris, pH 7.4, 150-mM NaCl, 2-mM CaCl2, 1-mM MgCl2, 1-mM MnCl2) was coated onto 96-well plates (Nunc-Immuno Plate with MaxiSorp) (1.5 h at 37°C). The plates were then blocked (1.5 h at 37°C) with bovine serum albumin (BSA) (3% in coating buffer). After washing twice with binding buffer (0.1% BSA, 0.05% Tween 20 in coating buffer), biotinylated vitronectin, with and without serially diluted peptides (0.05–5000 nM), was allowed to bind to the αvβ3 integrin (2 h at 37°C). After washing (three times in binding buffer), bound biotinylated vitronectin was detected by binding ExtrAvidin alkaline phosphatase (Sigma) (1/35,000 dilution, 1 hour at room temperature) using the p-nitrophenyl phosphate liquid substrate system (Sigma) as the chromogen. Each concentration data point was done in triplicate, and each binding experiment was performed at least twice. Nonlinear regression was used to fit binding curves and calculate inhibitory concentrations of 50% (IC50 values) (Prism; GraphPad).
Radiolabeling chemistry
CB-TE2A-c(RGDyK) was radiolabeled with Cu-64 as previously described.17–19 For Cu-64 labeling experiments of AP-c(RGDyK) and KP-c(RGDyK), 64CuCl2 (5–10 μL in 0.5 M HCl) was first diluted with 0.1 M ammonium acetate buffer pH 6 (50–100 μL). KP- or AP-c(RGDyK) (1 μg) was mixed with 64Cu-acetate (0.4–2 mCi) to a final volume of 100 μL. The mixture was incubated at 40°C for 20 min for AP-c(RGDyK) and 5 min for KP-c(RGDyK). After incubation, the radiochemical purity of the 64Cu-labeled KP/AP-c(RGDyK) was monitored by radio-TLC (Rf ∼0.1, C18 silica plates, eluant: methanol/0.1 M ammonium acetate 1:1, Rf ∼0.8 for 64Cu-EDTA).
Cell line and animal model
The 4T1 cell line transfected with green fluorescent protein (GFP) and firefly luciferase (Fluc) (4T1) was developed in the laboratory of Dr. Katherine Weilbaecher, Washington University. 4T1 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen-Gibco, Groningen, The Netherlands) supplemented with 10% FCS (Hyclone, Logan, UT), 100 IU/mL penicillin (Invitrogen-Gibco), and 100 μg/mL streptomycin (Invitrogen-Gibco). Cell cultures were incubated at 37°C in a humidified atmosphere and a CO2 content of 5% in air.
All animal experiments were conducted in compliance with the Guidelines for the Care and Use of Research Animals established by the Institutional Animal Care and Use Committee at the University of Pittsburgh. 4T1 cells (1 × 105) in 20 μL of saline were injected into the left heart ventricle of 4–6-week-old, 18–22 g, female BALB/cAnNCrl (BALB/c) mice (Charles River Laboratories, Wilmington, MA). Development of metastases was monitored at 2, 4, 6, and 10 d by bioluminescence imaging (BLI) before PET/CT.
Bioluminescence imaging
Mice were injected intraperitoneally with d-luciferin potassium salt (Gold Biotechnology, St. Louis, MO) (15 mg/mL in saline) 10 min before imaging. Mice were anesthetized with 2% isoflurane and were imaged in ventral positions using an IVIS Lumina XR (Perkin-Elmer) to monitor tumor growth and metastases. After acquiring a grayscale photograph, a bioluminescent image and X-ray image were collected with a binning (resolution) factor of 8, and a 1/f stop-and-open filter. Exposure times of up to 1 min were used and total photon flux was determined.
Biodistribution studies and in vivo metabolic studies
Ten d postinjection of 4T1 cells into the left ventricle, mice were injected intravenously (tail vein) with 1.1–1.7 MBq of Cu-64-labeled c(RGDyK) analogs. Mice were sacrificed at selected time points after injection (1, 2, 4, and 24 h). Organs of interest were removed, weighed, and counted. The percent injected dose per gram (%ID/g) was calculated.
