Abstract
Background:
Corneal stromal cells (CSCs) are components of the corneal endothelial microenvironment that can be induced to form a functional tissue-engineered corneal endothelium. Adipose-derived mesenchymal stem cells (ADSCs) have been reported as an important component of regenerative medicine and cell therapy for corneal stromal damage. We have demonstrated that the treatment with ADSCs leads to phenotypic changes in CSCs in vitro. However, the underlying mechanisms of such ADSC-induced changes in CSCs remain unclear.
Methods:
ADSCs and CSCs were isolated from New Zealand white rabbits and cultured in vitro. An Exosome Isolation Kit, Western blotting, and nanoparticle tracking analysis (NTA) were used to isolate and confirm the exosomes from ADSC culture medium. Meanwhile, the optimal exosome concentration and treatment time were selected. Cell Counting Kit-8 and annexin V-fluorescein isothiocyanate/propidium iodide assays were used to assess the effect of ADSC- derived exosomes on the proliferation and apoptosis of CSCs. To evaluate the effects of ADSC- derived exosomes on CSC invasion activity, Western blotting was used to detect the expression of matrix metalloproteinases (MMPs) and collagens.
Results:
ADSCs and CSCs were successfully isolated from New Zealand rabbits. The optimal concentration and treatment time of exosomes for the following study were 100 μg/ml and 96 h, respectively. NTA revealed that the ADSC-derived exosomes appeared as nanoparticles (40–200 nm), and Western blotting confirmed positive expression of CD9, CD81, flotillin-1, and HSP70 versus ADSC cytoplasmic proteins (all P < 0.01). ADSC-derived exosomes (50 μg/ml and 100 μg/ml) significantly promoted proliferation and inhibited apoptosis (mainly early apoptosis) of CSCs versus non-exosome-treated CSCs (all P < 0.05). Interestingly, MMPs were downregulated and extracellular matrix (ECM)-related proteins including collagens and fibronectin were upregulated in the exosome-treated CSCs versus non-exosome-treated CSCs (MMP1: t = 80.103, P < 0.01; MMP2: t = 114.778, P < 0.01; MMP3: t = 56.208, P < 0.01; and MMP9: t = 60.617, P < 0.01; collagen I: t = −82.742, P < 0.01; collagen II: t = −72.818, P < 0.01; collagen III: t = −104.452, P < 0.01; collagen IV: t = −133.426, P < 0.01, and collagen V: t = −294.019, P < 0.01; and fibronectin: t = −92.491, P < 0.01, respectively).
Conclusion:
The findings indicate that ADSCs might play an important role in CSC viability regulation and ECM remodeling, partially through the secretion of exosomes.
Keywords: Adipose-derived Mesenchymal Stem Cell, Corneal Stromal Cells, Exosomes, Extracellular Matrix Synthesis
摘要
背景:
角膜基质细胞(CSCs)是角膜内皮微环境的重要组分,可诱导构建功能性组织工程角膜。脂肪来源干细胞(ADSCs)被认为是角膜基质损伤的再生医学细胞治疗的重要方法之一。我们已经发现ADSCs在体外能诱导CSCs表型的改变,但其机制仍不明确。
方法:
ADSCs与CSCc分离自新西兰大白兔并在体外培养。使用外泌体提取试剂盒、蛋白免疫印迹法、纳米颗粒跟踪分析技术提取验证脂肪干细胞培养基中的外泌体,同时确定最佳的外泌体浓度与处理时间。使用细胞增殖检测试剂盒和流式双染色法分析脂肪干细胞外泌体对角膜基质细胞增殖和凋亡的影响。使用细胞免疫印迹法检测基质金属蛋白酶和胶原的表达,评估脂肪干细胞外泌体对角膜基质细胞迁徙活性的作用。
结果:
成功分离ADSCs与CSCs,发现最佳外泌体实验浓度为100μg/ml,对角膜基质细胞处理最佳时间为96小时。脂肪干细胞外泌体为直径在40-200nm纳米颗粒,与ADSC胞质蛋白相比,CD9、CD81、脂阀结构蛋白1和 HSP70表达阳性(P 均 <0.01)。50 μg/ml和100 μg/ml浓度的外泌体处理CSCs与单纯CSCs培养相比,外泌体显著促进CSCs增殖、抑制凋亡(主要为早期凋亡;P均<0.05)。在外泌体处理CSCs组中,基质金属蛋白酶(MMPs)下调,包括MMP1(t=80.103, P<0.01)、MMP2(t=114.778, P<0.01)、MMP3(t=56.208, P<0.01)、MMP9(t=60.617,P<0.01),细胞外基质蛋白增多,包括I型(t=-82.742, P<0.01)、II型(t=-72.818, P<0.01)、III型(t=-104.452,P<0.01)、IV型(t=-133.426, P<0.01)、V型胶原(t=-294.019, P<0.01)、纤维连接蛋白(t=-92.491, P<0.01)。
结论:
本研究发现提示ADSCs在CSCs活性调控和胞外基质重塑中有重要作用,与其分泌外泌体有一定相关。
INTRODUCTION
In humans, the corneal stroma occupies 90% of the corneal thickness and plays an important role in maintaining corneal transparency and strength.[1] Although promising treatment strategies have been reported, the dismal outcome of corneal stromal damage remains unchanged. Human corneal stroma in a healthy cornea is composed of highly organized lamellae and mitotically quiescent keratocytes with dendritic morphology.[2,3] Damage from topical drugs, trauma, and infection often leads to a loss of intercellular contact and stimulates keratocyte-fibroblast-myofibroblast transition, increasing the risk of corneal scar formation, corneal opacification, and visual impairment.[2,4]
Adipose-derived stem cells (ADSCs) are mesenchymal stem cells and have been successfully isolated from adipose tissue in large amounts.[5] ADSCs have the ability to self-renew and the potential to differentiate into one or more cell lineages via induction.[5] ADSCs double every 2–4 days, depending on the culture medium and passage number.[6] The characteristics of ADSCs, including cell interactions between themselves and other cells, as well as interactions between stem cells and the extracellular matrix (ECM), can be regulated by several factors. ADSCs are also able to stimulate the proliferation of stem cells that can differentiate into specialized lineages.[5] Moreover, ADSCs can produce antioxidants, free radical scavengers, and heat-shock proteins (HSPs) in the ischemic area to rescue damaged cells. ADSCs have the potential to differentiate into adipose, bone, cartilage, neuron, and other cell lineages.[5,6,7] In addition, a large number of ADSCs can be easily acquired from liposuction surgeries without ethical issues. These characteristics make ADSCs ideal for cell therapy.
