Abstract
Background
The aim of this study was to evaluate the effects of the phosphodiesterase-5 (PDE-5) inhibitors, zaprinast and avanafil, on NO signalling pathway, bone mineral density (BMD), epiphyseal bone width, bone marrow angiogenesis, and parameters of oxidative stress in a rat model of glucocorticoid-induced osteoporosis (GIOP).
Material/Methods
Twenty-four 8-month-old male rats in four groups were given a single daily treatment during a 30-day period: an (untreated) control group (n=6): a dexamethasone-treated group (120 μ/kg) (n=6); a group treated with dexamethasone (120 μ/kg) and zaprinast (10 mg/kg) (n=6): and a group treated with dexamethasone (120 μ/kg) and avanafil (10 mg/kg) (n=6). Rat whole body bone mineral density (BMD) was measured by dual-energy X-ray absorptiometry (DEXA), and bone histology was performed. Also, selected oxidative stress parameters by HPLC method and the other biochemical parameters by ELISA method were measured.
Results
The GIOP model rats treated with zaprinast and avanafil showed a significant increase in NO, cyclic guanosine monophosphate (cGMP), and protein kinase G (PKG) (NO/cGMP/PKG) signaling-pathway components, and in C-terminal telopeptide of type I collagen (CTX-1), bone marrow angiogenesis, BMD, and epiphyseal bone width, compared with the (untreated) control rats (p<0.05). Levels of pyridinoline (PD) and deoxypyridinoline (DPD) were significantly reduced in the dexamethasone + zaprinast, and dexamethasone + avanafil treatment groups (p<0.05). Malondialdehyde (MDA), ubiquinone-10 (CoQ10), ubiquinol CoQ10 (CoQ10H), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) were significantly increased in the dexamethasone-treated group, compared with the (untreated) controls (p<0.05).
Conclusions
In the GIOP rat model, markers of oxidative stress and bone atrophy were significantly reduced by treatment with the PDE-5 inhibitors, zaprinast and avanafil.
MeSH Keywords: Bone Density, Dexamethasone, Osteoporosis, Phosphodiesterase 5 Inhibitors
Background
Glucocorticoids are frequently used in the treatment of inflammatory or degenerative diseases, such as rheumatoid arthritis and bronchial asthma [1]. However, the use of glucocorticoids can cause osteoporosis [2]. Osteoporosis is a very common disease that is characterized by alterations of bone tissue microstructure, decreased bone mineral density (BMD), and increased bone fragility [3–5]. Because of the bone changes, osteoporosis is a most important clinical problem, which is expected to become even more prevalent in an increasingly aging population [6]. The prevention and treatment of osteoporosis have become an important clinical goal [7,8]. Current therapy for the prevention and treatment of osteoporosis include bisphosphonates, selective estrogen receptor (ER) modulators, hormone-replacement therapy (HRT), and calcium and vitamin D supplementation [9,10]. However, due to the side effects of hormone replacement therapy or the susceptibility of some patients to the side effects of bisphosphonates, alternative therapies and drugs continue to be investigated for the treatment of osteoporosis.
A vascular response is required for the healing of bone injuries and fractures, and this response occurs within days of a bone injury when regional blood flow is high, and bone marrow vascular differentiation occurs to aid the repair of fractures [11]. Nitric oxide (NO) is a highly reactive molecule, synthesized by the nitric oxide synthase (NOS) enzyme family, from L-arginine [12–14]. NO has an effective role in regulating perfusion pressure and blood flow in vascular beds [8,15]. Cyclic guanosine monophosphate (cGMP) mediates the intracellular effects of NO, and both NO and cGMP are controlled by the activation of protein kinase-G (PKG) [16]. NO, has also been shown to have an important regulatory role in osteoblast metabolism, which contributes to the healing of bone damage [11].
The ability to maintain the NO intracellular signaling pathway depends on cGMP activity [17]. However, phosphodiesterase-5 (PDE-5) selectively hydrolyzes cGMP [18]. For this reason, it is possible that PDE-5 inhibitors, including avanafil [19], zaprinast [20], sildenafil [21], vardenafil [22], tadalafil [23] and udenafil [24] may be effective in converting osteoclasts to osteoblasts, increasing the maturation of osteoblasts and increasing bone calcification, and increasing angiogenesis in the bone tissue via preservation of increased cGMP levels [25]. Based on the findings from previously published studies, the PDE-5 inhibitors, avanafil [19] and zaprinast [20] may have a positive effect on bone calcification [26]. We have previously shown that PDE-5 inhibition had a positive effect on bone healing in rats with bone damage [8,27].
Currently, following the review of the previously published literature, there have been no studies on bone mineralization, mechanisms of bone remodeling, and oxidative stress and the use of the PDE-5 inhibitors, avanafil and zaprinast in humans or experimental animals with osteoporosis. In this study, the aim was to examine the effects of the PDE-5 inhibitors, avanafil and zaprinast on endothelial nitric oxide synthase (eNOS), NO, cGMP, PKG, pyridinoline (PD) and deoxypyridinoline (DPD) as markers of bone damage and remodeling in samples of rat urine, and to examine the bone formation markers, C-terminal telopeptide of type I collagen (CTX-1) and procollagen type I carboxy-terminal extension peptide (PICP) in plasma and bone tissue from rats with glucocorticoid-induced osteoporosis (GIOP).
NO is known to cause oxidative stress by leading to the formation of reactive oxygen species (ROS) such as the peroxynitrite ion (ONOO−), as well as having beneficial effects on recovery from bone fracture [28–30]. To determine whether the PDE-5 inhibitors, avanafil and zaprinast maintain the activity of the NO metabolic pathway, it was assumed that, for this study, it was important to measure how NO levels and oxidative stress in rats with GIOP were affected, a view which is supported by several previous studies on the positive relationship between osteoporosis and oxidative stress [31–36]. Therefore, the effect of oxidative damage due to activation of the NO signaling pathway was also investigated, by measuring the lipid peroxidation product malondialdehyde (MDA), the indicator of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine (8-OHdG), and indicators of mitochondrial damage, including the ratio of ubiquinone-10 (CoQ10) to ubiquinol CoQ10 (CoQ10H), in rats with GIOP.
Material and Methods
Chemicals and reagents
All chemicals, reagents, and drugs were commercially purchased and were of analytical grade. The phosphodiesterase-5 (PDE-5) inhibitor, zaprinast, thiobarbituric acid, 1,1,3,3-tetraethoxypropane, ubiquinone-10 (CoQ10), 8-hydroxy-2′-deoxyguanosine (8-OHdG), deoxyguanosine (dG), 10% formalin and phosphate buffered saline (PBS) were purchased from Sigma (Catalog numbers: Z0878, T5500, T9889, C9538, H5653, 854999, HT501128, P4417, respectively) (Sigma Aldrich, USA). Osteosoft solution (HC 313331) was purchased from Merck (Merck, Germany) and the PDE-5 inhibitor, avanafil (CID: 330784-47-9) was purchased from PubChem.
Experimental design and development of the rat model of glucocorticoid-induced osteoporosis (GIOP)
For this study, approval was obtained from the Ethics Committee of the Yuzuncu Yıl University Experimental Animals Local Ethics Committee Presidency (REC: 28.11.2013, 2013/13). Twenty-four 8-month-old male rats in four groups were given a single daily treatment during a 30-day period: an (untreated) control group (n=6): a dexamethasone-treated group (120 μ/kg) (n=6); a group treated with dexamethasone (120 μ/kg) and zaprinast (10 mg/kg) (n=6): and a group treated with dexamethasone (120 μ/kg) and avanafil (10 mg/kg) (n=6). During the study, the rats in all groups were fed with standard pellet feed and water with 12-hour light and dark conditions.
