Abstract
Background
In 2009, the United States Preventive Services Task Force (USPSTF) recommended against routine mammography screening for women aged 40–49 years. This revised recommendation was widely criticized and has sparked off intense debate. The objectives of this study are to examine the impact of the revised recommendation on the proportion of women receiving mammograms and how the effect varied by age.
Methods
We identified women who had continuous health insurance coverage and who did not have breast cancer between 2008 and 2011 in the Truven Health MarketScan Commercial Claims Databases using mammogram procedure codes. Using women aged 50–59 years as a control group, we used a differences-in-differences approach to estimate the impact of the revised recommendation on the proportion of women ages 40–49 years who received at least one mammogram. We also compared the age-specific changes in the proportion of women ages 35–59 years who were screened before and after the release of the revised recommendation.
Results
The proportion of women screened among the 40–49 and 50–59 age groups were 58.5 and 62.5%, respectively, between 2008 and 2009, and 56.9 and 62.0%, respectively, between 2010 and 2011. After 2009, the proportion of women screened declined by 1.2 percentage point among women aged 40–49 years (P < 0.01). The proportion of women screened decreased for all ages, and decreases were larger among women closer to the 40-year threshold.
Conclusions
The 2009 USPSTF breast cancer recommendation was followed by a small reduction in the proportion of insured women aged 40–49 years who were screened. Reductions were larger among women at the younger end of the age range, who presumably had less prior experience with mammography than women nearing 50.
Keywords: Breast cancer, USPSTF, Mammography screening, Prevention guideline, Claims data
Introduction
In November 2009, the United States Preventive Services Task Force (USPSTF) released a new breast cancer recommendation that does not recommend routine breast cancer screening for average-risk women aged 40–49 years. The USPSTF recommended that decisions about screening in this age group be individualized: “the decision to start regular, biennial screening mammography before age 50 years should be an individual one and should take into account patient context, including the patient’s values regarding specific benefits and harms [1].” The previous version of the recommendation, issued in 2002, endorsed annual or biennial mammography screening for women aged 40 and older [2]. In January 2016, the USPSTF reaffirmed the 2009 revised recommendation by emphasizing the potential harms of screening and lower benefit of screening among women in their 40 s [3, 4]. The 2016 recommendation for women aged 40–49 stated that “women who place a higher value on the potential benefit than the potential harms may choose to begin biennial screening between the ages of 40 and 49 years [5].”
The 2009 recommendation was widely covered in the media [6] and subject to fierce criticism from patient advocates [7], clinicians, and policy makers after the release. Some radiologists appealed to the USPSTF to rescind the revised recommendation [8, 9]. After the release, the American Cancer Society, the American College of Radiology, and the Society of Breast Imaging continued to recommend annual screening for women aged 40 and older and strongly criticized the USPSTF recommendation [10–13]. In 2015, the American Cancer Society updated their recommendation and advised women to start annual mammography screening at age 45 and transition to biennial screening at age 55 [14].
In this study, we evaluate the impact of the revised USPSTF recommendation on the proportion of women receiving mammograms using data from a large private insurance claims database. The change in the recommendation is of inherent interest, given the prevalence and cost of breast cancer, and provides an opportunity to study how physicians and patients respond to changes in guidelines. In a national telephone survey conducted one year after the revised USPSTF recommendation was issued, 51% of women aged 40–49 years were aware of the new recommendations [15]. However, only 34% had a favorable opinion of the changes. We compare changes in the proportion of women who received a mammogram between women ages 40 and 49 years and 50 and 59 years and evaluate changes in age-specific rates among women ages 35–59 years.
