Abstract
MABLE investigated the efficacy and safety of rituximab plus bendamustine or rituximab plus chlorambucil in fludarabine-ineligible patients with chronic lymphocytic leukemia. Patients received rituximab plus bendamustine or rituximab plus chlorambucil every four weeks for six cycles. Rituximab plus chlorambucil-treated patients without a complete response after Cycle 6 received chlorambucil monotherapy for at least six additional cycles or until complete response. The primary endpoint was complete response rate (confirmed by bone marrow biopsy) after Cycle 6 in first-line patients. Secondary endpoints included progression-free survival, overall survival, minimal residual disease, and safety. Overall, 357 patients were randomized (rituximab plus bendamustine, n=178; rituximab plus chlorambucil, n=179; intent-to-treat population), including 241 first-line patients (n=121 and n=120, respectively); 355 patients received treatment (n=177 and n=178, respectively; safety population). In first-line patients, complete response rate after Cycle 6 (rituximab plus bendamustine, 24%; rituximab plus chlorambucil, 9%; P=0.002) and median progression-free survival (rituximab plus bendamustine, 40 months; rituximab plus chlorambucil, 30 months; P=0.003) were higher with rituximab plus bendamustine than rituximab plus chlorambucil. Overall response rate and overall survival were not different. In first-line patients with a complete response, minimal residual disease-negativity was higher with rituximab plus bendamustine than rituximab plus chlorambucil (66% vs. 36%). Overall adverse event incidence was similar (rituximab plus bendamustine, 98%; rituximab plus chlorambucil, 97%). Rituximab plus bendamustine may be a valuable first-line option for fludarabine-ineligible patients with chronic lymphocytic leukemia.
Introduction
Rituximab plus fludarabine and cyclophosphamide (R-FC) is standard treatment for medically fit chronic lymphocytic leukemia (CLL) patients,1,2 with high response rates in previously untreated patients (first-line; 1L) and treated patients (second-line; 2L).3–5 However, many CLL patients are elderly and have comorbidities, making them ineligible for fludarabine-based treatment.6 Chemotherapy options for these patients include bendamustine (B) and chlorambucil (Clb).
In treatment-naïve CLL patients, phase II studies showed promising efficacy with rituximab plus B (R-B)7 or Clb (R-Clb),8,9 while the phase III CLL11 study demonstrated improved efficacy with R-Clb and obinutuzumab plus Clb (G-Clb) versus Clb monotherapy.10,11 G-Clb also increased progression-free survival (PFS) and complete response (CR) rates versus R-Clb,10,11 although infusion-related reactions and neutropenia were more common; infection rates were not increased, however.10 While a phase III study demonstrated superior efficacy in terms of CR and PFS with B versus Clb in treatment-naïve CLL,12,13 the activity of R-B versus R-Clb has not been directly compared.
Herein, we present results from the randomized, open-label, multicenter, phase IIIb MABLE study, which aimed to investigate the efficacy and safety of R-B and R-Clb in fludarabine-ineligible CLL patients.
Methods
Study design
Patients received rituximab (intravenous 375 mg/m2 Day [D] 1, Cycle [C] 1 and 500 mg/m2 D1, C2-C6) plus B (intravenous 90 mg/m2 [1L] or 70 mg/m2 [2L] D1 and D2, C1-C6) or Clb (oral 10 mg/m2 D1-D7, C1-C6) every four weeks for six cycles. R-Clb patients without CR after C6 received Clb monotherapy for ≤6 additional cycles or until CR. After treatment completion, patients were followed every three months for one year, then every six months until data cut-off. Treatment was discontinued if the patient had progressive disease.
MABLE was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local laws, and approved by institutional review boards and ethics committees at participating centers. All patients provided written informed consent.
Patients
Patients were aged ≥18 years, with confirmed CLL requiring treatment as per the International Workshop on CLL (iwCLL) criteria,14 Binet stage B/C disease, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and investigator assessment of ineligibility for fludarabine-based treatment (Online Supplementary Information). Exclusion criteria included transformation to aggressive B-cell malignancy and previous malignancy within five years of enrollment (unless treated with curative intent). For full inclusion and exclusion criteria see the Online Supplementary Information.
During recruitment, the protocol was amended to permit inclusion of patients with progressive Binet stage A disease and to exclude 2L patients (due to slow recruitment). All 2L patients recruited before this amendment were included in the final analysis, as tumor response in this patient subpopulation was a secondary study endpoint; however, as the number of 2L patients enrolled was relatively small, there was insufficient power to show statistically significant differences between study treatments in this patient subpopulation.
Study endpoints
The primary endpoint was CR rate (confirmed by bone marrow [BM] biopsy) after C6 in 1L patients. Secondary endpoints included CR rate after C6 in 2L patients, PFS, overall survival (OS), time to next leukemic treatment, minimal residual disease (MRD), and safety. Response was assessed after C3 and C6 as per iwCLL 2008 guidelines.14 Response was also assessed in the R-Clb arm at C12, with treatment being discontinued for patients showing evidence of CR during C7-C12. Assessments are detailed in the Online Supplementary Information.
Safety
Adverse events (AEs) were monitored throughout the study and graded according to the National Cancer Institute Common Terminology Criteria for AEs v4.0 and coded according to the Medical Dictionary for Regulatory Activities v17.0.
Statistical analysis
Efficacy analyses were conducted on the intent-to-treat (ITT) population (all randomized patients). The safety population included all randomized patients who received treatment.
For 1L patients, the between-arm difference in response rates was tested using a one-sided continuity-corrected χ2 test. A two-sided continuity-corrected χ2 test assessed between-arm differences in overall response rates (ORRs) and molecular responses. PFS and OS were summarized by Kaplan–Meier estimates and compared via the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated based on the Cox proportional hazard model, with and without baseline Binet stage as a covariate.
