Abstract
Obesity at diagnosis is a negative prognostic indicator for several pediatric cancers including acute leukemia and bone tumors. Incidence of obesity in children has increased three fold over the past 2 decades and causes for this include poor diet, excessive caloric intake and lack of physical activity which collectively are referred to as energy balance related behaviors. Few energy balance interventions have been implemented in pediatric cancer patients during treatment, and here we will probe the rationale for pursuing such studies. The need to modify composition of calories consumed and identify specific beneficial exercise regimens will be discussed, relative to weight reduction or management.
Background on energy balance in the pediatric oncology setting
Energy balance, the balance between energy intake (calorie consumption) and energy expenditure (physical activity), has gained interest in pediatric oncology in recent years. This is largely due to the obesity epidemic in healthy children as well as children with cancer, and to the recognition in epidemiological and retrospective studies that obesity is a risk factor for several cancer types and for worse outcomes in some pediatric cancers. It is also due, in part, to increased interest from clinical care providers in improving quality of life for patients undergoing toxic anti-cancer treatments and to a growing understanding of how changes in energy balance affect the molecular biology of a tumor. Despite the relative ease of delivery of energy balance interventions, to date, there have been fewer than 30 clinical trials of physical activity interventions or diet/nutrition intervention studies in pediatric cancer patients during treatment regimens. While systematic reviews for topics related to energy balance and cancer have been and should continue to be done, there are not enough energy balance interventions conducted in pediatric cancer patients during therapy to conduct a systematic review. The goal of this article is to prompt consideration of specific diet and exercise regimens that can be delivered during treatment to potentially optimize the efficacy of therapy, and reduce risk of treatment related late effects. Many of these approaches may be ready for clinical implementation, such as moderate physical activity for prevention of functional decline, however, additional gaps in knowledge exist, such as determining the most appropriate calorie composition for patients with specific cancer types.
Over the past four decades the use of combination chemotherapy treatment strategies have raised cure rates for children and adolescents with cancer to 80% (1). This improvement in success of treatment has been attributed to the effectiveness of combination chemotherapy, and improved detection, classification and diagnosis of the type of malignancy, and the ability to identify presence of metastasis at the time of diagnosis (2). Efforts to design more specific and selective treatment modalities are still needed, and a majority of childhood cancer researchers are pursuing this goal in hopes of further raising cure rates. However, less attention has been given to factors which may influence treatment outcomes that are amenable to behavior modification.
Energy balance, or the relationship between intake of food and physical activity, is one such factor that is within the control of cancer patients and can be altered through behavioral interventions (3). Much of the work investigating energy balance in the context of cancer treatment and survivorship has focused on obesity, which can be caused by poor diet and sedentary lifestyles (4). Emerging data, primarily in adult cancers such as breast and prostate, highlight the relevance of obesity and energy balance to treatment outcomes and cure rates (5–7). Similar trends in pediatric oncology are developing. Recently it was shown that obesity and the overall nutritional status of children with cancer contributes to treatment response and patient outcome (8–13). Pediatric patients who are obese or overweight have inferior survival rates, a higher rate of relapse and are more resistant to therapy. Molecular explanations for this are postulated to be due to the altered pharmacokinetics of chemotherapy in overweight/obese patients and the inflammation-promoting interactions between adipocytes and cancer cells, which has been demonstrated in lab based studies but not in human studies as yet. Obesity rates in pediatric cancer patients have tracked with age matched controls globally, and have increased almost three fold in the past two decades (14). Based on these statistics, more newly diagnosed pediatric cancer patients are likely to be obese than ever before.
However, a key question is whether simply changing diet habits and/or exercise habits versus managing and reducing obesity will improve outcomes for these patients. Here we review the current knowledge of how obesity, nutritional status and PA affect the treatment and outcome of children and adults with cancer, and highlight studies which show the beneficial impact of implementing diet and PA regimens.
Diet – calories versus composition
Diet is certainly a component of the obesity problem and much data supports the notion that food habits of pediatric cancer patients and survivors are sub-optimal (15–17). This may be exacerbated with the use of steroid containing chemotherapy regimens (18). Weight trajectories in childhood ALL patients show that preschool children have a rapid rate of increase in overweight/obesity following diagnosis and this frequently persists after therapy is complete (19). A meta-analysis of over 1500 pediatric ALL patients in 16 studies concluded that BMI z-score increased early in treatment and in maintenance phase, and persisted beyond treatment into survivorship (20). These trends are seen in pediatric solid tumor and brain tumor patients as well, but the data on weight gain during treatment appears to be somewhat tumor type specific. A cohort of 133 newly diagnosed patients including brain and solid tumor patients showed increased BMI and fat mass within three months of and doubled by 12 months post diagnosis (21). In contrast, weight loss occurred in 37% of 468 patients with intermediate rhabdomyosarcoma, and low baseline BMI correlated with borderline inferior survival (22). Collectively, these data indicate that weight management early during cancer treatment is an opportunity for interventions, and to be successful, a nutritional program needs to be integrated into the clinical care model and must be sustained throughout treatment (23). However, research remains to be done to define precisely what “obesity management” or “good nutrition” means for these patients. Calorie reduction with no focus on the calorie composition or which foods the calories are coming from may not be adequate to improve outcomes. For example it is unclear whether reduced intake of sugars, carbohydrates, or fat versus increased protein intake or increased consumption of fruits and vegetables will be beneficial.