The rat model of glucocorticoid-induced osteoporosis (GIOP) was developed by using dexamethasone, derived from synthetic glucocorticoids, according to the method used by Mohd Ramli et al. [37]. For effective doses of dexamethasone and the phosphodiesterase-5 (PDE-5) inhibitors, zaprinast and avanafil, the effective dose levels were determined from previous studies and were administrated at daily doses of 10 mg/kg for PDE-5 inhibitors, and 120 μg/kg for dexamethasone [8,37,54]. The (GIOP) rat study groups were as follows:
Group 1: The non-treated (control) group (n=6).
Group 2: The dexamethasone-treated group, treated subcutaneously with 120 μg/kg dexamethasone, administered for 30 days as a single dose per day (n=6).
Group 3: The zaprinast + dexamethasone group, treated with 10 mg/kg oral zaprinast plus 120 μg/kg subcutaneous dexamethasone, administered for 30 days as a single dose per day (n=6).
Group 4: The avanafil + dexamethasone group 10 mg/kg oral avanafil plus 120 μg/kg subcutaneous dexamethasone, administered for 30 days as a single dose per day (n=6).
Measurement of bone mineral density (BMD) using dual-energy X-ray absorptiometry (DEXA)
Bone mineral density (BMD) was measured under anesthesia by a dual-energy X-ray absorptiometry (DEXA) device (Hologic, QDR-Discovery C Hologic, Inc., Waltham, MA, USA). The device used was suitable for the rats, and was used before and after the administration of dexamethasone, and treatment in all of the groups. The measurement of BMD was performed by whole-body scanning under anesthesia before and after the dexamethasone and PDE-5 inhibitor administration in the live experimental animals. The BMD results obtained were expressed as gm/cm2.
Rat urine sample analysis
Before and after the administration of dexamethasone and PDE-5 inhibitor therapy, 24-hour urine samples were collected from all the rats, by was collection using metabolic animal cages. Urine samples were stored at −80°C until further analysis. The values of the bone resorption markers, pyridinoline (PD) and deoxypyridinoline (DPD) were measured in the urine samples.
Blood and bone tissue samples
At the end of the 30-day treatment period, the rats were anesthetized and euthanized. Bone tissue and intracardiac blood samples were taken. Some of the whole blood was centrifuged at 2500×g for 15 min and the plasma samples obtained were divided and stored at −80°C until required for further study. The other part of the whole blood sample was reserved for the measurement of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and deoxyguanosine (dG). The right femoral bone tissues from the rats were preserved in 10% formalin for histopathological and immunohistochemical examination by light microscopy.
Biochemical analysis using the enzyme-linked immunosorbent assay (ELISA)
Endothelial nitric oxide synthase (eNOS), nitric oxide (NO), phosphodiesterase-5 (PDE-5), cyclic guanosine monophosphate (cGMP), and protein kinase G (PKG) associated with the nitric oxide (NO) signaling pathway, C-terminal telopeptide of type I collagen (CTX-1), and procollagen type I carboxy-terminal extension peptide (PICP) indicating bone activity in the plasma samples, and pyridinoline (PD) and deoxypyridinoline (DPD) which are bone destruction markers in the urine, as well as cortisol levels, were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer’s instructions (Hangzhou Eastbiopharm Co. Ltd.).
Measurement of plasma malondialdehyde (MDA) using high-pressure liquid chromatography (HPLC)
Plasma MDA levels were measured according to the method described by Khoschsorur et al. [38]. In this method, 750 μL and 0.44 M H3PO4, 250 μL and 0.25 mM thiobarbituric acid (TBA), and 450 μL distilled water were added to a 50 μL plasma sample. The tubes were tightly sealed and kept in a boiling water bath for 60 min and then cooled with tap water. Then, alkene methylation was performed with 50 mL methanol and 4.5 mL NaOH, added in a 1: 1 (v/v) ratio. The mixture was then centrifuged at 2,500×g for 3 min., and 200 μL of the supernatant remaining in the upper phase was removed and placed in a vial, where it was then transferred to for high-pressure liquid chromatography (HPLC) using an RP18 column, of 150×4.6 mm length and 5 μm particle size, as the measurement column. For the mobile phase, 400 mL of 50 mM phosphate buffer (pH: 6.8) and 600 mL of pure methanol were prepared by mixing. The flow rate of the device was set to 0.8 mL/min and the injection volume to 20 μL. The MDA and thiobarbituric acid (TBA) complex were measured and compared with standard samples prepared at different concentrations with 1,1,3,3 tetra-ethoxypropane, in the fluorescence detector at 527 nm excitation and 551 nm emission wavelengths. The results obtained were expressed in μM.
Measurement of plasma co-enzyme ubiquinone-10 (CoQ10) using HPLC
The measurement of plasma CoQ10 was performed according to the method of Litarru et al. [39,40]. The preparation of oxidized CoQ10 included 50 μL of benzoquinone (2 mg/mL), which was added to the 200 μL plasma sample and vortexed for 10 sec. After incubation at room temperature for 10 min, 1 mL of propanol was added. The mixture was vortexed for 10 sec, and then centrifuged at 4000×g for 6 min. 200 μL of the supernatant from the upper phase was placed in a vial and loaded into the HPLC apparatus. A mixture of 650 mL of ethanol and 350 mL of methanol was used for the mobile phase. A C18 column with a 5 μm particle size and a length of 25×4.6 mm was used. The flow rate of the device was set to 0.8 mL/min and the injection volume to 20 μL. The measurement was performed against the standards prepared at different concentrations, and using an ultraviolet (UV) detector at 275 nm.
Measurement of total CoQ10 and the ubiquinone-10 (CoQ10), ubiquinol CoQ10 (CoQ10H) ratio
The method used 50 μL of benzoquinone (2 mg/mL), which was added to the 200 μL plasma samples and vortexed for 10 sec. One milliliter of lithium perchlorate was then added to the mixture. One mililiter of propanol was added after incubation at room temperature for 10 min. The mixture was again vortexed for 10 sec and centrifuged at 4,000×g for 6 minutes and 200 μL of the supernatant was placed in a vial and loaded into the HPLC device. A mixture of pure ethanol, pure methanol, and lithium perchlorate (50 mM) in a volume ratio of 70: 20: 10 was used for the mobile phase. A C18 column, 25×4.6 mm in length and with a 5 μm particle size was used as the column. The flow rate of the device was set to 0.8 mL/min and the injection volume was set to 20 μL. The measurements were performed against the standards prepared at different concentrations in the UV detector at 275 nm. The results were expressed as a ubiquinone-10 (CoQ10), ubiquinol CoQ10 (CoQ10H) ratio with the help of the CoQ10/Total CoQ10 – oxidized CoQ10 equation.
Measurement of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and deoxyguanosine (dG) using HPLC with electrochemical detection (ECD)
Leukocyte DNA was isolated from whole blood samples using a DNA isolation kit (Invitrogen, CA, USA) in accordance with the manufacturer’s guidelines. According to the method of Kaur et al. [41], a 150 μL DNA sample was added to the same volume of pure formic acid and incubated at 150°C for 30 min. 100 μL of acetonitrile was then added, and 8-OHdG at 600 mV using HPLC with electrochemical detection (ECD), and dG at 275 nm in the UV detector were measured against different standards using the HPLC. The mobile phase was prepared by mixing 30 mL of acetonitrile in 970 mL of phosphate buffer (pH: 5.5 and 50 mM). A C18 column, with a length of 15×4.6 mm and 5 μm particle size was used. The flow rate of the device was set to 0.8 mL/min and the injection volume to 20 μL. The results were expressed as the number of 8-OHdG/106dG.