Results
Patients
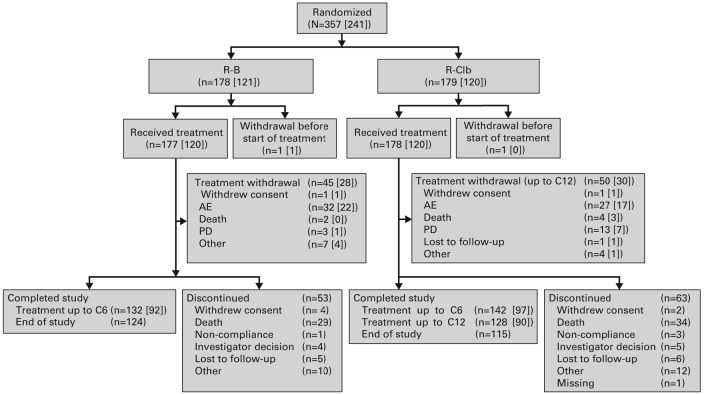
The study was conducted between 23 February 2010 and 31 March 2014. Of the 357 patients in the ITT population, comprising 241 1L patients (R-B, n=121; R-Clb, n=120) and 116 2L patients (R-B, n=57; R-Clb, n=59), 355 received treatment (R-B, n=177; R-Clb, n=178). Ninety-five patients (27%) withdrew from treatment during the study (Figure 1).
Figure 1.
Patient disposition. Numbers in parentheses represent the number of patients from the 1L subpopulation. In total, 118 patients (33%) discontinued the study prematurely, due to death (R-B 16%; R-Clb 19%), patient lost to follow-up (3% per arm), investigator decision (R-B 2%; R-Clb 3%), patient withdrew consent (R-B 3%; R-Clb 2%), patient non-compliance (R-B 1%; R-Clb 2%), and ‘other’ reasons (R-B 6%; R-Clb 7%). Reason for withdrawal was not available for one patient (R-Clb). AE: adverse event; C: cycle; N: number of patients; PD: progressive disease; R-B: rituximab plus bendamustine; R-Clb: rituximab plus chlorambucil.
Overall, 92/120 (76.7%) 1L patients treated with R-B and 97/120 (80.8%) 1L patients treated with R-Clb received six cycles of rituximab; 92 (76.7%) and 57 (47.5%) received six cycles of B and Clb, respectively. Twelve (10.0%) patients treated with R-Clb received 12 cycles of Clb. The median number of R, B and Clb doses was six for each. The median (interquartile range) dose of rituximab was 4780.5 mg (4222.5-5346.5) in 1L patients treated with R-B and 5028.5 mg (4546.0-5349.0) in 1L patients treated with R-Clb. In total, 6/121 (5.0%) patients treated with R-B and 2/120 (1.7%) patients treated with R-Clb had a reduction or delay in their treatment schedule due to treatment-emergent toxicities.
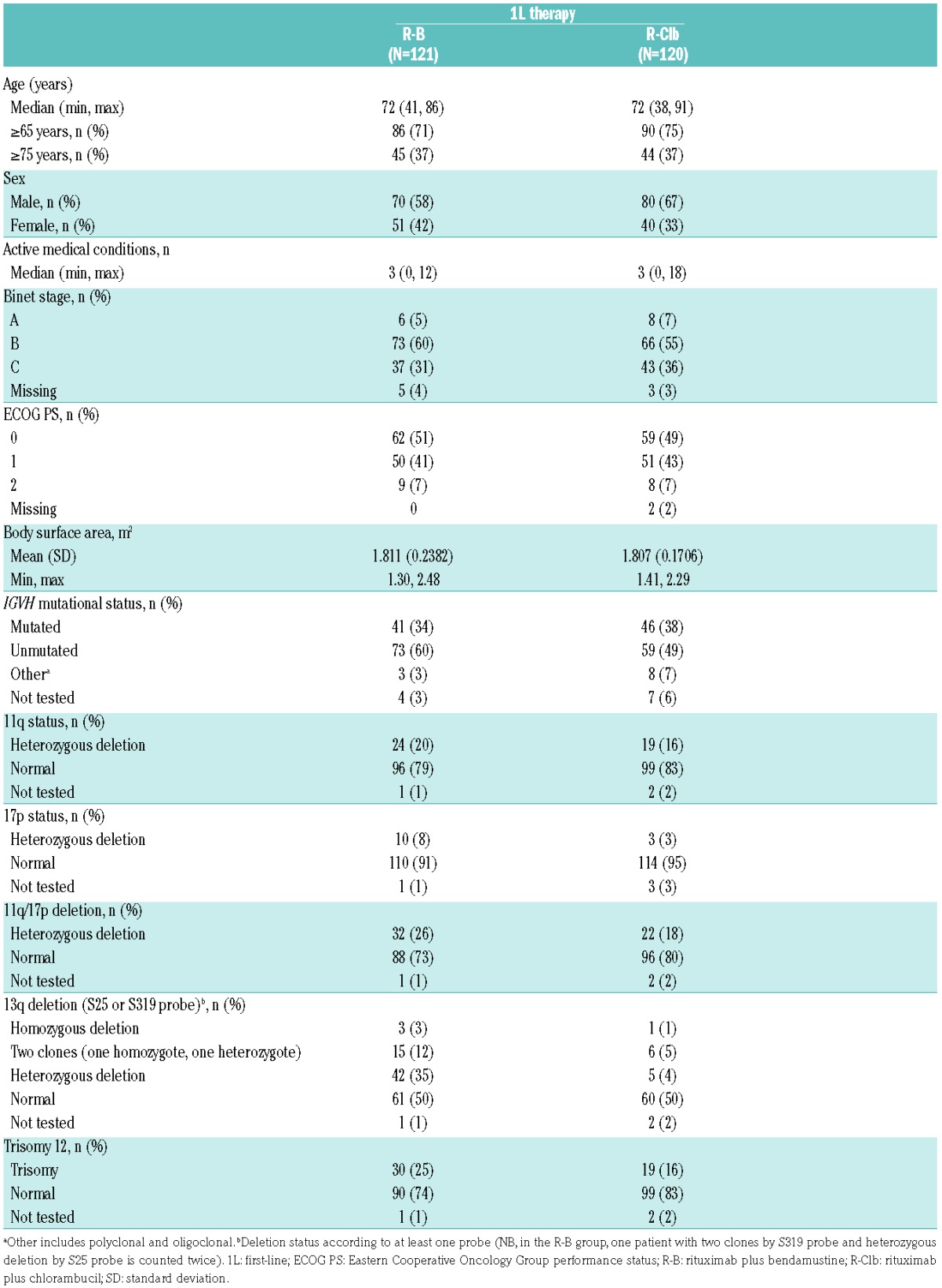