ADSCs play an important role in the regulation of the mesenchymal microenvironment. The tumor microenvironment is defined as a special kind of mesenchymal microenvironment that regulates many aspects of tumorigenesis.[8] Numerous reports have confirmed the complex and dynamic interplay between cancer cells and resident ADSCs.[8] It has been demonstrated that intravenous injection of ADSCs promotes the growth and metastasis of epithelial ovarian cancer.[9] However, another study has reported that implanted ADSCs have a suppressive effect on breast cancer and prostate cancer by inducing apoptosis.[6] These studies have shown that the effect of stem cells on the mesenchymal microenvironment is controversial, and that the complex regulation by ADSCs in vivo might depend on specific cell types. Investigators have reported that ADSCs are useful for the cell therapy of corneal stromal damage and postulated a number of nonexclusive mechanisms through which ADSCs may restore tissue integrity in disease states (e.g., via differentiation into somatic cells, secretion of cytokines and growth factors, and reduction under oxidative stress).[6] Corneal stromal cells (CSCs), which are mesenchymal-derived cells, are the principal cells of the corneal stroma. Most corneal diseases, including immune, infectious, and ectatic diseases, primarily or secondarily involve the corneal stroma, which accounts for 90% of the corneal thickness.[4] However, the regulatory roles and underlying mechanisms of the effects of ADSCs on CSCs remain unclear.
Exosomes are nanoparticles, sized 30–100 nm, produced by the reverse budding of multivesicular bodies upon fusion with plasma membranes.[10] Exosomes can be secreted from the surfaces of cells into the extracellular space and can enter the vascular system or various biological fluids.[10] The effects of exosomes depend on the specific cell types from which they arise. Exosomes from tumor cells may affect the immune system via the suppression of immune cells.[4] Exosomes from normal immune cells may trigger the inhibitory effects of cancer.[11,12,13] However, the effects of ADSC-derived exosomes on ECM synthesis of CSCs have not been investigated deeply.
In this study, we aimed to demonstrate the role of ADSC-derived exosomes in CSC viability regulation. We established exosome-treated CSCs to determine the effect and the underlying mechanism of ADSC-derived exosomes on proliferation, apoptosis, and especially ECM remodeling of CSCs.
METHODS
Ethics approval
All studies were performed under the American Association for the Accreditation of Laboratory Animal Care guidelines for the humane treatment of animals and adhered to national and international standards. In addition, the study was approved by the Ethics Committee of Zhejiang Provincial People's Hospital (No. KY2013053).
Isolation and culture of ADSCs
ADSCs were isolated from subcutaneous adipose tissue in the groin of New Zealand white rabbits (purchased from Wuxi Puhe Biomedical Co., Ltd., China) as performed previously.[14] The ADSC primary cultures were obtained by enzymatic digestion with 0.1% collagenase Type I (Invitrogen, Thermo Fisher Scientific Inc., USA) and treated with 10% fetal bovine serum (FBS; Wisent, Canada) to inactivate the collagenase. The primary cells were cultured for 14–16 days. Cells were passaged with 0.25% trypsin (Wisent, Canada) at a 1:2 ratio. After initial expansion, the obtained ADSCs were cultured up to passage 4 and then used for subsequent experiments.
Chondrogenic and osteogenic differentiation of ADSCs
The potential of ADSCs to be induced to osteoblasts and adipocytes was assessed. Briefly, passage 3 ADSCs (1 × 105 cells/well) adhering to coverslips were grown in a 6-well plate (Corning Life Sciences B.V., Netherlands) at 37°C with 5% CO2. After 24 h, cells were treated with adipogenic induction medium (AIM) consisting of 1 μmol/L dexamethasone, 10 mmol/L β-glycerophosphate, and 50 μg/ml ascorbic acid, as well as osteogenic induction medium (OIM) containing 10−6 mol/L dexamethasone, 10 μg/ml insulin, 60 μmol/L indomethacin, and 0.5 mmol/L 3-isobutyl-1-methylxanthine. Complete medium was changed after the AIM and OIM treatments for 3 days. Cells in the control group were cultured in the medium with 10% FBS. The above-mentioned reagents were purchased from Amresco (Amresco Inc., USA). At 14 and 21 days, osteoblast differentiation was verified using alkaline phosphatase detection (Jiancheng, Nanjing, China) and Alizarin Red staining (Hete, Xi'an, China). Two weeks after induction, adipocyte differentiation was identified by Oil Red O staining (Sigma, USA). The staining results were viewed under a light microscope (Leica, Germany).
Isolation and culture of corneal stromal cells
CSCs were obtained from the corneas of 1-month-old New Zealand rabbits. The cornea was incubated with 1.5% collagenase II (Invitrogen, Thermo Fisher Scientific Inc., USA) at 37°C for 45 min. Complete medium was then added to terminate digestion. The medium was collected and centrifuged at 112 ×g for 5 min, two times. The obtained CSCs were then resuspended in basal growth medium (DMEM/F12 supplemented with 20% FBS) and plated into cell culture flasks. Cells were maintained at 37°C in a 5% CO2 humidified atmosphere, and the culture medium was replaced with fresh medium every 2 days. When CSCs reached confluence, they were subcultured under the treatment of 0.25% trypsin-EDTA and seeded at a 1:2 ratio. At the 4th passage of CSCs, the medium was replaced with DMEM/F12 supplemented with 10% FBS. After the initial expansion, the achieved CSCs were cultured up to passage 2 and then used for subsequent experiments.
Exosome isolation, nanoparticle tracking analysis, and exosome protein quantification and characterization
The procedure for the isolation of exosomes from the culture medium of ADSCs (passage 3) was performed using an Exosome Isolation Kit (Invitrogen, USA). The exosomes isolated from ADSCs were pooled for bicinchoninic acid (BCA) assay, nanoparticle tracking analysis (NTA), protein separation and characterization, and Western blotting analysis. For the BCA assay (Thermo Scientific, USA) and NTA (JEM2100, JOEL Inc., Peabody, MA), the isolated exosomes were pelleted, fixed in 2.5% glutaraldehyde in cacodylate buffer at 20°C for 1 h, and stained with 2% uranyl acetate after three washes with phosphate buffered saline. The proteins in DMEM supplemented with 10% serum were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The exosomes produced by ADSCs in DMEM were purified, and the proteins in different exosome fractions (1 μg, 2 μg, 10 μg, and 50 μg) were also separated by SDS-PAGE. The gel was stained with Coomassie blue (Bio-Rad, Hercules, CA, USA) for analysis. For Western blotting analysis of the culture medium, conditioned medium, and exosome fractions, the following primary antibodies were used: mouse monoclonal anti-CD9 (Santa Cruz Biotechnology, USA), rabbit polyclonal anti-CD81 (TSG101; Abcam, USA), flotillin-1 (Abcam), and HSP70 (Abcam) antibodies.
Cell morphology
The cell morphology of individual cells was observed by culturing the cells (passage 2) on heat-sterilized cover slips at a concentration of 1 × 104 cells/ml. After reaching confluency, the cells were observed for different periods (3, 5, 7, or 9 days) using a microscope (Leica, Germany).