Previous studies have examined trends in the proportion of women ages 40–49 years screened before and after the release of the 2009 revision to the USPSTF’s breast cancer recommendation [16–21]. These studies have mostly found that changes in the proportion of women screened between 2008 and 2010 or 2011 were small and nonsignificant. For example, using Behavioral Risk Factor Surveillance System data, Block et al. [16] found that the proportion of women ages 40–49 years who were screened decreased from 53.2% in 2008 to 51.7% in 2010 (−2 percentage points). However, the proportion of women ages 50–74 years who were screened declined by a slightly larger amount, from 65.2 to 62.4% (−2.8 percentage points). Howard and Adams [18] used Medical Expenditure Panel Survey data and found that declines in the proportion of women screened were less than 1 percentage point in both groups. Using National Health Interview Survey data, Pace et al. [17] found that the proportion of women ages 40–49 years who were screened actually increased between 2008 and 2011, from 46.1 to 47.5% (1.4 percentage points). The proportion of women ages 50–74 years who were screened also increased from 57.2 to 59.1% (1.9 percentage points). Using private insurance claims, Wang et al. [20] found that there was a small difference in predicted versus observed screening rates among women ages 40–49 years in 2011, and Wharam et al. [19] found that the proportion of women ages 40–49 years who received a mammogram in 2012 was 4.3 percentage points below the predicted proportion, which takes pre-existing trends into account.
The ability of previous studies to draw conclusions about the impact of the 2009 revision to the screening recommendations has been limited by small sample sizes, which are sufficient for measuring mammography trends in broad age groups but not at a more granular level. In 2009, women ages 40–49 years would have varied in terms of their prior exposure to mammography. Women ages 49 years who were screened annually before 2009 would have had nine prior mammograms. Conversely, some 40-year-old women would not have had a mammogram. Faced with a change in screening recommendations in 2009, women in the 40–49 years age group may have interpreted the recommendation in light of their prior exposure to mammography. The size of our sample permits us to examine changes in screening by individual age group (for example, 39 vs. 40 years and 40 vs. 41 years) instead of across broad age groups. We hypothesize that the revision had a larger impact on screening among women closer to the 40-year-old age threshold compared to women close to the age of 50 years, many of whom were previously screened.
Methods
Data and sample
We measured the proportion of women screened by age and period using the Truven Health MarketScan Commercial Claims and Encounters Database over the period 2008–2011 [22]. The commercial database includes records for about 40 million insured persons ages 0–64 years, nearly one quarter of the privately insured population. The geographic distribution by US Census regions of insured persons is as follows: south, 40%; north central, 23%; west, 20%; and northeast 17%. The regional distribution of patients in the Truven claims can shift over time depending on the composition of employers using Truven to process their claims data.
Our sample includes women who were continuously enrolled with their insurer for at least 24 months in either the pre-recommendation period (2008–2009) or the post-recommendation period (2010–2011). We excluded women with one or more claims listing a diagnosis code for breast cancer. We measured changes in mammography rates among women ages 40–49 years, the age group of interest, and women ages 50–59 years, a concurrent control group. We measured changes in age-specific mammography rates among women aged 35–59 years. In our preliminary analyses, we found that there was a sharp increase in mammography rates among women who were around age 40, and so we selected age 35 as the starting point of the age range to help better understand the trends around age 40.
Variable coding
We identified mammograms using Healthcare Common Procedure Coding System (HCPCS) codes for screening mammograms (76083, 76085, 76092, 77052, 77057, G0202, and G0203) on outpatient and physician office claims [23]. In a sensitivity analysis, we identified mammograms using codes for screening mammograms as well as codes for diagnostic mammograms (76090, 76091, 77051, 77055, 77056, G0204, G0205, G0206, G0207, 76082). Some physicians may bill for screening mammograms using diagnostic codes [24, 25].
We measured the receipt of mammography biennially beginning 1 January 2008. We counted a woman as having received a mammogram if she had at least one claim listing one of the aforementioned HCPCS codes during a two-year interval.
