Abstract
Transcription factor IKZF1 (IKAROS) acts as a critical regulator of lymphoid differentiation and is frequently deleted or mutated in B-cell precursor acute lymphoblastic leukemia. IKZF1 gene defects are associated with inferior treatment outcome in both childhood and adult B-cell precursor acute lymphoblastic leukemia and occur in more than 70% of BCR-ABL1-positive and BCR-ABL1-like cases of acute lymphoblastic leukemia. Over the past few years, much has been learned about the tumor suppressive function of IKZF1 during leukemia development and the molecular pathways that relate to its impact on treatment outcome. In this review, we provide a concise overview on the role of IKZF1 during normal lymphopoiesis and the pathways that contribute to leukemia pathogenesis as a consequence of altered IKZF1 function. Furthermore, we discuss different mechanisms by which IKZF1 alterations impose therapy resistance on leukemic cells, including enhanced cell adhesion and modulation of glucocorticoid response.
Introduction
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is the most common malignancy in children and involves uncontrolled expansion of B-lymphoid progenitors in the bone marrow. The disease is frequently initiated by a chromosomal translocation but becomes manifest only when leukemic progenitors in the bone marrow have accumulated a number of additional gene deletions and mutations that drive disease progression. With current treatment protocols long-term survival approaches 90%;1 however, relapses still pose a significant clinical challenge due to resistance to chemotherapy of the recurrent disease.1 Both in pediatric and adult BCP-ALL, specific genetic subtypes with distinct prognostic outcomes can be identified2 Some of these subtypes, such as hyperdiploid ALL and ETV6-RUNX1-rearranged ALL are associated with a favorable outcome, while other genetic hallmarks, such as MLL gene rearrangements, hypodiploidy, intrachromosomal translocation of chromosome 21 (iAMP21), or the presence of the t(9;22) BCR-ABL1 translocation predict poor outcome. Moreover, the presence of a gene expression profile similar to that of BCR-ABL1-positive ALL, which frequently involves genetic alterations that deregulate cytokine receptor and/or tyrosine kinase signaling, is similarly associated with poor outcome.2 In addition to these gross chromosomal rearrangements, deletions or mutations affecting the B-cell transcription factor IKZF1, are a strong and independent predictor of poor outcome in BCP-ALL.3,4 Together with its role as a critical regulator of B-cell development and a leukemia tumor suppressor, there is mounting evidence that IKZF1 loss also affects signaling pathways that modulate therapy response.
Here, we provide an overview of the complex role of transcription factor IKZF1 during normal lymphopoiesis and consequences of IKZF1 loss for the pathogenesis of BCP-ALL. Finally, we discuss some of the molecular mechanisms by which IKZF1 gene alterations may contribute to therapy resistance.
Transcription regulation by IKAROS zinc-finger protein 1
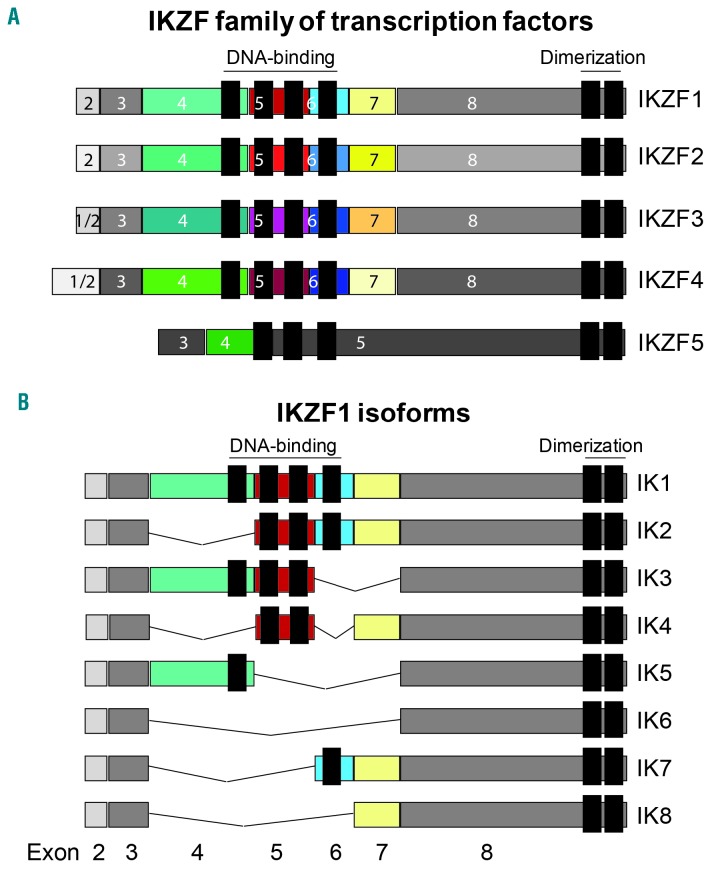
The IKAROS family of transcription factors consists of five different IKAROS zinc-finger proteins (IKZF1-IKZF5) that are able to bind DNA directly at the core motif A/GGGAA through their N-terminal zinc-finger domain.5,6 Furthermore, all IKAROS family members harbor two additional C-terminal zinc-fingers required for homo- and heterodimerization between the different IKZF proteins (Figure 1A). The formation of homo- or heterodimers between IKAROS zinc-finger proteins with a functional DNA binding domain strongly enhances their DNA affinity and transcriptional activity. However, a common feature of IKZF1 and related family members is the presence of shorter variants due to alternative splicing. These variants often lack DNA binding activity but retain the ability to interact with full-length IKZF1-IKZF5, thereby creating dominant-negative isoforms. A well-known splice variant of both the mouse and human IKZF1 gene is the IK6 isoform, which lacks exons 4 to 7 that encode the four N-terminal zinc-fingers representing the DNA binding domain (Figure 1B).
Figure 1.
Overview of the human family of IKAROS zinc-finger (IKZF) transcription factors and IKZF1 isoforms. (A) Schematic representation of the five IKZF proteins (IKZF1-IKZF5), including the N-terminal zinc-fingers that define the DNA-binding domain and the two C-terminal zinc-fingers representing the dimerization domain. The colored boxes indicate the individual regions within the protein that are encoded by distinct exons. (B) The common IKZF1 splice variants (IK1-IK8) are shown, including the shorter isoforms that are generated by alternative splicing. The splice variants lacking exons 4 and 5 (IK6-IK8) represent dominant-negative isoforms of IKZF1.
IKZF1 mainly regulates gene expression through association with the nucleosome remodeling and deacetylase complex,7–10 which includes histone deacetylases HDAC1, HDAC2 and the ATP-dependent chromatin remodeling proteins CHD3 and CHD4. The nucleosome remodeling and deacetylase complex is involved in both transcriptional repression as well as gene activation by IKZF1.11,12 Gene silencing by IKZF1 is also facilitated through interaction with Polycomb repressive complex 2, which promotes histone H3 lysine 27 trimethylation to maintain genes in an inactive state.13,14 Other transcriptional co-factors that can associate with IKZF1 and mediate gene regulation include CtBP, CtIP and SWI/SNF-related complex.15–17 On the other hand, IKZF1 may itself participate in transcription initiation through direct interactions with the general transcription factors TFIIB and TBP.16 IKZF1 also controls transcription elongation via association with protein phosphatase 1α and cyclin-dependent kinase 9 (CDK9), the enzymatic component of the positive transcription elongation factor b.18–20 IKZF1-mediated transfer of protein phosphatase 1α to CDK9 promotes activation of positive transcription elongation factor b and recruitment to gene regulatory regions, thereby facilitating transcription elongation of IKZF1-target genes in hematopoietic cells.18
Distinct post-translational modifications are able to modify the function of IKZF1. Phosphorylation of IKZF1 at multiple serine and threonine residues by casein kinase II impairs its function as a transcription factor.20–22 Conversely, casein kinase II inhibition enhances the transcriptional repressor function of IKZF1.23 On the other hand, dual-specificity kinases BTK and SYK both phosphorylate IKZF1 on specific serine residues in close proximity of the DNA binding domain to augment its nuclear localization and DNA binding activity.24,25 Sumoylation of IKZF1 on lysine residues occurs within the nucleus and seems to interfere with transcriptional repression.26,27 It was previously shown that IKZF1 is also subject to ubiquitination,20 and there is now renewed interest in this pathway, since both IKZF1 and IKZF3 are targets of the immunomodulatory drugs thalidomide, lenalidomide, pomalidomide and CC-122.28 These immunomodulatory drugs promote proteosomal degradation of IKZF1 and IKZF3 by redirecting the substrate specificity of the CRL4CRBN ubiquitin ligase complex.29,30 Immunomodulatory drugs show therapeutic effects in a broad range of hematologic malignancies through their ability to target the malignant cells and modulate the immune system and its microenvironment.