Histopathological and immunohistochemical evaluation of bone tissue from the right femur of the rats in the GIOP model
At the end of the study, the rats were euthanized and necropsy was performed to remove the right femoral bone from all rats. Bone specimens were fixed in a 10% formalin solution for 48 hours. The bone tissues were decalcified by incubated in osteosoft solution (MERC, HC313331, Germany) for between 96−120 hours. The tissues were then washed in tap water for 24 hours. After routine tissue processing and sectioning for histopathological evaluation, the right femoral bone samples were embedded in paraffin wax blocks and 4 μm thick sections were cut from each block and onto glass slides. The sections were stained with hematoxylin and eosin (H&E) for histopathologic examination and to examine the epiphyseal bone width of the right femoral head and trabecular bone thickness by light microscopy (Leica DM1000).
Immunohistochemical staining was performed according to the method of Shi et al. [42]. After deparaffinization and dehydration, the tissue sections were microwaved in an antigen-retrieval solution (citrate buffer, pH 6.1), four times for 5 min. After the preparations were cooled and washed, they were dried and the boundaries of the sections were drawn with a glass marker pen. For the inhibition of endogenous peroxidase activity, the sections were washed with a phosphate buffer solution (PBS, pH 7.2) for 5 minutes and incubated in a 3% H2O2 solution for 10 minutes. After the sections were washed in PBS, they were incubated with a protein solution for 5 minutes to prevent nonspecific antibody staining.
Then, the sections were incubated with the primary antibody to the endothelial cell marker, CD31 (PA5-16301) (Thermo Scientific, USA) at room temperature for 1 hour. The sections were washed with PBS for 10 min, and were then incubated with the specific anti-polyvalent horse-radish peroxidase (HRP)-conjugated immunohistochemistry (IHC) detection kit (CAT: TP-060-HL) (Thermo Scientific, USA) at room temperature for between 10−30 min, as recommended by the manufacturer. After repeated washing, 3-amino-9-ethyl-carbazole (AEC) was used as the chromogen and incubated with the sections for between 5−10 min. The sections were incubated with Mayer’s hematoxylin for between 1−2 minutes and washed in tap water. The tissue sections were then covered with glass coverslips using water-based mountant and examined for angiogenesis in bone marrow of the right femur of each rat, by light microscopy (Leica DM1000).
Statistical analysis
Numerical data were expressed as the mean ± standard deviation (SD). Repeated analysis of variance (ANOVA) was performed to compare the means of the measurements in each of the four of groups of rats before and after ovariectomy and after treatment with the PDE-5 inhibitors, avanafil and zaprinast. The Duncan multiple comparison test was used to identify the different groups. The Pearson correlation coefficients were calculated separately for each group by determining the relationship between the variables after the administration of dexamethasone and the PDE-5 inhibitor. The level of statistical significance in the calculations was taken as p<0.05. The SPSS statistical package program, version 15, was used for the calculations.
Results
Body weight of the animals in the rat model of glucocorticoid-induced osteoporosis (GIOP)
The weight of the rats before the administration of dexamethasone and ‘the PDE-5 inhibitors, avanafil and zaprinast, were measured. Before the start of the study, the mean weights of the rats in the control, dexamethasone, dexamethasone + zaprinast, and dexamethasone + avanafil groups were 329±16 g, 325±17 g, 332±19 g, and 356±14 g, respectively; there was no significant differences between the groups. At the end of the study, the mean weights of the control, dexamethasone, dexamethasone + zaprinast, and dexamethasone + avanafil were 341±16 g, 344±13 g, 357±17 g, and 374±19 g, respectively; there were no significant differences between the groups. Also, there was only a partial change in the weights of the rats in the same groups before and after the application dexamethasone and PDE-5 inhibitors during the 30-day study.
Dexamethasone and the nitric oxide (NO) signaling pathway
Some important biochemical markers of the nitric oxide (NO) signaling pathway, bone formation and degradation, and oxidative stress parameters are shown in Table 1. After the administration of dexamethasone and PDE-5 inhibitor, the levels of endothelial nitric oxide synthase (eNOS) and NO levels in all the rat groups treated with dexamethasone were significantly increased compared with the control group (p<0.05). However, there was no significant difference between the eNOS levels in the group treated with dexamethasone. Also, NO levels were significantly lower in the dexamethasone + zaprinast and dexamethasone + avanafil groups compared with the dexamethasone-treated group (p<0.05). While there was no significant difference between PDE-5 values in the (non treated) control, and the dexamethasone-treated groups, these values in the dexamethasone + zaprinast and dexamethasone + avanafil groups were significantly lower when compared with the control and dexamethasone groups (p<0.05).
Table 1.
The comparison of NO signaling pathway, some bone formation and degradation markers, cortisol and some oxidative stress parameter values in rats with glucocorticoid-induced osteoporosis.
| Control | DEX | DEX + Zaprinast | DEX + Avanafil | |
|---|---|---|---|---|
| eNOS (ng/mL) | 51.804±0.333 | 56.038±0.660* | 56.460±0.500* | 56.279±0.421* |
| NO (μmol/L) | 186.554±2.290 | 202.109±3.394* | 196.561±3.966# | 199.212±3.522# |
| PDE-5 (ng/mL) | 5.247±0.046 | 5.038±0.377 | 4.656±0.605# | 3.877±0.554#,@ |
| cGMP (pmol/L) | 37.901±0.440 | 40.752±0.645* | 42.515±0.348# | 45.481±0.511#,@ |
| PKG (ng/mL) | 11.821±0.164 | 11.744±0.161 | 11.914±0.238 | 14.710±0.332@ |
| PICP (ng/mL) | 68.105±0.781 | 72.595±0.434* | 63.625±0.317# | 62.897±0.724# |
| CTCP (ng/mL) | 5.668±0.026 | 5.702±0.061 | 5.813±0.058# | 6.389±0.023@ |
| Cortisol (ng/L) | 92.817±2.872 | 42.742±2.685* | 41.282±1.731* | 46.037±3.712* |
| MDA (μM) | 1.702±0.047 | 2.150±0.124* | 2.006±0.101# | 1.980±0.104# |
| CoQ10/CoQ10H | 0.301±0.032 | 0.402±0.026* | 0.331±0.044 | 0.332±0.029 |
| 8-OHdG/106dG | 0.666±0.097 | 0.831±0.073* | 0.500±0.094# | 0.486±0.095# |
When compared to the control group in each line (p<0.05).
When compared to the control and DEX groups in each line (p<0.05).
When compared to the other groups in each line (p<0.05).
eNOS – endothelial nitric oxide synthase; NO – nitric oxide; PDE-5 – phosphodiesterase-5; cGMP – cylic guanosine mono phosphate; PKG – protein kinase G; PICP – procollagen peptide; CTCP – cterminal collagen peptide; MDA – malondialdehyde; CoQ10 – ubiquinone 10; 8-OHdG – 8-hydroxy-2-deoxyguanosine; dG – deoxyguanosine.
Levels of cyclic guanosine monophosphate (cGMP) were significantly increased in all of the rat groups treated with dexamethasone (p<0.05). This increase was greater in the dexamethasone + zaprinast group, and particularly high in the dexamethasone + avanafil group (p<0.05). There was no significant difference in the protein kinase G (PKG) values of the (untreated) control, dexamethasone, and dexamethasone + zaprinast groups. However, the PKG value was significantly increased in the dexamethasone + avanafil group compared with the other groups (p<0.05).