Age was measured using 2008 and 2010 enrollment records for women in the pre-period and women in post-period (after the 2009 recommendation), respectively. We also used enrollment records to measure whether the patient was the primary policyholder or spouse of the primary policyholder, the type of plan (health maintenance organization [HMO]/preferred provider organization [PPO]/consumer-directed health plan [CDHP], point-of-service plan [POS], or other), and patients’ region of residence (northeast, north central, south, or west). We used inpatient, outpatient, and physician office claims to measure Elixhauser comorbidities [26, 27].
Statistical analysis
Difference-in-difference analysis
Previous studies have used a difference-in-difference approach to estimate the impact of the 2009 revision to the USPSTF breast cancer screening recommendation on the proportion of women screened. We applied a difference-in-difference estimator to our data to facilitate comparison between our results with those from prior studies. The unadjusted difference-in-difference estimator is the change in the proportion of women screened among women ages 50–59 years subtracted from the change among women ages 40–49 years. Difference-in-difference estimators control for underlying trends in the proportion of women screened that are common to both age groups and are robust to differences in unobserved, time-invariant enrollee characteristics between groups.
We also constructed a regression-based difference-in-difference estimator that adjusts for observed patient characteristics. Observations were at the enrollee-period (pre or post) level. Enrollees had either one or two observations depending on whether they were enrolled for only one or both of the periods.
Receipt of at least one mammogram over a two-year period was the outcome variable. The independent variables were age group (40–49 years vs. 50–59 years), period (2008–2009 vs. 2010–2011), an interaction term between age group and period, and controls for the relationship of the woman to the policyholder, plan type, region, and comorbidities.
The sample for the difference-in-difference analysis had over five million observations, so we used a linear probability model rather than a logit or probit regression to reduce the model run time. We used enrollee-level random effects to adjust standard errors for repeated observations.
Age-specific analysis
We conducted a separate analysis to assess age-specific changes in the proportion of women ages 35–59 years who were screened. We restricted the sample to women who met the inclusion criteria in both the pre- and post-period. We compared the proportion of women screened by age and assessed the significance of differences in the proportion of women screened by age using two-proportion, two-tailed z-tests for proportions. Data were analyzed using SAS version 9.3 (SAS Institute Inc, Cary, NC) and Stata 12 (StataCorp, College Station, Texas).
Results
We excluded 96,083 women who did not meet the enrollment criteria and 1,922 women who had a claim listing a breast cancer diagnosis from the 2008 to 2009 sample. We excluded 116,960 women who did not meet the enrollment criteria and 2,892 women who had a claim listing a breast cancer diagnosis from the 2010 to 2011 sample.
There were 1,947,409 women ages 40–49 years who met the sample inclusion criteria in 2008–2009, of whom 58.5% received at least one screening mammogram during this period. There were 2,451,673 women ages 40–49 years who met the inclusion criteria in 2010–2011, of whom 56.9% received a screening mammogram. The difference in the proportions between 2008–2009 and 2010–2011 was −1.6 percentage points (p < 0.001).
There were 2,064,883 women ages 50–59 years who met the sample inclusion criteria in 2008–2009, of whom 62.5% received at least one screening mammogram during this period. There were 2,607,280 women ages 50–59 years who met the inclusion criteria in 2010–2011, of whom 62.0% received a screening mammogram. The difference in the proportions between 2008–2009 and 2010–2011 was −0.5 percentage points (p <0.001). The unadjusted difference-in-difference estimator is −1.1 percentage points (= −1.6 − [−0.5]; p < 0.001).
Table 1 displays the characteristics of the sample. Most differences in patient characteristics between time periods within each age group are statistically significant but small. The composition of the sample with respect to employee relation (employee vs. dependent) is similar across time periods. There has been a small shift away from point-of-service plans to plans in the “Other” category, but this may reflect the vagaries of characterizing health plans rather than substantive changes in plan design. There was a large decline in the proportion of enrollees living in the south and a corresponding increase in the proportion living in the northeast and west regions. The proportion of enrollees with selected comorbidities was similar across time periods.
Table 1.