PET/CT imaging
Ten days after left ventricle injection of 4T1 cells, mice were injected intravenously (tail vein) with 5.5–7.4 MBq of Cu-64-labeled c(RGDyK) analogs. After 2, 4, and 24 h postinjection, mice were anesthetized with 1%–2% isoflurane and imaged on an Inveon small-animal PET/CT (Siemens Molecular Imaging). The imaging parameters were as follows: 600 s PET acquisition time, OSEM2D reconstruction algorithm, voxel size 0.7 mm3; CT exposure settings: 80 kV, 500 mA, 120 ms exposure time, 220° rotation with 120 steps, low magnification, bin 4, voxel size 0.8 mm3. For tail vein injection and throughout imaging, mice were anesthetized with 2%–3% isoflurane with oxygen at a flow rate of 2 L/minute. PET images were manually registered with CT, analyzed, and prepared using the Inveon Research Workplace (IRW) software (Siemens Molecular Imaging). Data were decay corrected from the time of 64Cu-peptide administration. Standardized uptake values (SUVs) were determined using the formula SUV 5 ([nCi/mL] × [animal weight (g)]/injected dose [nCi]).
Histology
After biodistribution and imaging studies, the femur and tibia (knee) were removed, fixed in 10% formalin for 48 h, decalcified in acid-free 14% EDTA solution for 10–14 d, embedded in paraffin, and sectioned at a thickness of 5 μ. Sections were stained with tartrate-resistant acid phosphatase (TRAP) to identify osteoclasts. Slides were scanned by an Aperio ePathology System (Leica, Buffalo Grove, IL), a high-resolution pathology scanner, at 20 × magnification, and analyzed using ImageScope software (Leica). The field of analysis was a standard measurement of 3.5 mm from the head of the tibia and femur into the shaft, including cortical and trabecular bone. TRAP-positive osteoclasts were enumerated and divided by total bone tissue area using Bioquant Osteo (Bioquant Image Analysis Corporation, Nashville, TN) high-throughput software for histomorphometric analysis.
Statistical analyses
All data are presented as mean ± standard deviation. A Student's t-test (two tailed, unpaired) was used to compare two data sets and p-values less than 0.05 were considered significant. When more than two data sets were compared, one-way ANOVA with Tukey's post-test was used with α = 0.05 (95% confidence intervals). All statistical analyses were performed using PRISM software (GraphPad).
Results
Synthesis and radiolabeling of peptide conjugates
c(RGDyK) was synthesized via standard solid-phase F-moc-based peptide synthesis and N3-PEG4-NHS was coupled to the amino side chain of lysine. After cleavage and HPLC purification, isolated N3-PEG4-c(RGDyK) was reacted in 0.1 M ammonium acetate buffer pH 6.8 at 37°C for 2 h with CB-TE1A1P-DBCO to produce CB-TE1A1P-DBCO-PEG4-c(RGDyK) [AP-c(RGDyK); 30% yield] and with CB-TE1K1P-PEG4-DBCO to produce CB-TE1K1P-PEG4-DBCO-PEG5-c(RGDyK) [KP-c(RGDyK); 7.5% yield]. Based on similar chemistry to produce Tyr3-octreotate (Y3-TATE) analogs, stereoisomers of the AP and KP-c(RGDyK) analogs are formed23; however, for this work, the authors did not separate and evaluate individual isomers. Structures of CB-TE2A-c(RGDyK), AP-c(RGDyK), and KP-c(RGDyK) are shown in Figure 1.
FIG. 1.
Structures of CB-TE2A-c(RGDyK) (A), AP-c(RGDyK) (B), and KP-c(RGDyK) (C).
The radiolabeling conditions for the AP-c(RGDyK) and KP-c(RGDyK) were optimized (Table 1), and the optimal buffer solution was 0.1 M NH4OAc (pH 6). Under the same conditions, AP-c(RGDyK) was labeled within 20 m at 40°C and KP-c(RGDyK) was labeled within 5 min at 40°C. In contrast, the CB-TE2A-c(RGDyK) was labeled at 95°C for 1 h in 0.1 M NH4OAc (pH 8). The relatively mild labeling conditions achievable with CB-TE1A1P and CB-TE1K1P chelators are consistent with previously published data on the same chelators conjugated to somatostatin analogs (Y3-TATE).23
Table 1.