Procollagen type I carboxy-terminal extension peptide (PICP) values were significantly increased in the control group compared with all the other groups given dexamethasone (p<0.05). However, PICP values were significantly lower in the dexamethasone + zaprinast and dexamethasone + avanafil groups compared with the dexamethasone group (p<0.05). While there was no significant difference between the C-terminal telopeptide of type I collagen (CTX-1) values of the control and dexamethasone groups, these values were significantly increased in the dexamethasone + zaprinast and dexamethasone + avanafil groups compared with the control and the dexamethasone-treated groups (p<0.05).
Cortisol and the oxidative stress parameters, malondialdehyde (MDA), ubiquinone-10 (CoQ10), ubiquinol CoQ10 (CoQ10H), and 8-hydroxy-2′-deoxyguanosine (8-OHdG)
The plasma cortisol levels of all of the groups treated with dexamethasone were reduced by about 50% when compared with the control group (p<0.05) (Table 1). Also, malondialdehyde (MDA) and ubiquinone-10 (CoQ10), ubiquinol CoQ10 (CoQ10H) levels were significantly increased in all of the groups given dexamethasone (p<0.05) (Table 1). However, the increase in the CoQ10 and CoQ10H ratio was not significantly increased in the dexamethasone + zaprinast and the dexamethasone + avanafil groups and were similar to the control group. The dexamethasone + zaprinast and dexamethasone + avanafil groups also had reduced MDA levels when compared with the dexamethasone-treated group. The rates of 8-hydroxy-2′-deoxyguanosine (8-OHdG), measured to determine oxidative DNA damage, were significantly increased in the dexamethasone group when compared with the control group, while levels were significantly decreased in the dexamethasone + zaprinast group and the dexamethasone + avanafil group (p<0.05).
Histopathological and immunohistochemical evaluation of bone tissue from the right femur in the rat model of glucocorticoid-induced osteoporosis (GIOP)
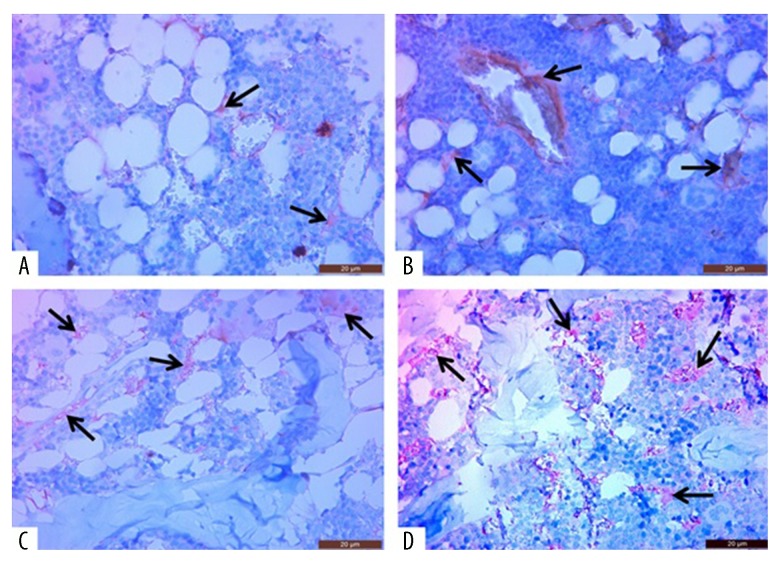
Immunohistochemical examination of the experimental animals showed that angiogenesis (CD31-positive cells) in the right femoral bone marrow was significantly increased in all groups given dexamethasone when compared with the control group. However, this increase in bone marrow angiogenesis was increased, in parallel to the activation of the NO signaling pathway, in the dexamethasone + zaprinast and dexamethasone + avanafil groups (Figure 1; Table 2). The dexamethasone + zaprinast group, and particularly the dexamethasone + avanafil group, had a significantly increased right femur trabecular bone thickness and epiphyseal bone width when compared with the dexamethasone-treated group on histopathologic examination in the right femoral tissue, and these were similar to the control group (Figures 2, 3; Table 2).
Figure 1.
Photomicrographs of new vessel formation (angiogenesis) in the bone marrow of the right femur in the rat model of glucocorticoid-induced osteoporosis (GIOP) treated with dexamethasone or the phosphodiesterase-5 (PDE-5) inhibitors, avanafil and zaprinast. (A) The control (untreated) group. (B) The dexamethasone-treated group. (C) The dexamethasone + zaprinast-treated group. (D) The dexamethasone + avanafil-treated group. The histological tissue sections viewed by light show new vessel formation. Scale bar: 20 μm. Hematoxylin and eosin (H&E).
Table 2.
The comparison of right femur trabecular bone density, new vascular formation and epiphyseal bone width of all groups in rats with glucocorticoid-induced osteoporosis.
| Groups | Right femur visual trabecular density (mm2) | Right femur visual new vessel density (mm2) | Right femur epiphyseal width (px) |
|---|---|---|---|
| Control | + + + + | ++ | 1565.357±33.640* |
| DEX | + | + + | 983.433±34.633* |
| DEX+Zaprinast | + + + | + + + | 1124.123±32.196* |
| DEX+Avanafil | + + + + | + + + + | 1217.607±33.102* |
p<0.001 – when compared to the other groups;
+ – It is a semicantitavie evaluation as visual.
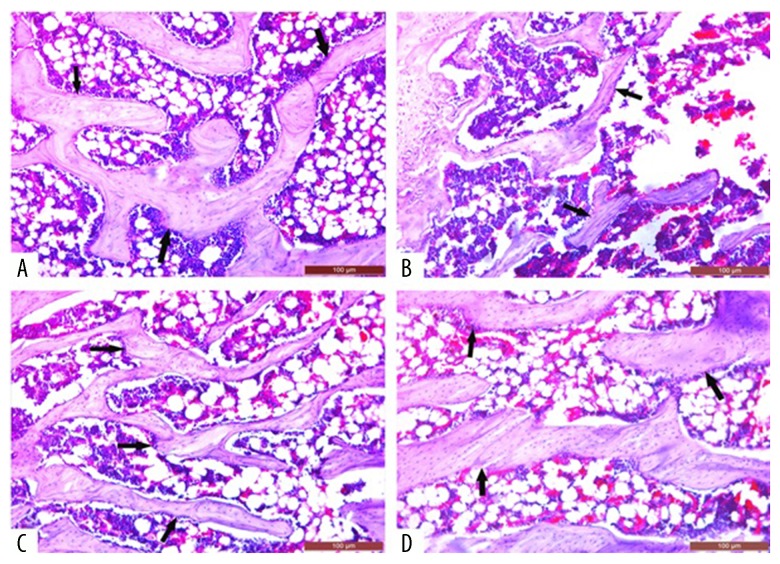
Figure 2.
Images showing the bone mineral density (BMD) of the right femoral head in rats with glucocorticoid-induced osteoporosis (GIOP). (A) The control (untreated) group. (B) The dexamethasone-treated group. (C) The dexamethasone + zaprinast-treated group. (D) The dexamethasone + avanafil-treated group. The channels indicate mineral deposits. Scale bar: 100 μm.
