Abstract
Diabetes severity may influence breast cancer treatment choices. We examined whether receipt of guideline-concordant breast cancer treatment varied with diabetes severity. Cancer registry data from seven states regarding 6,912 stage I–III breast cancers were supplemented by medical record abstraction and physician verification. We used logistic regression models to examine associations of diabetes severity with guideline-concordant locoregional treatment, adjuvant chemotherapy, and hormonal therapy adjusted for sociodemographics, comorbidity, and tumor characteristics. We defined guideline concordance using National Comprehensive Cancer Network guidelines, and diabetes and comorbidities using the Adult Comorbidity Evaluation-27 index. After adjustment, there was significant interaction of diabetes severity with age for locoregional treatment (p = 0.001), with many diabetic women under age 70 less frequently receiving guideline-concordant treatment than non-diabetic women. Among similarly aged women, guideline concordance was lower for women with mild diabetes in their late fifties through mid-sixties, and with moderate/severe diabetes in their late forties to early sixties. Among women in their mid-seventies to early eighties, moderate/severe diabetes was associated with increased guideline concordance. For adjuvant chemotherapy, moderate/severe diabetes was less frequently associated with guideline concordance than no diabetes [OR 0.58 (95 % CI 0.36–0.94)]. Diabetes was not associated with guideline-concordant hormonal treatment (p = 0.929). Some diabetic women were less likely to receive guideline-concordant treatment for stage I–III breast cancer than non-diabetic women. Diabetes severity was associated with lower guideline concordance for locoregional treatment among middle-aged women, and lower guideline concordance for adjuvant chemotherapy. Differences were not explained by comorbidity and may contribute to potentially worse breast cancer outcomes.
Keywords: Breast cancer, Diabetes, Cancer treatment, Surgery, Radiation, Chemotherapy, Hormonal therapy
Introduction
Breast cancer is the most commonly diagnosed non-skin cancer among U.S. women, with more than 200,000 women diagnosed in 2010 [1]. Diabetes afflicts 11.3 % of U.S. adults, including 27 % of those aged ≥65 (www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf), and rates of diabetes are increasing over time [2]. Diabetes is also common among breast cancer patients. In a systematic review, 8–32 % of breast cancer patients had diabetes [3], and breast cancer incidence may be higher among women with diabetes [4–8]. Furthermore, diabetic women may have lower breast cancer survival and greater breast cancer mortality [9–14], raising questions about whether differences in cancer treatment might contribute [10, 15].
Diabetes may influence breast cancer treatment. First, diabetes may increase tumor aggressiveness [4, 15, 16]. Second, diabetes-associated comorbid conditions may affect breast cancer treatment. For example, impaired renal, cardiac, or neurologic function in diabetic women may alter decisions concerning radiation or chemotherapy in order to minimize toxicities to these or other systems. Complications of breast cancer treatment are more frequent among diabetic women [10]. Concerns about glucocorticoid use may also lead to changes in treatment. An association of diabetes severity with receipt of guideline-concordant breast cancer care would suggest increased risk for recurrence or poor outcomes for some diabetic women and need for additional vigilance.
Two studies that examined the association of diabetes with breast cancer treatment in U.S. populations found that diabetic women were less likely to receive chemotherapy, including anthracyclines or taxanes [10], and axillary lymph node dissections [17] after adjusting for age (and for other sociodemographics in chemotherapy analyses), comorbidity and tumor characteristics, and they were also less likely to get radiation therapy and breast-conserving surgery without adjustment in one of the studies [10]. These analyses examined patterns of care, and with the exception of the analysis of lymph node dissection, did not necessarily examine whether treatment received by each woman was concordant with guidelines from expert organizations. Guideline-concordant treatment reflects evidence-based care (http://www.nccn.org/clinical.asp) likely to influence outcomes [18]. Furthermore, it is currently unknown whether breast cancer treatment differs by diabetes severity. The purpose of this study was to examine whether diabetes severity influences receipt of guideline-concordant locoregional and adjuvant therapies for breast cancer.
Methods
As part of the National Program of Cancer Registries (NPCR) Patterns of Care for Breast and Prostate Cancer Study (POCBP), we used data from seven population-based cancer registries (Georgia, North Carolina, Kentucky, Louisiana, Wisconsin, Minnesota, and California) to identify women diagnosed with breast cancer in 2004. Cases were randomly sampled after stratifying by race/ethnicity (all states), Appalachian versus non-Appalachian regions (North Carolina, Kentucky), facility type and patient volume (Wisconsin), and urban/rural status (Georgia). Cancer registry data were enhanced by medical record abstraction of sociodemographic, comorbidity, tumor, and treatment information from hospital and non-hospital facilities, and from physicians’ offices when facility information was incomplete. Data were merged with Census tract-level information on poverty and education from the 2000 Census.
We included 6,912 women diagnosed with stage I–III breast cancer (International Classification of Diseases for Oncology third edition C50.0–C50.9) in this study; those with prior cancers, sarcoma, fibromyxosarcoma, stromal sarcoma, hemangiosarcoma, inflammatory cancer, or diagnosis by autopsy or death certificate only were excluded.
Dependent variables included receipt of guideline-concordant locoregional treatment (breast cancer surgery, radiation therapy), adjuvant chemotherapy, and hormonal therapy. Guideline concordance was defined by whether the treatment received by each woman was consistent with the 2003 National Comprehensive Cancer Network (NCCN) guidelines (www.nccn.org). When guidelines indicated that a treatment should be “considered,” the treatment was categorized as guideline-concordant regardless of whether received because in these cases either treatment or no treatment may be appropriate. We programmed an algorithm in SAS to determine guideline concordance for each woman based on tumor characteristics and prior treatments.
Guideline-concordant locoregional treatment generally included receiving radiation after breast-conserving surgery, or after mastectomy with ≥4 positive axillary nodes, tumor ≥5 cm, or positive margins (www.nccn.org). Exclusions from treatment analyses are shown in Fig. 1. For women with T1N0, T0N1, or T1N1 disease, preoperative chemotherapy (pCTX) is not recommended. Because breast-conserving surgery with radiation is a recommended option both for women with these TN combinations who did not receive pCTX as well as for women with non-locally advanced disease who received pCTX, we considered women in these groups who received pCTX guideline-concordant for locoregional treatment if they received breast-conserving surgery with radiation. We excluded women with these TN combinations who received pCTX and mastectomy because it was unknown whether radiation after mastectomy would have been recommended.