IKZF1 is essential for normal lymphopoiesis
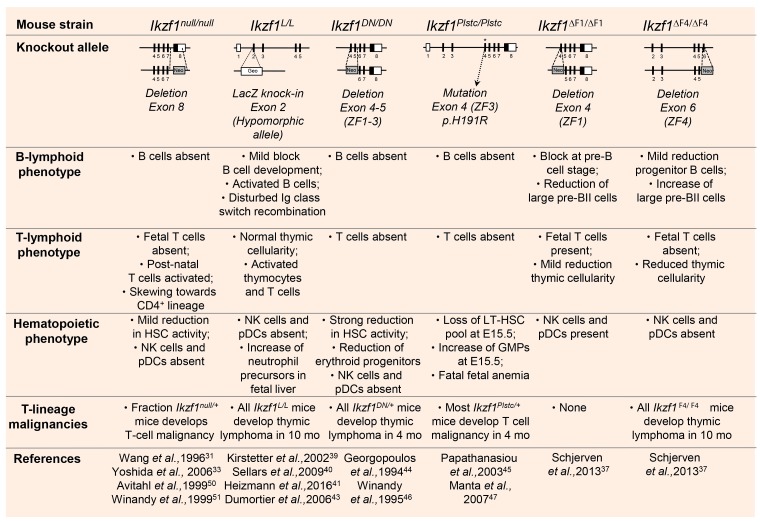
Studies performed in both constitutive and conditional Ikzf1 knockout mouse models have demonstrated that IKZF1 function is not only required at different stages of lymphopoiesis,12,31,32 but also for normal myeloid, megakaryocyte and erythroid differentiation.33–36 Ikzf1-deficient mice (Ikzf1null/null) lack all B cells, natural killer cells, plasmacytoid dendritic cells and fetal T cells31,37 (Figure 2). Nonetheless, post-natal Ikzf1-null mice harbor early T lineage progenitors within the thymus and export mature T cells to the periphery.38 Mice homozygous mutant for a hypomorphic allele of Ikzf1 (Ikzf1L/L) show reduced B-cell progenitors in the bone marrow compartment, but still generate normal counts of mature B2 cells.39 These splenic B cells display alterations in isotype selection during immunoglobulin class switch recombination and a hyperproliferation phenotype upon antigenic stimulation.40,41 Although spontaneous progression to B-cell ALL is not observed in Ikzf1L/L mice, haplodeficient Ikzf1L/+ animals demonstrate an accelerated onset of B-cell leukemia in combination with a BCR-ABL1 transgene.42 Moreover, all Ikzf1L/L mice develop thymic lymphoma within a period of 10 months through activation of the Notch pathway.43 Ikzf1 mutant mice expressing dominant-negative isoforms of IKZF1 (Ikzf1DN/DN and Ikzf1Plstc/Plstc) demonstrate a widespread failure of hematopoiesis,44,45 highlighting the importance of IKAROS transcription factors in hematolymphoid differentiation. Notably, heterozygous Ikzf1 mutant mice develop T-cell malignancies with very high penetrance and short latency in the case of the dominant-negative isoforms,46,47 while this phenotype is less obvious in Ikzf1+/− mice.48
Figure 2.
Summary of the observed phenotypes in the different constitutive Ikzf1 knockout mouse models. The knockout allele shows a schematic representation at which position the deletion or mutation is present in the mouse Ikzf1 gene. DN: dominant negative; Plstc: ENU-induced dominant-negative point mutation, called Plastic; Neo: neomycin gene; βGeo: fusion between LacZ and neomycin gene; ZF: zinc-finger; HSC: hematopoietic stem cell; NK: natural killer; pDCs: plasmacytoid dendritic cells; LT-HSC: long-term hematopoietic stem cell; GMPs: granulocyte-macrophage precursors; mo: months.
Detailed gene expression profiling has revealed that IKZF1 is essential for the generation of common lymphoid progenitors by priming lymphoid lineage-specific signatures in hematopoietic stem cells and lymphoid-primed multipotent progenitors.49 At different stages of T-lineage differentiation and development, IKZF1 is engaged by setting thresholds for (pre-)T-cell receptor-controlled checkpoints as well as T-cell activation downstream of interleukin-2 receptor signaling.50,51 In B-cell progenitors, Ikzf1 is required to induce Rag1 and Rag2 expression, and mediates chromatin accessibility during immunoglobulin gene rearrangement and allelic exclusion at the Igk locus.12,32,52 During pre-B-cell differentiation, IKZF1 regulates the transcription of genes implicated in pre-B-cell receptor signaling, cell survival, stromal-cell adhesion and B-cell commitment, such as Pax5, Foxo1 and Ebf1.12,32,53 Many of those regulatory activities during B-lineage differentiation are navigated by super-enhancer networks controlled by IKZF1 and other B-cell master transcription factors.54 Besides regulating expression of B-lymphoid genes, IKZF1 is actively involved in repression of a lineage-inappropriate transcriptional program normally prevalent in epithelial and mesenchymal precursors.54
To further delineate the function of the individual zinc-fingers within the DNA-binding domain of IKZF1 in B-lymphopoiesis, Ikzf1 mouse mutants have been generated with targeted deletion of exon 4, which encodes zinc-finger 1 (Ikzf1ΔF1/ΔF1), or exon 6 encoding zinc-finger 4 (Ikzf1ΔF4/ΔF4).37 Germline deletion of either exon 4 or 6 results in decreased B-cell precursors with a stronger developmental block in Ikzf1ΔF1/ΔF1 mice, especially at the pre-B-cell stage.37 In contrast, the fraction of large pre-B cells is strongly increased in Ikzf1ΔF4/ΔF4 mice as compared to wild-type control animals. Interestingly, deletion of zinc-finger 4, but not zinc-finger 1, accelerates the onset of BCR-ABL1-mediated B-cell leukemia.37,55 Conditional deletion of exon 5 (Ikzf1E5Δ/Δ), which encodes zinc-fingers 2 and 3, at the stage of common lymphoid progenitors also results in an expansion of large pre-B cells within the bone marrow compartment, which is followed by a subsequent block in the transition to small pre-B cells.56 These findings indicate that N-terminal zinc-fingers 2, 3 and 4 of IKZF1 limit cell proliferation and survival at the time of active pre-B-cell receptor signaling, while zinc-fingers 1, 2 and 3 are absolutely required for the transition to the pre-B-cell stage.