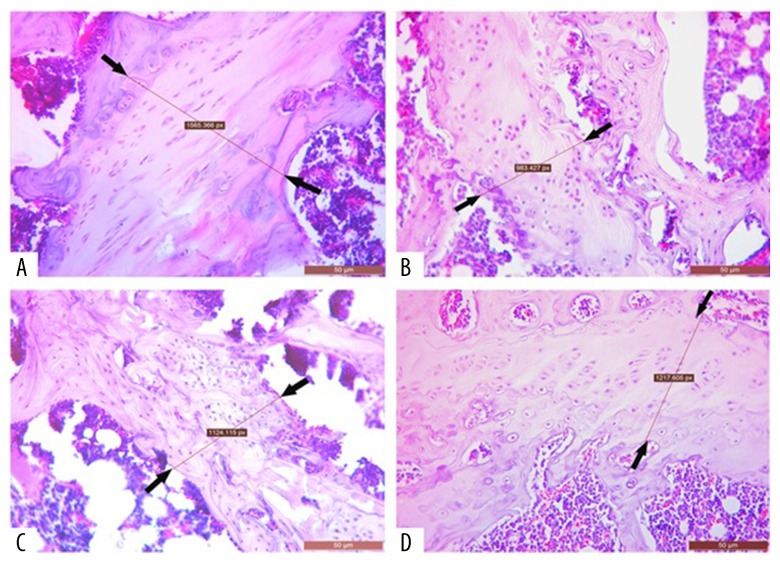
Figure 3.
Images showing the right femur epiphyseal bone width in rats with glucocorticoid-induced osteoporosis (GIOP). (A) The control (untreated) group. (B) The dexamethasone-treated group. (C) The dexamethasone + zaprinast-treated group. (D) The dexamethasone + avanafil-treated group. The area or distance between of the two arrows indicates the epiphyseal area (bone growth plate). Scale bar: 50 μm.
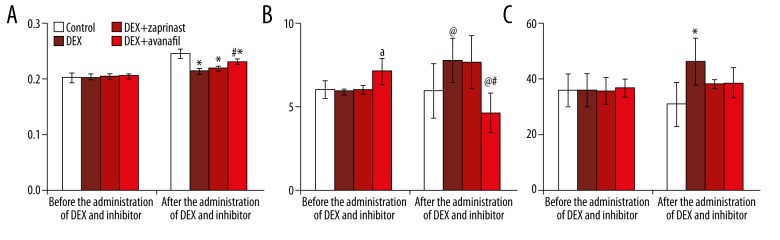
Whole-body bone mineral density (WB-BMD)
The whole-body bone mineral density (WB-BMD) values measured radiologically were very close to each other in all of the rat groups before the administration of dexamethasone and the PDE-5 inhibitors, as follows: control or untreated group, 0.203±0.008 g/cm2; dexamethasone-treated group, 0.204±0.005 g/cm2; dexamethasone + zaprinast group, 0.205±0.004 g/cm2; and the dexamethasone + avanafil group, 0.206±0.005 g/cm2.
The bone mineral density (BMD) values were partially elevated due increasing age of the animals during the 30-day period of the study in all groups, after the application of dexamethasone and PDE-5 inhibitor, as follows: control or untreated group, 0.246±0.008 g/cm2; the dexamethasone-treated group, 0.215±0.005 g/cm2; the dexamethasone + zaprinast group, 0.220±0.003 g/cm2; and the dexamethasone + avanafil group, 0.232±0.005 g/cm2. Also, the dexamethasone + avanafil group had the highest BMD value in all groups given dexamethasone treatment, and these values were similar to the control (untreated) group values (Figure 4A).
Figure 4.
Comparison of bone mineral density, pyridinoline (PD), and deoxypyridinoline (DPD) values before and after treatment with dexamethasone and the phosphodiesterase-5 (PDE-5) inhibitors, avanafil and zaprinast. (A) The radiologically measured values of bone mineral density (BMD) (g/cm2). (B) Urine pyridinoline (PD) values (nmol/mL). (C) Urine deoxypyridinoline (DPD) values (nmol/mL). * p<0.05: when compared with the control group after the administration of dexamethasone and the phosphodiesterase-5 (PDE-5) inhibitor. # p<0.05: when compared with the other groups after the administration of dexamethasone and the phosphodiesterase-5 (PDE-5) inhibitor. @ p<0.05: when compared with the administration of dexamethasone and the phosphodiesterase-5 (PDE-5) inhibitor in the same group. a p<0.05: when compared with the other groups before the administration of dexamethasone and the PDE-5 inhibitor in the same group.
Mean pyridinoline (PD) and deoxypyridinoline (DPD) values
The mean pyridinoline (PD) values, measured in the rat urine, as markers of bone resorption, were found to vary between 5.872± 0.157 and 7.061± 0.784 nmol/mL before the application of dexamethasone and PDE-5 inhibitor, and changed to between 4.601± 1.179 and 7.701±1.313 nmol/mL after the administration of dexamethasone and PDE-5 inhibitor. Also, the mean PD values of the dexamethasone + avanafil group were lowest compared with the other groups after the application of dexamethasone and PDE-5 inhibitor (Figure 4B).
The findings for the deoxypyridinoline (DPD) levels were similar. The mean DPD values changed between of 35.858±4.921 and 36.684±3.321 nmol/mL before the application of dexamethasone and PDE-5 inhibitor, and there was no significant difference between the groups. However, the DPD values of all of the groups after the application of dexamethasone and PDE-5 inhibitor changed to between of 30.938±7.966 and 46.452±8.623 nmol/mL and these values were lower in the dexamethasone + zaprinast and dexamethasone + avanafil groups compared with the dexamethasone-treated group (Figure 4C).
Discussion
The long-term use of glucocorticoids, such as dexamethasone, as anti-inflammatory and immunosuppressive drugs, can increase the risk of developing osteoporosis and its complications [43]. Glucocorticoids suppress bone formation by reducing the lifespan of osteoblasts and inhibiting the formation of new osteoblast cells [44].
Kann et al. found that the administration of dexamethasone, a synthetic glucocorticoid, to healthy subjects and patients with primary estrogen-related osteoporosis, had serum cortisol levels that were suppressed by about 15.4% [45]. In the present study, plasma cortisol levels in the all of the groups given dexamethasone were about 53.29% lower when compared with the control group. This result may be due to the physiological response of the rat model of glucocorticoid-induced osteoporosis (GIOP) to the high concentrations of dexamethasone.
There are several potential factors that may that prevent the development of osteoporosis. One of these possible factors is nitric oxide (NO), which regulates bone regeneration in vivo [46,47]. Previously published studies have demonstrated that the endothelial nitric oxide synthase (eNOS) and NO signaling pathway activation can reduce bone loss and improve osteoblastic activity and bone turnover [48–53]. It has previously been reported that phosphodiesterase-5 (PDE-5) inhibitors may positively contribute to increasing bone mineral density (BMD) and angiogenesis by reducing the hydrolysis of cyclic guanosine monophosphate (cGMP) in the NO signaling pathway [8,26,54]. There have some previous studies on the effect of the PDE-5 inhibitors on osteoporosis [8,25,54] and there are no studies on the therapeutic effects of the PDE-5 inhibitors, zaprinast or avanafil, in humans or animals with osteoporosis. In these previously published studies, PDE-5 inhibitors, including sildenafil, vardenafil, tadalafil and udenafil increased angiogenesis in the bone marrow by activating the NO/cGMP/protein kinase G signaling pathway components in rats with osteoporosis, as well as increasing BMD, epiphyseal bone width, and bone healing [8,25,54]. In the present study, the PDE-5 inhibitor, zaprinast, and especially avanafil, increased BMD and growth plate width as well as angiogenesis in the bone marrow of rats with GIOP. This data supports the view that increasing angiogenesis in bone marrow may contribute positively to increasing bone tissue and bone turnover.