Immunofluorescence
For immunofluorescence (IF), cells (1 × 104 cells/ml) adhering to coverslips were seeded into a 24-well plate. And 24 h after induction, the cells were then fixed with cold methanol for 10 min and blocked with 5% FBS. The coverslips were incubated with primary antibodies (CD29-PE, CD44-PE, CD90-PE, CD34-PE, and CD45-PE) overnight at 4°C. The cells were viewed via a fluorescence microscope (Nikon, Japan).
Cell proliferation and cell apoptosis
Exponentially growing CSCs (1 × 104 cells/ml, 100 μl) were seeded into 96-well plates and incubated with exosomes from passage 3 ADSCs at different concentrations (Group 1: 0 μg/ml exosome, Group 2: 12.5 μg/ml exosome, Group 3: 25.0 μg/ml exosome, Group 4: 50.0 μg/ml exosome, and Group 5: 100.0 μg/ml exosome) for different time periods (0, 1, 2, 3, 4, or 5 days). Subsequently, a Cell Counting Kit (CCK-8; Dojindo, Japan) was used to evaluate cell proliferating activity according to the manufacturer's instructions. Proliferation was analyzed by measuring optical density, where the absorbance of 100 μl of the cell suspension was read by a spectrophotometer at 450 nm (BioTek ELx800, BioTek, USA). All assays were performed in triplicates.
For the apoptosis analysis, cells were added to a 96-well culture plate (1 × 105 cells/well) and treated as mentioned above. Cell apoptosis was then analyzed by propidium iodide (PI) staining and annexin V-fluorescein isothiocyanate (FITC)/PI staining (Bestbio, Shanghai, China).
Western blotting
Radioimmunoprecipitation assay buffer (Beyotime, Shanghai, China) was used to lyse cells for 10 min on ice. Cells were then centrifuged at 11,200 ×g at 4°C to remove cell debris. After SDS-PAGE, equal amounts (30 μg) of cell extracts were transferred onto polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA), followed by incubation with primary rabbit monoclonal antibodies against aldehyde-3-dehydrogenase (ALDH); matrix metalloproteinase (MMP) 1, MMP2, MMP3, and MMP9; collagen I, II, III, IV, and V; and fibronectin. Proteins were detected using peroxidase-conjugated affinipure secondary IgG antibody (1:2000; Proteintech Group, Inc., Chicago, IL, USA) and visualized using a chemiluminescence detection system. Blots were detected using ImageLab™ Software, version 5.1 (Bio-Rad).
Statistical analysis
Data are presented as the mean ± standard deviation (SD). All statistical analyses were performed using one-way or factorial analysis of variance (ANOVA) or a two-tailed Student's t-test. All assays were repeated in triplicates. A value of P < 0.05 was considered statistically significant.
RESULTS
Phenotypic characterization of ADSCs and corneal stromal cells
Primary ADSCs showed a fibroblastoid, adherent, and typical spindle-shaped morphology [Figure 1]. The presence of CD29, CD44, and CD90 and the absence of hematopoietic and endothelial markers, CD34 and CD45, were used for ADSC identification. IF analysis revealed that the ADSCs had high expression of CD29, CD44, and CD90 and low expression of CD34 and CD45 [Figure 1].
Figure 1.
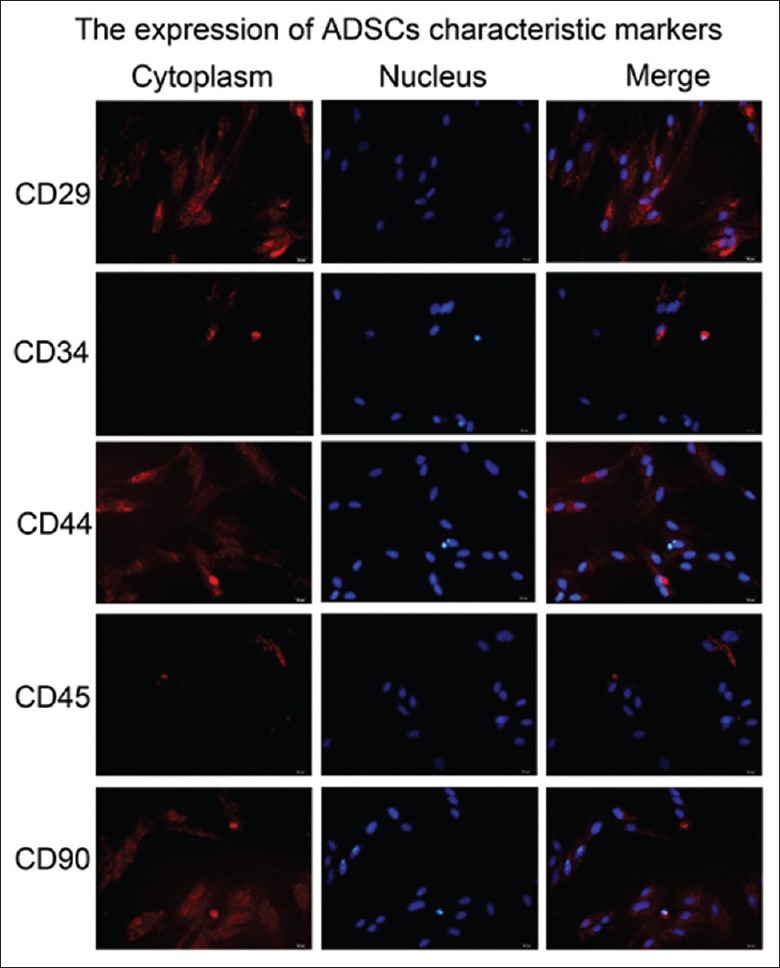
The culture and identification of adipose-derived stem cells in vitro. The ADSCs morphology was observed under the microscope, the cells were spindle shaped and growing vigorously, and the mitotic figures were visible. The expression of the markers (CD29, CD90, CD34, and CD45) for ADSCs was shown as red fluorescence within the cells using IF analyses. The nuclei of the cells were stained blue with DAPI. Original magnification, ×400. ADSCs: Adipose-derived stem cells; IF: Immunofluorescence; DAPI: 4–6 diamidino-2-phenylinole.
We next examined the capacity of ADSCs to differentiate into prototypical mesenchymal cell types: osteoblasts and adipocytes [Figure 2a and 2b]. The data indicated that ADSCs with multipotent properties had been successfully isolated.
Figure 2.