Characteristics of the sample
| Ages 40—49 | Ages 50—59 | |||
|---|---|---|---|---|
|
|
|
|||
| 2008–2009 | 2010–2011 | 2008–2009 | 2010–2011 | |
| Relation to policyholder | ||||
| Employee | 1,113,918 (57.2%) | 1,387,647 (56.6%) | 1,240,995 (60.1%) | 1,582,619 (60.7%) |
| Spouse/child/other | 833,491 (42.8%) | 1,064,026 (43.4%) | 823,888 (39.9%) | 1,027,268 (39.4%) |
| Plan type | ||||
| HMO/PPO/CDHP | 1,635,824 (84.0%) | 2,059,405 (84.0%) | 1,693,204 (82.0%) | 2,124,933 (81.5%) |
| POS | 206,425 (10.6%) | 176,520 (7.2%) | 233,332 (11.3%) | 216,404 (8.3%) |
| Other/missing | 105,160 (5.4%) | 218,199 (8.9%) | 138,347 (6.7%) | 265,943 (10.2%) |
| Region | ||||
| Northeast | 171,372 (8.8%) | 394,719 (16.1%) | 154,866 (7.5%) | 406,736 (15.6%) |
| North central | 482,957 (24.8%) | 593,305 (24.2%) | 545,129 (26.4%) | 646,605 (24.8%) |
| South | 962,020 (49.4%) | 953,701 (38.9%) | 1,007,663 (48.8%) | 998,588 (38.3%) |
| West | 325,217 (16.7%) | 505,045 (20.6%) | 351,030 (17.0%) | 552,743 (21.2%) |
| Missing | 7,790 (0.4%) | 4,903 (0.2%) | 8,260 (0.4%) | 5,215 (0.2%) |
| Comorbidities | ||||
| Cardiac disease | 138,850 (7.1%) | 169,901 (6.9%) | 228,996 (11.1%) | 274,807 (10.5%) |
| Hypertension | 431,935 (22.2%) | 514,361 (21.0%) | 788,372 (38.2%) | 934,449 (35.8%) |
| COPD | 199,999 (10.3%) | 238,793 (9.7%) | 246,547 (11.9%) | 294,362 (11.3%) |
| Diabetes | 145,471 (7.5%) | 181,914 (7.4%) | 286,399 (13.9%) | 347,811 (13.3%) |
| Cancera | 67,965 (3.5%) | 85,809 (3.5%) | 126,577 (6.1%) | 159,566 (6.1%) |
| Rheumatoid arthritis | 67,965 (3.5%) | 90,712 (3.7%) | 97,875 (4.7%) | 126,714 (4.9%) |
| Obesity | 103,018 (5.3%) | 156,662 (6.4%) | 99,321 (4.8%) | 151,222 (5.8%) |
| Anemia | 87,049 (4.5%) | 115,474 (4.7%) | 75,368 (3.7%) | 96,730 (3.7%) |
| Alcohol/drug abuse | 18,695 (1.0%) | 25,743 (1.1%) | 16,313 (0.8%) | 24,508 (0.9%) |
| Depression | 253,358 (13.0%) | 338,086 (13.8%) | 243,450 (11.8%) | 325,910 (12.5%) |
| N | 1,947,409 | 2,451,673 | 2,064,883 | 2,607,280 |
HMO health maintenance organization, PPO preferred provider organization, CDHP consumer-directed health plan, POS point-of-service plan, COPD chronic obstructive pulmonary disease
Excluding breast cancer
Table 2 displays estimates of the impact of patient characteristics and time period on the likelihood of receiving a mammogram over a two-year period. The coefficient on age group (ages 40–49 vs. 50–59 years) indicates that the proportion of women ages 40–49 years who received a mammogram was 3.1 percentage points lower than the proportion among women ages 50–59 years. The coefficient on time period (2010–2011 vs. 2008–2009) indicates that there was a secular decline of 0.7 percentage points in the proportion of women screened. The coefficient on the interaction of age group and time period indicates that the differential change in the proportion of women ages 40–49 years who were screened was −1.2 percentage points, similar to the unadjusted difference-in-difference estimator of −1.1.