IKZF1 gene lesions drive leukemia development and relapse
In the past decade, complementary genome-wide approaches have been employed to identify the genetic drivers implicated in the pathogenesis of ALL. Those studies revealed that the IKZF1 gene, which is located on chromosome band 7p12.2, is recurrently affected by different types of genetic alterations in BCP-ALL. Analysis of copy number alterations has demonstrated that IKZF1 gene deletions are present in about 15% of cases of childhood BCP-ALL and 40%-50% of adult patients with BCP-ALL.57–60 These deletions frequently involve the whole gene (DEL1-8) that results in loss of expression of wild-type IKZF1, as well as focal deletions that alter the function of IKZF1, such as the dominant-negative isoform IK6 (DEL4-7). Other common variants include deletions affecting exons 2-3, exons 2-7 and exons 4-8.61 In most cases these are monoallelic IKZF1 deletions where one functional copy of IKZF1 is retained, although biallelic deletions are also observed in a fraction of BCP-ALL cases.62,63 In addition, IKZF1 function is compromised by insertions, frameshift and missense mutations, which represent ~7% of IKZF1 alterations in BCP-ALL.63 Furthermore, rare in-frame gene fusions involving IKZF1 have been identified by RNA sequencing in BCP-ALL, including IKZF1-NUTM1, IKZF1-SETD5 and the reciprocal SETD5-IKZF1.64 However, it remains to be established whether these IKZF1 gene fusions are pathogenic and contribute to leukemia development.
An interesting feature is the strongly increased prevalence of IKZF1 deletions and mutations in high-risk BCP-ALL cases with an activated tyrosine kinase profile, particularly BCR-ABL1-positive ALL (~85%),65 and BCR-ABL1-like ALL (~70%), which is characterized by a range of genetic alterations driving cytokine receptor and kinase signaling.3,66–68 Similarly, IKZF1 deletions and mutations are highly abundant in chronic myeloid leukemia that has progressed to lymphoid blast crises, but IKZF1 alterations are virtually absent in chronic-phase and myeloid blast crisis chronic myeloid leukemia.65,69,70 IKZF1 deletions are also rarely detected in ETV6-RUNX1-positive BCP-ALL (3%), TCF3-rearranged (~3%) and MLL-rearranged (~5%) B-cell ALL.58,71,72 The distribution of IKZF1 deletions among the remaining subtypes, including hyperdiploid and B-other leukemia, ranges from 15%-20%.72
IKZF1 acts as a critical tumor suppressor in mouse T-lymphoid malignancies,43,46,47 but IKZF1 gene lesions are not very prevalent in T-ALL. Copy number alterations and mutations affecting the IKZF1 gene can be detected in ~4% of T-ALL.58,65,71,73 Notably, IKZF1 alterations occur in ~13% of early T-cell precursor ALL, a high-risk subtype of T-ALL characterized by recurrent mutations activating tyrosine kinases (FLT3, JAK1, JAK3) and cytokine signaling (IL7R).74 IKZF1 alterations have also been reported in myeloproliferative neoplasms,75 and both pediatric and adult acute myeloid leukemia harbor IKZF1 deletions that affect its function.76,77 Thus, the tumor suppressive activity of IKZF1 is not uniquely restricted towards the lymphoid lineage and extends to a broader range of hematologic malignancies.
Besides its critical role in the pathogenesis of leukemia, IKZF1 alterations are also associated with adverse prognosis in BCP-ALL.3,4,78 even within the high-risk group of BCR-ABL1-positive ALL.79,80 Notably, the occurrence and prognostic impact of IKZF1 alterations is not restricted to high-risk cases, but is also observed in standard-risk B-ALL subtypes,72 including high hyperploidy.81 Indeed, IKZF1 deletion represents one of the strongest independent predictors of poor treatment outcome in childhood BCP-ALL.71,72,82 Similar data have been reported in adult BCP-ALL, where loss-of-function gene deletions of IKZF1 predict poor treatment outcome in BCR-ABL1-negative cases.83–86 Interestingly, the presence of other co-occurring gene lesions may either enhance or negate the prognostic value of IKZF1 deletions. For instance, focal deletions affecting both transcriptional regulator BTG1 and IKZF1 represent a high-risk group with a worse outcome than those with IKZF1 alterations alone.48 On the other hand, the BCP-ALL subtype characterized by deregulation of transcription factors ERG and DUX4 has a favorable outcome, despite the presence of IKZF1 deletions in approximately 40% of these patients.64,87–90 An explanation for this latter observation remains elusive.
Genetic alterations that cooperate with IKZF1 deletions in B-cell precursor acute lymphoblastic leukemia
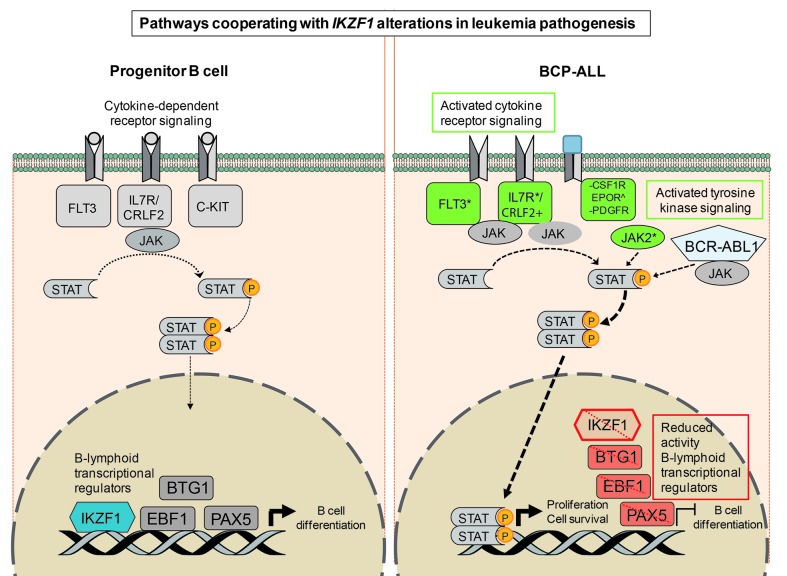
There is accumulating evidence that recurrent chromosomal aberrations present in BCP-ALL, such as BCR-ABL1 translocations or CRLF2 rearrangements, act as driver lesions and represent early events in leukemia development. Genome-wide analysis has established that several other genetic alterations cooperate before B-cell leukemia becomes manifest. Gene lesions that inactivate the lymphoid transcription factor IKZF1 are frequently observed in BCR-ABL1-positive and CRLF2-rearranged BCP-ALL.65,69,91,92 The latter group is associated with concomitant JAK1 and JAK2 activating mutations.91 Similarly, IKZF1 alterations are highly prevalent in tyrosine kinase-activating lesions that define BCR-ABL1-like ALL.67,68 These include rearrangements involving ABL1/ABL2, CSF1R, EPOR, JAK2 and PDGFRB, or sequence mutations affecting FLT3, IL7R or SH2B3. Indeed, loss of IKZF1 may permit more effective STAT5 target gene regulation downstream of these pathways.93 Collectively, these findings argue that loss of IKZF1 function strongly cooperates with activated tyrosine kinase signaling pathways linked to enhanced progenitor B-cell proliferation and immortalization (Figure 3).
Figure 3.
Pathways cooperating with IKZF1 alterations in leukemia pathogenesis. Pathways involving cytokine receptor signaling and B-cell differentiation by lymphoid transcriptional regulators in normal progenitor B cells are schematically indicated on the left. Alterations of these pathways co-occur frequently with IKZF1 deletions and mutations in B-cell progenitor acute lymphoblastic leukemia (BCP-ALL) as indicated on the right. These include, activating mutations in FLT3, IL7R, JAK2 (*), upregulation of CRLF2 (+), C-terminal truncations or upregulation of EPOR (^), chromosomal translocations generating fusion proteins with PDGFR or CSF1R (−), and BCR-ABL1, which collectively results in activated cytokine receptor and tyrosine kinase signaling leading to STAT activation. In addition, IKZF1 alterations co-occur with gene deletions affecting the activity of B-lymphoid transcriptional regulators EBF1, PAX5 and BTG1, which results in a block of B-cell differentiation. FLT3: FMS related tyrosine kinase 3; IL7R: interleukin 7 receptor; CRLF2: cytokine receptor like factor 2; C-KIT: mast/stem cell growth factor receptor Kit; JAK, Janus kinase; STAT: signal transducer and activator of transcription; BTG1: B-cell translocation gene 1; EBF1: early B-cell factor 1; PAX5: paired box 5; IKZF1: IKAROS family zinc finger 1; CSF1R: colony-stimulating factor 1 receptor; EPOR: erythropoietin receptor; PDGFR: platelet-derived growth factor receptor.