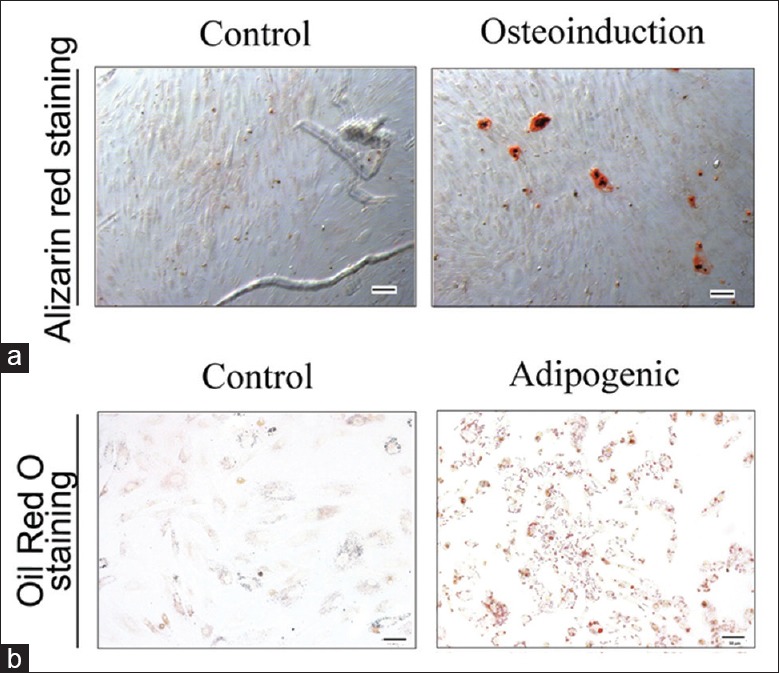
Adipogenic and osteogenic differentiations of adipose-derived stem cells. Alizarin Red staining (a, original magnification, ×200) and Oil Red O (b, original magnification, ×200) staining were used to detect the differentiations of ADSCs after induction of differentiation. ADSCs: Adipose-derived stem cells.
In vitro, CSCs were successfully isolated from New Zealand rabbits. After 7 days of inoculation at 37°C, the cells showed confluent growth [Figure 3]. The results of the IF assays showed that the CSCs had a high level of vimentin and low level of CK12 [Figure 4]. These data indicate that CSCs were isolated in high purity.
Figure 3.
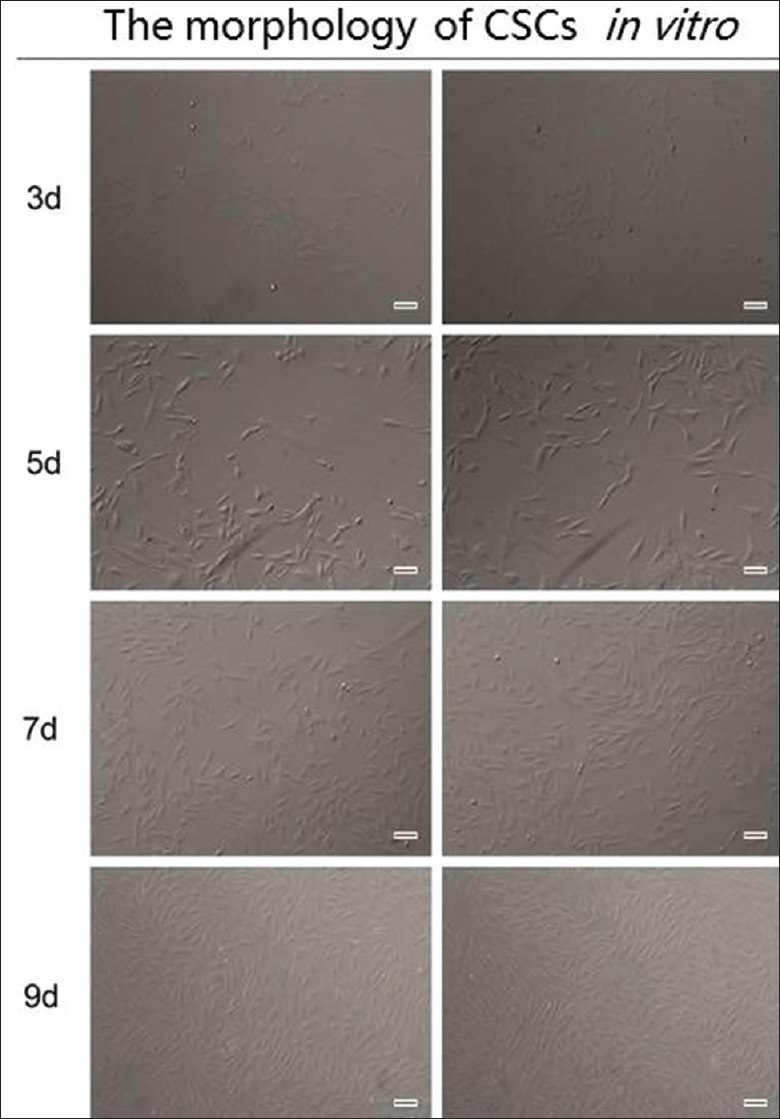
Morphology of CSCs under the microscope. The cells were spindle shaped and growing vigorously. Original magnification, ×200. CSCs: Corneal stromal cells.
Figure 4.
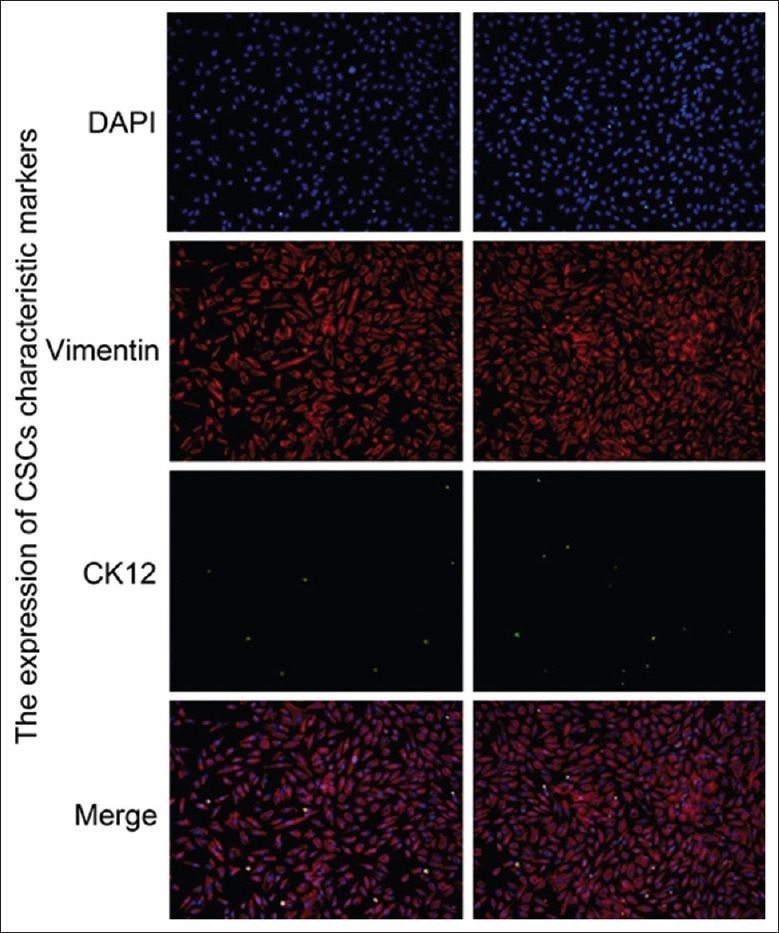
The identification of corneal stromal cells by IF. IF analysis revealed that the CSCs expressing vimentin were shown as red fluorescence and the CSCs expressing CK12 were shown as green fluorescence within the cells. The nuclei of the cells were stained blue with DAPI. Original magnification, ×200. IF: Immunofluorescence; CSCs: Corneal stromal cells; DAPI: 4-6 diamidino-2-phenylinole.