Baseline characteristics were balanced in the 1L population (Table 1; baseline characteristics for all patients are presented in Online Supplementary Table S1). Deletion of 17p was not an exclusion criterion due to a lack of efficacy data relating to 17p deletion for the treatment combinations used at the time of study design. Thus, 13 1L patients (R-B, 10; R-Clb, 3) with 17p deletion were included. Median follow-up was 23.5 months (R-B) and 23.3 months (R-Clb). Median age in 1L patients was 72 years in both treatment arms; the majority of patients were aged 65 years or more. The median number of comorbidities (active medical conditions) in 1L patients was three in both arms (Table 1); the most common comorbidities were vascular disorders and metabolism disorders affecting 49% and 37% of patients, respectively (Online Supplementary Table S2). The great majority of all patients in the study (including 2L patients) used concomitant medication during the study (R-B, 96%; R-Clb, 94%). Colony-stimulating factors were taken more frequently by patients treated with R-B (111/178 [62.4%] vs. 71/179 [39.7%] for R-Clb). ECOG performance status scores were ≥1 in approximately half the study population. At baseline, 108/177 (61.0%), 56/177 (31.6%) and 4/177 (2.3%) patients treated with R-B, and 102/178 (57.3%), 60/178 (33.7%) and 3/178 (1.7%) patients treated with R-Clb had normal; abnormal, non-clinically significant; and abnormal clinically significant calculated creatinine clearance, respectively.
Table 1.
Demographic characteristics for patients receiving 1L therapy.
Efficacy
In 1L patients, the CR rate after C6 was higher with R-B versus R-Clb (24% [n=29/121] vs. 9% [n=11/120]; P=0.002; Table 2). Logistic regression analysis supported the R-B treatment effect after adjusting for baseline covariates (odds ratio 4.18, 95% CI 1.77-9.87; P=0.001). None of the covariates had a statistically significant impact on the CR rate.
Table 2.
CR and PRs at C6 in 1L patients.
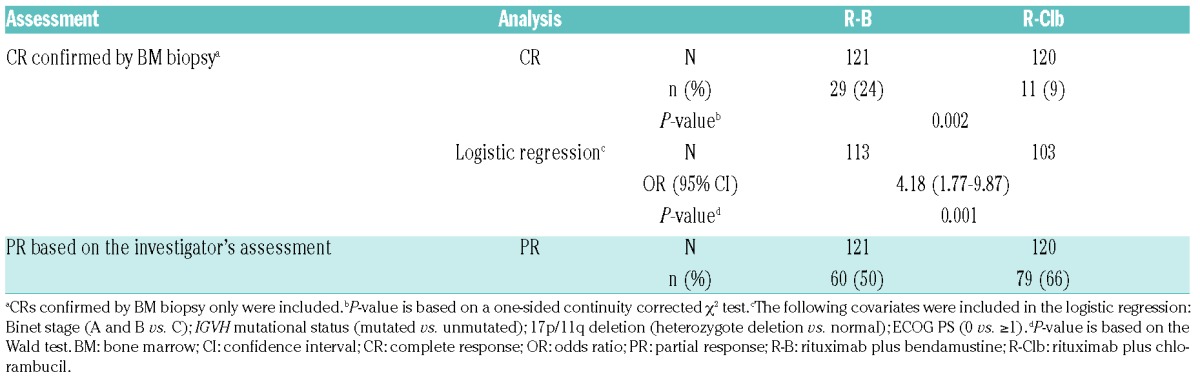
ORRs (based on the investigator’s assessment) at the end of rituximab treatment were similar for R-B and R-Clb (91% vs. 86%; P=0.304). The proportion of patients with stable disease (3% vs. 6%) and progressive disease (3% vs. 2%) at the end of treatment were also similar for R-B vs. R-Clb, respectively.