A recent study examining diet in 640 newly diagnosed pediatric acute lymphocytic leukemia patients found that caloric consumption was in excess of daily recommended intake, and that consumption of vitamin E and D was below recommended daily allowance(24). These nutritional deficiencies appear to extend beyond treatment since in pediatric cancer survivors spanning diagnoses and age groups, fruit and vegetable consumption is low (25). ALL survivors consumed less vitamin D, calcium, fiber and potassium than recommended guidelines, but exceed saturated fat and sodium intake guidelines (17). In an effort to improve bone mineral density in childhood ALL survivors which is compromised due to corticosteroids and methotrexate treatment regimens, a randomized supplementation trial using calcium and vitamin D or placebo was carried out in 275 participants over a two year span(26). Although bone density did not improve in the intervention group, the dosing of Vitamin D, and the timing of supplementation relative to chemotherapy exposure are variables that require consideration before drawing negative conclusions regarding the potential benefits of supplementation. Also, rather than using supplements, whole foods that are nutrient dense across food groups per the 2015–2020 Dietary Guidelines for Americans, could be employed during delivery of steroid or methotrexate intensive treatment regimens for pediatric cancer patients.
The obesity problem has been attributed in part to increased consumption of sugar and fat (27). Whether intake of sugar and fat contributes to poor outcomes in cancer patients independent of obesity is unclear. For example, epidemiological data shows that sugar intake correlates with increased cancer incidence, and mouse models for breast and liver cancer indicate that sucrose-rich diets promote tumor progression and metastasis in normal weight non-obese mice (28–32). Molecular explanations for sugar’s impact on tumor progression have been put forth from patient data and mouse models. Specific metabolites associated with the lipoxygenase pathway have also been correlated with sugar consumption. The lipoxygenase pathway, as well as activation of the IGF pathway, are linked to increased levels of intracellular and extracellular reactive oxygen species (ROS). Similarly, high levels of protein and fat consumption generate oxidative stress, which can promote cancer progression (33). A series of studies have documented that elevated serum levels of IGFBP2 are predictive of increased risk of recurrence in pediatric leukemia patients (34–36). Little is known about dietary influences on IGFBP2, however, a cross-sectional analysis of 4,731 men and women enrolled in the European Prospective Investigation into Cancer and Nutrition study showed an association between dairy protein consumption and circulating IGFBP2 concentrations (37). These data suggest that for patients on therapy, high sugar diets should be avoided and protein and fat consumption needs to be monitored. In stark contrast to this concept, recent data shows that obese melanoma patients or those with a high BMI had a better response to kinase inhibitor therapy (38) and that in mouse models, a ketogenic, high fat, low carbohydrate diet inhibited the growth and progression of glioblastoma (39). Therefore tailoring of diet recommendations to tumor type and therapy type is critical and suggests that perhaps the calorie composition rather than the total calories or the patient’s BMI should be the focus of behavioral interventions aimed at changing diet.
Mouse modeling of energy balance interventions using pediatric models will yield data necessary to optimize a diet regimen with the potential to improve treatment outcomes, but delivery in patients poses additional challenges. Few dietary interventions have been implemented in pediatric cancer patients and survivors and emphasis has primarily been on healthy lifestyles and nutrition education rather than use of a specific or prescribed diet (40–42). Surprisingly, the strongest data in this field has come from ketogenic diets(43). Long used for epilepsy patients, ketogenic diet improves therapy efficacy for brain tumors in mouse models (39). Only one small case study of two pediatric patients with astrocytoma tumors has been published to date and demonstrated decreased glucose uptake and improved mood in one patient using ketogenic diets(44,45). However, the feasibility of delivery of these diets to children is questionable, as is the drastic caloric restriction diets that show increased efficacy in mouse models for prostate, breast and ovarian cancers(46). Limited data in adult glioma patients(47)indicates the safety and feasibility of the ketogenic diet, caloric restriction, as well as minor modifications of these regimens and encourages its use as an adjunctive therapy. As described above, high sugar and high fat diets are being documented to negatively impact treatment outcomes while enrichment for other macro or micronutrients could have beneficial effects. In children, a major determinant of diet interventions involves parental practices. While short term diet interventions in a hospital setting could be controlled through collaboration between physicians and dieticians, diet change in an outpatient setting requires education and perhaps even interventions designed for parents (48). These types of interventions must be matched culturally, socio-economically, and with attention to time required for food preparation and unique palates of children with cancer, which may be changed due to side effects of treatment such as nausea and altered senses of taste and smell. Despite these challenges, a recent systematic review found that adherence to lifestyle interventions improves when a parental component is included(49).