Specific Activity, Radiolabeling Yield, and Log p-Values of 64Cu-Labeled Chelator-c(RGDyK) Analogs
| Compound | μCi/μg | % Radiolabeling yield | Log p |
|---|---|---|---|
| KP-c(RGDyK) | 333 | 97.9 | −1.79 ± 0.02 |
| AP-c(RGDyK) | 333 | 98.4 | −1.91 ± 0.14 |
| CB-TE2A-c(RGDyK) | 333 | 99.1 | −2.12 ± 0.27 |
Binding affinity
The effects of chelator on integrin binding affinity of c(RGDyK) conjugated with the three chelators were assessed. The affinities of c(RGDyK), CB-TE2A-c(RGDyK), AP-c(RGDyK), and KP-c(RGDyK) for αvβ3 integrin were determined using a competitive-binding assay with biotinylated vitronectin as the competing ligand. Conjugation of different cross-bridged chelators to c(RGDyK) peptide did not significantly alter peptide affinity (Table 2). Replicate experiments produced IC50 values within the same confidence intervals.
Table 2.
IC50 Values of Chelator-RGDyK Analogs in Competition with Biotinylated Vitronectin (n = 3)
| Compound | IC50 (nM) | 95% Confidence interval (nM) |
|---|---|---|
| c(RGDyK) | 8 | 3.4–19 |
| CB-TE2A-c(RGDyK) | 3.1 | 1.3–7.3 |
| AP-c(RGDyK) | 2.8 | 1.7–4.6 |
| KP-c(RGDyK) | 8.1 | 3.4–19.1 |
In vivo biodistribution and small-animal imaging
Biodistribution studies were performed on control mice at 1, 2, 4, and 24 h after injection of 64Cu-labeled CB-TE2A-c(RGDyK), AP-c(RGDyK), and KP-c(RGDyK) (Table 3). At 2 h postinjection in nontumor-bearing mice, 64Cu-CB-TE2A-c(RGDyK) showed faster blood kinetics with low blood retention and higher accumulation in the liver, kidneys, and the small and large intestine compared with 64Cu-AP-c(RGDyK) and 64Cu-KP-c(RGDyK) (Fig. 2B). 64Cu-AP-c(RGDyK) and 64Cu-KP-c(RGDyK) showed similar accumulation in those organs. Maximum intensity projection (MIP) PET/CT images in normal mice indicate a lower uptake in the liver for 64Cu-AP-c(RGDyK) and 64Cu-KP-c(RGDyK) compared with 64Cu-CB-TE2A-c(RGDyK) (Fig. 2A).
Table 3.
Biodistribution of 64Cu-CB-TE2A-c(RGDyK), 64Cu-AP-c(RGDyK), and 64Cu-KP-c(RGDyK) in Nontumor-Bearing BALB/c Female Mice at 1, 2, 4, and 24 H Postinjection
| 64Cu-CB-TE2A-c(RGDyK) | 64Cu-AP-c(RGDyK) | 64Cu-KP-c(RGDyK) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ | 1 h | 2 h | 4 h | 24 h | 1 h | 2 h | 4 h | 24 h | 1 h | 2 h | 4 h | 24 h |
| Blood | 0.14 ± 0.001 | 0.006 ± 0.008 | 0.005 ± 0.001 | 0.005 ± 0.002 | 0.08 ± 0.02 | 0.10 ± 0.03 | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.10 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.04 | 0.03 ± 0.01 |
| Lung | 0.51 ± 0.13 | 0.63 ± 0.07 | 0.56 ± 0.11 | 0.52 ± 0.29 | 0.57 ± 0.11 | 0.55 ± 0.16 | 0.29 ± 0.22 | 0.32 ± 0.15 | 0.41 ± 0.33 | 0.63 ± 0.37 | 0.49 ± 0.07 | 0.83 ± 0.48 |