The predilection for IKZF1 gene alterations in BCR-ABL1-mediated lymphoid versus myeloid malignancies has been further corroborated in mouse studies. In a bone marrow transplantation model using lineage-negative hematopoietic progenitor cells, it was shown that expression of IK6 skews BCR-ABL1-mediated leukemia from an exclusive myeloproliferative disease towards a combined myeloid and B-lymphoid disease.63 Introducing p19Arf-deficiency further strengthens this trend towards uniformly induced B-cell ALL. This is in agreement with the finding that BCR-ABL1-positive BCP-ALL is characterized by the co-occurrence of IKZF1 and CDKN2A gene deletions.65
Another group of genetic changes that frequently co-occur with IKZF1 alterations in BCP-ALL include gene deletions affecting lymphoid transcription factors, such as EBF1 and PAX5, and the transcriptional co-factor BTG148,58 (Figure 3). BTG1 belongs to the BTG/TOB antiproliferative (APRO) family of proteins,94 which control gene transcription by their ability to interact with specific transcription factors, such as nuclear receptors and homeobox proteins.95,96 the CCR4-NOT transcriptional regulatory complex,97 or through recruitment of protein arginine methyl transferase PRMT1.98 In addition, BTG1 through interaction with the CCR4-NOT, may also regulate mRNA deadenylation and consequently mRNA decay.99,100 Mice deficient for Btg1 show a partial block in B-cell development, which is even more evident in Btg1−/−;Btg2−/− mice.101 These studies have demonstrated that BTG1, together with BTG2, is required to suppress a T-lineage inappropriate expression program in progenitor B cells. Thus, monoallelic gene deletions of IKZF1 in combination with EBF1, PAX5 or BTG1 may contribute to a more prominent block in B-cell development and increased proliferative expansion of precursor B cells. Indeed, intercrossing haplodeficient Ikzf1 animals with heterozygous Ebf1 or Pax5 knockout mice promotes the onset of ALL, giving rise to both BALL and T-ALL.102 On the other hand, Btg1-deficiency specifically accelerates the development of T-ALL in Ikzf1+/− mice, which suggests that B-lineage-restricted mouse models will be required to establish their synergistic action in the pathogenesis of B-ALL.
Effector pathways downstream of IKZF1 involved in leukemia pathogenesis
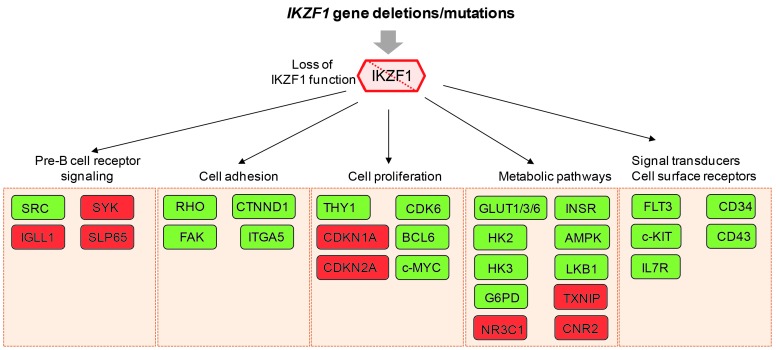
Since lymphoid transcription factors are commonly deleted in BCP-ALL, the tumor suppressive functions of IKZF1 and other B-cell master regulators, such as EBF1 and PAX5, have been mostly linked to the suppression of their B-cell differentiation programs in these leukemic cells. However, this would not fully explain the predilection of IKZF1 alterations in BCR-ABL1-positive and BCR-ABL1-like leukemia, suggesting that IKZF1 also regulates other molecular pathways. Furthermore, loss of IKZF1 function probably affects different target genes in human leukemic cells as compared to mouse progenitor B cells, which could even be distinct from those deregulated by expression of dominant-negative isoforms, such as IK6. Nonetheless, mouse studies performed over the past 5 years have been very instrumental in deciphering the transcriptional networks downstream of IKZF1. Thus, gene expression profiling in different Ikzf1 knockout mouse models combined with genome-wide chromatin immuno-precipitation studies has uncovered IKZF1-specific targets that are not only linked to lymphoid lineage commitment and B-cell differentiation, but also to leukemia development.
A large group of those Ikzf1-target genes can be classified as signal transducers, some of which drive early lymphoid differentiation, such as c-Kit, Flt3 and Il7r.12,32,37,53 Adult ALL samples harboring IKZF1 deletions display increased expression of IL7R together with reduced expression of SH2B3, which represents a defined subset of high-risk B-ALL.103 Other genes differentially expressed in Ikzf1-mutant mice are important for pre-B-cell receptor signaling, and several of these IKZF1 targets appear to be deregulated in BCR-ABL1-positive B-ALL, including IGLL1, SYK, and SLP65.104,105 Indeed, defective pre-B-cell receptor function is a hallmark of BCR-ABL1-positive ALL, and loss of IKZF1 function enhances SRC phosphorylation at the expense of the SYK/SLP65 pathway activation, which is required for pre-B-cell differentiation.104 Besides transcriptional regulation of signal transducers, Ikzf1 controls the expression of cell surface receptors, such as CD34 and CD43, and these molecules confer a leukemic growth advantage to IKZF1-mutated BCR-ABL1-positive B-ALL cells.55
Another group of IKZF1 target genes identified in mouse progenitor B cells seems to converge on a cellular network coupling cell surface protein expression with intracellular Wnt and Rho signaling as well as catenin-driven gene regulation inside the nucleus.55,106 A critical target gene within this subgroup includes Ctnnd1 encoding p120-catenin. This is a multifunctional protein that regulates cadherin stability at the cell membrane, activation of the Rho family of GTPases in the cytoplasm and Wnt/β-catenin target genes within the nucleus by interacting with Kaiso.107 Activation of CTNND1 expression is observed in samples from patients with IKZF1 deletions,108 and inactivation of p120-catenin reduces the proliferative capacity of BCR-ABL1-positive leukemic cells.55,106 A related downstream effector pathway of IKZF1 that plays an eminent role during mouse B-cell development is integrin-dependent survival signaling, which involves activation of focal adhesion kinase (FAK).12,56 In mouse models of BCR-ABL1-positive B-ALL, perturbation of Ikzf1, including loss-of-function deletions and expression of IK6, leads to activation of an adhesive phenotype, which correlates with overexpression of FAK.63,109 FAK pathway upregulation is also observed in BCR-ABL1-positive BCP-ALL, especially in the context of IK6 expression.109 Moreover, FAK inhibition potentiates the responsiveness to the ABL inhibitor dasatinib in a xenograft model system and improves survival.109
Recently, it has been proposed that the B-lymphoid transcriptional program regulated by IKZF1, as well as PAX5, acts as a metabolic barrier against malignant transformation of B-cell precursor cells.110 Inducible reconstitution of functional IKZF1 in patient-derived IKZF1-deleted B-ALL cells results in activation of the LKB1-AMPK energy-stress-sensor pathway, and decreased protein levels of the insulin receptor, the glucose transporters GLUT1, GLUT3 and GLUT6, as well as the effectors of glucose metabolism, such as HK2, HK3, and G6PD. On the other hand, the expression of glucose-transport inhibitors, such as TXNIP and CNR2, are strongly induced by IKZF1. Consequently, these IKZF1-reconstituted B-ALL cells transit into a state of chronic energy deficit. Thus, this ‘metabolic gatekeeper’ function of IKZF1 may force silent pre-leukemic clones that carry potentially oncogenic lesions to remain in a latent state.