Fig. 1.
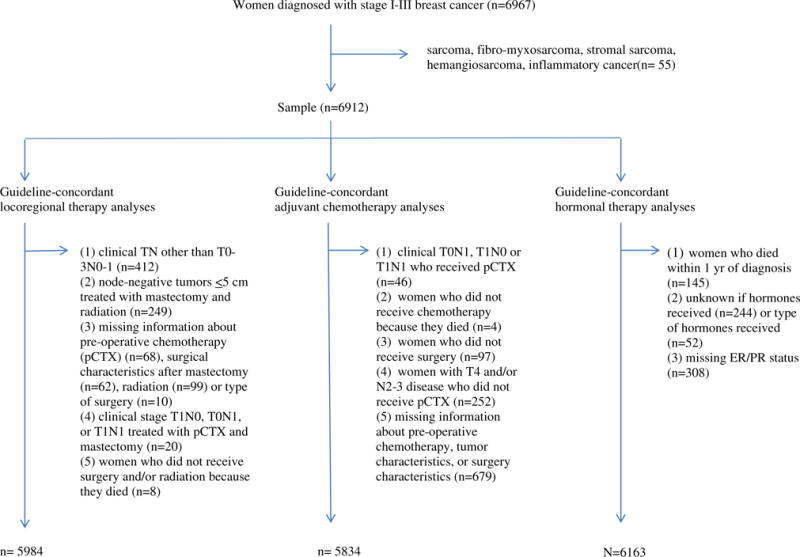
Flow chart of exclusions by analysis
For adjuvant chemotherapy, NCCN guidelines indicate that for women over age 70 years evidence for chemotherapy is insufficient and treatment should be individualized considering comorbid conditions (www.nccn.org). We included these women as in prior analyses [19] because findings were adjusted for comorbidity. Hormonal therapy was generally considered guideline-concordant if administered for ER+ and/or PR+ tumors and not for ER−/PR− tumors (www.nccn.org).
Diabetes and other comorbidity information were collected using the Adult Comorbidity Evaluation-27 index (ACE-27) [20], a comorbidity index specific to cancer patients. The ACE-27 includes 26 comorbid conditions, with three levels of severity for most. The ACE-27 defines mild diabetes as adult-onset diabetes well-controlled with oral agents and moderate diabetes as adult-onset disease poorly controlled using oral agents or uncomplicated insulin-dependent diabetes. Poor control is defined as elevated glucose levels while using oral agents, or frequent visits for monitoring glucose and treatment modifications. Severe diabetes is defined as involving end-organ impairment or recent hospitalization for diabetic ketoacidosis [20]. Because of small numbers of severe diabetes cases, we categorized severe diabetes with the moderate group.
Explanatory variables (covariates) included age, race/ethnicity, education and income (census tract-level), insurance, registry, body mass index (BMI, from medical records), and comorbidity score (from the ACE-27 excluding diabetes, obesity, and index breast cancers). For tumor characteristics, we included AJCC stage, tumor size, nodal status, histology, grade, ER/PR status, and human epidermal growth factor receptor 2 (HER2) status.
We presented frequencies and weighted proportions and used Rao-Scott Chi square tests [21] to examine the associations of diabetes with sociodemographic and tumor characteristics, and guideline-concordant treatment. Multivariable logistic regression models were employed to examine the independent association of diabetes with guideline-concordant treatment after adjusting for covariates. Separate models were created for each treatment type (locoregional, adjuvant chemotherapy, and hormonal therapy). In all models, age was treated as a continuous variable and transformed using restricted cubic spline functions to allow for non-linearity. We included an interaction term for age and diabetes to examine whether the association of diabetes with treatment varied by age. Non-significant interaction terms (p ≥ 0.05) were removed from models. To examine the effect of covariates on the association of diabetes with treatment, we ran four models for each treatment outcome to sequentially adjust for covariate groups (first age, then additionally adjusting for comorbidity and BMI, then adding tumor characteristics, and finally sociodemographic factors). The influence of individual covariates was determined by examining the change in diabetes coefficients.
We imputed missing information for education (n = 19), income (n = 19), insurance (n = 233), BMI (n = 1,495), and grade (n = 399) using multiple imputation (ten imputations) via the aregImpute function from the Hmisc package (version 3.10-1.1) in R, which performed multiple imputation using predictive mean matching. ER/PR status was unknown for both receptors for 386 women and for one receptor for 57 women; HER2 status was unknown for 1,204 women. For treatment analyses, we imputed unknown information for ER/PR and HER2 except in cases where medical records indicated that the test was not done [neither ER nor PR test done (n = 129); HER2 not done (n = 828)]. This was because treating physicians would not have had this information at the point of decision. All descriptive analyses were performed using SAS version 9.3 and SUDAAN version 11.0.0 to account for the complex sample design and allow for weighted estimates. Statistical modeling was performed using the rms package (version 4.1-0) and survey package (version 3.28-2) in R (version 3.0.2).
Results
Approximately 10 % of women had diabetes, with 8.6 % having mild diabetes and <2 % having moderate/severe diabetes (Table 1). About one quarter of women were younger than 50 years with a similar proportion 70 years or older. Most women were non-Hispanic white with 14 % black, 6 % Hispanic, <4 % Asian or American Indian/Alaska Native (AIAN). Almost one-third of women resided in census tracts with low education levels and almost one-fifth resided in low income areas. More than 60 % were privately insured although more than one-third had only public insurance. Almost half of women had no comorbid conditions; 9 % had moderate to severe comorbidity. Stage I disease was present in approximately half of women, with 14 % stage III. Two-thirds of cancers were node negative, less than one quarter were ER−/PR− and 16.5 % were HER2+. Almost 40 % were poorly differentiated or undifferentiated.
Table 1.