| Liver | 1.21 ± 0.19 | 2.06 ± 0.22 | 2.28 ± 0.53 | 1.02 ± 0.13 | 0.64 ± 0.58 | 1.08 ± 0.39 | 0.73 ± 0.60 | 0.43 ± 0.22 | 0.77 ± 0.03 | 0.99 ± 0.29 | 0.94 ± 0.17 | 1.25 ± 0.78 |
| Spleen | 0.81 ± 0.09 | 1.39 ± 0.10 | 1.22 ± 0.38 | 1.32 ± 0.32 | 0.86 ± 0.13 | 1.12 ± 0.19 | 0.60 ± 0.48 | 0.94 ± 0.32 | 0.71 ± 0.05 | 1.04 ± 0.29 | 1.01 ± 0.08 | 0.90 ± 0.08 |
| Kidney | 1.88 ± 0.40 | 3.17 ± 0.13 | 3.01 ± 0.10 | 1.38 ± 0.49 | 1.39 ± 0.19 | 1.77 ± 0.46 | 1.50 ± 0.52 | 0.93 ± 0.38 | 1.56 ± 0.09 | 1.62 ± 0.14 | 1.71 ± 0.17 | 2.56 ± 0.79 |
| Muscle | 0.12 ± 0.01 | 0.10 ± 0.04 | 0.13 ± 0.003 | 0.10 ± 0.03 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.003 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.06 |
| Heart | 0.21 ± 0.02 | 0.21 ± 0.07 | 0.33 ± 0.12 | 0.29 ± 0.17 | 0.26 ± 0.03 | 0.27 ± 0.06 | 0.12 ± 0.09 | 0.16 ± 0.07 | 0.24 ± 0.03 | 0.51 ± 0.52 | 0.26 ± 0.01 | 0.56 ± 0.23 |
| Stomach | 0.37 ± 0.05 | 0.43 ± 0.07 | 0.56 ± 0.19 | 0.22 ± 0.05 | 0.28 ± 0.09 | 0.50 ± 0.11 | 0.28 ± 0.18 | 0.23 ± 0.08 | 0.27 ± 0.05 | 0.52 ± 0.26 | 0.36 ± 0.10 | 0.97 ± 0.5 |
| Sm int | 0.82 ± 0.17 | 1.26 ± 0.20 | 1.00 ± 0.06 | 0.25 ± 0.05 | 0.55 ± 0.19 | 0.77 ± 0.24 | 0.53 ± 0.28 | 0.45 ± 0.15 | 0.67 ± 0.04 | 0.72 ± 0.14 | 0.78 ± 0.25 | 0.97 ± 0.57 |
| Lg int | 0.44 ± 0.04 | 2.16 ± 0.44 | 2.11 ± 0.63 | 0.19 ± 0.23 | 0.41 ± 0.11 | 1.36 ± 0.10 | 0.88 ± 0.38 | 0.32 ± 0.15 | 0.34 ± 0.08 | 0.73 ± 0.23 | 1.13 ± 0.61 | 1.05 ± 0.69 |
Values are expressed as %ID/g (n = 3).
FIG. 2.
(A) MIP PET/CT images in nontumor-bearing mice at 2 h postinjection; (B) biodistribution of 64Cu-CB-TE2A-c(RGDyK), 64Cu-AP-c(RGDyK), and 64Cu-KP-c(RGDyK) in nontumor-bearing mice at 2 h postinjection.
To examine osteoclast-mediated bone uptake in vivo, the authors used the 4T1 breast tumor bone metastasis model by intracardiac injection of tumor cells in the left ventricle, which seeds the breast cancer cells directly into the circulation, bypassing the early steps in the metastatic process, enabling rapid metastases to the bone. They used BLI to monitor bone metastasis development. Metastases in the leg bones become detectable 10–12 d after inoculation (Fig. 3). Representative mouse PET/CT and BLI images at baseline and 10 d postinjection of 4T1 cells in the left ventricle are presented in Figure 4. Bone metastases in the spine and legs were visualized by BLI and PET/CT imaging (Fig. 4B), which were not present at baseline. Quantification of uptake of the three 64Cu-labeled c(RGDyK) analogs (2 h postinjection) in bones pre- and postintracardiac injection of 4T1 cells shows a significantly increased uptake of all three PET tracers after 10 d of tumor growth (p ≤ 0.05) (Fig. 4C).
FIG. 3.
Schematic describing imaging experiments in left ventricle injected 4T1 tumor-bearing mice.
FIG. 4.