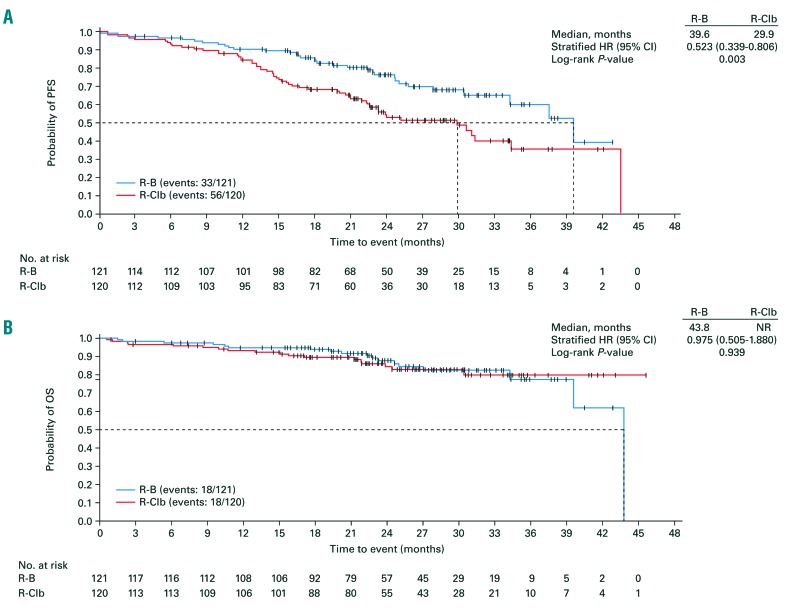
A statistically significant ten-month extension in median PFS was observed with R-B versus R-Clb (39.6 vs. 29.9 months; HR [adjusted for baseline Binet stage] 0.523, 95% CI 0.339-0.806; P=0.003; Figure 2A); median OS was not significantly different (43.8 months vs. not reached; HR [adjusted for baseline Binet stage] 0.975, 95% CI 0.505-1.880; P=0.939; Figure 2B). During the study, 11/121 (9.1%) patients treated with R-B and 22/120 (18.3%) patients treated with R-Clb had a documented intake of any new leukemia treatment. Due to the low numbers, the median time to next leukemic treatment could not be calculated for either treatment arm (log-rank test for comparison between treatment arms: P=0.037).
Figure 2.
Efficacy in 1L patients. (A) PFS and (B) OS. CI: confidence interval; HR: hazard ratio; NR: not reached; OS: overall survival; PFS: progression-free survival; R-B: rituximab plus bendamustine; R-Clb: rituximab plus chlorambucil.
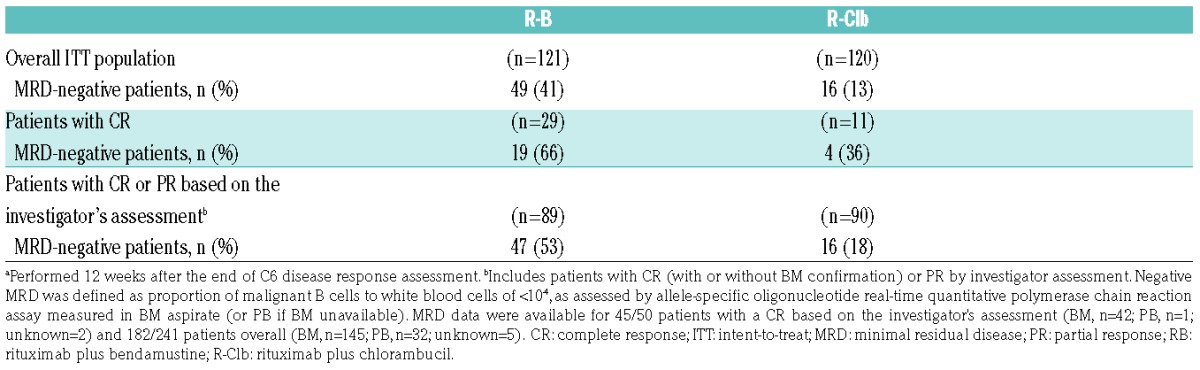
MRD data were available for 45/50 patients (90%) who had a CR based on the investigator’s assessment and 182/241 patients (76%) overall. BM aspirates were available for 42/45 patients (93%) and 145/182 patients (80%), respectively. MRD-negativity rates at the confirmation-of-response visit (ITT population) were higher for R-B than for R-Clb (41% vs. 13%; Table 3). In 1L patients with a CR after C6, MRD-negativity rates at the confirmation-of-response visit were higher in the R-B group than in the R-Clb group (66% vs. 36%). A similar pattern was seen in those with a CR or PR according to investigator’s assessment (53% vs. 18%).
Table 3.
MRD negativity at the confirmation-of-response visita in 1L patients.
Efficacy results in 2L patients are presented in the Online Supplementary Information.
Safety
Safety results are presented for the pooled population (1L and 2L patients). AEs were similar between arms (R-B, 98%; R-Clb, 97%; Table 4). The most common AEs by System Organ Class (SOC) were ‘blood and lymphatic system disorders’ (R-B, 75%; R-Clb, 64%); the most commonly reported AE was neutropenia (R-B, 56%; R-Clb, 49%). AEs in the SOC ‘skin and subcutaneous tissue disorders’ were more frequent in the R-B versus R-Clb arm (36% vs. 23%), driven by a higher incidence of rash (16% vs. 5%).
Table 4.
Summary of AEs (safety population).
