Abstract
Background:
Cesarean section (CS) is an independent risk factor for placenta accreta. Some researchers think that the timing of primary cesarean delivery is associated with placenta accreta in subsequent pregnancies. The aim of this study was to investigate the risk of placenta accreta following primary CS without labor, also called primary elective CS, in a pregnancy complicated with placenta previa.
Methods:
A retrospective, single-center, case-control study was conducted at Peking University Third Hospital. Relevant clinical data of singleton pregnancies between January 2010 and September 2017 were recorded. The case group included women with placenta accreta who had placenta previa and one previous CS. Control group included women with one previous CS that was complicated with placenta previa. Maternal age, body mass index, gestational age, fetal birth weight, gravity, parity, induced abortion, the rate of women received assisted reproductive technology, other uterine surgery, and primary elective CS were analyzed between the two groups.
Results:
The rate of primary elective CS (90.1% vs. 69.9%, P < 0.001) was higher, and maternal age was younger (32.7 ± 4.7 years vs. 34.6 ± 4.0 years, P < 0.001) in case group, compared with control group. Case group also had higher gravity and induced abortions compared with the control group (both P < 0.05). Primary CS without labor was associated with significantly increased risk of placenta accreta in a subsequent pregnancy complicated with placenta previa (odds ratio: 3.32; 95% confidential interval: 1.68–6.58).
Conclusion:
Women with a primary elective CS without labor have a higher chance of developing an accreta in a subsequent pregnancy that is complicated with placenta previa.
Keywords: Cesarean Section, Placenta Accreta, Placenta Previa
摘要
目的:
剖宫产(CS)是胎盘植入的独立危险因素。有研究表明,在随后的妊娠中,初次剖宫产的时机与胎盘植入有关。本研究探讨了初次剖宫产时机与再次妊娠合并前置胎盘者发生胎盘植入的关系。
方法:
本单中心病例对照回顾性研究选择了2010年1月至2017年9月在北京大学第三医院的单胎分娩数据。诊断为胎盘植入合并前置胎盘且只有一次剖宫产史者被纳入病例组,对照组则与病例组相匹配纳入了合并前置胎盘且只有一次剖宫产史者。比较两组间孕妇年龄、体重指数 (BMI)、分娩孕周、胎儿体重、孕次、产次、人工流产次数、接受辅助生殖技术 (ART) 的比率、其他子宫手术史的比率和既往选择性剖宫产的比率的差别。
结果:
与对照组相比,病例组初次择期剖宫产率更高 (90.1% vs. 69.9%, P<0.001)、孕妇的年龄更低 (32.7±4.7 岁 vs. 34.6±4.0岁,P<0.001)。病例组孕次及流产次数则明显高于对照组 (均为P<0.05)。初次剖宫产手术为临产前者再次妊娠合并前置胎盘后发生胎盘植入的风险明显升高 (OR: 3.32; 95% CI: 1.68-6.58)。
结论:
初次剖宫产手术为临产前的孕妇再次妊娠合并前置胎盘时,发生胎盘植入的风险明显升高。
INTRODUCTION
Placenta accreta, also known as abnormally invasive placenta, is characterized by the excessive invasion of chorionic villi to the myometrium or even to the uterine serosa and neighboring organs. Abnormalities that are more serious include placenta increta, where there is villous invasion to the deep myometrium, and placenta percreta, in which there is complete villous invasion to the uterine serosa and even neighboring organs.[1] The incidence of an abnormally invasive placenta is reported to occur in 2–90/10,000 births and has increased over the past 30 years and is still increasing.[2,3,4] Placenta accreta, as one of the most severe obstetric complications, threatens maternal life because it leads to massive hemorrhage during delivery. Moreover, there are increased rates of hysterectomy, neighboring organ damage, blood product transfusion, Intensive Care Unit admission, and prolonged hospitalization in these cases.