BLI and MIP PET/CT images of mouse postinjection of 64Cu-KP-c(RGDyK) (A) and 10 d after injection (B) of 4T1 cells in the left ventricle. BLI, bioluminescence imaging. * indicates p < 0.05. (C) Quantification of uptake of c(RGDyK) analogs in bones pre- and postintracardiac injection of 4T1 tumor cells.
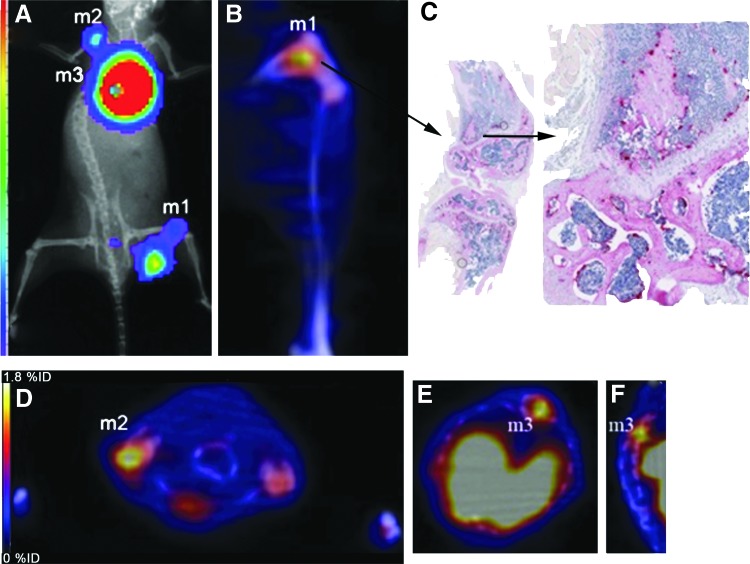
After imaging at 10 d post-tumor cell injection, mice were sacrificed and bones were dissected for histological analysis. Figure 5 shows BLI and PET/CT images of a representative mouse with bone metastases after imaging with 64Cu-AP-c(RGDyK). Histological analysis and TRAP staining confirmed increased numbers of osteoclasts in leg bones (Fig. 5C). Histomorphometry was performed on bone sections of nontumor-bearing and tumor-bearing mice to determine the percentage of osteoclasts (OC) per surface area (mm2), and these data were correlated with the %ID/g from PET images (Fig. 6A–E). A linear correlation (Pearson correlation, two tailed) between percentage osteoclast surface area and %ID/g was obtained (Fig. 6F). The percentage of osteoclasts/mm2 on bone increased and the uptake of all three 64Cu-labeled c(RGDyK) analogs in the bone also increased, suggesting osteoclast-specific uptake in bone due to metastases. The Pearson correlations (r2) were similar for all three 64Cu-labeled compounds [CB-TE2A-c(RGDyK): 0.59; AP-c(RGDyK): 0.69; KP-c(RGDyK): 0.65].
FIG. 5.
Representative BLI and MIP PET/CT images of mouse postinjection of 64Cu-AP-c(RGDyK) at 10 d after intracardiac injection of 4T1 cells in the left ventricle. (A) BLI image showing three bone metastases (m1, m2, m3); (B) PET/CT image of m1 in the knee; (C) histology of knee ex vivo showing osteoclasts that stain for TRAP; (D) transaxial PET/CT image showing m2; (E, F) transaxial and coronal PET/CT images showing m3. TRAP, tartrate-resistant acid phosphatase.
FIG. 6.
Bone uptake of 64Cu-labeled c(RGDyK) analogs from the PET images correlates to osteoclast numbers by histomorphometry. (A–C) Coronal bone images of control (nontumor) and bone metastasis after injection of 64Cu-labeled c(RGDyK) analogs; (D, E) histology showing TRAP staining of osteoclasts in control and tumor-bearing bones; (F) correlation of %ID/g from PET imaging data with numbers of osteoclasts (OC) per mm2 on bone slides for the three 64Cu-labeled c(RGDyK) analogs, demonstrating that the uptake of the PET tracers increases with greater numbers of osteoclasts.