Table 2.
Least squares regression of the impact of the age, period, the interaction between age and period, and other enrollee characteristics on the likelihood of receiving a mammogram
| Effect | 95% CI | p value | |
|---|---|---|---|
| Ages 40–49 (Ref. 50–59) | −0.031 | (−0.032, −0.030) | <.0001 |
| Year 2010–2011 (Ref: 2008–2009) | −0.007 | (−0.008, −0.006) | <.0001 |
| Interaction: ages 40–49 and year 2010–2011 | −0.012 | (−0.013, −0.010) | <.0001 |
| Relation to policyholder | |||
| Employee | 0.033 | (0.0324, 0.0336) | <.0001 |
| Spouse/child/other | Reference group | ||
| Plan type | |||
| HMO/PPO/CDHP | −0.008 | (−0.009, −0.006) | <.0001 |
| Other/missing | −0.023 | (−0.025, −0.021) | <.0001 |
| POS | Reference group | ||
| Region | |||
| Northeast | 0.001 | (−0.000, 0.0015) | 0.3048 |
| North central | 0.031 | (0.0294, 0.0318) | <.0001 |
| South | −0.017 | (−0.018, −0.016) | <.0001 |
| West | Reference group | ||
| Comorbidity | |||
| Cardiac disease | 0.033 | (0.0322, 0.0346) | <.0001 |
| Hypertension | 0.062 | (0.0612, 0.0628) | <.0001 |
| COPD | 0.010 | (0.0091, 0.0111) | <.0001 |
| Diabetes | −0.019 | (−0.020, −0.018) | <.0001 |
| Cancera | −0.068 | (−0.070, −0.066) | <.0001 |
| Rheumatoid arthritis | 0.049 | (0.0469, 0.0501) | <.0001 |
| Obesity | 0.032 | (0.0304, 0.0332) | <.0001 |
| Anemia | 0.056 | (0.0541, 0.0573) | <.0001 |
| Alcohol/drug abuse | −0.102 | (−0.105, −0.099) | <.0001 |
| Depression | 0.045 | (0.0436, 0.0456) | <.0001 |
CI confidence interval, HMO health maintenance organization, PPO preferred provider organization, CDHP consumer-directed health plan, POS point-of-service plan, COPD chronic obstructive pulmonary disease
Excluding breast cancer
We analyzed the proportion of women screened by age to determine whether declines in the proportion of women screened between 2008–2009 and 2010–2011 were larger among women near age 40 years. We restricted the sample to women who met the sample inclusion criteria in both periods.
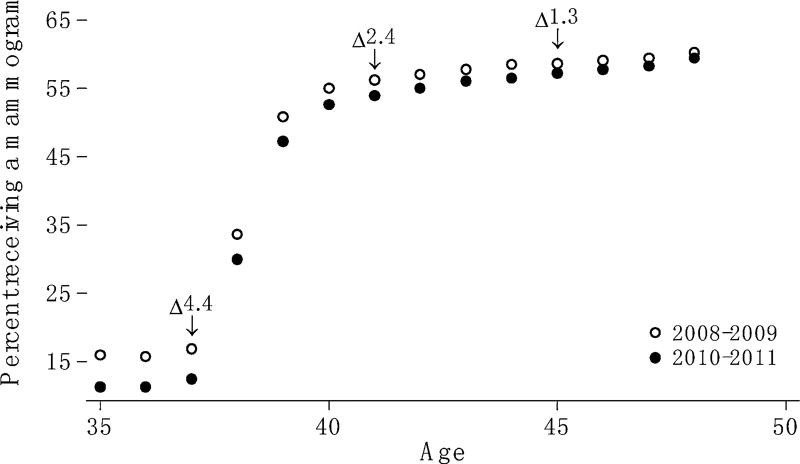
Figure 1 shows the proportion of women ages 35–49 years screened between 2008–2009 and 2010–2011. Women who were 37 years old in 2008 and 2009 were 39 years old in 2010 and 2011, women who were 38 years old in 2008 and 2009 were 40 years old in 2010 and 2011, and so forth. The number of women in each one year age group ranged from 90,197 (women age 40) to 123,493 (women age 51).