The pathogenesis of placenta accreta is still unclear. However, increasing number of cesarean section (CS), endometrial damage, and placenta previa were reported as risk factors.[5] CS is an independent risk factor with at least a twofold increase in the incidence of placenta accreta.[5] CS delivery rates have risen significantly in recent years. Importantly, accumulating data indicate that the current high rates of CS are in large part due to an increasing number of planned elective or nonmedically indicated CS.[6,7]
Although CS is a widely reported and accepted risk factor for placenta accreta, few studies have reported the effect of primary CS timing on the subsequent accreta occurrences. CS timing consists of elective CS, which means that the CS is performed without prior labor, and emergency CS, which are performed after established labor. The aim of this study was to investigate the relationship between primary emergency CS and placenta accreta.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Peking University Third Hospital. As this was a retrospective study, written informed consent was not obtained, but all patients’ records/information were anonymized before analysis.
Subjects
A retrospective single-center case-control study was performed in a tertiary hospital (Peking University Third Hospital). Medical data of over 33,000 singleton pregnancies with childbirth occurring between January 2010 and September 2017 were reviewed. Only women with placenta accreta, placenta previa, and one previous CS were included in the case group. Placenta accreta cases and controls were matched by one CS and placenta previa to control for the high risk of placenta accreta due to placenta previa and CS. Controls were selected in women with one previous CS that was complicated with placenta previa. This allowed us to investigate the association between the type of primary CS and placenta accreta in a pregnancy complicated with placenta previa.
Case ascertainment
Placenta accreta was defined as follows: the placenta adhered to the uterine wall and could not be easily separated. In more severe cases, manual removal of the placenta, blood transfusion, or even hysterectomy was conducted, if necessary. In the worst cases, the placenta can penetrate through the myometrium to the uterine serosa and may invade adjacent organs. Most of these patients require massive transfusions and a hysterectomy. The diagnosis was made based on clinical findings or combined with histological findings. Placenta previa was defined as when the lower edge of the placenta reaches or covers the internal os. Primary CS was defined as the first CS delivery, regardless of whether the women have a vaginal delivery before or after the first CS. The indication for primary CS was provided in detailed records. CS because of cephalopelvic disproportion, fetal distress, and labor stagnation was considered an emergency CS as it was performed after the onset of labor. The remaining was considered an elective CS.
Exclusion criteria were as follows: nulliparous women, women who had more than one CS, or those with an accreta in the absence of a prior CS delivery.
Statistical analysis
Data were analyzed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). Continuous data with normal distribution are presented as a mean ± standard deviation (SD). Independent samples t-test was used for comparisons between the two groups. Discrete variables are shown as median (range) and were compared with the Mann-Whitney U-test. The Chi-square test was performed to ascertain differences in qualitative variables. Binary logistic regression analysis was carried out to analyze the effect of primary CS on placenta accreta. A two-tailed P < 0.05 was considered statistically significant.
RESULTS
Among all recorded cases, 839 women were diagnosed with placenta accreta. Among these placenta accreta cases, 141 were complicated with placenta previa and one previous CS and were included as cases in this study. Potential controls were selected from women who had no placenta accreta. The cases and controls in the study were matched by one CS and placenta previa. At first, we planned to match each placenta accreta case with two controls. One hundred and sixty-six participants with placenta previa and one previous CS were identified; however, it was hard to obtain a complete matched set of two controls per placenta accreta cases. Finally, a total of 141 women with placenta accreta met the inclusion criteria as cases, and 166 women were qualified as controls [Figure 1]. There were 122 women in the case group and 134 in control group after 2014, and 78 (58.2%) women with advanced maternal age (>35 years) were found in the control group, which was significantly more than that of case group (39, 32.0%; P < 0.001). There were no significant differences in body mass index preconception, and at delivery between the case and control groups, and no difference in the number of women who had received assisted reproductive technology or other uterine surgery was observed (All P > 0.05). The case group had a younger maternal age (P < 0.001), lower gestational age (P < 0.001) and fetal birth weights (P < 0.001), less parity (P = 0.029), more gravity (P = 0.005), and increased induced abortions (P < 0.001), compared with the control group. The case group had more elective CS deliveries than the control group (90.1% vs. 69.9%, P < 0.001; Table 1).
Figure 1.