Discussion
The high level of αvβ3 integrin expression in certain tumors and in angiogenic blood vessels is well known, and has been studied extensively as a target for molecular imaging and therapy (for reviews, see Refs.4,27). Specific imaging of osteoclasts, as they relate to bone/joint diseases or cancer, occurred much later.17–19 More recently, human studies of PET tracers targeting αvβ3 integrin in bone metastases have been reported. Beer et al. imaged prostate cancer patients with bone metastases with [18F]Galacto-RGD.28 Although there was uptake of this tracer in bone metastases, SUVs in lymph nodes and the primary tumor were similar or higher. Prostate tumors are typically osteoblastic in nature rather than osteolytic, and therefore, these data are reflective of this.29 In a human SPECT imaging study comparing 99mTc-labeled PRGD2 with 99mTc-MDP (bone scan) in lung cancer patients with bone metastases, 99mTc-labeled PRGD2 had higher accuracy and specificity than the bone scan, which gave many false-positive results.30 The authors point out that αvβ3 integrin is expressed in osteoclasts and osteoblasts; however, αvβ3 integrin is highly expressed and is the main integrin in osteoclasts.31 Withofs et al. recently reported a human study where 18F-FPRGD2 uptake was significantly higher than FDG in joints known to have osteoarthritis.32 This uptake was attributed to high αvβ3 integrin expression in dedifferentiated chondrocytes and osteoarthritic cartilage, and it was mentioned that osteoclasts are also a major contributor to osteoarthritis development.
Previous studies showed specific uptake of 64Cu-CB-TE2A-c(RGDyK) by osteoclasts both in vivo and ex vivo, where blocking with cold c(RGDyK) decreased uptake in parathyroid hormone-treated calvaria in mice, which induced osteolysis.17 A goal of this study was to compare the CB-TE2A chelator with phosphonate-based cross-bridged chelators CB-TE1A1P33 and CB-TE1K1P24 conjugated to c(RGDyK) via copper-free click chemistry, as previously described for the somatostatin analog, Y3-TATE.23 The phosphonate-based cross-bridged chelator, CB-TE1A1P, showed improved biodistribution compared with CB-TE2A conjugates of Y3-TATE and the α4β1 integrin targeting ligand, LLP2A.34,35 Due to poor yields of direct conjugation of CB-TE1A1P through the carboxylate group, click chemistry was used via a DBCO analog of CB-TE1A1P, where the chelator to N3-c(RGDyK) coupling yield was 30%, compared with 7% for preparation of CB-TE2A-c(RGDyK).17 However, the coupling of a DBCO analog of CB-TE1K1P to N3-c(RGDyK) was significantly lower, at 7.5% yield after purification. The authors previously reported that the chelator coupling chemistry applied here produced diastereomers of Y3-TATE analogs that were isolated and analyzed for binding affinity and lipophilicity.23 Because no major differences in both binding affinity and lipophilicity were observed for the chelator-Y3-TATE diastereomers, in this study, the authors did not separate these isomers for individual evaluation in vitro and in vivo.
The biodistribution of the three 64Cu-labeled chelator-c(RGDyK) analogs (Fig. 2) shows a significantly lower liver and kidney uptake for the phosphonate chelator analogs compared to the CB-TE2A agent; although at 2 h postinjection, there is significantly more clearance of 64Cu-CB-TE2A-c(RGDyK) from the blood than for the other two analogs. The uptake of the three tracers in bone, both normal and after intracardiac injection of 4T1 tumor cells, correlates well with osteoclast surface area based on ex vivo histomorphometry (Fig. 6F). Of the three analogs, 64Cu-AP-c(RGDyK) shows the highest uptake at 40% osteoclasts/mm2 from TRAP staining. The AP analog was synthesized in the highest yield and showed the greatest clearance through the liver and kidneys (Table 3). Dumont et al. compared 64Cu-labeled c(RGDfK) peptides conjugated to NODAGA and CB-CB2A in U87MG tumor-bearing mice and demonstrated the superiority of the CB-TE2A chelator with respect to tumor:nontumor ratios (blood, kidney, and liver) at 18 h postinjection.36 Cai et al. evaluated 64Cu-labeled c(RGDyK) peptides conjugated to the chelator AmBaSar, which is a carboxylate derivative of the sarcophagine-based chelator diamSar.37 The biodistribution of the AP and KP-c(RGDyK) analogs has similar tumor uptake and kidney/liver clearance to the diamSar agent, although different tumor-bearing mouse models were used (4T1 vs. U87MG).