Nanoparticle tracking analysis and exosome protein quantification and characterization
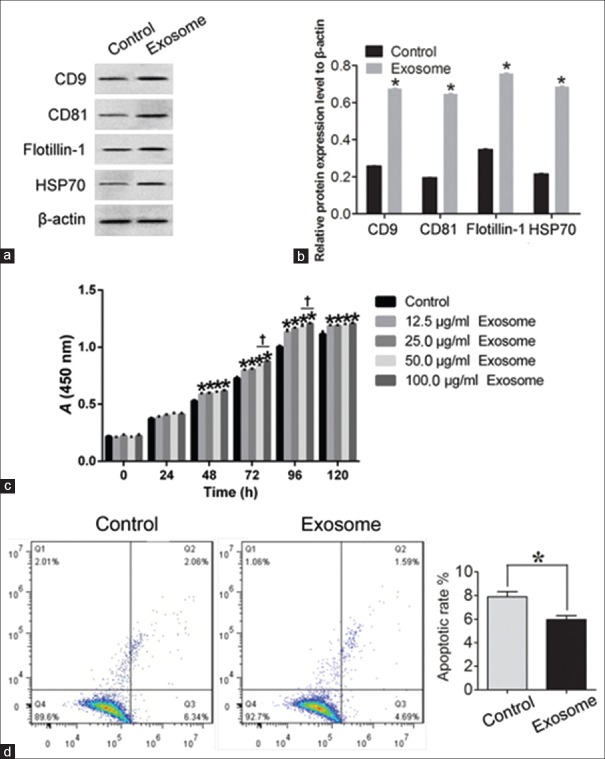
The BCA method was performed to quantify the protein concentration. The protein concentration standard curve is shown in Figure 5a. After calculation, the protein concentration of the extracted exosomes was determined to be 5.54 μg/μl. NTA revealed the presence of nanovesicles (40–200 nm, especially 113 nm) in the sample isolated using the Exosome Isolation Kit [Figure 5b]. Western blotting analysis confirmed the expression of CD9 (t = −295.483, P < 0.01), CD81 (t = −304.686, P < 0.01), flotillin-1 (t = −158.569, P < 0.01), and HSP70 (t = −175.036, P < 0.01) in the exosome fractions, compared with the ADSC cytoplasmic proteins [Figure 6a and 6b].
Figure 5.
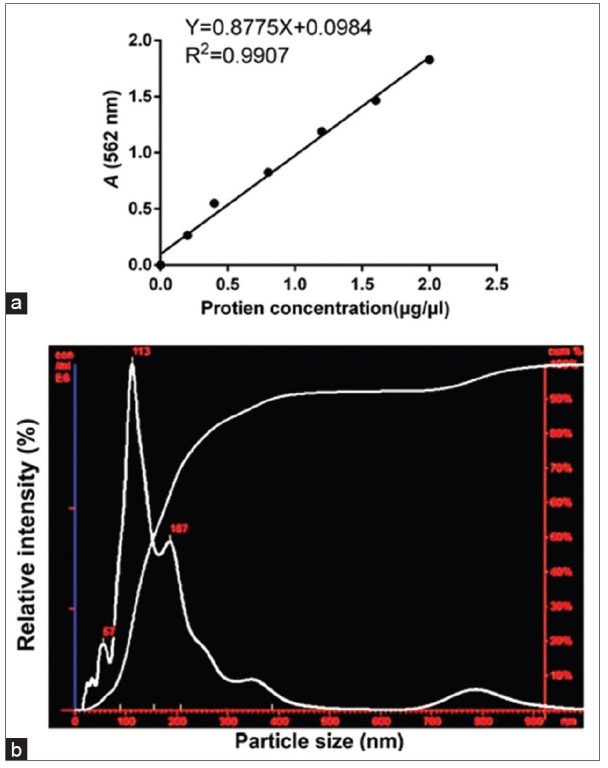
The quantification and characterization of exosomes. (a) BCA method was performed to quantify protein concentration. Protein concentration standard curve was shown. After calculation, the extraction of the protein concentration of exosomes was 5.54 μg/μl. (b) NTA evaluation shows small vesicles within the expected range of exosomes (40–200 nm) in the sample isolated from the ADSCs culture medium by ultracentrifugation. BCA: Bicinchoninic acid; NTA: Nanoparticle tracking analysis; ADSCs: Adipose-derived stem cells.
Figure 6.
Identification of exosomes with Western blotting and proliferation and apoptosis analyses of corneal stromal cells treated with exosomes in vitro using CCK-8 and annexin V-FITC methods. (a and b) Western blotting was used to analyze CD9, CD81, flotillin-1, and HSP70 in exosome. ADSCs alone were used as the control. The exosome-treated CSCs were defined as the exosome group. *P < 0.01 versus control. (c) CCK-8 Detection Kit was used to detect the proliferation of CSCs with exosomes. *P < 0.05 versus control. †P < 0.05 versus 50 μg/ml exosomes. (d) Annexin V-FITC Apoptosis Detection Kit was used to detect the apoptotic cells under control and exosome-treated conditions. All the bar graphs show the mean ± standard deviation in independent transfection experiments. CSCs alone were used as the control group, and the exosome-treated CSCs were defined as the exosome group. *P < 0.05 versus control. ADSCs: Adipose-derived stem cells; CSCs: Corneal stromal cells; CCK-8: Cell Counting Kit-8; FITC: Fluorescein isothiocyanate; PI: Propidium iodide.