Sociodemographic, health, and tumor characteristics of women with stage I–III breast cancer, National Program of Cancer Registries Patterns of Care for Breast and Prostate Cancer Study (n = 6,912)
| N | Weighted % | |
|---|---|---|
| Diabetesa | ||
| None | 6,060 | 89.8 |
| Any diabetes | 852 | 10.2 |
| Mild diabetes | 712 | 8.6 |
| Moderate/severe diabetes | 140 | 1.6 |
| Age at diagnosis | ||
| <50 | 2,012 | 26.6 |
| 50–59 | 1,774 | 25.8 |
| 60–69 | 1,419 | 21.2 |
| 70+ | 1,707 | 26.4 |
| Race/ethnicity | ||
| Non-Hispanic white | 3,995 | 76.6 |
| Non-Hispanic black | 1,877 | 13.7 |
| Hispanic | 600 | 6.0 |
| Asian | 382 | 3.4 |
| AIAN | 58 | 0.3 |
| Census tract educationb | ||
| Low | 2,687 | 31.0 |
| High | 4,206 | 69.0 |
| Census tract incomec | ||
| Low | 1,730 | 17.4 |
| High | 5,163 | 82.6 |
| Insurance | ||
| Private | 3,993 | 63.3 |
| Public only | 2,480 | 34.5 |
| None | 206 | 2.2 |
| Registryd | ||
| A | 414 | 9.3 |
| B | 689 | 10.6 |
| C | 1,348 | 9.5 |
| D | 1,289 | 23.7 |
| E | 791 | 17.8 |
| F | 785 | 11.5 |
| G | 1,596 | 17.7 |
| BMI | ||
| <25 | 1,716 | 34.3 |
| 25 to <30 | 1,609 | 30.3 |
| 30 to <40 | 1,700 | 29.6 |
| 40+ | 392 | 5.9 |
| Comorbidity scoree | ||
| None | 3,314 | 48.0 |
| Minor | 2,987 | 42.7 |
| Moderate | 432 | 6.8 |
| Severe | 179 | 2.5 |
| Cancer Stage | ||
| I | 3,246 | 49.2 |
| II | 2,644 | 37.2 |
| III | 1,022 | 13.6 |
| Tumor size (cm) | ||
| T0/<1.0 | 1,252 | 19.6 |
| 1.0 to <3.0 | 4,003 | 59.3 |
| 3.0 to <5.0 | 1,031 | 14.6 |
| ≥5.0 | 537 | 6.5 |
| Nodal Status | ||
| N0 | 4,475 | 66.9 |
| N1mi, 0.2–2.0 mm | 273 | 3.9 |
| N1, >2.0 mm | 1,358 | 18.4 |
| N2 | 523 | 7.1 |
| N3 | 281 | 3.7 |
| Histology type | ||
| Tubular/colloid | 254 | 3.8 |
| Ductal/lobular/mixed | 6,392 | 92.7 |
| Other | 266 | 3.5 |
| ER/PR status | ||
| ER+ and/or PR+ | 4,886 | 75.6 |
| ER−/PR− | 1,640 | 22.6 |
| Neither test done | 136 | 1.9 |
| HER2 status | ||
| Positive | 1,061 | 16.5 |
| Negative | 4,354 | 67.0 |
| Equivocal | 293 | 4.1 |
| Test not done | 828 | 12.4 |
| Grade | ||
| Well differentiated | 1,214 | 20.4 |
| Moderately differentiated | 2,647 | 41.2 |
| Poorly/undifferentiated | 2,652 | 38.4 |
As determined by ACE-27 index
Low/high education defined as ≥25 % versus <25 % of census tract residents with less than high school education
Low/high income defined as ≥20 % versus <20 % of census tract residents below the federal poverty level
Participating registries include Georgia, North Carolina, Kentucky, Louisiana, Wisconsin, Minnesota, and California
As determined by ACE-27 index, excluding diabetes, obesity, and index breast cancer
Greater diabetes prevalence and severity was associated with age, race/ethnicity, education, poverty, insurance, BMI, and comorbidity (Table 2). The proportion of women with moderate/severe diabetes was greatest among women who were age ≥70, black, residents of census tracts with low education or low income or who were publicly insured, and who had BMI ≥40 or severe comorbidity.
Table 2.
Unadjusted associations of sociodemographic characteristics and comorbidity with diabetes status and severity among women with stage I–III breast cancer, National Program of Cancer Registries Patterns of Care for Breast and Prostate Cancer Study (n = 6,912)
| Severity of diabetes
|
Any diabetes
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No diabetes
|
Mild
|
Mod/severe
|
N | Wtd row %a | pc | |||||
| N | Wtd row %a | N | Wtd row %a | N | Wtd row %a | pb | ||||
| Age at diagnosis | <0.0001 | <0.0001 | ||||||||
| <50 | 1,914 | 95.7 | 83 | 3.5 | 15 | 0.7 | 98 | 4.3 | ||
| 50–59 | 1,573 | 91.5 | 164 | 6.9 | 37 | 1.6 | 201 | 8.5 | ||
| 60–69 | 1,198 | 86.9 | 194 | 11.7 | 27 | 1.3 | 221 | 13.1 | ||
| 70+ | 1,375 | 84.3 | 271 | 13.0 | 61 | 2.8 | 332 | 15.7 | ||
| Race/ethnicity | <0.0001 | <0.0001 | ||||||||
| Non-Hispanic white | 3,624 | 91.6 | 306 | 7.0 | 65 | 1.4 | 371 | 8.4 | ||
| Non-Hispanic black | 1,527 | 81.0 | 292 | 16.0 | 58 | 3.0 | 350 | 19.0 | ||
| Hispanic | 522 | 87.0 | 66 | 11.6 | 12 | 1.3 | 78 | 13.0 | ||
| Asian | 342 | 89.8 | 36 | 9.4 | 4 | 0.8 | 40 | 10.2 | ||
| AIAN | 45 | 77.8 | 12 | 20.7 | 1 | 1.5 | 13 | 22.2 | ||
| Census tract educationd | <0.0001 | <0.0001 | ||||||||
| Low | 2,252 | 85.5 | 366 | 12.6 | 69 | 1.9 | 435 | 14.5 | ||
| High | 3,790 | 91.6 | 345 | 6.9 | 71 | 1.5 | 416 | 8.4 | ||
| Census tract incomee | <0.0001 | <0.0001 | ||||||||
| Low | 1,449 | 85.1 | 239 | 12.7 | 42 | 2.2 | 281 | 14.9 | ||
| High | 4,593 | 90.7 | 472 | 7.8 | 98 | 1.5 | 570 | 9.3 | ||
| Insurance | <0.0001 | <0.0001 | ||||||||
| Private | 3,654 | 93.2 | 294 | 5.8 | 45 | 0.9 | 339 | 6.8 | ||
| Public only | 2,021 | 83.7 | 375 | 13.7 | 84 | 2.6 | 459 | 16.3 | ||
| None | 182 | 90.4 | 20 | 7.7 | 4 | 1.9 | 24 | 9.6 | ||
| Registryf | 0.0119 | 0.4587 | ||||||||
| A | 367 | 90.1 | 40 | 8.6 | 7 | 1.3 | 47 | 9.9 | ||
| B | 616 | 89.6 | 49 | 6.9 | 24 | 3.6 | 73 | 10.4 | ||
| C | 1,171 | 87.5 | 157 | 11.2 | 20 | 1.3 | 177 | 12.5 | ||