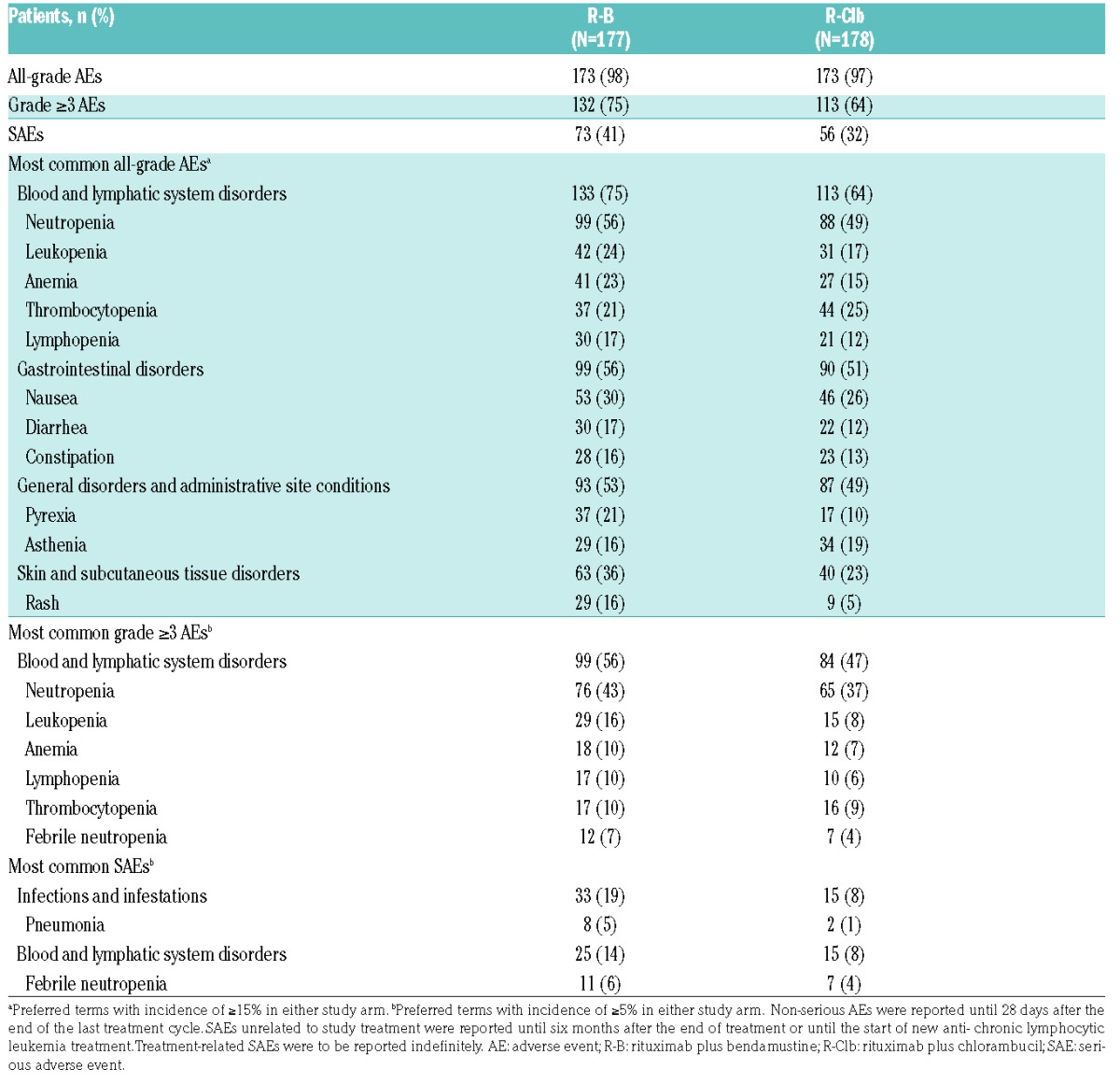
Grade ≥3 AEs were higher with R-B (75%) than R-Clb (64%), mainly due to a higher incidence of serious AEs (SAEs) of the SOC ‘infections and infestations’. The most common grade ≥3 AEs were of the SOC ‘blood and lymphatic system disorders’ (R-B, 56%; R-Clb, 47%). SAEs were experienced by 41% (R-B) and 32% (R-Clb) of patients, and were most frequently of the SOC ‘infections and infestations’ (R-B, 19%; R-Clb, 8%; Table 4).
Rituximab-related AEs were experienced by 81% (R-B) and 73% (R-Clb) of patients. B/Clb-related AEs were reported for 92% (R-B) and 80% (R-Clb) of patients. Drug-related AEs (rituximab, B, and Clb) were most commonly of the SOC ‘blood and lymphatic system disorders’. AEs leading to rituximab discontinuation were experienced by 18% (R-B) and 11% (R-Clb) of patients. AEs leading to discontinuation of B or Clb were reported for 19% and 18% of patients, respectively. In 1L patients, AEs leading to treatment discontinuation were experienced by 22 R-B patients (18%) and 14 R-Clb patients (12%).
Overall, 65 patients died (R-B, n=30, 17%; R-Clb, n=35, 20%) due to CLL (R-B, n=14, 8%; R-Clb, n=20, 11%) and AEs (R-B, n=16, 9%; R-Clb, n=14, 8%). Cause of death was missing for one patient (R-Clb arm). AEs leading to death were infection (n=4 per arm), acute myeloid leukemia or myelodysplastic syndrome (n=1 per arm), other neoplasm (R-Clb, n=1), and other causes (R-B, n=11; R-Clb, n=8). Treatment-related AEs leading to death included thrombocytopenia, neutropenic sepsis and febrile neutropenia (n=1 each) in the 1L population and multi-organ failure, pneumonia, acute myeloid leukemia and sepsis (n=1 each) in the 2L population.