Conclusions
The data presented here suggest that PET imaging with 64Cu-AP-c(RGDyK), which performed the best of the three agents evaluated, could be highly sensitive for imaging osteolytic bone metastases resulting from specific cancers such as breast cancer. High uptake of this tracer could also suggest that treatment with antiosteoclast agents such as bisphosphonates or anti-RANKL antibodies would be effective.18,19 In addition, PET imaging with 64Cu-AP-c(RGDyK) could be informative for diagnosis and/or monitoring treatment for other diseases resulting in high levels of osteoclasts, such as osteoarthritis.
Acknowledgments
The authors acknowledge Kathryn Day for assistance with PET/CT imaging, Jalpa Modi for assistance with cell culture and binding assays, Crystal Darby at Washington University for expert histologic sample preparation, and A. Nicole Myers for help with histomorphometry. This research project used the Animal Facility and the In Vivo Imaging Facility supported, in part, by a grant from the NCI grant (P30 CA047904; Principal Investigator—Dr. Robert L. Ferris) and was funded by the University of Pittsburgh and UPMC.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gotthardt M, Bleeker-Rovers CP, Boerman OC, et al. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med 2010;51:1937. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Basu S, Torigian D, et al. Role of modern imaging techniques for diagnosis of infection in the era of 18F-fluorodeoxyglucose positron emission tomography. Clin Microbiol Rev 2008;21:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruoslahti E. Integrins as signaling molecules and targets for tumor therapy. Kidney Int 1997;51:1413. [DOI] [PubMed] [Google Scholar]
- 4.Danhier F, Le Breton A, Preat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm 2012;9:2961. [DOI] [PubMed] [Google Scholar]
- 5.Tateishi U, Oka T, Inoue T. Radiolabeled RGD peptides as integrin alpha(v)beta3-targeted PET tracers. Curr Med Chem 2012;19:3301. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Chakraborty S, Liu S. Radiolabeled cyclic RGD peptides as radiotracers for imaging tumors and thrombosis by SPECT. Theranostics 2011;1:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roodman GD. Advances in bone biology: The osteoclast. Endocr Rev 1996;17:308. [DOI] [PubMed] [Google Scholar]
- 8.Teti A, Migliaccio S, Baron R. The role of the alphaVbeta3 integrin in the development of osteolytic bone metastases: A pharmacological target for alternative therapy? Calcif Tissue Int 2002;71:293. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura I, Pilkington MF, Lakkakorpi PT, et al. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J Cell Sci 1999;112 (Pt 22):3985. [DOI] [PubMed] [Google Scholar]
- 10.Takayama S, Ishii S, Ikeda T, et al. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res 2005;25:79. [PubMed] [Google Scholar]
- 11.Chambers TJ, Fuller K, Darby JA, et al. Monoclonal antibodies against osteoclasts inhibit bone resorption in vitro. Bone Miner 1986;1:127. [PubMed] [Google Scholar]
- 12.Davies J, Warwick J, Totty N, et al. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol 1989;109:1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton MA, Taylor ML, Arnett TR, et al. Arg-Gly-Asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res 1991;195:368. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura I, Gailit J, Sasaki T. Osteoclast integrin alphaVbeta3 is present in the clear zone and contributes to cellular polarization. Cell Tissue Res 1996;286:507. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Garsky V, Majeska RJ, et al. Structure-activity studies of the s-echistatin inhibition of bone resorption. J Bone Miner Res 1994;9:1441. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Sardana MK, Grasser WA, et al. Echistatin is a potent inhibitor of bone resorption in culture. J Cell Biol 1990;111:1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprague JE, Kitaura H, Zou W, et al. Noninvasive imaging of osteoclasts in parathyroid hormone-induced osteolysis using a 64Cu-labeled RGD peptide. J Nucl Med 2007;48:311. [PMC free article] [PubMed] [Google Scholar]