C-terminal telopeptide of type I collagen (CTX-1) and procollagen type I carboxy-terminal extension peptide (PICP) are the most important biomarkers in bone formation and turnover indicating osteoblastic activity, whereas pyridinoline (PD) and deoxypyridinoline (DPD) levels in the urine are markers of bone destruction [54,55]. In a previously published study from our group, we found that vardenafil, tadalafil, and udenafil treatment significantly increased the levels of procollagen type I carboxy-terminal extension peptide (PICP) and reduced PD and DPD levels in rats with ovariectomy-induced osteoporosis [55]. In the present study, while the PICP values were elevated in the dexamethasone-treated group, they were reduced in the dexamethasone + zaprinast and dexamethasone + avanafil groups. However, CTX-1 values were significantly increased in the dexamethasone + zaprinast and dexamethasone + avanafil groups when compared with the control and the dexamethasone-treated groups. The high CTX-1 as well as low DP and DPD values in the dexamethasone + zaprinast group and especially in the dexamethasone + avanafil group, when compared with the dexamethasone-treated group, indicated that osteoblastic activity was increased and bone destruction was decreased by treatment with the PDE-5 inhibitors, zaprinast and avanafil, in the GIOP rat model.
Dual-energy X-ray absorptiometry (DEXA) measurements have previously been reported to show that a low BMD is one of the most important findings indicating a high risk of osteoporotic vertebral fractures, and is used in the diagnosis and follow-up of osteoporosis [56,57]. There have been several studies that have reported that BMD was reduced in control or sham groups consisting of ovariectomized female rats receiving glucocorticoids [58–60]. In our previous study, we also showed that BMD was significantly reduced in ovariectomized (OVX) female rats, but was increased and similar to the control levels in the OVX + PDE-5 inhibitor-treated groups [55]. In the present study, the low BMD in the dexamethasone-treated groups indicated that osteoporosis developed in the dexamethasone-treated rats; the fact that BMD in the dexamethasone +zaprinast group, and especially the dexamethasone + avanafil group was greater than that of the dexamethasone-treated group and similar to the control group, indicating that PDE-5 inhibitors may have a positive effect on increasing BMD.
It has been previously demonstrated that, following the administration of dexamethasone, antioxidant depletion, increased levels of reactive oxygen species (ROS), and lipid peroxidation may play an important role in the pathogenesis of osteoporosis in the GIOP rat model [61]. Several previous studies have shown that oxidative damage is increased in response to exposure to high-dose dexamethasone [62–68]. From review of the published literature, there have been no previously published studies on the lipid peroxidation or oxidative stress parameters of the PDE-5 inhibitors, zaprinast and avanafil in GIOP.
In the present study, it was shown that malondialdehyde (MDA) levels from lipid peroxidation products [69–71], were significantly increased in the rat groups treated with dexamethasone. MDA levels were significantly increased in the dexamethasone-treated group when compared with groups treated with the PDE-5 inhibitors, zaprinast or avanafil together with dexamethasone. Ubiquinone-10 (CoQ10), ubiquinol CoQ10 (CoQ10H), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) are now known to be some of the most important markers of mitochondrial damage [72] and oxidative DNA damage, respectively [73,74]. In the present study, CoQ10 and 8-OHdG were increased in all of the groups of rats treated with dexamethasone. However, the levels of the ratios of CoQ10 and CoQ10H, and of 8-OHdG and dG were significantly lower in the zaprinast and avanafil + dexamethasone treated groups compared with the dexamethasone-treated groups. These results indicated that oxidative stress was significantly increased with the use of dexamethasone, but that the PDE-5 inhibitors, zaprinast and especially avanafil, significantly inhibited oxidative stress when given with dexamethasone in the GIOP rat model.
Conclusions
The findings this study, in a rat model of glucocorticoid-induced osteoporosis (GIOP), showed that dexamethasone administration significantly increased bone atrophy, reduced bone mineral density (BMD), and oxidative stress in the rat femur. However, treatment with the phosphodiesterase-5 (PDE-5) inhibitors, zaprinast and avanafil, significantly increased angiogenesis in bone tissue via the activation of components of the nitric oxide (NO), cyclic guanosine monophosphate (cGMP), and protein kinase G (PKG) (NO/cGMP/PKG) signaling-pathway and significantly decreased dexamethasone-induced loss in BMD, bone atrophy, and oxidative stress. Further molecular studies and controlled clinical studies are required to determine how PDE-5 inhibitors exert the effects found in this animal model study of osteoporosis. Specifically, the effects of zaprinast and avanafil on the effects of the parathyroid hormone analog, teriparatide, and on bone morphogenetic protein (BMP)2 and BMP4 that are known to have a positive effect on BMD, are recommended in patients with osteoporosis.
Acknowledgements
The authors thank Dr. Levent Ediz for help with the use of the dual-energy X-ray absorptiometry (DEXA) device to measure whole-body bone mineral density (WB-BMD).
Footnotes
Source of support: This study was supported by opportunities from the Department of Biochemistry, Faculty of Medicine, Yuzuncu Yıl and Ataturk University, Turkey
Conflict of interests
None.
References
- 1.Shen G, Ren H, Qiu T, et al. Effect of glucocorticoid withdrawal on glucocorticoid inducing bone impairment. Biochem Biophy Res Commun. 2016;477:1059–64. doi: 10.1016/j.bbrc.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZF, Min JK, Wang D, et al. Pinoresinol diglucoside exhibits protective effect on dexamethasone-induced osteoporosis in rats. Trop J Pharm Res. 2016;15:2451–57. [Google Scholar]
- 3.Sun X, Fengbo L, Xinlong MA, et al. The effects of combined treatment with naringin and treadmill exercise on osteoporosis in ovariectomized rats. Sci Rep-UK. 2015;5:1–9. doi: 10.1038/srep13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halici Z, Borekci B, Ozdemir Y, et al. Protective effects of amlodipine and lacidipine on ovariectomy-induced bone loss in rats. Eur J Pharmacol. 2008;579:241–45. doi: 10.1016/j.ejphar.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Polat B, Halici Z, Cadirci E, et al. The effect of alpha-lipoic acid in ovariectomy and inflammation-mediated osteoporosis on the skeletal status of rat bone. Eur J Pharmacol. 2013;718:469–74. doi: 10.1016/j.ejphar.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 6.Hui-Ping M, Lei-Guo M, Bao-Feng G, et al. Icarin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. J Cell Biochem. 2011;112:916–23. doi: 10.1002/jcb.23007. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita S, Kobayashi S, Ebara Y, et al. Phosphodiesterase inhibitors, Pentoxifylline and Rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone. 2000;8:811–17. doi: 10.1016/s8756-3282(00)00395-1. [DOI] [PubMed] [Google Scholar]