Besides imposing a change on pre-B-cell receptor signaling, cell adhesion and metabolic state, IKZF1 alterations in combination with BCR-ABL1 expression also result in acquisition of stem cell-like features and enhanced self-renewal of progenitor B cells63,105 (Figure 4). Activation of THY1 expression has been linked to enhanced self-renewal,63 and Ikzf1 has been shown to regulate expression of multiple genes involved in cell cycle regulation, including Cdkn1a, Cdkn2a, and Cdk6.53,55 In mouse progenitor B cells and human B-ALL, BCL6 and MYC have been identified as IKZF1 targets,32,111–113 and probably both contribute to enhanced cell proliferation of IKZF1-deleted B-ALL. However, it remains to be established whether targeting these pathways has therapeutic potential in high-risk BALL patients.
Figure 4.
Effector pathways downstream of IKZF1 involved in leukemia pathogenesis. Loss of IKZF1 function due to IKZF1 gene deletions and mutations affects multiple pathways, including pre-B-cell receptor signaling, cell adhesion and proliferation, metabolic pathways and signal transducers and cell surface receptors. IKZF1 affects the expression of defined key molecules within each of these pathways, as indicated in the boxes. Green boxes define targets that are upregulated upon loss of IKZF1 function, while red boxes represent repressed targets. SRC: sarcoma proto-oncogene tyrosine kinase; SYK: spleen tyrosine kinase; IGLL1: immunoglobulin lambda-like polypeptide 1; SLP65: B-cell linker; RHO: RHO family of GTPases; CTNND1: catenin delta 1/p120 catenin; FAK: focal adhesion kinase; ITGA5: integrin subunit alpha 5; THY1: thymus cell antigen 1; CDK6: cyclin dependent kinase 6; CDKN1A: cyclin dependent kinase inhibitor 1A; BCL6: B-cell lymphoma 6; CDKN2A: cyclin dependent kinase inhibitor 2A; c-MYC: cellular myelocytomatosis oncogene; GLUT1/3/6: glucose transporter 1/3/6; INSR: insuline receptor; HK2: hexokinase 2; HK3: hexokinase 3; AMPK: AMP-activated protein kinase; LKB1: liver kinase B1; G6PD: glucose-6-phosphate dehydrogenase; TXNIP: thioredoxin interacting protein; NR3C1: nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor); CNR2: cannabinoid receptor 2; FLT3: FMS related tyrosine kinase 3; CD34: hematopoietic progenitor cell antigen; c-KIT: KIT receptor tyrosine kinase; CD43: sialophorin; IL7R: interleukin 7 receptor.
IKZF1 alterations mediate therapy resistance
The presence of IKZF1 gene lesions in BCR-ABL1-positive B-ALL results in inferior treatment outcome and mouse xenograft models suggest that IKZF1 loss contributes to resistance to tyrosine kinase inhibitor-based therapy.63,80 Reactivation of cell adhesion pathways by perturbation of IKZF1 function leads to elevation of key adhesion molecules, such as integrins (ITGA5) and CD90, and adhesion regulators, such as FAK, as well as increased phosphorylation of FAK itself, which permits relocalization of leukemic cells to the bone marrow niche. Indeed, FAK inhibition resensitizes BCR-ABL1 leukemic cells to tyrosine kinase inhibitor therapy.109 Similar results are observed after treatment with retinoids, specifically retinoid X receptor agonists, which induce expression of wild-type IKZF1, but not IK6, thereby abrogating expression of stem cell and adhesion molecules.63 Although these studies have provided important clues about how IKZF1 deletions alter treatment response especially in the context of BCR-ABL1-positive ALL, alternative mechanisms of therapy resistance may exist besides protection through cell interactions within the bone marrow microenvironment.
Synthetic glucocorticoids, such as prednisolone, constitute essential drugs in the treatment of ALL patients and glucocorticoid resistance remains a substantial problem in the treatment of BCP-ALL. There is accumulating evidence that IKZF1 deletions mediate prednisolone resistance in vivo,114,115 but different mechanisms have been proposed. IKZF1 actively represses genes of the phosphatidylinositol-3 kinase pathway, including PIK3CD and PIK3C2B.23 Disruption of IKZF1 function, and subsequent activation of the PI3K/AKT/mTOR pathway can promote glucocorticoid resistance.116,117 IKZF1 controls expression of several genes involved in glucose and energy supply.110 This metabolic program may alter the threshold for responses to glucocorticoids in BCP-ALL. Specifically, the glucocorticoid receptor NR3C1 was reported to be a target of IKZF1 in pre-B ALL cells, and downregulation of NR3C1 protein levels could be observed upon expression of IK6.110 However, studies performed in murine Ikzf1+/− B cells and human BCP-ALL cell lines with short hairpin-mediated IKZF1 knockdown have demonstrated that loss of IKZF1 function induces glucocorticoid resistance independently of altered NR3C1 mRNA and protein expression.114 Indeed, IKZF1 itself appears to regulate NR3C1-dependent gene transcription.114 The transcriptional regulator BTG1 has been identified as a modifier of IKZF1-mediated resistance to glucocorticoid therapy and the combined loss of BTG1 and IKZF1 leads to an even stronger inhibition of glucocorticoid-induced cell death.48 Finally, IKZF1 target gene EMP1,106 which itself represents a poor prognostic factor in pediatric ALL, was shown to regulate the response to prednisolone, but also, on the other hand, to affect normal leukemic cell viability and proliferation.118 Collectively, these findings demonstrate that IKZF1, through modulation of different signaling pathways and acting directly on glucocorticoid target genes, alters treatment response, thereby mediating therapy resistance in BCP-ALL (Figure 5).
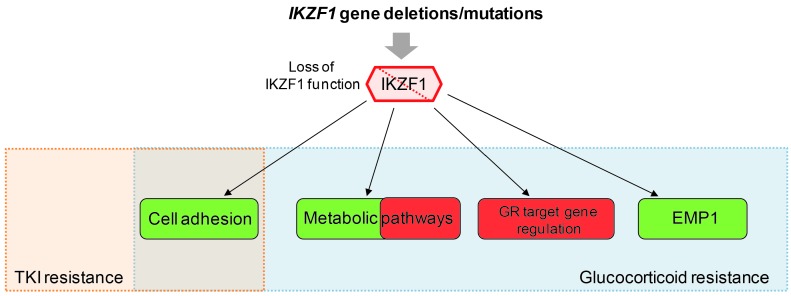
Figure 5.
IKZF1 alterations mediate therapy resistance. Overview of IKZF1-affected pathways contributing to tyrosine kinase inhibitor (TKI) resistance and glucocorticoid (GC) resistance. Enhanced cell adhesion due to loss of IKZF1 function has been shown to contribute to both TKI and GC resistance. Deregulation of metabolic pathways, such as LKB1/AMPK signaling and glucose metabolism, attenuated glucocorticoid receptor (GR) target gene regulation and upregulation of epithelial membrane protein 1 (EMP1) have been implicated in mediating GC resistance of IKZF1-deleted BCP-ALL. Green boxes indicate activated targets or pathways, while red boxes define attenuated pathways. Targets within the metabolic pathway can either promote or inhibit GC resistance.
Conclusions and perspectives
From this review it becomes clear that loss of IKZF1 function affects a broad variety of biological pathways which may all contribute to leukemia development. Moreover, the recently established roles for IKZF1 in cell adhesion, metabolism and glucocorticoid-dependent target gene regulation seem to be important determinants of therapy resistance. Preclinical studies are helping with the identification of molecular pathways that can be exploited for targeted therapy of IKZF1-deleted BCP-ALL.
