Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD) are clonal disorders of the monocyte/macrophage and dendritic cell lineages characterized by infiltration of histiocytes, acute and chronic inflammation, and fibrosis that can involve multiple organ systems and cause a wide range of clinical manifestations. Even though LCH and ECD are clinically and pathologically distinct, both harbor BRAFV600E mutations in ~50% of cases,1 and nearly 20% of patients with ECD have a diagnosis of both ECD and LCH simultaneously [mixed histiocytosis (MH)].2 Several retrospective cases and one prospective clinical trial have demonstrated that BRAFV600E -mutated ECD/LCH can be treated with vemurafenib with responses that are robust and durable.3–5 Vemurafenib recently obtained approval by the Food and Drug administration for treatment of BRAFV600E -mutated ECD on the basis of the VE-BASKET trial.6 However, many patients require dose reductions of vemurafenib, or discontinuation, due to toxicity.3,4 While there are no direct comparisons of vemurafenib to dabrafenib, several studies report a lower incidence of adverse events with dabrafenib.7,8 There have been three single cases reported of treatment of BRAFV600E -mutated ECD9,10 with dabrafenib, an alternative BRAF inhibitor approved for treatment of BRAFV600E-mutant metastatic melanoma. However, thus far there have been no LCH patients treated with dabrafenib, and there has been only one report of treatment with dabrafenib in the setting of vemurafenib intolerance.11 Herein, we report a series of 11 patients, from three institutions, with ECD or ECD/LCH treated with single agent dabrafenib as (1) initial histiocytosis therapy, (2) following failure of chemotherapy or radiation, or (3) following discontinuation of vemurafenib therapy because of toxicity or intolerance.
This is a retrospective study of patients with ECD or ECD/LCH treated between January 1st, 2014 and October 31st, 2017, at the Memorial Sloan Kettering Cancer Center (MSKCC), NY, USA, the Department of Hematology at Shaare Zedek Medical Center in Jerusalem, Israel, and the University of Florida Health Cancer Center at Orlando Health, FL, USA. This retrospective review was approved with a waiver of consent by the Institutional Review Board.
ECD was diagnosed based on criteria which has been previously published,12 and in all cases tissue biopsy demonstrated CD68+/CD1a− histiocytic infiltration in the context of skeletal abnormalities on 99-Technetium bone scintigraphy or 18-fluorodeoxyglucose positron emission tomographic/computed tomography (FDG-PET/CT), and at least one other characteristic manifestation of ECD (xanthelasma, perinephric infiltration, “coated aorta”, pericardial infiltration, right atrial pseudo-tumor, or brain or dural infiltration). Genomic analysis of biopsy tissue was performed using the Memorial Sloan Kettering Cancer Center - Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) assay or pyrosequencing, as previously described.13
Dabrafenib monotherapy (Table 1) was initially dosed from 50mg BID to 150mg BID. Adverse events were documented and dose adjustments were made at the treating physician’s discretion. FDG-PET/CT scans were performed prior to starting dabrafenib and in follow up to measure disease response in nearly all cases; in addition, organ-specific imaging, such as cranial magnetic resonance imaging (MRI), was performed when appropriate. The primary response assessment was by FDG PET/CT using modified PET Response Criteria in Solid Tumors (PERCIST), as published.6 In brief, PERCIST criteria14 were used with the following modifications: up to 5 lesions were selected, SUVs were normalized for body weight, and the FDG avidity of each lesion was calculated as SUVmax lesion − SUVmax liver background = SUVcorrected for background, or simply “SUV.” For brain lesions, brain background was used in lieu of liver background. Values less than zero were treated as 0, which allowed the FDG avidity of a lesion to be considered as the excess avidity above background. Complete metabolic response (CMR) was defined as all lesions decreased to or below background; partial metabolic response (PMR) was defined as a 50% or greater decrease from baseline in the sum SUV of all target lesions; progressive metabolic disease (PMD) was defined as a 50% or greater increase from the nadir in the sum of SUV all target lesions or the appearance of new evaluable lesions; stable metabolic disease (SMD) was when the response did not meet other criteria.
Table 1.