- 8.Yaman F, Atılgan S, Günes N, et al. Phosphodiesterase-5 inhibitors may facilitate bone defect recovery. Eur Rev Med Pharmacol. 2011;15:1301–5. [PubMed] [Google Scholar]
- 9.Siris ES, Pasquale MK, Wang YT, et al. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older. J Bone Miner Res. 2011;26:3–11. doi: 10.1002/jbmr.189. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with Raloksifene: Results from a 3 year randomized clinical trial. J Am Med Assoc. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 11.Corbett SA, Hukkanen M, Batten J, et al. Nitric oxide in fracture repair. Differential localisation, expression and activity of nitric oxide synthases. J Bone Joint Surger Br. 1999;81:531–37. doi: 10.1302/0301-620x.81b3.8852. [DOI] [PubMed] [Google Scholar]
- 12.Rodeberg D, Chaet MS, Bass RC, et al. Nitric oxide: An oerview. Am J Surg. 1995;170:292–303. doi: 10.1016/s0002-9610(05)80017-0. [DOI] [PubMed] [Google Scholar]
- 13.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 14.Gülçin İ. Antioxidant activity of food constituents – an overview. Arch Toxicol. 2012;86:345–91. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 15.Clark AS, Meerts SH, Guarraci FA. Zaprinast, a phosphodiesterase type-5 inhibitor, alters paced mating behavior in female rats. Physiol Behav. 2009;96:289–93. doi: 10.1016/j.physbeh.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kass DA, Takimoto E, Nagayama T, et al. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 2007;75:303–14. doi: 10.1016/j.cardiores.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Mancini N, Moradi-Bidhendi L, Becherini V, et al. The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem Biophy Res Commun. 2000;274:477–81. doi: 10.1006/bbrc.2000.3164. [DOI] [PubMed] [Google Scholar]
- 18.Wie J, Jeong SJ, Kwak M, et al. The regulation of transient receptor potential canonical 4 (TRPC4) channel by phosphodiesterase 5 inhibitor via the cyclic guanosine 3′,5′-monophosphate. Pflug Arch Eur J Phy. 2017;469:693–702. doi: 10.1007/s00424-017-1937-7. [DOI] [PubMed] [Google Scholar]
- 19.Jun K, Hideki M, Hirotaka I, et al. Avanafil, a potent and highly selective phosphodiesterase-5 inhibitor for erectile dysfunction. J Urology. 2012;188:668–74. doi: 10.1016/j.juro.2012.03.115. [DOI] [PubMed] [Google Scholar]
- 20.Cheryl AF, Madeline ER. Zaprinast, a phosphodiesterase-5 inhibitor, overcomes sexual dysfunction produced by Fluoxetine, a selective serotonin reuptake inhibitor in hamsters. Neuropsychopharmacol. 2003;28:310–16. doi: 10.1038/sj.npp.1300051. [DOI] [PubMed] [Google Scholar]
- 21.Kaleta B, Boguska A. Sildenafil, a phosphodiesterase type 5 inhibitor, downregulates osteopontin in human peripheral blood mononuclear cells. Arch Immunol Ther Exp. 2017;65:347–53. doi: 10.1007/s00005-017-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noel S, Panin N, Beka M, et al. Vardenafil reduces macrophage pro-inflammatory overresponses in cystic fibrosis through PDE5-and CFTR-dependent mechanisms. Clin Sci. 2017;131:1107–21. doi: 10.1042/CS20160749. [DOI] [PubMed] [Google Scholar]
- 23.Park HJ, Kim SW, Kim JJ, et al. Multi-center therapeutic confirmatory study to evaluate the safety and efficacy of avanafil in Korean patients with erectile dysfunction. J Korean Med Sci. 2017;32:1016–23. doi: 10.3346/jkms.2017.32.6.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg DJ, Zak V, Goldstein BH, et al. Results of a phase I/II multi-center investigation of udenafil in adolescents after fontan palliation. Am Heart J. 2017;188:42–52. doi: 10.1016/j.ahj.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toğral G, Arıkan ŞM, Korkusuz P, et al. Positive effect of tadalafil, a phosphodiesterase-5 inhibitor, on fracture healing in rat femur. Eklem Hast Cerrahisi. 2015;26:137–44. doi: 10.5606/ehc.2015.29. [DOI] [PubMed] [Google Scholar]
- 26.Rahal A, Kumar A, Singh V, et al. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Histing T, Marciniak K, Scheuer C, et al. Sildenafil accelerates fracture healing in mice. J Orthop Res. 2011;29:867–73. doi: 10.1002/jor.21324. [DOI] [PubMed] [Google Scholar]
- 28.Akgül T, Alemdaroğlu B. Phosphodiesterase 5 inhibitors may facilitate bone fracture recovery. Med Hypotheses. 2008;70:461–62. doi: 10.1016/j.mehy.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Gülçin İ. Antioxidant activity of eugenol: A structure – activity relationship study. J Med Food. 2011;14:975–85. doi: 10.1089/jmf.2010.0197. [DOI] [PubMed] [Google Scholar]
- 30.Şıktar E, Ekinci D, Şıktar E, et al. Protective role of L-carnitine supplementation against exhaustive exercise-induced oxidative stress in rats. Eur J Pharmacol. 2011;668:407–13. doi: 10.1016/j.ejphar.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 31.Zhang DW, Deng H, Qi W, et al. Osteoprotective effect of cordycepin on estrogen deficiency-induced osteoporosis in vitro and in vivo. BioMed Res Int. 2015;6:1–6. doi: 10.1155/2015/423869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almeida M, Han L, Martin-Millan M, et al. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting-catenin from T cell factor- to Forkhead box O-mediated transcription. J Biol Chem. 2007;37:27298–305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- 33.Sendur OF, Turan Y, Tastaban E, et al. Antioxidant status in patients with osteoporosis: A controlled study. Joint Bone Spine. 2009;76:514–18. doi: 10.1016/j.jbspin.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Hale GE, Robertson DM. Burger HGL The perimenopausal women: Endocrinology and management. J Steroid Biochem. 2014;142:121–31. doi: 10.1016/j.jsbmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Manolagas SC. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muthusami S, Ramachandran I, Muthusamy B, et al. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360:81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Mohd Ramli ES, Suhaimi F, Mohamad Asri SF, et al. Glycyrrhizic acid (GCA) as 11beta-hydroxysteroid dehydrogenase inhibitor exerts protective effect against glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31:262–73. doi: 10.1007/s00774-012-0413-x. [DOI] [PubMed] [Google Scholar]
- 38.Khoschsorur GA, Winklhofer-Roob BM, Rab H, et al. Evaluation of a sensitive HPLC method for the determination of malondialdehyde, and application of the method to different biological materials. Chromatographia. 2000;52:181–84. [Google Scholar]
- 39.Littarru GP, Mosca F, Fattorini D, et al. Assay of coenzyme Q10 in plasma by a single dilution step. Meth Enzymol. 2004;378:170–76. doi: 10.1016/S0076-6879(04)78014-3. [DOI] [PubMed] [Google Scholar]
- 40.Littarru GP, Langsjoen P. Coenzyme Q(10) and statins: Biochemical and clinical implications. Mitochondrion. 2007;7:168–74. doi: 10.1016/j.mito.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Kaur H, Halliwell B. Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem J. 1996;318:21–23. doi: 10.1042/bj3180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi SR, Gu J, Krishan LK, et al. A novel approach to immunohistochemistry on routinely processed tissue sections. In: Gu J, editor. Analitical morphology theory, applications and protocols. 1st ed. Basel: Birkhauser; 1996. pp. 1–40. [Google Scholar]