Exercise – feasibility and timing
While diet modification can play a role in controlling obesity and BMI, there can be adverse effects of diet-induced weight loss on muscle strength (50). There can be a significant loss of fat-free mass depending on the diet modification and the calorie reduction. Strategies such as weight training or aerobic exercise can combat the loss of muscle mass and strength (50). Physical activity (PA) can also play a role in controlling BMI and obesity as energy balance is defined as calories consumed – energy expended. Therefore, it is important for a nutritional program for cancer patients to also include a PA component to increase the number of calories burned and prevent loss of muscle mass. Exercise has been shown to be both feasible and safe in Pediatric cancer patients undergoing chemotherapy (51). Exercise has also been shown to improve quality of life of cancer patients and reduce the risk of cardiovascular disease in cancer survivors (52,53). However, there is still hesitancy by clinicians to use PA in the cancer care setting, largely due to uncertainties about how to appropriately prescribe it. First, it is unclear when PA is most beneficial. Most epidemiological studies have been used to support exercise as part of a healthy lifestyle for cancer prevention, while most clinical studies have used PA/exercise in the survivorship setting as part of obesity management. One growing field of research focuses on the use of exercise during active therapy for a cancer patient, the topic of this review. Additionally, it is unclear whether exercise quantity, quality or both is most important. Due to the large variability in experimental design in published studies in terms of type of exercise, frequency, and duration of the intervention program, identifying the “best” exercise program is challenging. Finally, the relationship between obesity and exercise efficacy is not well-defined. It remains unknown whether exercise can have beneficial effects even in the absence of weight control.
Exercise or PA during cancer treatment can be used for three main purposes: to improve the quality of life of a cancer patient (psychosocial benefits), to prevent decline of physical functioning and secondary medical complications (obesity prevention, atrophy prevention), and to improve the efficacy of chemotherapy. The data for each of these broad categories is described below, with an emphasis on data specific to pediatric patients. Studies in adults have unequivocally demonstrated improved quality of life (QOL) for cancer patients who are physically active, reporting decreased fatigue, increased mobility, and decreased depression(54,55). The pediatric literature is much less robust, as fewer studies using PA/exercise have been performed in pediatric cancer patients. Those that have been performed support a positive effect of PA on QOL, with varying levels of significance, and have been well reviewed (56,57). Importantly, a reduction in pain and perceived procedure anxiety was achieved by a combined PA and psychosocial intervention for patients within one year of therapy (58). Similarly, a 6-month group exercise program for outpatients of mixed diagnoses was shown to significantly improve overall motor performance, activity level, and emotional well-being (59). While improved QOL is an accepted benefit of PA/exercise for cancer patients, other benefits are also supported by research. The six randomized, prospective clinical trials that have been performed in pediatric patients undergoing treatment for Acute Lymphoblastic Leukemia (ALL) have recently been summarized (60). In all studies, cardiorespiratory fitness was significantly improved by exercise, as measured by the 9-minute walk test, despite heterogeneity in exercise programs. Similarly, in a small study of 27 ALL participants receiving maintenance therapy in their first remission, a home based exercise program was shown to achieve significant improvement in 6 minute walk test performance for 75% of participants. The mean BMI change for participants in this study was a decrease of 4.2%, indicating that a physical activity plan performed while on chemotherapy can reduce obesity (61). It’s unclear, however, whether a reduction in obesity is necessary to achieve the numerous other benefits associated with PA. In a study of 60 children with any type of malignancy, either actively on therapy or within a year of completion, PA was again demonstrated to be significantly correlated to cardiorespiratory fitness. This randomized controlled trial consisted of a 12-week exercise and psychosocial program. Each additional activity count per minute correlated with an increase of 0.05 ml/kg/min in VO2peak. Conversely, each sedentary minute reduced VO2peak by 0.06 ml/kg/min. This study also confirmed that pediatric cancer patients are less physically fit than their age-matched healthy counterparts, with significantly lower VO2peak (62). These studies demonstrate that exercise for pediatric cancer patients is feasible. However, the most effective type of exercise (aerobic vs resistance) has not been compared in pediatric populations.