Patients with BRAFV600E-mutated histiocytosis treated with dabrafenib.
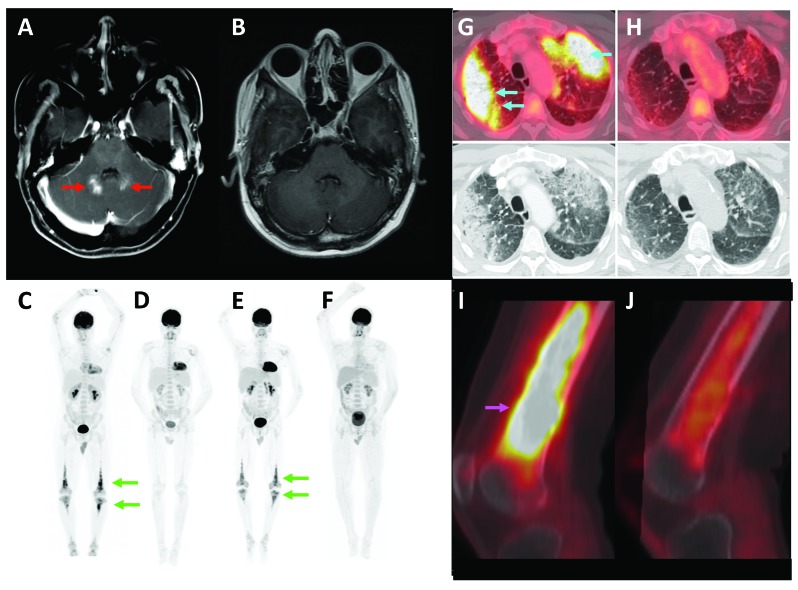
Eleven patients were treated, seven men and four women (Table 1). Seven had ECD and four had overlap ECD/LCH. The age at diagnosis ranged from 31–77 years (median 59). Sites of ECD and LCH involvement, clinical symptoms, prior therapies, toxicities of therapy, and duration of dabrafenib therapy are described in Table 1. For the five patients treated with dabrafenib as initial therapy or following failure of conventional therapy, three had a PMR and two had a CMR by FDG-PET; all had a complete clinical response (resolution of bone pain, dizziness, hearing loss, dysarthria, skin lesions and/or dyspnea). One patient had a complete resolution of a cerebellar lesion by MRI that had been refractory to treatment with cytarabine (Figure 1). These patients have continued treatment with minimal toxicities (one Grade 3 event) for three, four, five, 11, and 13 months. Formal response assessment by way of anatomic measurement of tumors was not performed for this series, although overt regression of cerebellar masses and lung infiltrates (Figure 1), as well as skin lesions and a dural mass were observed in patients treated with dabrafenib as initial therapy. For the six patients treated with vemurafenib prior to dabrafenib, treatment with vemurafenib was stopped after four and nine months because of Grade 4 transaminitis (two patients), after 15 and 16 months as a result of intolerable Grade 2 arthralgia (two patients), after four months due to intolerable Grade 2 fatigue (one patient), and after 14 days for Grade 2 photosensitivity (one patient). Two of these six patients had relapsed ECD (both clinically and by FDG-PET) within 12 weeks of cessation of vemurafenib for transaminitis, however, both recaptured a response with dabrafenib (Figure 1), without hepatotoxicity, and both remained on treatment for 16 and 43 months with sustained response. In three of these six patients, dabrafenib maintained their clinical and metabolic response to vemurafenib; the sixth patient had been treated with interferon-α with poor clinical response, and dabrafenib achieved a sustained clinical and metabolic response. Two patients who stopped vemurafenib for arthralgia or fatigue stopped dabrafenib for similar intolerance after four and nine months, respectively.
Figure 1.