Fig. 1.
The proportion of women screened by age and period
In both periods, there is a sharp increase in the proportion of women screened between ages 37 and 39 years. The proportion of women screened decreased between 2008–2009 and 2010–2011 for all ages. Differences are significant at the 1% level for each age. Decreases are larger among women at the younger end of the age range. For example, among women age 37 years, the proportion screened declined by 4.4 percentage points, from 16.9% in 2008–2009 to 12.5% in 2010–2011. Among women 45 years old, the proportion screened declined by 1.3 percentage points, from 58.6% in 2008–2009 to 57.3% in 2010–2011.
In a sensitivity analysis, we examined the proportion of women receiving mammograms by age group and time period counting both mammograms billed using screening and diagnostic billing codes. The unadjusted difference-in-difference estimator is −0.3 percentage points (p < 0.001).
Discussion
Consistent with previous studies, we find that the 2009 revision to the USPSTF breast cancer screening recommendation led to a small reduction (−1.2 percentage points) in the proportion of insured women ages 40–49 years who were screened. Over 50% of women age 40 received a screening mammogram in the period 2010–2011, suggesting that the 2009 revision had little immediate impact on clinical care for insured women. Screening rates measured using claims data are generally lower than those measured using surveys where respondents self-report receipt of mammograms [25]. If mammograms are not completely captured in claims data, then our results would understate screening rates. However, it seems more likely that survey data overstate screening rates.
The relationships between patient characteristics other than age and receipt of mammograms were not a focus of the study, but the estimated relationships were in the expected direction and had a reasonable magnitude. Women who are primary policyholders are more likely to receive a mammogram compared to women who are covered through their spouses, perhaps reflecting the unobserved influence of education or personality.
Unlike previous studies, we examined the proportion of women screened for each age in the neighborhood of age 40. The size of the effect varied by age, and the pattern of age-specific declines suggests that women’s responses to the 2009 revision were subject to inertia: Women who were screened previously were probably more likely to continue receiving mammograms. We observed the smallest declines among women at the upper end of the 40–49 years age range, many of whom were probably screened multiple times in the past. We observed the largest declines among women at the lower end of the age range, many of whom had limited prior experience with screening. However, even among women age 39–41 years, decreases were small in magnitude.
Our study has a number of limitations. We present screening rates from a population comprised mainly of women with group health insurance, and so the results may not be generalizable to women who are uninsured, purchase insurance directly, or have Medicare or Medicaid. However, the MarketScan insurance claims database captures a sizable share (about 25%) of the privately insured population. Many previous studies of the impact of the 2009 revision to the USPSTF breast cancer screening recommendation relied on survey data where receipt of mammography was measured via self-report. Compared to self-reports of screening mammograms, insurance claims can measure screening rates more accurately [28, 29]. The data do not include information about breast cancer risk. Guidelines generally apply to average-risk patients, and we may have observed a larger impact of the revision to the USPSTF recommendation in a sample that excludes above-average-risk women. However, as long as the distribution of risk did not change over time, our estimate of the impact will be unbiased.
The ability to accurately distinguish between mammograms performed for screening versus diagnosis is unknown [30]. We would not expect the number of diagnostic mammograms to change over the relatively short time frame of our study independent of any changes related to declines in the use of screening mammography, and our sensitivity analysis including diagnostic mammograms also showed a significant reduction in screening.
We used women aged 50–59 years as a control group. The 2009 USPSTF breast cancer screening recommendation advised women in this age range to have a mammogram every one to two years. The previous version recommended that women receive an annual mammogram. The change in the recommended screening frequency may have contributed to a decline in the mammography rate among women ages 50–59 years. By not taking this change into account, we may have underestimated the impact of the 2009 revision among women ages 40–49.