In a recent study comparing aerobic exercise (motorized wheel running) to resistance training (weighted ladder climbing) as a means of preventing cancer associated cachexia using the C26 mouse colon cancer model, Khamoui et al. demonstrated that aerobic exercise preserved relative grip strength, sensorimotor function and muscle mass while resistance training did not. In fact, 25% of mice undergoing resistance training became moribund before the end of the study while none of the mice performing aerobic exercise became moribund. However, all mice lost total mass, suggesting that these exercise regimens alone are not sufficient to protect against cachexia (63). In stark contrast to the findings in mice, a recent study in breast cancer patients undergoing chemotherapy compared three times weekly aerobic exercise to three times weekly resistance training. In this study, resistance exercise training improved sarcopenia and dynapenia significantly more than aerobic exercise (64). These nearly opposite findings accentuate the need for more controlled clinical trials comparing exercise types, intensities, and frequencies, as each of these variables is likely to cause different outcomes.
Exercise during therapy is feasible and safe for pediatric cancer patients, and improves physical fitness (56). PA or exercise may also improve therapeutic efficacy. To date, no clinical trials in humans have examined the effect of PA on treatment efficacy. Animal studies using tumor-bearing mice inform our understanding of the usefulness of exercise as an adjuvant to chemotherapy. Unfortunately, the majority of animal studies have been done using models of adult cancers, but these may have relevance to pediatric oncology. The effect of exercise on tumor growth in the absence of other therapies appears to be tumor-type dependent. For example, wheel running inhibited growth of A549 lung tumors and ET1 mammary tumors in mice, but had no effect on growth of orthotopic prostate tumors (65–67). Wheel running and treadmill exercise both promoted the growth of tumors in p53+/−;MMTV-Wnt-1 transgenic mice (68). While exercise alone appears to affect different tumor types differently, exercise has consistently been demonstrated to improve chemotherapy efficacy. Five days per week of high intensity treadmill running alone had no effect on tumor growth in mice bearing orthotopic glioblastoma tumors, but increased survival and decreased tumor growth when combined with temozolomide (TMZ) compared to TMZ alone (69). Similarly, treadmill running at a more moderate intensity increased the efficacy of gemcitabine against pancreatic ductal adenocarcinoma and of doxorubicin against melanoma in mice (70). Voluntary wheel running also significantly increased the efficacy of chemotherapy in multiple models. Wheel running significantly increased the anti-tumor effect of cyclophosphamide against mammary tumors and of celecoxib against prostate tumors in mice (65,71). The heterogeneity in exercise prescriptions between these studies suggests that there may be a wide range of aerobic exercise that is beneficial to improve outcome during chemotherapy. No weight loss due to exercise was reported for mice in any of these studies, indicating that exercise may be efficacious as a therapeutic adjuvant regardless of changes in weight. However, it must be noted that these studies did not use obese mice, and therefore the importance of reducing obesity cannot be evaluated with current data.
Conclusions
In summary, the studies cited above suggest that diet quality and/or specific exercise parameters, which can contribute to obesity prevention and management, are important factors for improving outcomes for pediatric cancer patients. These studies provide impetus for investigation of focused nutritional and PA programs which manage or prevent obesity and promote healthy lifestyles in the context of the treatment of pediatric cancer patients. Patients with genetic predisposition to obesity will likely require different approaches. For example, mutations in the leptin gene (which encodes a hormone synthesized primarily by adipocytes that acts on its receptor in the hypothalamus and conveys feelings of satiety), in mice and in humans are associated with mild to severe forms of obesity(72,73). Experiments using mice bearing mutations in the leptin-melanocortin pathway showed that caloric restriction and exercise were non-identical to strain matched lean mice in terms of body fat composition, gene expression and blood lipid levels(74). These data suggest that screening patients for monogenic or polygenic obesity could be useful in determining whether energy balance interventions alone will exert beneficial effects or whether pharmacological approaches would be required to target these pathways.
Collectively, answering questions raised in this review will require concerted efforts of clinicians, researchers and behavioral scientists, but has potential to improve chemotherapy efficacy without raising the dose and accompanying toxicities/late effects, and represents a relatively inexpensive supportive care intervention. Cooperative groups and consortiums are an ideal setting for carrying out these trials, however, require that a consensus be reached regarding exactly what exercise and/or diet modification would be implemented during standard of care regimens for the most common pediatric cancers in order to determine whether improved outcomes are seen. With regard to exercise this may be easier, given the many reports of feasibility of moderate exercise regimens in pediatric cancer patients and the proliferation of activity trackers which can be used to monitor compliance to prescribed PA. For diet, challenges to implementation of a specific nutritional regimen during treatment includes a lack of consensus regarding how to assess adequate nutrition, and a lack of measures for diet compliance. Self-report of diet is inherently flawed, and further complicated in pediatric populations, and reliable biomarkers of diet modification (such as inflammation or oxidative stress related biomarkers) are confounded by factors related to cancer type, modality of treatment and patient to patient variability. These challenges provide impetus for future work in this area to clarify exactly how energy balance can leveraged in the treatment setting for pediatric oncology patients. ….