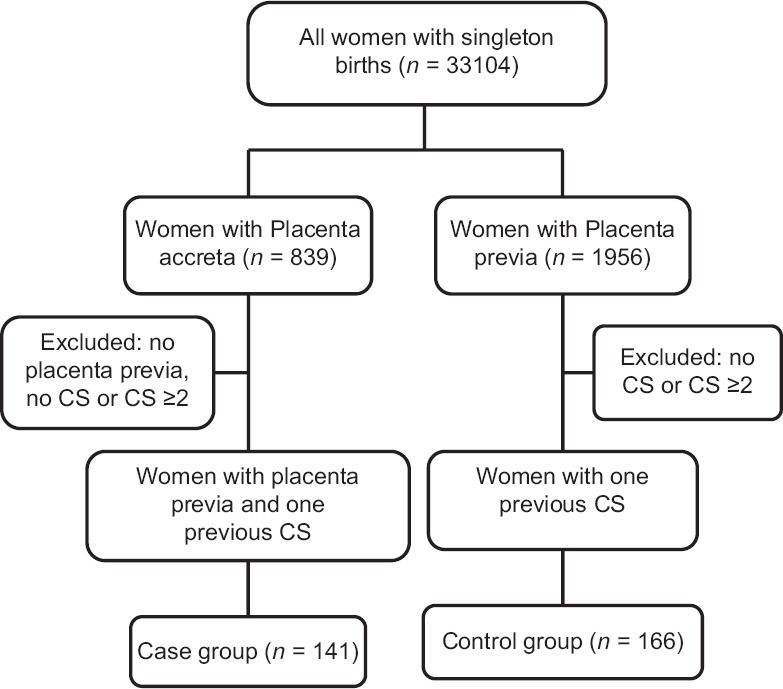
Schematic flow chart of study design, showing the identification of case and control groups. CS: Cesarean section.
Table 1.
Clinical characteristics of all women in case and control groups
| Clinical characteristics | Case group (n = 141) | Control group (n = 166) | Statistical values | P |
|---|---|---|---|---|
| Maternal age (years) | 32.7 ± 4.7 | 34.6 ± 4.0 | −3.788* | <0.001 |
| BMI preconception (kg/m2) | 22.7 ± 3.5 | 23.3 ± 3.4 | −1.587* | 0.114 |
| BMI at delivery (kg/m2) | 27.1 ± 4.0 | 27.9 ± 4.0 | −1.754* | 0.080 |
| Gestational age (weeks) | 36.1 ± 5.3 | 37.9 ± 1.9 | −3.955* | <0.001 |
| Fetal birth weight (g) | 2689.2 ± 597.6 | 3197.4 ± 567.5 | −7.516* | <0.001 |
| Gravity | 2 (2–7) | 2 (2–8) | 2.777† | 0.005 |
| Parity | 1 (1–2) | 1 (1–5) | −2.184† | 0.029 |
| Induced abortion | 1 (0–4) | 1 (0–4) | 3.608† | <0.001 |
| Received ART | 3 (2.1) | 3 (1.8) | 0.041‡ | 0.840 |
| Other uterine surgery | 9 (6.4) | 10 (6.0) | 0.017‡ | 0.897 |
| Elective CS | 127 (90.1) | 116 (69.9) | 18.837‡ | <0.001 |
Data are shown as mean ± SD, median (range) or n (%). *t value; †Z value; ‡Chi-square value. ART: Assisted reproductive technology; CS: Cesarean section; SD: Standard deviation; BMI: Body mass index.
The univariate analysis showed that maternal age, gravity, parity, induced abortion, and the primary CS timing were significantly associated with placenta accreta. Then, a binary logistic regression analysis was performed to analyze the effects of the women's age, gravity, parity, induced abortion, and elective CS on the presence of placenta accrete, and the results showed that the variables did have relationships with placenta accreta. Women in case group were younger in age (odds ratio [OR]: 0.89, 95% confidential interval [CI]: 0.84–0.95), had higher gravities (OR: 1.26, 95% CI: 1.24–1.52), and had more induced abortions (OR: 1.50, 95% CI: 1.16–1.94), compared with the control group. Compared with the women who had a primary emergency CS after labor onset, the women whose primary CS was performed without labor were more likely to develop a subsequent placenta accreta (OR: 3.32, 95% CI: 1.68–6.58; Table 2).