Our study examines only the short-term effect of the revised USPSTF recommendations. When changes in guidelines affect practice patterns, the effect is often apparent immediately [31, 32]. The 2009 USPSTF recommendations received a lot of attention in the media, so it was not as if physicians were unaware the revisions. However, the recommendations addressed a repeated behavior: screening. If patients’ and physicians’ responses are influenced by their past actions (i.e., subject to inertia), then it may take some time for the impact of changes in practice patterns to become apparent. We find some evidence consistent with inertia: Declines in the proportion of women screened were larger among women at the younger end of the 40–49 age range, whom we would expect have less prior exposure to mammography. However, many women at the younger end of the 40–49 age range received mammograms in 2010–2011. A study that analyzes screening among ages 40–49 in the future may find a larger effect than studies that examined screening behavior shortly after the release of the 2009 revision.
Acknowledgments
Dr. Howard is partially supported by the Centers for Disease Control and Prevention under an Interagency Personnel Agreement.
Footnotes
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
References
- 1.US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Am Fam Physician. 2002;65(12):2537–2544. [PubMed] [Google Scholar]
- 3.Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):256–267. doi: 10.7326/M15-0970. [DOI] [PubMed] [Google Scholar]
- 4.Siu AL, Bibbins-Domingo K, Grossman DC, LeFevre ML. Convergence and Divergence Around Breast Cancer Screening. Ann Intern Med. 2016;164(4):301–302. doi: 10.7326/M15-3065. [DOI] [PubMed] [Google Scholar]
- 5.Siu AL. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement Screening for Breast Cancer. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 6.Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA. 2010;303(2):162–163. doi: 10.1001/jama.2009.1989. [DOI] [PubMed] [Google Scholar]
- 7.DeAngelis CD, Fontanarosa PB. US Preventive Services Task Force and breast cancer screening. JAMA. 2010;303(2):172–173. doi: 10.1001/jama.2009.1990. [DOI] [PubMed] [Google Scholar]
- 8.Kopans DB. The recent US Preventive Services Task Force guidelines are not supported by the scientific evidence and should be rescinded. J Am Coll Radiol. 2010;7(4):260–264. doi: 10.1016/j.jacr.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196(2):W112–W116. doi: 10.2214/AJR.10.5609. [DOI] [PubMed] [Google Scholar]
- 10.Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, Monticciolo D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7(1):18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society. [cited 2013 Sep 22];American Cancer Society Responds to Changes to USPSTF Mammography Guidelines [Internet] 2009 http://pressroom.cancer.org/index.php?s=43&item=201.
- 12.American College of Radiology. [cited 2013 Sep 22];USPSTF mammography recommendations will result in countless unnecessary breast cancer deaths each year [Internet] 2009 http://www.acr.org/About-Us/Media-Center/Position-Statements/Position-Statements-Folder/USPSTF-Mammography-Recommendations-Will-Result-in-Countless-Unnecessary-Breast-Cancer-Deaths.
- 13.Society of Breast Imaging. [cited 2013 Sep 22];ACR-SBI call for exclusion of USPSTF recommendations from healthcare reform Legislation [Internet] 2009 http://sbi-online.org/displaycommon.cfm?an=1&subarticlenbr=119.