| D | 1,125 | 89.4 | 134 | 8.8 | 30 | 1.7 | 164 | 10.6 | ||
| E | 671 | 89.5 | 102 | 9.0 | 18 | 1.4 | 120 | 10.5 | ||
| F | 703 | 91.5 | 69 | 7.4 | 13 | 1.2 | 82 | 8.5 | ||
| G | 1,407 | 90.4 | 161 | 8.4 | 28 | 1.2 | 189 | 9.6 | ||
| BMI | <0.0001 | <0.0001 | ||||||||
| <25 | 1,612 | 95.6 | 78 | 3.1 | 26 | 1.3 | 104 | 4.4 | ||
| 25 to <30 | 1,419 | 90.4 | 162 | 8.3 | 28 | 1.3 | 190 | 9.6 | ||
| 30 to <40 | 1,403 | 84.1 | 255 | 14.0 | 42 | 1.9 | 297 | 15.9 | ||
| 40+ | 298 | 76.3 | 80 | 20.4 | 14 | 3.2 | 94 | 23.7 | ||
| Comorbidity scoreg | <0.0001 | <0.0001 | ||||||||
| None | 3,205 | 97.5 | 96 | 2.3 | 13 | 0.2 | 109 | 2.5 | ||
| Minor | 2,393 | 83.6 | 522 | 14.5 | 72 | 1.9 | 594 | 16.4 | ||
| Moderate | 335 | 79.6 | 67 | 14.6 | 30 | 5.8 | 97 | 20.4 | ||
| Severe | 127 | 75.4 | 27 | 12.9 | 25 | 11.7 | 52 | 24.6 | ||
Data are presented as frequencies and weighted percentages. Statistical testing for differences in weighted percentages was performed using the Rao-Scott Pearson Chi square test
p value for testing for differences across three levels of diabetes severity
p value for testing for differences in diabetes (yes/no)
Low/high education defined as >25 % versus <25 % of census tract residents with less than high school education
Low/high income defined as >20 % versus <20 % of census tract residents below the federal poverty level
Participating registries include Georgia, North Carolina, Kentucky, Louisiana, Wisconsin, Minnesota, and California
As determined by ACE-27 index, excluding diabetes, obesity, and index breast cancer
In unadjusted analyses (Table 3), compared with women with no or mild diabetes, women with moderate/severe diabetes were more likely to have stage III disease (19 % vs. 12–14 %, p = 0.056), and tumor size ≥5 cm (13 % vs. 5–7 %, p = 0.044). Other associations of diabetes with tumor characteristics were not significant.
Table 3.
Unadjusted associations of diabetes status and severity with stage and tumor characteristics among women with stage I–III breast cancer, National Program of Cancer Registries Patterns of Care for Breast and Prostate Cancer Study (n = 6,912)
| Severity of diabetes
|
Any diabetes
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No diabetes
|
Mild
|
Mod/severe
|
pb | N | Wtd column %a | pc | ||||
| N | Wtd column %a | N | Wtd column %a | N | Wtd column %a | |||||
| Cancer stage | 0.0565 | 0.3533 | ||||||||
| I | 2,878 | 49.5 | 305 | 45.4 | 63 | 52.6 | 368 | 46.5 | ||
| II | 2,291 | 36.9 | 307 | 42.1 | 46 | 28.3 | 353 | 39.9 | ||
| III | 891 | 13.6 | 100 | 12.6 | 31 | 19.1 | 131 | 13.6 | ||
| Tumor size (cm) | 0.0436 | 0.1314 | ||||||||
| T0/<1.0 | 1,123 | 19.9 | 107 | 16.5 | 22 | 17.3 | 129 | 16.6 | ||
| 1.0 to <3.0 | 3,508 | 59.3 | 429 | 60.9 | 66 | 51.2 | 495 | 59.3 | ||
| 3.0 to <5.0 | 885 | 14.3 | 118 | 17.2 | 28 | 18.6 | 146 | 17.4 | ||
| ≥5.0 | 468 | 6.5 | 48 | 5.5 | 21 | 12.9 | 69 | 6.7 | ||
| Nodal status | 0.8366 | 0.5220 | ||||||||
| N0 | 3,942 | 67.0 | 444 | 65.8 | 89 | 66.0 | 533 | 65.8 | ||
| N1mi, 0.2–2.0 mm | 249 | 4.0 | 22 | 2.4 | 2 | 4.4 | 24 | 2.7 | ||
| N1, >2.0 mm | 1,176 | 18.3 | 156 | 19.9 | 26 | 16.9 | 182 | 19.5 | ||
| N2 | 451 | 7.0 | 59 | 7.9 | 13 | 8.4 | 72 | 8.0 | ||
| N3 | 241 | 3.7 | 31 | 3.9 | 9 | 4.3 | 40 | 4.0 | ||
| Histology type | 0.9115 | 0.8798 | ||||||||
| Tubular/colloid | 227 | 3.8 | 21 | 3.6 | 6 | 5.6 | 27 | 3.9 | ||
| Ductal/lobular/mixed | 5,602 | 92.8 | 663 | 92.4 | 127 | 90.8 | 790 | 92.2 | ||
| Other | 231 | 3.5 | 28 | 3.9 | 7 | 3.7 | 35 | 3.9 | ||
| ER/PR status | 0.9933 | 0.9313 | ||||||||
| ER+ and/or PR+ | 4,303 | 75.7 | 486 | 74.2 | 97 | 75.5 | 583 | 74.4 | ||
| ER−/PR− | 1,419 | 22.5 | 186 | 23.8 | 35 | 23.0 | 221 | 23.7 | ||
| Neither test done | 114 | 1.8 | 12 | 2.0 | 3 | 1.5 | 15 | 1.9 | ||
| HER2 status | 0.1564 | 0.4543 | ||||||||
| Positive | 942 | 16.8 | 107 | 15.9 | 12 | 5.7 | 119 | 14.4 | ||
| Negative | 3,794 | 66.7 | 459 | 67.8 | 101 | 76.7 | 560 | 69.2 | ||
| Equivocal | 250 | 4.1 | 37 | 4.7 | 6 | 4.3 | 43 | 4.6 | ||
| Test not done | 738 | 12.4 | 74 | 11.6 | 16 | 13.3 | 90 | 11.9 | ||
| Grade | 0.1844 | 0.0906 | ||||||||
| Well differentiated | 1,088 | 20.8 | 103 | 15.7 | 23 | 21.4 | 126 | 16.6 | ||
| Moderately differentiated | 2,322 | 41.1 | 275 | 43.1 | 50 | 39.1 | 325 | 42.5 | ||
| Poorly/undifferentiated | 2,293 | 38.1 | 302 | 41.1 | 57 | 39.5 | 359 | 40.9 | ||
Data are presented as frequencies and weighted percentages. Statistical testing for differences in weighted percentages was performed using the Rao-Scott Pearson Chi square test
p value for testing for differences across three levels of diabetes severity
p value for testing for differences in diabetes (yes/no)