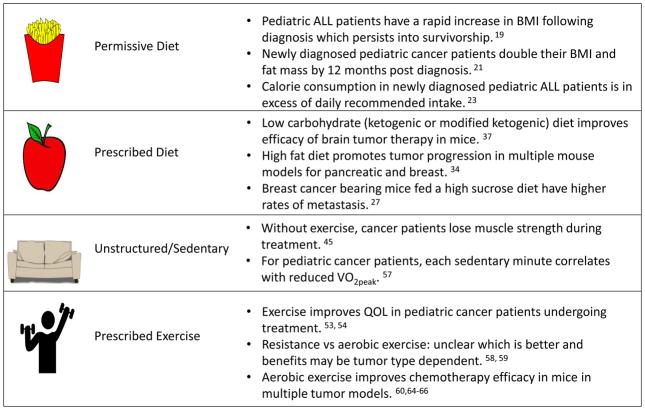
Figure 1. Rationale for structured energy balance interventions in pediatric cancer patients.
Permissive diets are associated with rapidly increased BMI and excess calorie consumption in pediatric cancer patients undergoing treatment. In contrast, prescribed diet in mouse models of brain, breast and pancreatic cancer may improve therapy outcomes. Sedentary behaviors during cancer treatment are associated with loss of muscle strength and reduced cardiorespiratory endurance. In contrast, prescribed exercise enhances chemotherapy efficacy in mouse models, and improves quality of life in patients.
Acknowledgments
Statement of financial support: Support from a Multidisciplinary Research Project from the MD Anderson Cancer Foundation to Drs. Kleinerman, Chandra and Schadler is gratefully acknowledged.
Footnotes
Disclosure statement: The authors have no relevant financial disclosures.
Category of study: Regular review
References
- 1.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–34. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaman GH. Pediatric cancer research from past successes through collaboration to future transdisciplinary research. J Pediatr Oncol Nurs. 2004;21:123–7. doi: 10.1177/1043454204264406. [DOI] [PubMed] [Google Scholar]
- 3.Ligibel JA, Alfano CM, Hershman D, et al. Recommendations for Obesity Clinical Trials in Cancer Survivors: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;33:3961–7. doi: 10.1200/JCO.2015.63.1440. [DOI] [PubMed] [Google Scholar]
- 4.Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2016.120. [DOI] [PubMed] [Google Scholar]
- 5.Wang LS, Murphy CT, Ruth K, et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2015;121:3010–7. doi: 10.1002/cncr.29472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews SB, Thompson HJ. The Obesity-Breast Cancer Conundrum: An Analysis of the Issues. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurent V, Guerard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orgel E, Tucci J, Alhushki W, et al. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood. 2014;124:3932–8. doi: 10.1182/blood-2014-08-595389. [DOI] [PubMed] [Google Scholar]
- 9.Sala A, Rossi E, Antillon F, et al. Nutritional status at diagnosis is related to clinical outcomes in children and adolescents with cancer: a perspective from Central America. Eur J Cancer. 2012;48:243–52. doi: 10.1016/j.ejca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Orgel E, Sposto R, Malvar J, et al. Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol. 2014;32:1331–7. doi: 10.1200/JCO.2013.52.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange BJ, Gerbing RB, Feusner J, et al. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293:203–11. doi: 10.1001/jama.293.2.203. [DOI] [PubMed] [Google Scholar]
- 12.Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25:2063–9. doi: 10.1200/JCO.2006.07.7792. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein G, Shemesh E, Frenkel T, Jacobson JM, Toren A. Abnormal body mass index at diagnosis in patients with Ewing sarcoma is associated with inferior tumor necrosis. Pediatr Blood Cancer. 2015;62:1892–6. doi: 10.1002/pbc.25589. [DOI] [PubMed] [Google Scholar]
- 14.Rogers PC, Meacham LR, Oeffinger KC, Henry DW, Lange BJ. Obesity in pediatric oncology. Pediatr Blood Cancer. 2005;45:881–91. doi: 10.1002/pbc.20451. [DOI] [PubMed] [Google Scholar]
- 15.Badr H, Chandra J, Paxton RJ, et al. Health-related quality of life, lifestyle behaviors, and intervention preferences of survivors of childhood cancer. Journal of cancer survivorship: research and practice. 2013;7:523–34. doi: 10.1007/s11764-013-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J, Wakefield CE, Fleming CA, Gawthorne R, Tapsell LC, Cohn RJ. Dietary intake after treatment in child cancer survivors. Pediatr Blood Cancer. 2012;58:752–7. doi: 10.1002/pbc.23280. [DOI] [PubMed] [Google Scholar]
- 17.Zhang FF, Saltzman E, Kelly MJ, et al. Comparison of childhood cancer survivors’ nutritional intake with US dietary guidelines. Pediatr Blood Cancer. 2015;62:1461–7. doi: 10.1002/pbc.25521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang FF, Rodday AM, Kelly MJ, et al. Predictors of being overweight or obese in survivors of pediatric acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2014;61:1263–9. doi: 10.1002/pbc.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler MR, Hockenberry MJ, McCarthy KS, Silva SG. Trajectories of Obesity and Overweight Rates Among Survivors of Childhood Acute Lymphoblastic Leukemia. Oncology nursing forum. 2015;42:E287–93. doi: 10.1188/15.ONF.E287-E293. [DOI] [PubMed] [Google Scholar]