- 14.Oeffinger KC, Fontham EH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiviniemi MT, Hay JL. Awareness of the 2009 US Preventive Services Task Force recommended changes in mammography screening guidelines, accuracy of awareness, sources of knowledge about recommendations, and attitudes about updated screening guidelines in women ages 40–49 and 50+ BMC Public Health. 2012;12:899. doi: 10.1186/1471-2458-12-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block LD, Jarlenski MP, Wu AW, Bennett WL. Mammography use among women ages 40–49 after the, US Preventive Services Task Force recommendation. J Gen Intern Med. 2009;2013:1–7. doi: 10.1007/s11606-013-2482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pace LE, He Y, Keating NL. Trends in mammography screening rates after publication of the 2009 US Preventive Services Task Force recommendations. Cancer. 2013;119(14):2518–2523. doi: 10.1002/cncr.28105. [DOI] [PubMed] [Google Scholar]
- 18.Howard DH, Adams EK. Mammography rates after the 2009 US Preventive Services Task Force breast cancer screening recommendation. Prev Med. 2012;55(5):485–487. doi: 10.1016/j.ypmed.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Wharam JF, Landon B, Zhang F, Xu X, Soumerai S, Ross-Degnan D. Mammography Rates 3 Years After the 2009 US Preventive Services Task Force Guidelines Changes. J Clin Oncol [Internet] 2015 2015 Feb 9; doi: 10.1200/JCO.2014.56.9848. http://www.ncbi.nlm.nih.gov/pubmed/25667290. [DOI] [PubMed]
- 20.Wang AT, Fan J, Van Houten HK, Tilburt JC, Stout NK, Montori VM, et al. Impact of the 2009 US Preventive Services Task Force guidelines on screening mammography rates on women in their 40s. PLoS ONE. 2014;9(3):e91399. doi: 10.1371/journal.pone.0091399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehkordy SF, Hall KS, Roach AL, Rothman ED, Dalton VK, Carlos RC. Trends in Breast Cancer Screening: impact of U.S. Preventive Services Task Force Recommendations. Am J Prev Med. 2015;49(3):419–422. doi: 10.1016/j.amepre.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson Reuters. Thomson Reuters Marketscan Research Databases. Thomson Reuters; New York: 2012. [Google Scholar]
- 23.Howard DH, Huang YL. Serious health events and discontinuation of routine cancer screening. Med Decis Mak. 2012;32(4):627–635. doi: 10.1177/0272989X11434600. [DOI] [PubMed] [Google Scholar]
- 24.Fenton JJ, Zhu W, Balch S, Smith-Bindman R, Fishman P, Hubbard RA. Distinguishing screening from diagnostic mammograms using Medicare claims data. Med Care. 2014;52(7):e44–e51. doi: 10.1097/MLR.0b013e318269e0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randolph WM, Mahnken JD, Goodwin JS, Freeman JL. Using Medicare data to estimate the prevalence of breast cancer screening in older women: comparison of different methods to identify screening mammograms. Health Serv Res. 2002;37(6):1643–1657. doi: 10.1111/1475-6773.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Burchill KTC. Elixhauser Comorbidity Index Macro-ICD9CM Codes [Internet] 20062006 http://mchp-appserv.cpe.umanitoba.ca/Upload/SAS/_ElixhauserICD9CM.sas.txt.
- 28.Fiscella K, Holt K, Meldrum S, Franks P. Disparities in preventive procedures: comparisons of self-report and Medicare claims data. BMC Health Serv Res. 2006;6:122. doi: 10.1186/1472-6963-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomark Prev. 2008;17(4):748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 30.Freeman JL, Klabunde CN, Schussler N, Warren JL, Virnig BA, Cooper GS. Measuring breast, colorectal, and prostate cancer screening with medicare claims data. Med Care. 2002;40(8):36–42. doi: 10.1097/00005650-200208001-00005. [DOI] [PubMed] [Google Scholar]
- 31.Howard DH, Tangka FK, Guy GP, Ekwueme DU, Lipscomb J. Prostate cancer screening in men ages 75 and older fell by 8 percentage points after task force recommendation. Health Aff Millwood. 2013;32(3):596–602. doi: 10.1377/hlthaff.2012.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvocoressi L, Sun A, Kasl SV, Claus EB, Jones BA. Mammography screening of women in their 40s: impact of changes in screening guidelines. Cancer. 2008;112(3):473–480. doi: 10.1002/cncr.23210. [DOI] [PMC free article] [PubMed] [Google Scholar]