Before adjusting for covariates, increasing diabetes severity was significantly associated with less frequent receipt of guideline-concordant locoregional treatment (p = 0.030 and adjuvant chemotherapy (p < 0.0001; Table 4). For locoregional treatment, most women received guideline-concordant treatment regardless of diabetes severity (79–86 %). However, guideline concordance declined with increasing severity. Among women aged 40–64, approximately 30 % of those with moderate/severe diabetes did not receive guideline-concordant locoregional care, a significant difference compared with non-diabetic women 71.0 % (95 % CI 52.1–84.6 %) versus 89.4 % (95 % CI 88.0–90.6 %, not shown). For adjuvant chemotherapy, differences by diabetes severity were greater, with more than 40 % of diabetic women not receiving guideline-concordant care, including 50 % of women with moderate/severe diabetes. Guideline concordance for hormonal therapy was about the same across diabetes severity groups.
Table 4.
Associations of diabetes severity with guideline-concordant breast cancer treatment among women with stage I–III breast cancer, National Program of Cancer Registries Patterns of Care for Breast and Prostate Cancer Study
| Unadjusted % guideline concordant | Unadjusted OR (95 % CI) |
Model 1a OR (95 % CI) |
Model 2b OR (95 % CI) |
Model 3c OR (95 % CI) |
Model 4d OR (95 % CI) |
|
|---|---|---|---|---|---|---|
| Locoregional treatmente | ||||||
| Diabetes × age | na | p = 0.0005 | p = 0.0008 | p = 0.0003 | p = 0.0012 | |
| Diabetes severityf | p = 0.0279 | p = 0.0304 | p = 0.0005 | p = 0.0009 | p = 0.0004 | p = 0.0035 |
| None | 86.3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mild | 82.7 | 0.75 (0.58–0.98) | 0.60 (0.40–0.91) | 0.56 (0.35–0.90) | 0.52 (0.32–0.86) | 0.61 (0.37–1.00) |
| Moderate/severe | 79.3 | 0.61 (0.35–1.05) | 0.23 (0.11–0.50) | 0.19 (0.08–0.46) | 0.18 (0.07–0.47) | 0.17 (0.06–0.51) |
| Adjuvant chemotherapyg | ||||||
| Diabetes severity | p < .0001 | p < 0.0001 | p = 0.0436 | p = 0.0636 | p = 0.0204 | p = 0.0422 |
| None | 69.6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mild | 57.6 | 0.59 (0.48–0.74) | 0.83 (0.66–1.04) | 0.81 (0.64–1.03) | 0.79 (0.61–1.02) | 0.83 (0.64–1.07) |
| Moderate/severe | 50.7 | 0.45 (0.27–0.74) | 0.60 (0.37–0.98) | 0.63 (0.38–1.05) | 0.58 (0.36–0.92) | 0.58 (0.36–0.94) |
| Hormonal therapyh | ||||||
| Diabetes severity | p = 0.9455 | p = 0.9481 | 0.9653 | p = 0.9501 | p = 0.9400 | 0.9293 |
| None | 82.1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mild | 82.1 | 1.00 (0.76–1.30) | 1.00 (0.76–1.32) | 0.97 (0.73–1.29) | 0.95 (0.71–1.29) | 1.05 (0.77–1.43) |
| Moderate/severe | 80.6 | 0.90 (0.49–1.68) | 0.92 (0.49–1.72) | 0.92 (0.49–1.73) | 0.95 (0.50–1.79) | 0.94 (0.51–1.73) |
Adjusted for age. Age was transformed in each model using a 4-knot restricted cubic spline function to allow for nonlinearity
Adjusted as in Model 1 and additionally adjusted for comorbidity score and BMI. Comorbidity was determined by ACE-27 index, excluding diabetes, obesity, and index breast cancer
Adjusted as in Model 2 and additionally adjusted for tumor characteristics. Tumor characteristics included tumor size, nodal status, histology, ER/PR status, HER2 status, and grade
Adjusted as in Model 3 and additionally adjusted for sociodemographic factors. Sociodemographic factors included race/ethnicity, area-level education (≥25 % vs.<25 % of census tract residents with less than high school education), area-level income (≥20 % vs.<20 % of census tract residents below the federal poverty level), insurance, and registry
The number of women included in the model and receiving guideline-concordant treatment was 5,984 and 5,096, respectively, for models 1 and 2, and 5,979 and 5,092 for models 3 and 4
Diabetes odds ratios for locoregional treatment represent comparisons at the median age of 58 years old and p values are from the simultaneous test that the main effect and interaction coefficients are all equal to zero
The number of women included in the model and receiving guideline-concordant treatment was 5,834 and 4,058, respectively, for models 1 and 2, and 5,828 and 4,054 for models 3 and 4. Non-significant diabetes × age interactions were removed from models
The number of women included in the model and receiving guideline-concordant treatment was 6,163 and 5,062, respectively, for all 4 models. Non-significant diabetes × age interactions were removed from models
After adjusting for all factors, there was a significant interaction of diabetes severity with age for locoregional treatment (p = 0.001; Fig. 2; Table 4). Compared with similarly aged women without diabetes, receipt of guideline-concordant locoregional treatment was significantly reduced for women in their late fifties through mid-sixties with mild diabetes, and was considerably lower for women between their late forties and early sixties with moderate/severe diabetes. For example, compared with no diabetes the odds ratio for receiving guideline-concordant locoregional treatment for a 60-year-old woman with mild diabetes was 0.59 (95 % CI 0.38–0.92) and with moderate/severe diabetes was 0.26 (95 % CI 0.10–0.68) (not shown). Guideline concordance was higher among women in their mid-seventies to early eighties with moderate/severe diabetes.