Over the past decade, a large series of studies conducted in both childhood and adult ALL have provided clear evidence that IKZF1 alterations predict adverse outcome in BCP-ALL, both in BCR-ABL1-positive and -negative B ALL. However, more recently the role of IKZF1 deletions as an independent prognostic marker has been challenged,119 as has the specific contributions of whole gene versus intragenic dominant-negative IKZF1 deletions.86 One potential explanation for such disparities may relate to differences in scheduling and dosing of specific therapeutic agents between different treatment protocols. It will, therefore, be important to study these protocol-dependent differences in order to define what is currently the most efficient treatment for IKZF1-deleted ALL. Certain adjustments, such as the addition of vincristine-steroid pulses during maintenance therapy,81 may already prevent relapses. For the near future, more systematic screens aimed at determining specific vulnerabilities of IKZF1-deleted ALL may lead to the identification of targeted therapies that can re-sensitize this high-risk ALL subgroup to curative treatment.
Supplementary Material
Acknowledgments
This work was supported by Stichting Kinderen Kankervrij (KiKa; grant numbers KiKa 2009-55 and KiKa 2010-77) and Stichting KOC Nijmegen.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/4/565
References
- 1.Hunger SP, Mullighan CG. Acute lymphoblastic Leukemia in children. N Engl J Med. 2015;373(16):1541–1552. [DOI] [PubMed] [Google Scholar]
- 2.Iacobucci I, Mullighan CG. Genetic Basis of Acute Lymphoblastic Leukemia. J Clin Oncol. 2017;35(9):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper RP, Waanders E, van der Velden VH, et al. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24(7):1258–1264. [DOI] [PubMed] [Google Scholar]
- 5.John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48(9–10):1272–1278. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida T, Georgopoulos K. Ikaros fingers on lymphocyte differentiation. Int J Hematol. 2014;100(3):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Sif S, Jones B, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10(3):345–355. [DOI] [PubMed] [Google Scholar]
- 8.Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999;18(11):3090–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J Biol Chem. 2007;282(41):30227–30238. [DOI] [PubMed] [Google Scholar]
- 10.Liang Z, Brown KE, Carroll T, et al. A high-resolution map of transcriptional repression. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Jackson AF, Naito T, et al. Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nat Immunol. 2011;13(1):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwickert TA, Tagoh H, Gultekin S, et al. Stage-specific control of early B cell development by the transcription factor Ikaros. Nat Immunol. 2014;15(3):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross J, Mavoungou L, Bresnick EH, Milot E. GATA-1 utilizes Ikaros and polycomb repressive complex 2 to suppress Hes1 and to promote erythropoiesis. Mol Cell Biol. 2012;32(18):3624–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oravecz A, Apostolov A, Polak K, et al. Ikaros mediates gene silencing in T cells through Polycomb repressive complex 2. Nat Commun. 2015;6:8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koipally J, Georgopoulos K. Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J Biol Chem. 2000;275(26):19594–19602. [DOI] [PubMed] [Google Scholar]
- 16.Koipally J, Georgopoulos K. Ikaros-CtIP interactions do not require C-terminal binding protein and participate in a deacetylase-independent mode of repression. J Biol Chem. 2002;277(26):23143–23149. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill DW, Schoetz SS, Lopez RA, et al. An ikaros-containing chromatin-remodeling complex in adult-type erythroid cells. Mol Cell Biol. 2000;20(20):7572–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottardi S, Mavoungou L, Pak H, et al. The IKAROS interaction with a complex including chromatin remodeling and transcription elongation activities is required for hematopoiesis. PLoS Genet. 2014;10(12): e1004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bottardi S, Zmiri FA, Bourgoin V, et al. Ikaros interacts with P-TEFb and cooperates with GATA-1 to enhance transcription elongation. Nucleic Acids Res. 2011;39(9):3505–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popescu M, Gurel Z, Ronni T, et al. Ikaros stability and pericentromeric localization are regulated by protein phosphatase 1. J Biol Chem. 2009;284(20):13869–13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gowda C, Song C, Kapadia M, et al. Regulation of cellular proliferation in acute lymphoblastic leukemia by Casein Kinase II (CK2) and Ikaros. Adv Biol Regul. 2017;63:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-del Arco P, Maki K, Georgopoulos K. Phosphorylation controls Ikaros’s ability to negatively regulate the G(1)-S transition. Mol Cell Biol. 2004;24(7):2797–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song C, Gowda C, Pan X, et al. Targeting casein kinase II restores Ikaros tumor suppressor activity and demonstrates therapeutic efficacy in high-risk leukemia. Blood. 2015;126(15):1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma H, Qazi S, Ozer Z, et al. Regulatory phosphorylation of Ikaros by Bruton’s tyrosine kinase. PloS one. 2013;8(8):e71302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uckun FM, Ma H, Zhang J, et al. Serine phosphorylation by SYK is critical for nuclear localization and transcription factor function of Ikaros. Proc Natl Acad Sci USA. 2012;109(44):18072–18077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-del Arco P, Koipally J, Georgopoulos K. Ikaros SUMOylation: switching out of repression. Mol Cell Biol. 2005;25(7):2688–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apostolov A, Litim-Mecheri I, Oravecz A, et al. Sumoylation inhibits the growth suppressive properties of Ikaros. PloS one. 2016;11(6):e0157767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindner S, Kronke J. The molecular mechanism of thalidomide analogs in hematologic malignancies. J Mol Med (Berl). 2016;94(12):1327–1334. [DOI] [PubMed] [Google Scholar]
- 29.Kronke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JH, Nichogiannopoulou A, Wu L, et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5(6):537–549. [DOI] [PubMed] [Google Scholar]
- 32.Heizmann B, Kastner P, Chan S. Ikaros is absolutely required for pre-B cell differentiation by attenuating IL-7 signals. J Exp Med. 2013;210(13):2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida T, Ng SY, Zuniga-Pflucker JC, Georgopoulos K. Early hematopoietic lineage restrictions directed by Ikaros. Nat Immunol. 2006;7(4):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez RA, Schoetz S, DeAngelis K, O’Neill D, Bank A. Multiple hematopoietic defects and delayed globin switching in Ikaros null mice. Proc Natl Acad Sci USA. 2002;99(2): 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao KN, Smuda C, Gregory GD, Min B, Brown MA. Ikaros limits basophil development by suppressing C/EBP-alpha expression. Blood. 2013;122(15):2572–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malinge S, Thiollier C, Chlon TM, et al. Ikaros inhibits megakaryopoiesis through functional interaction with GATA-1 and NOTCH signaling. Blood. 2013;121(13): 2440–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schjerven H, McLaughlin J, Arenzana TL, et al. Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nat Immunol. 2013;14(10):1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allman D, Sambandam A, Kim S, et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4(2):168–174. [DOI] [PubMed] [Google Scholar]
- 39.Kirstetter P, Thomas M, Dierich A, Kastner P, Chan S. Ikaros is critical for B cell differentiation and function. Eur J Immunol. 2002;32(3):720–730. [DOI] [PubMed] [Google Scholar]