ADSC-derived exosomes promote corneal stromal cell growth and inhibit the apoptosis of corneal stromal cells in vitro
To determine the effect of exosomes on the proliferative kinetics of CSCs, we examined deoxyribonucleic acid synthesis in CSCs with exosomes at different concentrations and different time points. CSCs cultured alone were also used as negative controls. Significant differences in growth were observed between the treatment group and the control group. The increase in growth of the exosome-treated group was observed as early as the 3rd day, and a higher exosome concentration (50 μg/ml and 100 μg/ml) resulted in the significant promotion of cell proliferation (Figure 6c; F = 84.197, P < 0.05, exosomes-treated CSCs vs. non-exosome-treated CSCs). In addition, 100 μg/ml and 96 h of treatment with exosomes were found to be the optimal concentration and treatment time, respectively, for studying the biological behaviors of the CSCs in response to exosomes.
To determine how ADSCs contribute to the apoptosis of CSCs, we performed apoptotic assays using CSCs. The results revealed that the presence of ADSCs inhibited the apoptosis of CSCs, compared with the negative control group. After treatment with exosomes for 5 days, the percentage of apoptotic cells was quantified using an annexin V-FITC Apoptosis Detection Kit. The percentage of (early and late) apoptotic and dead cells was calculated. A significantly lower percentage of apoptotic cells (mainly early apoptosis) was found in the exosome-treated CSCs compared to the non-exosome-treated CSCs (t = 9.608, P = 0.01; Figure 6d).
ADSCs regulate the expression of matrix metalloproteinases and collagen in corneal stromal cells and promote extracellular matrix synthesis by secreting exosomes
Our previous studies have shown that ADSCs elevate ECM synthesis in CSCs in vitro. However, the underlying mechanism is still unclear. As the above results indicate, ADSC-derived exosomes promote proliferation and inhibit apoptosis in CSCs in vitro. Thus, we assumed that ADSC-derived exosomes participate in the ECM synthesis process. We evaluated the levels of MMP1, MMP2, MMP3, and MMP9 using Western blotting. The expression of MMP1, MMP2, MMP3, and MMP9 were downregulated under the treatment of exosomes (MMP1: t = 80.103, P < 0.01; MMP2: t = 114.778, P < 0.01; MMP3: t = 56.208, P < 0.01; and MMP9: t = 60.617, P < 0.01, exosomes-treated CSCs vs. non-exosome-treated CSCs, respectively; Figure 7). A 3.04-fold, 2.86-fold, 3.00-fold, 2.73-fold, and 2.80-fold increase in collagen I, II, III, IV, and V band intensity, respectively, as well as an increase in fibronectin band intensity, was observed in the exosome-treated CSCs group (collagen I: t = −82.742, P < 0.01; collagen II: t = −72.818, P < 0.01; collagen III: t = −104.452, P < 0.01; collagen IV: t = −133.426, P < 0.01; and collagen V: t = −294.019, P < 0.01; fibronectin: t = −92.491, P < 0.01, exosomes-treated CSCs vs. non-exosome-treated CSCs, respectively; Figure 7). Based on these results, we suggest that ADSCs may be responsible for the changes in MMP and ECM (collagen and fibronectin) protein expression in CSCs through exosome secretion, which in turn promotes ECM synthesis.
Figure 7.
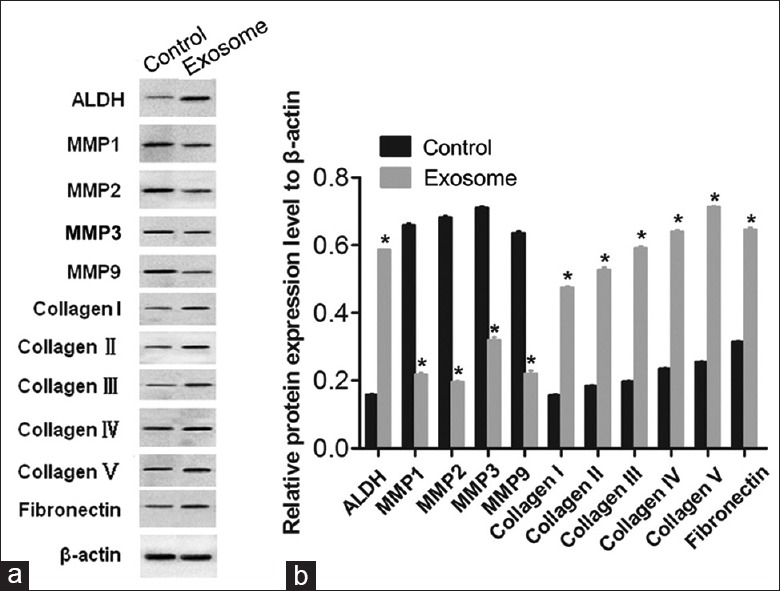
Adipose-derived stem cells-derived exosomes suppressed the expression of matrix metalloproteinases and promoted extracellular matrix synthesis in corneal stromal cells with Western blotting. Representative Western blotting images (a) and quantification (b) of the protein expression levels of ALDH, MMPs (MMP1, MMP2, MMP3, and MMP9), and collagen type I, II, III, IV, and V in CSCs and exosomes-treated CSCs (β-actin was used as the internal control). The bar graph shows the mean ± standard deviation in three independent transfection experiments. *P < 0.05 versus the CSCs alone. ADSCs: Adipose-derived stem cells; CSCs: Corneal stromal cells; MMPs: Matrix metalloproteinases; ALDH: Aldehyde-3-dehydrogenase.
In adult corneal stroma, CSCs are mitotically quiescent, with a flat, dendritic morphology, and are positive for CD34 and ALDH expression.[2] During corneal wound healing, CSCs are activated and transformed into fibroblasts, myofibroblasts, or both, losing their dendritic morphology[1] and resulting in a reduction in ALDH levels[15] and corneal transparency. The results of this study indicate that treatment with ADSC-derived exosomes increases the expression of ALDH in CSCs [Figure 7]. This suggests that ADSC-derived exosomes might promote CSC transformation into fibroblasts or myofibroblasts, thereby playing a protective role in CSC plasticity and ultimately contributing to ECM synthesis.
DISCUSSION
Most corneal diseases primarily or secondarily involve the corneal stroma, which accounts for 90% of the corneal thickness. Cell-based therapy is a promising approach to overcome the current disadvantages of corneal transplantation.[16] ADSCs are adult stem cells derived from adipose tissues and have the capacity to differentiate into multiple cell lines.[17] Thus, adipose tissue and ADSCs represent one of the major research topics in regenerative medicine. In the present study, we provide insight into the molecular mechanisms accounting for the protective role of ADSCs in CSCs. We also investigated the role of ADSC-derived exosomes in the biological behavior of CSCs. The results indicate that ADSC-derived exosomes enhance the growth and plasticity of CSCs, which may be at least partially due to the inhibition of MMP expression and stimulation of collagen expression. These findings provide a novel insight into the mechanism of how ADSCs fuel the spread of CSCs through the secretion of exosomes.