Fig. 2.
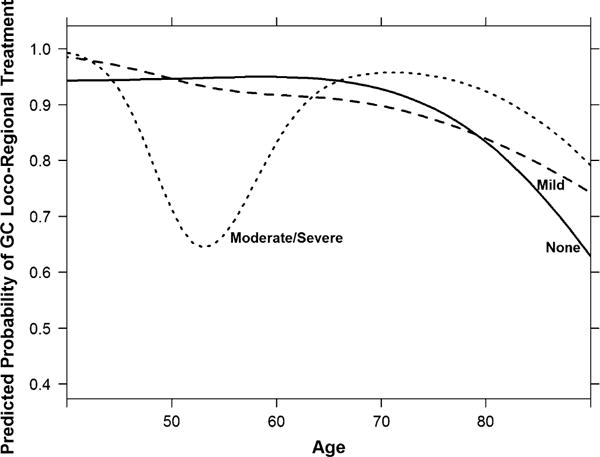
Adjusted relationship between age and guideline-concordant locoregional treatment by diabetes severity
Diabetes severity was not associated with guideline-concordant hormonal treatment after adjusting for covariates (Table 4), and the diabetes by age interaction was not significant (p = 0.130). For adjuvant chemotherapy, unadjusted differences by diabetes severity were largely explained by age, which was the greatest confounder of diabetes severity (Table 4; Fig. 3). After further adjusting for comorbidity and BMI, the association was no longer significant. When tumor characteristics and sociodemographic factors were added, differences by diabetes severity regained significance (p = 0.042), with women with moderate/severe diabetes less likely to receive guideline-concordant care than non-diabetic women. Findings from the fully adjusted model were almost identical to those adjusted only for age. The interaction between diabetes and age was not significant (p = 0.061).
Fig. 3.
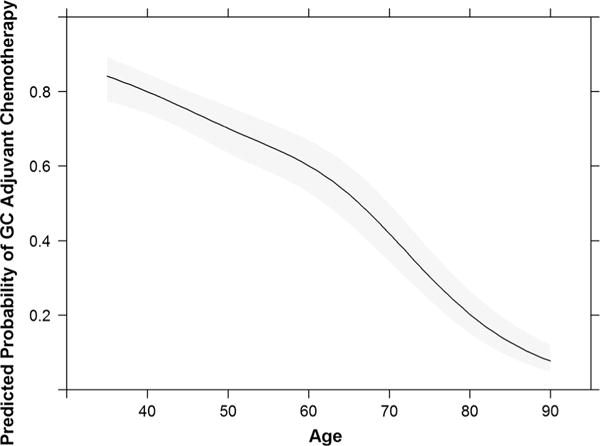
Adjusted relationship between age and guideline-concordant adjuvant chemotherapy
Discussion
Diabetes has been associated with lower breast cancer survival and greater breast cancer mortality [9–14], and some have postulated that differences in cancer treatment may contribute to such potentially worse outcomes [10, 15]. After adjusting for sociodemographic factors, comorbidity, BMI, and tumor characteristics, findings from this large population-based sample suggest that some diabetic women were less likely to receive guideline-concordant breast cancer treatment than non-diabetic women. This includes women with moderate/severe diabetes, who were less likely to receive both guideline-concordant locoregional treatment (among those in their late forties to early sixties) and adjuvant chemotherapy. Women with mild diabetes were also less likely to receive guideline-concordant locoregional care among those in their late fifties to mid-sixties. For both locoregional treatment and chemotherapy, lower guideline concordance was not explained by increased comorbidity burden.
Others have also reported significant interaction between age and diabetes on breast cancer treatment. In a Dutch population, van de Poll-Franse [22] reported that diabetic women younger than 65 were more likely to receive surgery and hormonal therapy and less likely to receive chemotherapy than non-diabetic women, while older diabetic women were less likely to receive radiotherapy. Lower radiotherapy use among older diabetic women in their sample was reported to be related to less frequent receipt of breast-conserving surgery [22], and thus may not have reflected inappropriate care. Our findings suggest that after controlling for other factors, older diabetic women were not less likely to receive guideline-concordant locoregional treatment overall. In fact, among women in their mid-seventies to early eighties moderate/severe diabetes was associated with increased guideline concordance for locoregional treatment. Reasons for this are uncertain. Others have suggested that older women with diabetes may be more likely to receive mastectomy than breast-conserving surgery [10, 22–24], which for many may equate to guideline concordance. Among women who do receive breast-conserving surgery, older women have been shown to be less likely to receive radiotherapy than younger women [17, 24]. Taken together, these factors may contribute to the higher guideline concordance among some older women with moderate/severe diabetes in our sample.
Our finding of less frequent receipt of guideline-concordant locoregional treatment among younger diabetic women, as in Fig. 2, may have implications for recurrence risk and other outcomes [25, 26]. For example, failure to receive recommended radiation leads to higher rates of recurrence [24–26] and breast cancer mortality [25, 27], and possibly all-cause mortality [26–28]. Lower guideline concordance may reflect contraindications to or perceived risks of treatment. For younger women, more severe diabetes may have heightened concerns about potential adverse effects of surgery or radiation. For older women, rates of guideline-concordant care declined with age regardless of diabetes severity. Others have also reported lower rates of guideline-concordant locoregional treatment with older age [17, 24]. Surgical and radiation risks may be a concern in this age group [24]. Furthermore, potentially small reductions in recurrence with radiation among older women [29] may lead to less frequent use [17, 24], as noted above, and consequently less frequent guideline concordance. Alternatively, reduced performance status may explain some differences by age [30].