Table 2.
Logistic regression analysis for placenta accreta in case and control groups
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Maternal age | 0.89 | 0.84–0.95 | <0.001 |
| Gravity | 1.26 | 1.24–1.52 | 0.021 |
| Parity | 0.54 | 0.27–1.06 | 0.074 |
| Induced abortion | 1.50 | 1.16–1.94 | 0.002 |
| Elective versus emergency CS | 3.32 | 1.68–6.58 | 0.001 |
OR: Odds ratio; CI: Confidence interval; CS: Cesarean section.
Placenta accreta occurred more often in women who had a primary elective CS. The case group included placenta accreta, increta, and percreta according to the invasion depth of the chorionic villi to the myometrium. As the depth grew, the primary elective CS ratio increased (83.3% in accreta, 89.7% in increta, and 94.9% in percreta, respectively); however, they were not significantly different from each other [Table 3].
Table 3.
Distribution of abnormally invasive placenta in case group
| Abnormally invasive placenta | n | Frequency of elective CS, n (%) |
|---|---|---|
| Accreta | 24 | 20 (83.3) |
| Increta | 78 | 70 (89.7) |
| Percreta | 39 | 37 (94.9) |
CS: Cesarean section.
DISCUSSION
In the present study, primary CS performed without labor onset had increased risk of placenta accreta. This study observed that women with primary elective CS were over three times more likely to develop placenta accreta in a subsequent pregnancy complicated with placenta previa, compared with women whose primary CS was performed after labor onset. Furthermore, this study demonstrated that women who were younger in age, had higher gravities and induced abortions, also had a higher risk of developing subsequent placenta accreta.
The CS rate has increased worldwide in recent decades. In China, the CS rate was 46.2% in 2008 according to a WHO global survey,[8] and it has reportedly risen to 54.5%, recently.[9] Nonindicated CS, also known as cesarean delivery on maternal request, accounted for 38.4% of CS and may be one of the drivers of the skyrocketing CS rate in China.[10,11,12] Furthermore, many studies found that CS may increase maternal morbidity, neonatal mortality, and other complications in subsequent pregnancies including abnormal placentation and uterine rupture.[13,14,15] One prior CS may cause a sevenfold increase in the risk of abnormally invasive placenta.[4]
CS and placenta previa are widely accepted as independent risk factors for placenta accreta.[5] Therefore, the participants in case and control groups of this study were included with one previous CS and a coexisting placenta previa for the sake of correcting for the above factors. The present study demonstrated that women with primary elective CS had a higher chance of developing placenta accreta, which was similar to the results observed in Kamara et al.'s study.[16] Some researchers also found similar associations between the CS timing and placenta previa.[17,18] Why women with primary elective CS are at higher risk of placenta accreta? A possible reason might be that the incisions are different in position, length, and healing compared with emergency CS. In contrast to a uterus during labor, a quiescent uterus has thick myometrium whose lower segment is relatively high and thick because of a lack of contractions, and cutting into the lower segment might cause more bleeding as well as difficulty in suturing. Furthermore, a uterus during labor has been subjected to contractions, which might shorten the wound, diminish damage to the endometrium, and render the tissue with more potential for healing. It was reported that women with an elective CS history had thicker lower uterine segments at term, and this might imply better healing in elective CS compared with women who had an emergency CS.[19,20] A second hypothesis was that the immune status changes from tolerance to rejection following labor onset.[21,22] The microenvironment in the laboring uterus, which is immunological active, may stimulate restructuring and healing after CS. We speculated that the absence of this uterine activation might result in abnormal placentation in a subsequent pregnancy.
The results of this study also indicated that younger age, higher gravity, and more induced abortions were related to placenta accreta. The younger maternal age in the case group in this study was different from other studies, which showed that older age was associated with placenta accreta.[4,5] A possible reason was that the encouragement of the “two-child policy” after 2014 increased the number of women with advanced maternal age to over threefold in our medical center. The ratio of advanced maternal age was significantly increased in the control group compared with the case group after 2014. This special phenomenon caused by the “two-child policy” might attribute to the younger age in the case group in our study. More induced abortions, also called dilatation and curettage, are a widely known risk factor for placenta accreta.[23,24] Curettage may cause endomyometrial injury, leading to poor decidualization and placenta penetration, which subsequently causes accreta. Moreover, more induced abortions may be a major cause of the higher gravities observed in the case group of our study.