- 18.Wadas TJ, Deng H, Sprague JE, et al. Targeting the alphavbeta3 integrin for small-animal PET/CT of osteolytic bone metastases. J Nucl Med 2009;50:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheleznyak A, Wadas TJ, Sherman CD, et al. Integrin alpha(v)beta(3) as a PET imaging biomarker for osteoclast number in mouse models of negative and positive osteoclast regulation. Mol Imaging Biol 2012;14:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson CJ, Wadas TJ, Wong EH, et al. Cross-bridged macrocyclic chelators for stable complexation of copper radionuclides for PET imaging. Q J Nucl Med Mol Imaging 2008;52:185. [PMC free article] [PubMed] [Google Scholar]
- 21.Boswell CA, Sun X, Niu W, et al. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem 2004;47:1465. [DOI] [PubMed] [Google Scholar]
- 22.Sprague JE, Peng Y, Sun X, et al. Preparation and biological evaluation of copper-64-labeled tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin Cancer Res 2004;10:8674. [DOI] [PubMed] [Google Scholar]
- 23.Cai Z, Ouyang Q, Zeng D, et al. 64Cu-labeled somatostatin analogues conjugated with cross-bridged phosphonate-based chelators via strain-promoted click chemistry for PET imaging: In silico through in vivo studies. J Med Chem 2014;57:6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng D, Ouyang Q, Cai Z, et al. New cross-bridged cyclam derivative CB-TE1K1P, an improved bifunctional chelator for copper radionuclides. Chem Commun (Camb) 2014;50:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng D, Guo Y, White AG, et al. Comparison of conjugation strategies of cross-bridged macrocyclic chelators with cetuximab for copper-64 radiolabeling and PET imaging of EGFR in colorectal tumor-bearing mice. Mol Pharm 2014;11:3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haubner R, Wester HJ, Reuning U, et al. Radiolabeled alpha(v)beta3 integrin antagonists: A new class of tracers for tumor targeting. J Nucl Med 1999;40:1061. [PubMed] [Google Scholar]
- 27.Gaertner FC, Kessler H, Wester HJ, et al. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging 2012;39 Suppl 1:S126. [DOI] [PubMed] [Google Scholar]
- 28.Beer AJ, Schwarzenbock SM, Zantl N, et al. Non-invasive assessment of inter-and intrapatient variability of integrin expression in metastasized prostate cancer by PET. Oncotarget 2016;7:28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guise TA, Mohammad KS, Clines G, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 2006;12:6213s. [DOI] [PubMed] [Google Scholar]
- 30.Miao W, Zheng S, Dai H, et al. Comparison of 99mTc-3PRGD2 integrin receptor imaging with 99mTc-MDP bone scan in diagnosis of bone metastasis in patients with lung cancer: A multicenter study. PLoS One 2014;9:e111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes DE, Salter DM, Dedhar S, et al. Integrin expression in human bone. J Bone Miner Res 1993;8:527. [DOI] [PubMed] [Google Scholar]
- 32.Withofs N, Charlier E, Simoni P, et al. (1)(8)F-FPRGD(2) PET/CT imaging of musculoskeletal disorders. Ann Nucl Med 2015;29:839. [DOI] [PubMed] [Google Scholar]
- 33.Ferdani R, Stigers DJ, Fiamengo AL, et al. Synthesis, Cu(II) complexation, 64Cu-labeling and biological evaluation of cross-bridged cyclam chelators with phosphonate pendant arms. Dalton Trans 2012;41:1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Y, Ferdani R, Anderson CJ. Preparation and biological evaluation of (64)Cu labeled Tyr(3)-octreotate using a phosphonic acid-based cross-bridged macrocyclic chelator. Bioconjug Chem 2012;23:1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang M, Ferdani R, Shokeen M, et al. Comparison of two cross-bridged macrocyclic chelators for the evaluation of 64Cu-labeled-LLP2A, a peptidomimetic ligand targeting VLA-4-positive tumors. Nucl Med Biol 2013;40:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumont RA, Deininger F, Haubner R, et al. Novel (64)Cu- and (68)Ga-labeled RGD conjugates show improved PET imaging of alpha(nu)beta(3) integrin expression and facile radiosynthesis. J Nucl Med 2011;52:1276. [DOI] [PubMed] [Google Scholar]
- 37.Cai H, Li Z, Huang CW, et al. Evaluation of copper-64 labeled AmBaSar conjugated cyclic RGD peptide for improved microPET imaging of integrin alphavbeta3 expression. Bioconjug Chem 2010;21:1417. [DOI] [PubMed] [Google Scholar]