In this study, we first described the isolation and characterization of ADSCs. These cells fulfilled all the characteristics of stem cells, including self-renewal, multidifferentiation, and clonogenicity.[5,6] After five passages, the cultures consisted of rapidly dividing, ameboid-shaped ADSCs that were predominantly CD90+/CD29+/CD45−/CD34−.[5,6,18] To examine the multidifferentiation potential of the ADSCs, osteogenesis and adipogenesis were induced in the cells. Time-lapse experiments indicated that ADSC osteogenic and adipogenic abilities increased significantly, up to 69% compared to their relevant controls, indicating their multipotency.
ADSCs are demonstrated as a vital player in wound healing.[19,20,21,22] Previous reports indicate that ADSCs can transform into cells with endothelial cell characteristics,[9,23] but true endothelial cells derived from ADSCs have yet to be seen. In addition, most studies performed on ADSCs reveal that differentiation into other cells could take a longer period. Thus, we focused on the effect of ADSCs on CSCs and the underlying mechanisms. Along with others, we had proposed and indicated that (a) coculturing ADSCs with CSCs could enhance the viability and proliferation of the CSCs compared to CSCs that were not exposed to ADSCs,[16,24] and that (b) ADSCs also play a vital role in CSC plasticity.
Numerous articles have reported that ADSCs play a role in CSC plasticity via autocrine or paracrine factors.[25] In synergy with other cells, they recover damaged cells and promote the formation of new functional tissues.[25,26] Studies have also shown that ADSCs are usually placed as seed cells to produce biologically vital factors in engineered tissue constructs. A series of growth factors secreted by ADSCs are thought to be associated with their stimulating effects.[27,28] In this study, the changes in MMPs and collagens expression attracted our attention. Mounting evidence supports that MMPs and collagens mediate many changes in the ECM during wound healing to facilitate tissue regeneration.[29] The data indicate that ECM synthesis in CSCs could be promoted, which may be at least partially due to the changes in MMP and collagen levels in CSCs. These findings provide a novel mechanism for how ADSCs play a role in tissue regeneration. Exosomes are nanovesicles secreted from intracellular multivesicular bodies. Exosomes have complex molecular compositions, including common and cell type-specific proteins and lipids, messenger RNA, and microRNA, allowing them to act as a vectorized, multisignaling device.[11,30] In the present study, NTA and exosome protein quantification revealed the presence of protein-containing nanovesicles (40–200 nm) in samples positive for expression of specific markers for exosomes, CD9, flotillin-1, and HSP70, as well as CD81, a cellular protein that functions in the secretion of multivesicular bodies. All the data confirmed that the nanovesicles were exosomes. Several reports on exosomes derived from mesenchymal stem/stromal cells (MSCs) exist in the literature. Dismuke et al.[31] demonstrated that the majority of extracellular vesicles in the aqueous humor behind the cornea were in the exosomal size range, and that exosomes are a component of the human aqueous humor and contain miRNAs. In addition, it has been previously reported that exosomes secreted by CSCs can transport proteins, including MMP14, to vascular endothelial cells.[32] The results revealed that exosome-treated CSCs had a significant increase in proliferation and ECM synthesis, and a lower level of MMPs and a higher level of collagens, compared to the relevant control groups. These results indicate that exosomes contribute to the ECM-promoting effects of ADSCs in vitro to a similar extent, proposing that the promoting effects of ADSCs are mainly mediated by paracrine factors in ADSCs. To the best of our knowledge, this is the first in vitro study using ADSC-derived exosomes for the treatment of CSCs. However, the precise mechanisms involved in EMC synthesis in CSCs mediated by ADSC-derived exosomes remain elusive.
The main limitations of the present study were the lack of results on the underlying mechanisms of the antioxidant effect and further investigation using animal models. In this study, a rat model of corneal stromal damage would be useful for investigating the therapeutic impact of ADSCs. ADSCs might ameliorate corneal stromal damage, possibly via anti-oxidative effects. Furthermore, although ADSCs are a promising candidate for cell therapy in the field of corneal stromal damage, ADSCs have a limited life span during in vitro culture. Finally, vital factors may be involved in the effect of ADSCs on CSCs. We performed indirect coculturing of ADSCs and CSCs, and the interaction between these two cell types should be further studied.
In conclusion, ADSCs significantly contribute to the growth and the plasticity of CSCs, which may be associated with MMP downregulation, suggesting active participation of exosomes secreted by ADSCs. For the application of ADSCs in corneal stromal damage therapy, coculturing, the addition of growth factors, and exposing cocultures to ADSCs would significantly benefit the development of therapeutic methods for tissue engineering and regenerative medicine. Patients with CSC injuries might benefit from further studies on ADSCs.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81700795), and the Natural Science Foundation of Zhejiang Province (No. LY13H120007).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Määttä M, Väisänen T, Väisänen MR, Pihlajaniemi T, Tervo T. Altered expression of type XIII collagen in keratoconus and scarred human cornea: Increased expression in scarred cornea is associated with myofibroblast transformation. Cornea. 2006;25:448–53. doi: 10.1097/01.ico.0000183537.45393.1f. doi: 10.1097/01.ico.0000183537.45393.1f. [DOI] [PubMed] [Google Scholar]
- 2.Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, et al. The cellular basis of corneal transparency: Evidence for ‘corneal crystallins'. J Cell Sci. 1999;112(Pt 5):613–22. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 3.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–96. doi: 10.1056/NEJMoa040455. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 4.Beauregard C, Huq SO, Barabino S, Zhang Q, Kazlauskas A, Dana MR, et al. Keratocyte apoptosis and failure of corneal allografts. Transplantation. 2006;81:1577–82. doi: 10.1097/01.tp.0000209503.62204.c3. doi: 10.1097/01.tp.0000209503.62204.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang SJ, Fu RH, Shyu WC, Liu SP, Jong GP, Chiu YW, et al. Adipose-derived stem cells: Isolation, characterization, and differentiation potential. Cell Transplant. 2013;22:701–9. doi: 10.3727/096368912X655127. doi: 10.3727/096368912X655127. [DOI] [PubMed] [Google Scholar]