- 40.Sellars M, Reina-San-Martin B, Kastner P, Chan S. Ikaros controls isotype selection during immunoglobulin class switch recombination. J Exp Med. 2009;206(5): 1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heizmann B, Sellars M, Macias-Garcia A, Chan S, Kastner P. Ikaros limits follicular B cell activation by regulating B cell receptor signaling pathways. Biochem Biophys Res Commun. 2016;470(3):714–720. [DOI] [PubMed] [Google Scholar]
- 42.Virely C, Moulin S, Cobaleda C, et al. Haploinsufficiency of the IKZF1 (IKAROS) tumor suppressor gene cooperates with BCR-ABL in a transgenic model of acute lymphoblastic leukemia. Leukemia. 2010;24(6):1200–1204. [DOI] [PubMed] [Google Scholar]
- 43.Dumortier A, Jeannet R, Kirstetter P, et al. Notch activation is an early and critical event during T-Cell leukemogenesis in Ikaros-deficient mice. Mol Cell Biol. 2006;26(1):209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgopoulos K, Bigby M, Wang JH, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79(1):143–156. [DOI] [PubMed] [Google Scholar]
- 45.Papathanasiou P, Perkins AC, Cobb BS, et al. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity. 2003;19(1):131–144. [DOI] [PubMed] [Google Scholar]
- 46.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):289–299. [DOI] [PubMed] [Google Scholar]
- 47.Mantha S, Ward M, McCafferty J, et al. Activating Notch1 mutations are an early event in T-cell malignancy of Ikaros point mutant Plastic/+ mice. Leuk Res. 2007;31(3):321–327. [DOI] [PubMed] [Google Scholar]
- 48.Scheijen B, Boer JM, Marke R, et al. Tumor suppressors BTG1 and IKZF1 cooperate during mouse leukemia development and increase relapse risk in B-cell precursor acute lymphoblastic leukemia patients. Haematologica. 2017;102(3):541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng SY, Yoshida T, Zhang J, Georgopoulos K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity. 2009;30(4):493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avitahl N, Winandy S, Friedrich C, et al. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10(3):333–343. [DOI] [PubMed] [Google Scholar]
- 51.Winandy S, Wu L, Wang JH, Georgopoulos K. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. J Exp Med. 1999;190(8): 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynaud D, Demarco IA, Reddy KL, et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9(8):927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferreiros-Vidal I, Carroll T, Taylor B, et al. Genome-wide identification of Ikaros targets elucidates its contribution to mouse B-cell lineage specification and pre-B-cell differentiation. Blood. 2013;121(10):1769–1782. [DOI] [PubMed] [Google Scholar]
- 54.Hu Y, Zhang Z, Kashiwagi M, et al. Superenhancer reprogramming drives a B-cell-epithelial transition and high-risk leukemia. Genes Dev. 2016;30(17):1971–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schjerven H, Ayongaba EF, Aghajanirefah A, et al. Genetic analysis of Ikaros target genes and tumor suppressor function in BCR-ABL1+ pre-B ALL. J Exp Med. 2017;214(3): 793–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joshi I, Yoshida T, Jena N, et al. Loss of Ikaros DNA-binding function confers integrin-dependent survival on pre-B cells and progression to acute lymphoblastic leukemia. Nat Immunol. 2014;15(3):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuiper RP, Schoenmakers EF, van Reijmersdal SV, et al. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21(6):1258–1266. [DOI] [PubMed] [Google Scholar]
- 58.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–764. [DOI] [PubMed] [Google Scholar]
- 59.Paulsson K, Cazier JB, Macdougall F, et al. Microdeletions are a general feature of adult and adolescent acute lymphoblastic leukemia: Unexpected similarities with pediatric disease. Proc Natl Acad Sci USA. 2008;105(18):6708–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tokunaga K, Yamaguchi S, Iwanaga E, et al. High frequency of IKZF1 genetic alterations in adult patients with B-cell acute lymphoblastic leukemia. Eur J Haematol. 2013;91(3):201–208. [DOI] [PubMed] [Google Scholar]
- 61.Boer JM, van der Veer A, Rizopoulos D, et al. Prognostic value of rare IKZF1 deletion in childhood B-cell precursor acute lymphoblastic leukemia: an international collaborative study. Leukemia. 2016;30(1):32–38. [DOI] [PubMed] [Google Scholar]
- 62.Dupuis A, Gaub MP, Legrain M, et al. Biclonal and biallelic deletions occur in 20% of B-ALL cases with IKZF1 mutations. Leukemia. 2013;27(2):503–507. [DOI] [PubMed] [Google Scholar]
- 63.Churchman ML, Low J, Qu C, et al. Efficacy of retinoids in IKZF1-mutated BCR-ABL1 acute lymphoblastic leukemia. Cancer Cell. 2015;28(3):343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lilljebjorn H, Henningsson R, Hyrenius-Wittsten A, et al. Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat Commun. 2016;7:11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–114. [DOI] [PubMed] [Google Scholar]
- 66.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2): 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts KG, Morin RD, Zhang J, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22(2):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iacobucci I, Storlazzi CT, Cilloni D, et al. Identification and molecular characterization of recurrent genomic deletions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: on behalf of Gruppo Italiano Malattie Ematologiche dell’Adulto Acute Leukemia Working Party (GIMEMA AL WP). Blood. 2009;114(10):2159–2167. [DOI] [PubMed] [Google Scholar]
- 70.Grossmann V, Kohlmann A, Zenger M, et al. A deep-sequencing study of chronic myeloid leukemia patients in blast crisis (BC-CML) detects mutations in 76.9% of cases. Leukemia. 2011;25(3):557–560. [DOI] [PubMed] [Google Scholar]
- 71.Dorge P, Meissner B, Zimmermann M, et al. IKZF1 deletion is an independent predictor of outcome in pediatric acute lymphoblastic leukemia treated according to the ALL-BFM 2000 protocol. Haematologica. 2013;98(3): 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van der Veer A, Waanders E, Pieters R, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122(15): 2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, Easton J, Shao Y, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49(8):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jager R, Gisslinger H, Passamonti F, et al. Deletions of the transcription factor Ikaros in myeloproliferative neoplasms. Leukemia. 2010;24(7):1290–1298. [DOI] [PubMed] [Google Scholar]
- 76.Crescenzi B, La Starza R, Romoli S, et al. Submicroscopic deletions in 5q- associated malignancies. Haematologica. 2004;89(3): 281–285. [PubMed] [Google Scholar]
- 77.de Rooij JD, Beuling E, van den Heuvel-Eibrink MM, et al. Recurrent deletions of IKZF1 in pediatric acute myeloid leukemia. Haematologica. 2015;100(9):1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waanders E, van der Velden VH, van der Schoot CE, et al. Integrated use of minimal residual disease classification and IKZF1 alteration status accurately predicts 79% of relapses in pediatric acute lymphoblastic leukemia. Leukemia. 2011;25(2):254–258. [DOI] [PubMed] [Google Scholar]
- 79.Martinelli G, Iacobucci I, Storlazzi CT, et al. IKZF1 (Ikaros) deletions in BCR-ABL1-positive acute lymphoblastic leukemia are associated with short disease-free survival and high rate of cumulative incidence of relapse: a GIMEMA AL WP report. J Clin Oncol. 2009;27(31):5202–5207. [DOI] [PubMed] [Google Scholar]
- 80.van der Veer A, Zaliova M, Mottadelli F, et al. IKZF1 status as a prognostic feature in BCR-ABL1-positive childhood ALL. Blood. 2014;123(11):1691–1698. [DOI] [PubMed] [Google Scholar]
- 81.Clappier E, Grardel N, Bakkus M, et al. IKZF1 deletion is an independent prognostic marker in childhood B-cell precursor acute lymphoblastic leukemia, and distinguishes patients benefiting from pulses during maintenance therapy: results of the EORTC Children’s Leukemia Group study 58951. Leukemia. 2015;29(11):2154–2161. [DOI] [PubMed] [Google Scholar]
- 82.Olsson L, Ivanov Ofverholm I, Noren-Nystrom U, et al. The clinical impact of IKZF1 deletions in paediatric B-cell precursor acute lymphoblastic leukaemia is independent of minimal residual disease stratification in Nordic Society for Paediatric Haematology and Oncology treatment protocols used between 1992 and 2013. Br J Haematol. 2015;170(6):847–858. [DOI] [PubMed] [Google Scholar]