Discussion
R-FC is the standard 1L treatment for medically-fit patients with CLL. However, this regimen can cause significant myelosuppression and high rates of early and late infections, especially in elderly patients15 who may have comorbidities and be considered ineligible for fludarabine-based treatment.6 In MABLE, the efficacy and safety of R-B and R-Clb were investigated in fludarabine-ineligible CLL patients. B is an established treatment for CLL, and a previous phase III study of B versus Clb in treatment-naïve patients demonstrated improved CR rates and median PFS with B.12,13 R combined with chemotherapeutic agents prolongs OS in previously untreated, medically fit CLL patients.16,17 In unfit patients, survival was improved by the addition of G to Clb.10
Among 1L patients in MABLE, the rates of CR and of CR with MRD-negativity were higher for R-B than for R-Clb. ORRs were similar in the two arms. Whereas the CR rate in 1L R-Clb patients in MABLE (9%) was comparable with that in R-Clb patients in the phase III CLL11 study (7%),10 the ORR for R-Clb-treated patients was higher in MABLE than in CLL11 (86% vs. 58%). Two explanations for this might be the different Clb doses used (MABLE, 10 mg/m2; CLL11, 0.5 mg/kg), which resulted in a higher median cumulative dose (MABLE, 720 mg; CLL11, 366-400 mg), and differences in the study populations, with patients in MABLE having fewer active comorbidities than those in CLL11 (medians of 3 and 5, respectively) and a better performance status.10 Earlier phase II studies in elderly CLL patients treated 1L with R-Clb reported CR rates of 10-17% and ORRs of 82-84%.8,9 The CLL2M trial, a phase II study of CLL patients treated 1L with R-B, at the same B dosage as the current study, reported a CR rate of 23% and an ORR of 88%.7
In 1L patients in the study reported herein, the median PFS of 39.6 months in the R-B arm was significantly longer (by 10 months) than the value in the R-Clb arm. This result is similar to the median PFS of 43.2 months achieved in fit CLL patients treated with R-B in the CLL10 study,18 and is consistent with the findings of the CLL2M study, which enrolled fit and unfit 1L patients and reported a median event-free survival in R-B-treated patients of 33.9 months.7 The CLL2M study did not select patients by fitness, but a substantial proportion could be considered unfit based on age, Binet stage, and renal impairment.7 In MABLE, 33% of the 1L patients were Binet stage C and the median age was 72 years. The PFS result in the R-Clb arm in MABLE was almost twice as long as that in CLL11 (29.9 vs. 15.4 months),11 however, as noted above, a direct comparison of these studies is limited by differences in Clb doses and patient fitness.
In MABLE, 1L patients with CR had higher MRD-negativity rates with R-B versus R-Clb, indicating a greater depth of response with R-B. Of note, MRD was assessed primarily in BM from patients with CR based on the investigator’s assessment, whereas in CLL11 and previous phase II studies, MRD was measured in peripheral blood ([PB] or BM) from all patients.7,8,10 One phase II study reported a MRD-negativity rate of 12.5% (2/16 patients) using BM aspirates from R-Clb-treated patients who achieved a CR/unconfirmed CR.9
Median OS was not significantly different between treatment arms in 1L patients in MABLE and was not reached in previous phase II studies of R-B and R-Clb,7–9 or in the CLL11 and COMPLEMENT-1 studies.11,19 At current follow-up reported for CLL11 and COMPLEMENT-1, no significant OS benefit was observed for G-Clb versus R-Clb or ofatumumab plus Clb (Ofa-Clb) versus Clb, respectively,11,19 whereas a significant improvement was observed for G-Clb versus Clb alone.10 Further observation is required to determine if there is an OS benefit with G-Clb versus R-Clb or Ofa-Clb versus Clb.
Safety profiles (for pooled 1L and 2L patients) were similar for R-Clb and R-B, with no new or clinically relevant safety signals, and events were as expected for CLL patients receiving immunochemotherapy.7–9 Incidences of all-grade AEs, SAEs, and treatment-related AEs were similar across arms. Grade ≥3 AE incidence was slightly higher with R-B versus R-Clb, driven by a higher incidence of infections and infestations. Few patients in either arm discontinued therapy due to AEs. Treatment withdrawal due to AEs was reported for 18% of patients receiving 1L R-B and 12% receiving R-Clb.
As previously noted, the results of the CLL11 study show that, in unfit CLL patients, G-Clb was associated with higher response rates and longer PFS than R-Clb, with more frequent MRD eradication and an acceptable toxicity profile.10 In addition, current guidelines, (European Society for Medical Oncology and National Comprehensive Cancer Network), recommend Clb in combination with an anti-CD20 antibody as standard 1L therapy in unfit CLL patients.20,21 However, in MABLE and previous studies, R-B was associated with a good response rate and improved PFS compared with R-Clb. Currently, evidence to guide the choice between R-B and G-Clb in 1L unfit patients with CLL is limited, although a recent meta- analysis of PFS and OS results in five studies showed a trend towards better efficacy for G-Clb than R-B;22 however, the difference was not significant despite a significant difference between G-Clb and other comparators such as R-Clb, Ofa-Clb, Clb, and fludarabine. A randomized trial comparing these combinations could resolve this question.