- 6.Rigotti G, Marchi A, Sbarbati A. Adipose-derived mesenchymal stem cells: Past, present, and future. Aesthetic Plast Surg. 2009;33:271–3. doi: 10.1007/s00266-009-9339-7. doi: 10.1007/s00266-009-9339-7. [DOI] [PubMed] [Google Scholar]
- 7.Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76:130–44. doi: 10.1111/j.1432-0436.2007.00194.x. doi: 10.1111/j.1432-0436.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- 8.Ko SF, Yip HK, Zhen YY, Lee CC, Lee CC, Huang CC, et al. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: Apparent diffusion coefficient, natural killer T-cell responses, and histopathological features. Stem Cells Int. 2015;2015:853506. doi: 10.1155/2015/853506. doi: 10.1155/2015/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu Y, Tang H, Guo Y, Guo J, Huang B, Fang F, et al. Adipose-derived mesenchymal stem cells promote cell proliferation and invasion of epithelial ovarian cancer. Exp Cell Res. 2015;337:16–27. doi: 10.1016/j.yexcr.2015.07.020. doi: 10.1016/j.yexcr.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. doi: 10.1038/srep01197. doi: 10.1038/srep0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meehan K, Vella LJ. The contribution of tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin Lab Sci. 2016;53:121–31. doi: 10.3109/10408363.2015.1092496. doi: 10.3109/10408363.2015.1092496. [DOI] [PubMed] [Google Scholar]
- 12.Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–85. doi: 10.1016/j.ijcard.2016.04.061. doi: 10.1016/j.ijcard.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 13.Lin HC, Dhanani N, Tseng H, Souza G, Wang G, Cao YN, et al. The mechanisms of nanoparticle improving adipose derived stem cells therapy for erectile dysfuncton. J Urol. 2016;195:E1139. doi: 10.1016/j.juro.2015.10.129. doi: 10.1016/j.juro.2016.02.2468. [DOI] [PubMed] [Google Scholar]
- 14.Shen T, Shen J, Zheng QQ, Li QS, Zhao HL, Cui L, et al. Cell viability and extracellular matrix synthesis in a co-culture system of corneal stromal cells and adipose-derived mesenchymal stem cells. Int J Ophthalmol. 2017;10:670–8. doi: 10.18240/ijo.2017.05.02. doi: 10.18240/ijo.2017.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–37. doi: 10.1074/jbc.M303292200. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnalich-Montiel F, Pastor S, Blazquez-Martinez A, Fernandez-Delgado J, Nistal M, Alio JL, et al. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26:570–9. doi: 10.1634/stemcells.2007-0653. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- 17.Vachkova E, Bosnakovski D, Yonkova P, Grigorova N, Ivanova Zh, Todorov P, et al. Adipogenic potential of stem cells derived from rabbit subcutaneous and visceral adipose tissue in vitro. In Vitro Cell Dev Biol Anim. 2016;52:829–37. doi: 10.1007/s11626-016-0048-7. doi: 10.1007/s11626-016-0048-7. [DOI] [PubMed] [Google Scholar]
- 18.Noël D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575–84. doi: 10.1016/j.yexcr.2007.12.022. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Han T, Amsden B, Flynn L. Adipose-derived stem cells mediate in vivo soft tissue regeneration in decellularized adipose tissue bioscaffolds. Tissue Eng Pt A. 2014;20:S6–7. doi: 10.1016/j.biomaterials.2015.08.053. [Google Scholar]
- 20.Kalinina N, Lopatina T, Efimenko A, Rubina K, Parfyonova YE, Tkachuk V. Adipose-derived mesenchymal stem cells enhance tissue regeneration by inducing growth of blood vessels. Hum Gene Ther. 2010;21:1481. [Google Scholar]
- 21.Komiyama S, Sakakura C, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, et al. Adipose-derived stem cells enhance tissue regeneration of gastrotomy closure. J Surg Res. 2013;185:945–52. doi: 10.1016/j.jss.2013.05.017. doi: 10.1016/j.jss.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Yun YP, Kim SJ, Lim YM, Park K, Kim HJ, Jeong SI, et al. The effect of alendronate-loaded polycarprolactone nanofibrous scaffolds on osteogenic differentiation of adipose-derived stem cells in bone tissue regeneration. J Biomed Nanotechnol. 2014;10:1080–90. doi: 10.1166/jbn.2014.1819. doi: 10.1166/jbn.2014.1819. [DOI] [PubMed] [Google Scholar]
- 23.Mvula B, Abrahamse H. Differentiation potential of adipose-derived stem cells when cocultured with smooth muscle cells, and the role of low-intensity laser irradiation. Photomed Laser Surg. 2016;34:509–15. doi: 10.1089/pho.2015.3978. doi: 10.1089/pho.2015.3978. [DOI] [PubMed] [Google Scholar]
- 24.Ma XY, Bao HJ, Cui L, Zou J. The graft of autologous adipose-derived stem cells in the corneal stromal after mechanic damage. PLoS One. 2013;8:e76103. doi: 10.1371/journal.pone.0076103. doi: 10.1371/journal.pone.0076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida S, Yoshimoto H, Hirano A, Akita S. Wound healing and angiogenesis through combined use of a vascularized tissue flap and adipose-derived stem cells in a rat hindlimb irradiated ischemia model. Plast Reconstr Surg. 2016;137:1486–97. doi: 10.1097/PRS.0000000000002062. doi: 10.1097/Prs.0000000000002062. [DOI] [PubMed] [Google Scholar]
- 26.Makarevich PI, Boldyreva MA, Efimenko AY, Gluhanyuk EV, Dergilev KV, Gallinger JO, et al. Therapeutic angiogenesis by subcutaneous cell sheet delivery is superior to cell injection: A study of ADSC efficacy in a model of hind limb Ischemia. Mol Ther. 2016;24:S178. [Google Scholar]
- 27.Froelich K, Setiawan LE, Technau A, Tirado MR, Hackenberg S, Hagen R, et al. Influence of different growth factors on chondrogenic differentiation of adipose-derived stem cells in polyurethane-fibrin composites. Int J Artif Organs. 2012;35:1047–60. doi: 10.5301/ijao.5000132. doi: 10.5301/ijao.5000132. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Li PH, Hou DJ, Zhang AJ, Tao CB, Li XY, et al. EGF enhances ADSCs secretion via ERK and JNK pathways. Cell Biochem Biophys. 2014;69:189–96. doi: 10.1007/s12013-013-9769-3. doi: 10.1007/s12013-013-9769-3. [DOI] [PubMed] [Google Scholar]
- 29.Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177–83. doi: 10.3109/14756366.2016.1161620. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 30.Wahlgren J, Statello L, Skogberg G, Telemo E, Valadi H. Delivery of small interfering RNAs to cells via exosomes. Methods Mol Biol. 2016;1364:105–25. doi: 10.1007/978-1-4939-3112-5_10. doi: 10.1007/978-1-4939-3112-5_10. [DOI] [PubMed] [Google Scholar]
- 31.Dismuke WM, Challa P, Navarro I, Stamer WD, Liu Y. Human aqueous humor exosomes. Exp Eye Res. 2015;132:73–7. doi: 10.1016/j.exer.2015.01.019. doi: 10.1016/j.exer.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han KY, Dugas-Ford J, Seiki M, Chang JH, Azar DT. Evidence for the involvement of MMP14 in MMP2 processing and recruitment in exosomes of corneal fibroblasts. Invest Ophthalmol Vis Sci. 2015;56:5323–9. doi: 10.1167/iovs.14-14417. doi: 10.1167/iovs.14-14417. [DOI] [PMC free article] [PubMed] [Google Scholar]