- 83.Ribera J, Morgades M, Zamora L, et al. Prognostic significance of copy number alterations in adolescent and adult patients with precursor B acute lymphoblastic leukemia enrolled in PETHEMA protocols. Cancer. 2015;121(21):3809–3817. [DOI] [PubMed] [Google Scholar]
- 84.Yao QM, Liu KY, Gale RP, et al. Prognostic impact of IKZF1 deletion in adults with common B-cell acute lymphoblastic leukemia. BMC Cancer. 2016;16:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang W, Kuang P, Li H, Wang F, Wang Y. Prognostic significance of IKZF1 deletion in adult B cell acute lymphoblastic leukemia: a meta-analysis. Ann Hematol. 2017;96(2): 215–225. [DOI] [PubMed] [Google Scholar]
- 86.Kobitzsch B, Gokbuget N, Schwartz S, et al. Loss-of-function but not dominant-negative intragenic IKZF1 deletions are associated with an adverse prognosis in adult BCR-ABL-negative acute lymphoblastic leukemia. Haematologica. 2017;102(10): 1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasuda T, Tsuzuki S, Kawazu M, et al. Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nat Genet. 2016;48(5):569–574. [DOI] [PubMed] [Google Scholar]
- 88.Clappier E, Auclerc MF, Rapion J, et al. An intragenic ERG deletion is a marker of an oncogenic subtype of B-cell precursor acute lymphoblastic leukemia with a favorable outcome despite frequent IKZF1 deletions. Leukemia. 2014;28(1):70–77. [DOI] [PubMed] [Google Scholar]
- 89.Zaliova M, Zimmermannova O, Dorge P, et al. ERG deletion is associated with CD2 and attenuates the negative impact of IKZF1 deletion in childhood acute lymphoblastic leukemia. Leukemia. 2014;28(1):182–185. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, McCastlain K, Yoshihara H, et al. Deregulation of DUX4 and ERG in acute lymphoblastic leukemia. Nat Genet. 2016;48(12):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Russell LJ, Jones L, Enshaei A, et al. Characterisation of the genomic landscape of CRLF2-rearranged acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2017;56(5):363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katerndahl CDS, Heltemes-Harris LM, Willette MJL, et al. Antagonism of B cell enhancer networks by STAT5 drives leukemia and poor patient survival. Nat Immunol. 2017;18(6):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuda S, Rouault J, Magaud J, Berthet C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 2001;497(2–3):67–72. [DOI] [PubMed] [Google Scholar]
- 95.Busson M, Carazo A, Seyer P, et al. Coactivation of nuclear receptors and myogenic factors induces the major BTG1 influence on muscle differentiation. Oncogene. 2005;24(10):1698–1710. [DOI] [PubMed] [Google Scholar]
- 96.van Galen JC, Kuiper RP, van Emst L, et al. BTG1 regulates glucocorticoid receptor autoinduction in acute lymphoblastic leukemia. Blood. 2010;115(23):4810–4819. [DOI] [PubMed] [Google Scholar]
- 97.Prevot D, Morel AP, Voeltzel T, et al. Relationships of the antiproliferative proteins BTG1 and BTG2 with CAF1, the human homolog of a component of the yeast CCR4 transcriptional complex: involvement in estrogen receptor alpha signaling pathway. J Biol Chem. 2001;276(13):9640–9648. [DOI] [PubMed] [Google Scholar]
- 98.Lin WJ, Gary JD, Yang MC, Clarke S, Herschman HR. The mammalian immediate-early TIS21 protein and the leukemia-associated BTG1 protein interact with a protein-arginine N-methyltransferase. J Biol Chem. 1996;271(25):15034–15044. [DOI] [PubMed] [Google Scholar]
- 99.Bogdan JA, Adams-Burton C, Pedicord DL, et al. Human carbon catabolite repressor protein (CCR4)-associative factor 1: cloning, expression and characterization of its interaction with the B-cell translocation protein BTG1. Biochem J. 1998;336(Pt 2):471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rouault JP, Prevot D, Berthet C, et al. Interaction of BTG1 and p53-regulated BTG2 gene products with mCaf1, the murine homolog of a component of the yeast CCR4 transcriptional regulatory complex. J Biol Chem. 1998;273(35):22563–22569. [DOI] [PubMed] [Google Scholar]
- 101.Tijchon E, van Emst L, Yuniati L, et al. Tumor suppressors BTG1 and BTG2 regulate early mouse B-cell development. Haematologica. 2016;101(7):e272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farrar M, Harris LH, Kornblau S, et al. B cell transcription factors define a novel tumor suppressor gene network in acute lymphoblastic leukemia. J Immunol. 2013;190(1 Suppl):52–55. [Google Scholar]
- 103.Ge Z, Gu Y, Xiao L, et al. Co-existence of IL7R high and SH2B3 low expression distinguishes a novel high-risk acute lymphoblastic leukemia with Ikaros dysfunction. Oncotarget. 2016;7(29):46014–46027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trageser D, Iacobucci I, Nahar R, et al. Pre-B cell receptor-mediated cell cycle arrest in Philadelphia chromosome-positive acute lymphoblastic leukemia requires IKAROS function. J Exp Med. 2009;206(8):1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iacobucci I, Iraci N, Messina M, et al. IKAROS deletions dictate a unique gene expression signature in patients with adult B-cell acute lymphoblastic leukemia. PloS One. 2012;7(7):e40934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Witkowski MT, Hu Y, Roberts KG, et al. Conserved IKAROS-regulated genes associated with B-progenitor acute lymphoblastic leukemia outcome. J Exp Med. 2017;214(3): 773–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vitanza NA, Zaky W, Blum R, et al. Ikaros deletions in BCR-ABL-negative childhood acute lymphoblastic leukemia are associated with a distinct gene expression signature but do not result in intrinsic chemoresistance. Pediatr Blood Cancer. 2014;61(10):1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Churchman ML, Evans K, Richmond J, et al. Synergism of FAK and tyrosine kinase inhibition in Ph+ B-ALL. JCI Insight. 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan LN, Chen Z, Braas D, et al. Metabolic gatekeeper function of B-lymphoid transcription factors. Nature. 2017;542(7642): 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ge Z, Guo X, Li J, et al. Clinical significance of high c-MYC and low MYCBP2 expression and their association with Ikaros dysfunction in adult acute lymphoblastic leukemia. Oncotarget. 2015;6(39):42300–42311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ge Z, Zhou X, Gu Y, et al. Ikaros regulation of the BCL6/BACH2 axis and its clinical relevance in acute lymphoblastic leukemia. Oncotarget. 2017;8(5):8022–8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma S, Pathak S, Mandal M, et al. Ikaros and Aiolos inhibit pre-B-cell proliferation by directly suppressing c-Myc expression. Mol Cell Biol. 2010;30(17):4149–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marke R, Havinga J, Cloos J, et al. Tumor suppressor IKZF1 mediates glucocorticoid resistance in B-cell precursor acute lymphoblastic leukemia. Leukemia. 2016;30(7): 1599–1603. [DOI] [PubMed] [Google Scholar]
- 115.Imamura T, Yano M, Asai D, et al. IKZF1 deletion is enriched in pediatric B-cell precursor acute lymphoblastic leukemia patients showing prednisolone resistance. Leukemia. 2016;30(8):1801–1803. [DOI] [PubMed] [Google Scholar]
- 116.Piovan E, Yu J, Tosello V, et al. Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell. 2013;24(6):766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Evangelisti C, Cappellini A, Oliveira M, et al. Phosphatidylinositol 3-kinase inhibition potentiates glucocorticoid response in B-cell acute lymphoblastic leukemia. J Cell Physiol. 2018;233(3):1796–1811. [DOI] [PubMed] [Google Scholar]
- 118.Aries IM, Jerchel IS, van den Dungen RE, et al. EMP1, a novel poor prognostic factor in pediatric leukemia regulates prednisolone resistance, cell proliferation, migration and adhesion. Leukemia. 2014;28(9):1828–1837. [DOI] [PubMed] [Google Scholar]
- 119.Palmi C, Valsecchi MG, Longinotti G, et al. What is the relevance of Ikaros gene deletions as a prognostic marker in pediatric Philadelphia-negative B-cell precursor acute lymphoblastic leukemia? Haematologica. 2013;98(8):1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.