- 20.Zhang FF, Liu S, Chung M, Kelly MJ. Growth patterns during and after treatment in patients with pediatric ALL: A meta-analysis. Pediatr Blood Cancer. 2015;62:1452–60. doi: 10.1002/pbc.25519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinksma A, Roodbol PF, Sulkers E, et al. Changes in nutritional status in childhood cancer patients: a prospective cohort study. Clin Nutr. 2015;34:66–73. doi: 10.1016/j.clnu.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Burke ME, Lyden ER, Meza JL, et al. Does body mass index at diagnosis or weight change during therapy predict toxicity or survival in intermediate risk rhabdomyosarcoma? A report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Pediatr Blood Cancer. 2013;60:748–53. doi: 10.1002/pbc.24322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladas EJ, Arora B, Howard SC, Rogers PC, Mosby TT, Barr RD. A Framework for Adapted Nutritional Therapy for Children With Cancer in Low- and Middle-Income Countries: A Report From the SIOP PODC Nutrition Working Group. Pediatr Blood Cancer. 2016;63:1339–48. doi: 10.1002/pbc.26016. [DOI] [PubMed] [Google Scholar]
- 24.Ladas EJ, Orjuela M, Stevenson K, et al. Dietary intake and childhood leukemia: The Diet and Acute Lymphoblastic Leukemia Treatment (DALLT) cohort study. Nutrition. 2016;32:1103–9e1. doi: 10.1016/j.nut.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warner EL, Nam GE, Zhang Y, et al. Health behaviors, quality of life, and psychosocial health among survivors of adolescent and young adult cancers. Journal of cancer survivorship: research and practice. 2016;10:280–90. doi: 10.1007/s11764-015-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaste SC, Qi A, Smith K, et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2014;61:885–93. doi: 10.1002/pbc.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norat T, Scoccianti C, Boutron-Ruault MC, et al. European Code against Cancer 4th Edition: Diet and cancer. Cancer Epidemiol. 2015;39(Suppl 1):S56–66. doi: 10.1016/j.canep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Melkonian SC, Daniel CR, Ye Y, Pierzynski JA, Roth JA, Wu X. Glycemic Index, Glycemic Load, and Lung Cancer Risk in Non-Hispanic Whites. Cancer Epidemiol Biomarkers Prev. 2016;25:532–9. doi: 10.1158/1055-9965.EPI-15-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Pan Y, Rhea PR, et al. A Sucrose-Enriched Diet Promotes Tumorigenesis in Mammary Gland in Part through the 12-Lipoxygenase Pathway. Cancer Res. 2016;76:24–9. doi: 10.1158/0008-5472.CAN-14-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Healy ME, Lahiri S, Hargett SR, et al. Dietary sugar intake increases liver tumor incidence in female mice. Sci Rep. 2016;6:22292. doi: 10.1038/srep22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin B, Moorman PG, Alberg AJ, et al. Dietary carbohydrate intake, glycaemic load, glycaemic index and ovarian cancer risk in African-American women. Br J Nutr. 2016;115:694–702. doi: 10.1017/S0007114515004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sulaiman S, Shahril MR, Wafa SW, Shaharudin SH, Hussin SN. Dietary carbohydrate, fiber and sugar and risk of breast cancer according to menopausal status in Malaysia. Asian Pac J Cancer Prev. 2014;15:5959–64. doi: 10.7314/apjcp.2014.15.14.5959. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med. 2016;14:21. doi: 10.1186/s12967-016-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitszel A, Krawczuk-Rybak M. Are elevated serum levels of IGFBP-2 after intensive chemotherapy of childhood acute lymphoblastic leukemia a risk factor of relapse? Adv Med Sci. 2007;52:147–53. [PubMed] [Google Scholar]
- 35.Vorwerk P, Mohnike K, Wex H, et al. Insulin-like growth factor binding protein-2 at diagnosis of childhood acute lymphoblastic leukemia and the prediction of relapse risk. J Clin Endocrinol Metab. 2005;90:3022–7. doi: 10.1210/jc.2004-0461. [DOI] [PubMed] [Google Scholar]
- 36.Dawczynski K, Kauf E, Schlenvoigt D, Gruhn B, Fuchs D, Zintl F. Elevated serum insulin-like growth factor binding protein-2 is associated with a high relapse risk after hematopoietic stem cell transplantation in childhood AML. Bone Marrow Transplant. 2006;37:589–94. doi: 10.1038/sj.bmt.1705281. [DOI] [PubMed] [Google Scholar]