Some limitations of our study should be acknowledged. Since we relied only on investigator assessments of tumor response to evaluate efficacy, and did not include assessments by an independent review committee, this might have introduced a potential bias in the efficacy results. Comparison of our results with those of other studies in CLL patients is potentially complicated because the selection of patients based on fitness was based on a judgment made by the investigator that the patients were not eligible for fludarabine, according to a set of pre-defined criteria that were based on the prescribing information for fludarabine at the time of study design; the Cumulative Illness Rating Scale scoring was not used. However, a recent randomized study comparing the efficacy of ibrutinib with Clb used an age cut-off of 65 years as the only criterion for selecting “older” patients, and did not carry out any assessment of comorbidities.23
Although the efficacy of immunochemotherapy combinations such as R-B, Ofa-Clb, and G-Clb in CLL have been shown,7,11,19 obinutuzumab plus B (G-B) needs to be evaluated further, although it is likely that future clinical studies will focus more on the combination of anti-CD20 antibodies with novel agents such as ibrutinib, idelalisib, and venetoclax. Indeed, these agents have shown promising efficacy when combined with anti-CD20 monoclonal antibodies (including rituximab) in the relapsed/refractory CLL setting,24–29 and are now being evaluated in 1L.23 Furthermore, additional safety data for 1L B-cell receptor (BCR) signaling inhibitors are required. In March 2016, the US Food and Drug Administration alerted healthcare professionals about safety concerns with idelalisib when used in combination with rituximab or R-B in CLL and follicular lymphoma. This was related to a high rate of viral and fungal infections that led the sponsor to discontinue six trials.30
However, given the economic burden associated with new agents and the fact that BCR inhibitors are not yet available in many European countries, immunochemotherapy combinations are likely to continue to be valuable treatment options for 1L patients with CLL.31
In conclusion, in fludarabine-ineligible CLL patients, 1L R-B treatment significantly improved CR rates and median PFS versus R-Clb, and increased MRD-negativity rates, with no new safety signals reported. R-B may be a valuable 1L option for fludarabine-ineligible CLL patients and this combination continues to be widely used in clinical practice in Europe, reinforcing the interest of this large randomized study.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families, and the study investigators, study coordinators, and nurses who assisted with the rituximab clinical program. We would also like to thank Mundipharma for provision of bendamustine for use in this study. AS is supported by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the Department of Health’s National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health. MABLE was sponsored by F. Hoffmann-La Roche Ltd, with provision of bendamustine and financial support from Mundipharma. Third-party medical writing assistance, under the direction of the authors, was provided by Susan Browne, PhD, of Gardiner-Caldwell Communications (Macclesfield, UK) and funded by F. Hoffmann-La Roche Ltd.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/4/698
List of MABLE investigators and centers
Seppo Vanhatalo, Satakunta Central Hospital, Pori, Finland; Kimmo Porkka, HUS/Medisiininen tulosyksikkö/Hematologian klinikka, Haartmaninkatu, Finland; Anne-Sophie Michallet, Centre Hospitalier Lyon Sud, Pierre-Bénite, France; Bruno Royer, Hôpital Sud, Salouel, France; Olivier Boulat, Centre Hospitalier Henri Duffaut, Avignon, France; Christian Berthou, Hôpital Morvan – CHU Brest, France; Dominique Bordessoule, CHU de Limoges, France; Jean-Claude Eisenmann, Centre Hospitalier de Mulhouse, France; Jean-Michel Karsenti, Hôpital Archet 1, Nice, France; Eric Jourdan, CHU de Nîmes, France; Véronique Leblond, Hôpital de la Pitié-Salpêtrière, Paris, France; Laurence Sanhes, Hôpital Saint Jean, Perpignan, France; Mourad Tiab, Centre Hospitalier Départemental Vendée, La Roche Sur Yon, France; Kamel Laribi, Centre Hospitalier du Mans, Le Mans, France; Régis Costello, Hôpital la Conception, Marseille, France; Lysiane Molina, Hôpital Nord – Michallon, La Tronche, France; Laurent Sutton, CH Victor Dupouy, Argenteuil, France; Abderrazak El Yamani, Centre Hospitalier de Blois, France; João Raposo, Centro Hospitalar de Lisboa Norte, Lisbon, Portugal; Emília Cortesão, Centro Hospitalar e Universitário de Coimbra, Portugal; Cristina João, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Joaquim Andrade, Hospital de São João, Oporto, Portugal; Ângelo Martins, Instituto Português de Oncologia do Porto Francisco Gentil, Oporto, Portugal; José Antonio García Marco, Hospital Universitario Puerta de Hierro, Madrid, Spain; Marcos González Díaz, Hospital Universitario de Salamanca, Spain; Francisco Javier de la Serna Torroba, Hospital Universitario 12 de Octubre, Madrid, Spain; Carolina Moreno Atanasio, Hospital de la Sant Creu i Sant Pau, Barcelona, Spain; José María Moraleda Jiménez, Hospital Universitario Virgen de la Arrixaca, Murcia, Spain; M. Pilar Giraldo Castellano, Hospital Miguel Servet, Zaragoza, Spain; Macarena Ortiz Pareja, Hospital Regional Carlos Haya, Málaga, Spain; Alicia Rodríguez Fernández, Hospital Virgen Macarena, Sevilla, Spain; José Ignacio Olalla Antolín, Hospital Sierrallana, Cantabria, Spain; Emilio Montserrat Costa, Hospital Clínic de Barcelona, Spain; Christelle Ferra Coll, Hospital Universitari Germans Trias i Pujol, Barcelona, Spain; Kristina Wallman, Falu Iasarett, Falun, Sweden; Birgitta Lauri, Sunderby Sjukhus, Luleå, Sweden; Maria Strandberg, Sundsvall County Hospital, Sweden; Peter Johansson, Uddevalla County Hospital, Sweden; Honar Cherif, The Academic Hospital, Uppsala, Sweden; Alicja Markuszewska-Kuczynska, Norrlands Universietetssjukhus, Umeå, Sweden; Lars Timberg, Medicinkliniken, Kristianstad, Sweden; Balkis Meddeb, Hôpital Aziza Othmana, Tunis, Tunisia; Ilhan Osman, Ankara University Medical Faculty, Turkey; Filiz Vural and Seckin Cagirgan, Ege University Medical Faculty, Izmir, Turkey; Yagci Munci, Gazi University Medical Faculty, Ankara, Turkey; Undar Buient, Dokuzeylul University Medical Faculty, Izmir, Turkey; Nilgun Sayinalp, Hacettepe University Medical Faculty, Ankara, Turkey; Melih Aktan, Istanbul University Medical Faculty, Turkey; Mehmet Turgut, Ondokuzmayis University Medical Faculty, Samsun, Turkey; Muzaffer Demir, Trakya University Medical Faculty, Edirne, Turkey; Ali Unal, Erciyes Universitesi Medical Faculty, Kayseri, Turkey; Zafer Gulbas, Anadolu Health Center, Kocaeli, Turkey; Marion Macheta, Blackpool Victoria Hospital, UK; Stephen Devereux, Kings College Hospital, London, UK; Anna Schuh, Oxford Radcliffe Hospitals, UK; Adrian Bloor, Christie NHS Foundation Trust, Manchester, UK; Saad Rassam, Maidstone and Tunbridge Wells NHS Trust, Maidstone, UK; Julie Blundell, Royal Cornwall Hospital, Truro, UK; Renata Walewska, The Royal Bournemouth & Christchurch Hospital NHS Foundation Trust, Bournemouth, UK; Claire Hemmaway, Queens Hospital, Romford, UK; Peter Hillmen, St. James’ University Hospital, Leeds, UK.