Radiologic response to single-agent dabrafenib in BRAFV600E-mutant histiocytoses. (A) Axial post-gadolinium T1-weighted MRI demonstrates enhancing lesions in the cerebellar vermis bilaterally (red arrows), (B) that were resolved after four months of therapy. (C) Full-body FDG-PET demonstrates osseous (green arrows) ECD prior to treatment with vemurafenib, (D) partial metabolic response after six months of vemurafenib, (E) relapse of disease (green arrows) after 12 weeks of treatment interruption, and (F) complete metabolic response after six months of dabrafenib. (G) ECD of the lungs (light blue arrows) on FDG-PET and CT, (H) demonstrate partial metabolic response and radiologic near-resolution after four months of dabrafenib. (I) Osseous ECD of the femur (pink arrow) shows (J) partial metabolic response after two months of dabrafenib.
In this study, we report the largest series to date of treatment with single-agent dabrafenib for ECD or ECD/LCH harboring the BRAFV600E mutation. Treatment was effective as measured by FDG-PET in the context of newly diagnosed disease, disease refractory to conventional therapy, or following vemurafenib. Responses were observed in the nervous system, a site of disease often refractory to treatment. In addition, a sustained response of up to 43 months was observed in one case. Toxicities were limited (one Grade 3 event), and two out of the 11 patients stopped for intolerance while all others continued on treatment. This may be particularly relevant when considering BRAF inhibitor therapy in the pediatric population where toxicity and tolerability are paramount. Of note, treatment was tolerated in four out of six patients for whom vemurafenib was discontinued for toxicity or intolerance. The reason for the differential toxicities of dabrafenib and vemurafenib are not well understood, but possible explanations include differential potency for wild-type RAF and the fact that dabrafenib is dosed below its maximum tolerated dose (no MTD was reached in early trials).15 Our series is limited by its retrospective nature, but we believe these limitations are mitigated by the rarity of this disease, the relative uniformity of assessments, and the robustness of the observed responses. Optimal dosing of dabrafenib cannot be determined from this series, although we note that responses were seen and maintained at 50–100mg twice daily in many cases. Experience with vemurafenib in BRAFV600E -mutated histiocytosis, including a prospective trial, far outweighs experience with dabrafenib to date; therefore, our study cannot necessarily suggest that dabrafenib should replace vemurafenib in this context. The optimal duration of treatment with BRAF inhibitors and the consequences of interrupting therapy in this context is unknown, as is the efficacy of combined treatment with agents such as MEK inhibitors, although there are ongoing active studies of these questions (clinicaltrials.gov Identifiers: 02089724 and 02281760). Further studies may elucidate the role of dabrafenib in the treatment of these conditions.
Supplementary Material
Footnotes
Funding: this work was supported by the Erdheim-Chester Disease Global Alliance (E.L.D) and National Institutes of Health/National Cancer Institute Core Grant [P30 CA008748] awarded to Memorial Sloan Kettering Cancer Center.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. [DOI] [PubMed] [Google Scholar]
- 2.Hervier B, Haroche J, Arnaud L, et al. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124(7):1119–1126. [DOI] [PubMed] [Google Scholar]
- 3.Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121(9):1495–1500. [DOI] [PubMed] [Google Scholar]
- 4.Haroche J, Cohen-Aubart F, Emile JF, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411–418. [DOI] [PubMed] [Google Scholar]
- 5.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond EL, Subbiah V, Lockhart AC, et al. Efficacy of vemurafenib in BRAFV600-mutant Erdheim–Chester disease and Langerhans cell histiocytosis: the histology-independent phase II open-label VE-BASKET study. JAMA Oncol. 2017:November 29 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. [DOI] [PubMed] [Google Scholar]
- 8.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordmann TM, Juengling FD, Recher M, et al. Trametinib after disease reactivation under dabrafenib in Erdheim-Chester disease with both BRAF and KRAS mutations. Blood. 2017;129(7):879–82. [DOI] [PubMed] [Google Scholar]
- 10.Hyman DM, Diamond EL, Vibat CR, et al. Prospective blinded study of BRAFV600E mutation detection in cell-free DNA of patients with systemic histiocytic disorders. Cancer Discov. 2015;5(1):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt D, Milne P, Fernandes P, et al. Targeted treatment of brainstem neurohistiocytosis guided by urinary cell-free DNA. Neurol Neuroimmunol Neuroinflamm. 2017;4(1):e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PER-CIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menzies AM, Long GV, Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Devel Ther. 2012;6:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.