- 43.Zhang Z, Ren H, Shen G, et al. Animal models for glucocorticoid-induced postmenopausal osteoporosis: An updated rev. Biomed Pharmacother. 2016;84:438–46. doi: 10.1016/j.biopha.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Huang L, Zhu J, et al. Effects of short-term glucocorticoid administration on bone mineral density, biomechanics and microstructure in rats’ femur. Hum Exp Toxicol. 2017;36:287–94. doi: 10.1177/0960327116649674. [DOI] [PubMed] [Google Scholar]
- 45.Kann P, Laudes M, Piepkorn B, et al. Germany suppressed levels of serum cortisol following high-dose oral dexamethasone administration differ between healthy postmenopausal females and patients with established primary vertebral osteoporosis. Clin Rheumatol. 2001;20:25–29. doi: 10.1007/s100670170099. [DOI] [PubMed] [Google Scholar]
- 46.Evans DM, Ralston SH. Nitric oxide and bone. J Bone Miner Res. 1996;11:300–5. doi: 10.1002/jbmr.5650110303. [DOI] [PubMed] [Google Scholar]
- 47.Fan X, Roy E, Zhu L, et al. Nitric oxide regulates receptor activator of nuclear factor-kappa B ligand and osteoprotegerin expression in bone marrow stromal cells. Endocrinology. 2003;145:751–59. doi: 10.1210/en.2003-0726. [DOI] [PubMed] [Google Scholar]
- 48.Wimalawansa SM, Shankar VS, Simmins DJ, et al. The mechanism of bone resorption by cyclosporin: involvement of the NO-cGMP pathway. J Musculoskel Neuron. 2000;1:141–43. [PubMed] [Google Scholar]
- 49.Armour KA, Armour KJ, Gallagher ME, et al. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001;142:760–66. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- 50.Singh M, Singh P, Singh S, et al. A susceptibility haplotype within the endothelial nitric oxide synthase gene influences bone mineral density in hypertensive women. J Bone Miner Metab. 2014;32:580–87. doi: 10.1007/s00774-013-0533-y. [DOI] [PubMed] [Google Scholar]
- 51.Lu R, Hu CP, Wu XP, et al. Effect of age on bone mineral density and the serum concentration of endogenous nitric oxide synthase inhibitors in rats. Comparative Med. 2002;52:224–28. [PubMed] [Google Scholar]
- 52.Hao YJ, Tang Y, Chen FB, et al. Different doses of nitric oxide donor prevent osteoporosis in ovariectomized rats. Clin Orthop Relat Res. 2005;435:226–31. doi: 10.1097/01.blo.0000153990.74837.73. [DOI] [PubMed] [Google Scholar]
- 53.Chae HJ, Park RK, Chung HT, et al. Nitric oxide is a regulator of bone remodeling. J Pharm Pharmacol. 1997;49:897–902. doi: 10.1111/j.2042-7158.1997.tb06132.x. [DOI] [PubMed] [Google Scholar]
- 54.Alp HH, Huyut Z, Yildirim S, et al. The effect of PDE5 inhibitors on bone and oxidative damage in ovariectomy-induced osteoporosis. Exp Biol Med. 2017;242(10):1051–61. doi: 10.1177/1535370217703352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang DW, Deng H, Qi W, et al. Osteoprotective effect of cordycepin on estrogen deficiency-induced osteoporosis in vitro and in vivo. Biomed Res Int. 2015;2015:423869. doi: 10.1155/2015/423869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Böcker W, Khassawna T, Bauer N, et al. Short-term glucocorticoid treatment causes spinal osteoporosis in ovariectomized rats. Eur Spine J. 2014;23:2437–48. doi: 10.1007/s00586-014-3463-z. [DOI] [PubMed] [Google Scholar]
- 57.Ulivieri FM, Silva BC, Sardanelli F, et al. Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine. 2014;47:435–48. doi: 10.1007/s12020-014-0280-4. [DOI] [PubMed] [Google Scholar]
- 58.Li F, Sun X, Ma J, et al. Naringin prevents ovariectomy-induced osteoporosis and promotes osteoclasts apoptosis through the mitochondria-mediated apoptosis pathway. Biochem Biophy Res Commun. 2014;452:629–35. doi: 10.1016/j.bbrc.2014.08.117. [DOI] [PubMed] [Google Scholar]
- 59.Ogoshi T, Hagino H, Fukata S, et al. Influence of glucocorticoid on bone in 3-, 6-, and 12-month-old rats as determined by bone mass and histomorphometry. Mod Rheumatol. 2008;18:552–61. doi: 10.1007/s10165-008-0096-2. [DOI] [PubMed] [Google Scholar]
- 60.King CS, Weir EC, Gundberg CW, et al. Effects of continuous glucocorticoid infusion on bone metabolism in the rat. Calcified Tissue Int. 1996;59:184–91. doi: 10.1007/s002239900107. [DOI] [PubMed] [Google Scholar]
- 61.Feng YL, Tang XL. Effect of glucocorticoid-induced oxidative stress on the expression of Cbfa1. Chem-Biol Interact. 2014;207:26–31. doi: 10.1016/j.cbi.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Bera S, Greiner S, Choudhury A, et al. Dexamethasone-induced oxidative stress enhances myeloma cell radio sensitization while sparing normal bone marrow hematopoiesis. Neoplasia. 2010;12:980–92. doi: 10.1593/neo.101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato H, Takahashi T, Sumitani K, et al. Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J Clin Biochem Nutr. 2010;47:224–32. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu TJ, Shuai XH, Chen JR, et al. Protective effect of a Potentilla anserine polysaccharide on oxidative damages in mice. Int J Biol Macromol. 2009;45:279–83. doi: 10.1016/j.ijbiomac.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Assaf N, Shalby AB, Khalil WKB, et al. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: Protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68:77–90. doi: 10.1007/s13105-011-0121-3. [DOI] [PubMed] [Google Scholar]
- 66.Costantini D, Marasco V, Molle AP. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011;181:447–56. doi: 10.1007/s00360-011-0566-2. [DOI] [PubMed] [Google Scholar]
- 67.Suwanjang W, Abramov AY, Govitrapong P, et al. Melatonin attenuates dexamethasone toxicity-induced oxidative stress, calpain and caspase activation in human neuroblastoma SH-SY5Y cells. J Steroid Biochem. 2013;138:116–22. doi: 10.1016/j.jsbmb.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Qiao S, Okret S, Jondal M. Thymocyte-synthesized glucocorticoids play a role in thymocyte homeostasis and are down-regulated by adrenocorticotropic hormone. Endocrinology. 2009;150:4163–69. doi: 10.1210/en.2009-0195. [DOI] [PubMed] [Google Scholar]
- 69.Uslu C, Taysi S, Bakan N. Lipid peroxidation and antioxidant enzyme activities in experimental maxillary sinusitis. Ann Clin Lab Sci. 2003;3:18–22. [PubMed] [Google Scholar]
- 70.Huyut Z, Şekeroğlu MR, Balahoroğlu R, et al. The relationship of oxidation sensitivity of red blood cells and carbonic anhydrase activity in stored human blood; Effect of certain phenolic compounds. BioMed Res Int. 2016;2016:3057384. doi: 10.1155/2016/3057384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sekeroglu MR, Huyut Z, Him A. The susceptibility of erthrocytes to oxidation during stores of blood: Effects of melatonin and propofol. Clin Biochem. 2012;45:315–19. doi: 10.1016/j.clinbiochem.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 72.Macunluoglu B, Atakan A, Ari E, et al. Epicardial fat tissue thickness is correlated with diminished levels of co-enzyme Q10, a major antioxidant molecule among hemodialysis patients. Clin Biochem. 2014;47:1231–34. doi: 10.1016/j.clinbiochem.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 73.Geyik S, Altunısık E, Neyal AM, Taysi S. Oxidative stress and DNA damage in patients with migraine. J Headache Pain. 2016;17:10. doi: 10.1186/s10194-016-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alici D, Bulbul F, Virit O, et al. Evaluation of oxidative metabolism and oxidative DNA damage in patients with obsessive compulsive disorder. Psychiat Clin Neuros. 2016;70:109–15. doi: 10.1111/pcn.12362. [DOI] [PubMed] [Google Scholar]