Appropriate use of adjuvant chemotherapy improves survival [18, 31]. The inverse association between receipt of guideline-concordant adjuvant chemotherapy and diabetes severity was significant in this study, with women with moderate/severe disease tending toward less frequent guideline-concordant care. Others have reported less frequent receipt of chemotherapy among diabetic patients, but did not examine guideline concordance or include diabetes severity [10, 22]. Our findings suggest that compared with women without diabetes, guideline concordance may not be lower for women with mild diabetes, who represent most diabetic women in our sample (84 %). This in turn raises questions regarding whether differences in guideline-concordant chemotherapy as a contributing factor to potentially worse breast cancer outcomes among diabetic patients [9–11] might be limited to those with more severe diabetes. Other factors might contribute to potentially worse outcomes for diabetic women [9–11]. Different rates of chemotherapy complications among diabetic patients [10] might lead to differences in completing recommended treatments, which could impact outcomes. Furthermore, reduced doses or different regimens may contribute [32]. Obesity could also be a factor given its associations with diabetes, dose intensity, and worse cancer outcomes [33].
The unadjusted association between diabetes severity and guideline-concordant adjuvant chemotherapy was largely confounded by age. Diabetes prevalence and severity increased with age, and as our findings indicate, older women are less likely to receive guideline-concordant breast cancer care than younger women, consistent with previous evidence [30, 34–37]. Less is known about the effectiveness of adjuvant chemotherapy among women over age 70 and NCCN guidelines state that evidence was insufficient to make recommendations for that age group (www.nccn.org). Instead they advised individualizing chemotherapy decisions according to a woman’s comorbidities. Our findings were adjusted for comorbidity, suggesting that differences in chemotherapy administration among older women were not driven by greater comorbidity burden, consistent with other findings [30, 38]. However, specific individual comorbid conditions more common among older women, such as heart failure or dementia, may have influenced decisions regarding chemotherapy for this group, as may concerns about increased vulnerability to chemotherapy-induced toxicity [17, 39, 40] or performance status [30].
Our study included a large population-based sample from seven states, with routine cancer registry data enhanced by medical record review. This enabled us to incorporate detailed cancer treatment and comorbidity information with cancer registry information. We examined whether the care received by each woman was concordant with guidelines, and our data included information about diabetes severity not available in other studies [10, 22, 41]. We also included women younger than 55 years old unlike other studies of treatment differences [10, 17]. Despite these strengths, several factors should be considered. First, because of small numbers of women with severe diabetes, we combined moderate and severe diabetes, which may have obscured associations of diabetes severity with treatment. Second, we did not examine chemotherapy regimens. It is unknown whether diabetic patients received less aggressive [10] or non-recommended regimens (http://www.nccn.org), or reduced doses or cycles of chemotherapy [10, 22, 32]. Third, according to the ACE27, adult-onset diabetes controlled by diet alone is not coded [20]. Therefore, these women would have been combined with the no diabetes group. This might bias toward the null, although diabetic women well-controlled without medication may be most likely to be treated the same as non-diabetic women. Fourth, we excluded women for whom guideline concordance could not be determined. Finally, data are from seven states, which may affect generalizability.
In summary, among women with stage I–III breast cancer, some diabetic women were less likely to receive guideline-concordant care than non-diabetic women. Mild and moderate/severe diabetes were associated with less frequent receipt of guideline-concordant locoregional treatment for many women younger than 65 years old. Moderate/severe diabetes was also associated with a lower likelihood of receiving guideline-concordant adjuvant chemotherapy. For these groups of women, lower rates of guideline-concordant cancer treatment may contribute to potentially worse breast cancer outcomes.
Acknowledgments
The data used for this publication were collected by the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR) Patterns of Care Study for Breast and Prostate Cancers (POCBP), which was funded by CDC through cooperative agreements with the participating state cancer registries. Dr. Sabatino and Mr. Thompson are employees of the Centers for Disease Control and Prevention. This manuscript is written on behalf of the POCBP Group. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Dr. Trentham-Dietz reported receiving funding from the Centers for Disease Control and Prevention. Dr. Anderson reported serving in a consultant/advisory role for Bayer and Abbott. Dr. Kimmick reported receiving remuneration from Genomic Health, Astra Zeneca, Pfizer and Novartis, serving in a consultant/advisory role for Genomic Health, Astra Zeneca, Pfizer and Novartis, and receiving funding from Astra Zeneca, Roche, Wyeth, Bristol-Meyers-Squibb, GlaxoSmithKline, and Bionovo. Dr. Sabatino reported stock ownership in Pfizer and a healthcare index fund.
Footnotes
Conflict of interest The remaining coauthors declared no conflict of interest.
Contributor Information
Susan A. Sabatino, Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, 4770 Buford Highway MS-F76, Atlanta, GA 30341, USA
Trevor D. Thompson, Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, 4770 Buford Highway MS-F76, Atlanta, GA 30341, USA
Xiao-Cheng Wu, Epidemiology Program, School of Public Health, LSU Health Sciences Center, New Orleans, LA, USA.
Steven T. Fleming, Department of Epidemiology, University of Kentucky College of Public Health, Lexington, KY, USA
Gretchen G. Kimmick, Internal Medicine, Medical Oncology, Multidisciplinary Breast Program, Duke University Medical Center, Durham, NC, USA
Amy Trentham-Dietz, Department of Population Health Sciences and Paul P. Carbone Comprehensive Cancer Center, University of Wisconsin, Madison, WI, USA.
Rosemary Cress, Public Health Institute, Cancer Registry of Greater California, Sacramento, CA, USA; Department of Public Health Sciences, UC Davis School of Medicine, Davis, CA, USA.