There were several limitations in this study. First, this was a retrospective study, and all data and diagnoses were based on clinical records. There were inevitable biases in this retrospective study. Second, the total sample size was small. Despite reviewing every possible control, we were unable to match each participant in case group with two participants in control group. The relationships between the different types of creta (accreta, increta, and percreta) and CS timing are still unclear because of the small sample size. This might be another reason of bias.
In conclusion, this study suggests that the primary CS timing influenced the risk of placenta accreta in subsequent pregnancies that are complicated by placenta previa, and therefore, might inform clinical decision-making related to primary CS, especially in cases of a maternal request or nonmedically indicated CS.
Financial support and sponsorship
This study was supported by a grant from the National Key R&D Program of China (No. 2016YFC1000408).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Silver RM, Barbour KD. Placenta accreta spectrum: Accreta, increta, and percreta. Obstet Gynecol Clin North Am. 2015;42:381–402. doi: 10.1016/j.ogc.2015.01.014. doi: 10.1016/j.ogc.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: Twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458–61. doi: 10.1016/j.ajog.2004.12.074. doi: 10.1016/j.ajog.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 3.Belfort MA. Placenta accreta. Am J Obstet Gynecol. 2010;203:430–9. doi: 10.1016/j.ajog.2010.09.013. doi: 10.1016/j.ajog.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Thurn L, Lindqvist PG, Jakobsson M, Colmorn LB, Klungsoyr K, Bjarnadóttir RI, et al. Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: Results from a large population-based pregnancy cohort study in the Nordic countries. BJOG. 2016;123:1348–55. doi: 10.1111/1471-0528.13547. doi: 10.1111/1471-0528.13547. [DOI] [PubMed] [Google Scholar]
- 5.Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, Silver RM, et al. Risk factors for placenta accreta: A large prospective cohort. Am J Perinatol. 2014;31:799–804. doi: 10.1055/s-0033-1361833. doi: 10.1055/s-0033-1361833. [DOI] [PubMed] [Google Scholar]
- 6.Aminu M, Utz B, Halim A, van den Broek N. Reasons for performing a caesarean section in public hospitals in rural Bangladesh. BMC Pregnancy Childbirth. 2014;14:130. doi: 10.1186/1471-2393-14-130. doi: 10.1186/1471-2393-14- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maaløe N, Bygbjerg IC, Onesmo R, Secher NJ, Sorensen BL. Disclosing doubtful indications for emergency cesarean sections in rural hospitals in Tanzania: A retrospective criterion-based audit. Acta Obstet Gynecol Scand. 2012;91:1069–76. doi: 10.1111/j.1600-0412.2012.01474.x. doi: 10.1111/j.1600-0412.2012.01474.x. [DOI] [PubMed] [Google Scholar]
- 8.Lumbiganon P, Laopaiboon M, Gülmezoglu AM, Souza JP, Taneepanichskul S, Ruyan P, et al. Method of delivery and pregnancy outcomes in Asia: The WHO global survey on maternal and perinatal health 2007-08. Lancet. 2010;375:490–9. doi: 10.1016/S0140-6736(09)61870-5. doi: 10.1016/S0140-6736(09)61870-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Hellerstein S, Hou L, Zou L, Ruan Y, Zhang W. Caesarean deliveries in China. BMC Pregnancy Childbirth. 2017;17:54. doi: 10.1186/s12884-017-1233-8. doi: 10.1186/s12884-017-1233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Li G, Chen Y, Wang X, Ruan Y, Zou L, et al. A descriptive analysis of the indications for caesarean section in mainland China. BMC Pregnancy Childbirth. 