- 37.Crowe FL, Key TJ, Allen NE, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18:1333–40. doi: 10.1158/1055-9965.EPI-08-0781. [DOI] [PubMed] [Google Scholar]
- 38.McQuade J. The impact of obesity on outcomes in metastatic melanoma (MM) patients (pts) treated with dabrafenib and trametinib. Journal of Clinical Oncology. 2016;34:9566. [Google Scholar]
- 39.Klement RJ, Champ CE, Otto C, Kammerer U. Anti-Tumor Effects of Ketogenic Diets in Mice: A Meta-Analysis. PLoS One. 2016;11:e0155050. doi: 10.1371/journal.pone.0155050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selwood K, Ward E, Gibson F. Assessment and management of nutritional challenges in children’s cancer care: a survey of current practice in the United Kingdom. Eur J Oncol Nurs. 2010;14:439–46. doi: 10.1016/j.ejon.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Wu YP, Yi J, McClellan J, et al. Barriers and Facilitators of Healthy Diet and Exercise Among Adolescent and Young Adult Cancer Survivors: Implications for Behavioral Interventions. Journal of adolescent and young adult oncology. 2015;4:184–91. doi: 10.1089/jayao.2015.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyer-Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard-risk acute lymphoblastic leukemia during maintenance therapy: response to a home-based exercise and nutrition program. Journal of pediatric hematology/oncology. 2009;31:259–66. doi: 10.1097/MPH.0b013e3181978fd4. [DOI] [PubMed] [Google Scholar]
- 43.Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014;2:963–70. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nebeling LC, Lerner E. Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. J Am Diet Assoc. 1995;95:693–7. doi: 10.1016/S0002-8223(95)00189-1. [DOI] [PubMed] [Google Scholar]
- 45.Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14:202–8. doi: 10.1080/07315724.1995.10718495. [DOI] [PubMed] [Google Scholar]
- 46.Brandhorst S, Longo VD. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Recent Results Cancer Res. 2016;207:241–66. doi: 10.1007/978-3-319-42118-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz K, Chang HT, Nikolai M, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3. doi: 10.1186/s40170-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern M, Ewing L, Davila E, Thompson AL, Hale G, Mazzeo S. Design and rationale for NOURISH-T: a randomized control trial targeting parents of overweight children off cancer treatment. Contemporary clinical trials. 2015;41:227–37. doi: 10.1016/j.cct.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raber M, Swartz MC, Santa Maria D, et al. Parental involvement in exercise and diet interventions for childhood cancer survivors: a systematic review. Pediatric research. 2016 doi: 10.1038/pr.2016.84. [DOI] [PubMed] [Google Scholar]
- 50.Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Sainsbury A. Effect of diet-induced weight loss on muscle strength in adults with overweight or obesity - a systematic review and meta-analysis of clinical trials. Obes Rev. 2016 doi: 10.1111/obr.12422. [DOI] [PubMed] [Google Scholar]
- 51.Huang T-T. Exercise Interventions in Children with Cancer: A Review. International Journal of Pediatrics. 2011 doi: 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mishra SI, Scherer RW, Snyder C, Geigle P, Gotay C. Are exercise programs effective for improving health-related quality of life among cancer survivors? A systematic review and meta-analysis. Oncology nursing forum. 2014;41:E326–42. doi: 10.1188/14.ONF.E326-E342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirkham AA, Davis MK. Exercise Prevention of Cardiovascular Disease in Breast Cancer Survivors. J Oncol. 2015;2015:917606. doi: 10.1155/2015/917606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucia A, Earnest C, Perez M. Cancer-related fatigue: can exercise physiology assist oncologists? Lancet Oncol. 2003;4:616–25. doi: 10.1016/s1470-2045(03)01221-x. [DOI] [PubMed] [Google Scholar]
- 55.Buffart L, Ros W, Chinapaw M, Brug J, Knol D, Korstjens I. Mediators of physical exercise for improvement in cancer survivors’ quality of life. Psychooncology. 2014;23:330–8. doi: 10.1002/pon.3428. [DOI] [PubMed] [Google Scholar]
- 56.Baumann FT, Bloch W, Beulertz J. Clinical exercise interventions in pediatric oncology: a systematic review. Pediatric research. 2013;74:366–74. doi: 10.1038/pr.2013.123. [DOI] [PubMed] [Google Scholar]