Roger T. Anderson, Pennsylvania State College of Medicine, Hershey, PA, USA
References
- 1.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 incidence and mortality web-based report. Department of Health and Human Services Centers for Disease Control and Prevention and National Cancer Institute; Atlanta, GA, USA: 2013. [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, Wolff AC. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29(1):40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 5.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. Diabetes mellitus and breast cancer: a retrospective population-based cohort study. Breast Cancer Res Treat. 2006;98(3):349–356. doi: 10.1007/s10549-006-9172-5. [DOI] [PubMed] [Google Scholar]
- 6.Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, Manson JE. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses’ Health Study. Diabetes Care. 2003;26(6):1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 7.Sellers TA, Jensen LE, Vierkant RA, Fredericksen ZS, Brandt KR, Giuliano AR, Pankratz VS, Cerhan JR, Vachon CM. Association of diabetes with mammographic breast density and breast cancer in the Minnesota breast cancer family study. Cancer Causes Control. 2007;18(5):505–515. doi: 10.1007/s10552-007-0128-9. [DOI] [PubMed] [Google Scholar]
- 8.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86(3):s823–s835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 10.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27(13):2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louwman WJ, Janssen-Heijnen ML, Houterman S, Voogd AC, van der Sangen MJ, Nieuwenhuijzen GA, Coebergh JW. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: a population-based study. Eur J Cancer. 2005;41(5):779–785. doi: 10.1016/j.ejca.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–1844. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WW, Shao YY, Shau WY, Lin ZZ, Lu YS, Chen HM, Kuo RN, Cheng AL, Lai MS. The impact of diabetes mellitus on prognosis of early breast cancer in Asia. Oncologist. 2012;17(4):485–491. doi: 10.1634/theoncologist.2011-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100(11):1421–1429. doi: 10.1002/bjs.9229. [DOI] [PubMed] [Google Scholar]
- 15.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith U, Gale EA. Cancer and diabetes: are we ready for prime time? Diabetologia. 2010;53(8):1541–1544. doi: 10.1007/s00125-010-1815-8. [DOI] [PubMed] [Google Scholar]
- 17.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 18.Hebert-Croteau N, Brisson J, Latreille J, Rivard M, Abdelaziz N, Martin G. Compliance with consensus recommendations for systemic therapy is associated with improved survival of women with node-negative breast cancer. J Clin Oncol. 2004;22(18):3685–3693. doi: 10.1200/jco.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Wu XC, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30(2):142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 20.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 21.Rao J, Scott A. On Chi squared tests for multiway contingency tables with proportions estimated from survey data. Ann Stat. 1984;12:46–60. [Google Scholar]
- 22.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120(9):1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Enewold L, Zahm SH, Jatoi I, Shriver C, Anderson WF, Jeffery DD, Andaya A, Potter JF, McGlynn KA, Zhu K. Breast conserving surgery versus mastectomy: the influence of comorbidities on choice of surgical operation in the Department of Defense Health Care System. Am J Surg. 2013;206(3):393–399. doi: 10.1016/j.amjsurg.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Field TS, Bosco JL, Prout MN, Gold HT, Cutrona S, Pawloski PA, Ulcickas Yood M, Quinn VP, Thwin SS, Silliman RA. Age, comorbidity, and breast cancer severity: impact on receipt of definitive local therapy and rate of recurrence among older women with early stage breast cancer. J Am Coll Surg. 2011;213(6):757–765. doi: 10.1016/j.jamcollsurg.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2000;355(9217):1757–1770. [PubMed] [Google Scholar]
- 26.Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst. 2004;96(2):115–121. doi: 10.1093/jnci/djh013. [DOI] [PubMed] [Google Scholar]
- 27.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y, Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2006;366(9503):2087–2106. doi: 10.1016/s0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 28.Vinh-Hung V, Voordeckers M, Van de Steene J, Soete G, Lamote J, Storme G. Omission of radiotherapy after breast-conserving surgery: survival impact and time trends. Radiother Oncol. 2003;67(2):147–158. doi: 10.1016/s0167-8140(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 29.Smith IE, Ross GM. Breast radiotherapy after lumpectomy: no longer always necessary. N Engl J Med. 2004;351(10):1021–1023. doi: 10.1056/NEJMe048173. [DOI] [PubMed] [Google Scholar]
- 30.Bergman L, Dekker G, van Kerkhoff EH, Peterse HL, van Dongen JA, van Leeuwen FE. Influence of age and comorbidity on treatment choice and survival in elderly patients with breast cancer. Breast Cancer Res Treat. 1991;18(3):189–198. doi: 10.1007/BF01990035. [DOI] [PubMed] [Google Scholar]
- 31.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352(9132):930–942. [PubMed] [Google Scholar]
- 32.Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2(1):48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 33.Griggs JJ, Sabel MS. Obesity and cancer treatment: weighing the evidence. J Clin Oncol. 2008;26(25):4060–4062. doi: 10.1200/JCO.2008.17.4250. [DOI] [PubMed] [Google Scholar]
- 34.Giordano SH, Hortobagyi GN, Kau SW, Theriault RL, Bondy ML. Breast cancer treatment guidelines in older women. J Clin Oncol. 2005;23(4):783–791. doi: 10.1200/jco.2005.04.175. [DOI] [PubMed] [Google Scholar]
- 35.van de Water W, Bastiaannet E, Dekkers OM, de Craen AJ, Westendorp RG, Voogd AC, van de Velde CJ, Liefers GJ. Adherence to treatment guidelines and survival in patients with early stage breast cancer by age at diagnosis. Br J Surg. 2012;99(6):813–820. doi: 10.1002/bjs.8743. [DOI] [PubMed] [Google Scholar]
- 36.Griggs JJ, Culakova E, Sorbero ME, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25(18):2522–2527. doi: 10.1200/jco.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 37.Kimmick GG, Camacho F, Hwang W, Mackley H, Stewart J, Anderson RT. Adjuvant radiation and outcomes after breast conserving surgery in publicly insured patients. J Geriatr Oncol. 2012;3(2):138–146. doi: 10.1016/j.jgo.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boureau AS, Bourbouloux E, Retornaz F, Berrut G, de Decker L. Effect of burden of comorbidity on optimal breast cancer treatment in older adults. J Am Geriatr Soc. 2012;60(12):2368–2370. doi: 10.1111/jgs.12013. [DOI] [PubMed] [Google Scholar]
- 39.Du XL, Osborne C, Goodwin JS. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol. 2002;20(24):4636–4642. doi: 10.1200/JCO.2002.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crivellari D, Bonetti M, Castiglione-Gertsch M, Gelber RD, Rudenstam CM, Thurlimann B, Price KN, Coates AS, Hurny C, Bernhard J, Lindtner J, Collins J, Senn HJ, Cavalli F, Forbes J, Gudgeon A, Simoncini E, Cortes-Funes H, Veronesi A, Fey M, Goldhirsch A. Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: the International Breast Cancer Study Group Trial VII. J Clin Oncol. 2000;18(7):1412–1422. doi: 10.1200/JCO.2000.18.7.1412. [DOI] [PubMed] [Google Scholar]
- 41.Wolf I, Sadetzki S, Gluck I, Oberman B, Ben-David M, Papa MZ, Catane R, Kaufman B. Association between diabetes mellitus and adverse characteristics of breast cancer at presentation. Eur J Cancer. 2006;42(8):1077–1082. doi: 10.1016/j.ejca.2006.01.027. [DOI] [PubMed] [Google Scholar]