2014;14:410. doi: 10.1186/s12884-014-0410-2. doi: 10.1186/s12884-014-0410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Wang X, Zou L, Ruan Y, Zhang W. An analysis of variations of indications and maternal-fetal prognosis for caesarean section in a tertiary hospital of Beijing: A population-based retrospective cohort study. Medicine (Baltimore) 2017;96:e5509. doi: 10.1097/MD.0000000000005509. doi: 10.1097/MD.0000000000005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song G, Wei YM, Zhu WW, Yang HX. Cesarean section rate in singleton primiparae and related factors in Beijing, China. Chin Med J. 2017;130:2395–401. doi: 10.4103/0366-6999.216415. doi: 10.4103/0366-6999.216415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mascarello KC, Horta BL, Silveira MF. Maternal complications and cesarean section without indication: Systematic review and meta-analysis. Rev Saude Publica. 2017;51:105. doi: 10.11606/S1518-8787.2017051000389. doi: 10.11606/S1518-8787.2017051000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landon MB, Hauth JC, Leveno KJ, Spong CY, Leindecker S, Varner MW, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351:2581–9. doi: 10.1056/NEJMoa040405. doi: 10.1056/NEJMoa040405. [DOI] [PubMed] [Google Scholar]
- 15.Gilliam M. Cesarean delivery on request: Reproductive consequences. Semin Perinatol. 2006;30:257–60. doi: 10.1053/j.semperi.2006.07.005. doi: 10.1053/j.semperi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Kamara M, Henderson JJ, Doherty DA, Dickinson JE, Pennell CE. The risk of placenta accreta following primary elective caesarean delivery: A case-control study. BJOG. 2013;120:879–86. doi: 10.1111/1471-0528.12148. doi: 10.1111/1471-0528.12148. [DOI] [PubMed] [Google Scholar]
- 17.Downes KL, Hinkle SN, Sjaarda LA, Albert PS, Grantz KL. Previous prelabor or intrapartum cesarean delivery and risk of placenta previa. Am J Obstet Gynecol. 2015;212:669e1–6. doi: 10.1016/j.ajog.2015.01.004. doi: 10.1016/j.ajog.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu TL, Sadler L, Wise MR. Placenta praevia after prior caesarean section: An exploratory case-control study. Aust N Z J Obstet Gynaecol. 2013;53:455–8. doi: 10.1111/ajo.12098. doi: 10.1111/ajo.12098. [DOI] [PubMed] [Google Scholar]
- 19.Brahmalakshmy BL, Kushtagi P. Variables influencing the integrity of lower uterine segment in post-cesarean pregnancy. Arch Gynecol Obstet. 2015;291:755–62. doi: 10.1007/s00404-014-3455-6. doi: 10.1007/s00404-014-3455-6. [DOI] [PubMed] [Google Scholar]
- 20.Jastrow N, Gauthier RJ, Gagnon G, Leroux N, Beaudoin F, Bujold E, et al. Impact of labor at prior cesarean on lower uterine segment thickness in subsequent pregnancy. Am J Obstet Gynecol. 2010:202, 563e1–7. doi: 10.1016/j.ajog.2009.10.894. doi: 10.1016/j.ajog.2009.10.894. [DOI] [PubMed] [Google Scholar]
- 21.Sivarajasingam SP, Imami N, Johnson MR. Myometrial cytokines and their role in the onset of labour. J Endocrinol. 2016;231:R101–19. doi: 10.1530/JOE-16-0157. doi: 10.1530/JOE-16-0157. [DOI] [PubMed] [Google Scholar]
- 22.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: Integration of endocrine and mechanical signals. Reprod Sci. 2013;20:154–67. doi: 10.1177/1933719112446084. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 23.Greenbaum S, Wainstock T, Dukler D, Leron E, Erez O. Underlying mechanisms of retained placenta: Evidence from a population based cohort study. Eur J Obstet Gynecol Reprod Biol. 2017;216:12–7. doi: 10.1016/j.ejogrb.2017.06.035. doi: 10.1016/j.ejogrb.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Beuker JM, Erwich JJ, Khong TY. Is endomyometrial injury during termination of pregnancy or curettage following miscarriage the precursor to placenta accreta? J Clin Pathol. 2005;58:273–5. doi: 10.1136/jcp.2004.020602. doi: 10.1136/jcp.2004.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]