- 57.Tseng-Tien Huang KKN. Exercise Interventions in Children with Cancer: A Review. International Journal of Pediatrics. 2011;2011 doi: 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Dijk-Lokkart EM, Braam KI, van Dulmen-den Broeder E, et al. Effects of a combined physical and psychosocial intervention program for childhood cancer patients on quality of life and psychosocial functioning: results of the QLIM randomized clinical trial. Psycho-Oncology. 2016;25:815–22. doi: 10.1002/pon.4016. [DOI] [PubMed] [Google Scholar]
- 59.Beulertz J, Prokop A, Rustler V, Bloch W, Felsch M, Baumann FT. Effects of a 6-Month, Group-Based, Therapeutic Exercise Program for Childhood Cancer Outpatients on Motor Performance, Level of Activity, and Quality of Life. Pediatric Blood & Cancer. 2016;63:127–32. doi: 10.1002/pbc.25640. [DOI] [PubMed] [Google Scholar]
- 60.Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJL. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews. 2016 doi: 10.1002/14651858.CD008796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esbenshade AJ, Friedman DL, Smith WA, et al. Feasibility and Initial Effectiveness of Home Exercise During Maintenance Therapy for Childhood Acute Lymphoblastic Leukemia. Pediatric Physical Therapy. 2014;26:301–7. doi: 10.1097/PEP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braam KI, van Dijk-Lokkart EM, Kaspers GJL, et al. Cardiorespiratory fitness and physical activity in children with cancer. Supportive Care in Cancer. 2016;24:2259–68. doi: 10.1007/s00520-015-2993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khamoui AV, Park B-S, Kim D-H, et al. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metabolism. 2016;65:685–98. doi: 10.1016/j.metabol.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 64.Adams SC, Segal RJ, McKenzie DC, et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Research and Treatment. 2016;158:497–507. doi: 10.1007/s10549-016-3900-2. [DOI] [PubMed] [Google Scholar]
- 65.Betof AS, Lascola CD, Weitzel DH, et al. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. Journal of the National Cancer Institute. 2015:107. doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones LW, Antonelli J, Masko EM, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. Journal of Applied Physiology. 2012;113:263–72. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Higgins KA, Park D, Lee GY, Curran WJ, Deng X. Exercise-induced lung cancer regression: Mechanistic findings from a mouse model. Cancer. 2014;120:3302–10. doi: 10.1002/cncr.28878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.COLBERT LHW, KIM C, PERKINS SUSANN, HAINES DIANAC, BERRIGAN DAVID, DONEHOWER LAWRENCEA, FUCHS-YOUNG ROBIN, HURSTING STEPHEND. Exercise Effects on Tumorigenesis in a p53-Deficient Mouse Model of Breast Cancer. Medicine & Science in Sports & Exercise. 2009;41:1597–605. doi: 10.1249/MSS.0b013e31819f1f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lemke D, Pledl H-W, Zorn M, et al. Slowing down glioblastoma progression in mice by running or the anti-malarial drug dihydroartemisinin? Induction of oxidative stress in murine glioblastoma therapy. 2016 doi: 10.18632/oncotarget.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schadler KL, Thomas NJ, Galie PA, et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. 2016 doi: 10.18632/oncotarget.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng XI, Cui X-X, Gao ZHI, et al. Inhibitory effect of dietary atorvastatin and celecoxib together with voluntary running wheel exercise on the progression of androgen-dependent LNCaP prostate tumors to androgen independence. Experimental and Therapeutic Medicine. 2011;2:221–8. doi: 10.3892/etm.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer-Posovszky P, von Schnurbein J, Moepps B, et al. A new missense mutation in the leptin gene causes mild obesity and hypogonadism without affecting T cell responsiveness. J Clin Endocrinol Metab. 2010;95:2836–40. doi: 10.1210/jc.2009-2466. [DOI] [PubMed] [Google Scholar]
- 73.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 74.Chiu S, Fisler JS, Espinal GM, Havel PJ, Stern JS, Warden CH. The yellow agouti mutation alters some but not all responses to diet and exercise. Obes Res. 2004;12:1243–55. doi: 10.1038/oby.2004.158. [DOI] [PubMed] [Google Scholar]