Funding
This work was funded by F. Hoffmann-La Roche Ltd.
References
- 1.Roche Registration Ltd [Internet]. MabThera: Summary of product characteristics; Updated 2017 Nov 6 [cited 2018 Jan 9]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000165/WC500025821.pdf [Google Scholar]
- 2.Genentech Inc [Internet]. Rituxan: Highlights of prescribing information; Updated 2016 Apr [cited 2018 Jan 9]. Available from: https://www.gene.com/download/pdf/rituxan_prescribing.pdf. [Google Scholar]
- 3.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. [DOI] [PubMed] [Google Scholar]
- 4.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756–1765. [DOI] [PubMed] [Google Scholar]
- 5.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117(11):3016–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah N, Tam C, Seymour JF, Rule S. How applicable is fludarabine, cyclophosphamide and rituximab to the elderly? Leuk Lymphoma. 2015;56(6):1599–1610. [DOI] [PubMed] [Google Scholar]
- 7.Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30(26):3209–3216. [DOI] [PubMed] [Google Scholar]
- 8.Hillmen P, Gribben JG, Follows GA, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: final analysis of an open-label phase II study. J Clin Oncol. 2014;32(12):1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foà R, Del Giudice I, Cuneo A, et al. Chlorambucil plus rituximab with or without maintenance rituximab as first-line treatment for elderly chronic lymphocytic leukemia patients. Am J Hematol. 2014;89(5):480–486. [DOI] [PubMed] [Google Scholar]
- 10.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. [DOI] [PubMed] [Google Scholar]
- 11.Goede V, Fischer K, Engelke A, et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia. 2015;29(7):1602–1604. [DOI] [PubMed] [Google Scholar]
- 12.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27(26):4378–4384. [DOI] [PubMed] [Google Scholar]
- 13.Knauf WU, Lissitchkov T, Aldaoud A, et al. Bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia: updated results of a randomized phase III trial. Br J Haematol. 2012;159(1):67–77. [DOI] [PubMed] [Google Scholar]
- 14.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurenti L, Innocenti I, Autore F, et al. Bendamustine in combination with rituximab for elderly patients with previously untreated B-cell chronic lymphocytic leukemia: a retrospective analysis of real-life practice in Italian hematology departments. Leuk Res. 2015;39(10):1066–1070. [DOI] [PubMed] [Google Scholar]
- 16.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. [DOI] [PubMed] [Google Scholar]
- 18.Eichhorst B, Fink AM, Busch R, et al. Frontline chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) shows superior efficacy in comparison to bendamustine (B) and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): final analysis of an international, randomized study of the German CLL Study Group (GCLLSG) (CLL10 Study). Blood. 2014;124(21):19. [Google Scholar]
- 19.Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015;385(9980):1873–1883. [DOI] [PubMed] [Google Scholar]
- 20.Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v78–84. [DOI] [PubMed] [Google Scholar]
- 21.NCCN [Internet]. NCCN Guidelines for patients: chronic lymphocytic leukemia [cited 2018 Jan 11]. Available from: https://www.nccn.org/patients/guidelines/cll/files/assets/common/downloads/files/cll.pdf.
- 22.Städler N, Shang A, Bosch F, et al. A systematic review and network meta-analysis to evaluate the comparative efficacy of interventions for unfit patients with chronic lymphocytic leukemia. Adv Ther. 2016;33(10):1814–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chanan-Khan A, Cramer P, Demirkan F, et al. HELIOS investigators. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. [DOI] [PubMed] [Google Scholar]
- 26.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts AW, Ma S, Brander D, et al. Venetoclax (ABT-199/GDC-0199) combined with rituximab induces deep responses in patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica. 2015;100(suppl 1):154 (abstr S431). [Google Scholar]
- 28.Sharman JP, Coutre SE, Furman RR, et al. Second interim analysis of a phase 3 study of idelalisib (ZYDELIG®) plus rituximab (R) for relapsed chronic lymphocytic leukemia (CLL): efficacy analysis in patient subpopulations with del(17p) and other adverse prognostic factors. Blood. 2014;124(21):330. [Google Scholar]
- 29.Robak T, Wach M, Jones JA, et al. Results of a phase 3 randomized controlled study evaluating the efficacy and safety of idelalisib (idela) in combination with ofatumumab (ofa) for previously treated chronic lymphocytic leukemia (CLL). Haematologica. 2015;100(suppl 1):229 (abstr LB598). [Google Scholar]
- 30.US Food and Drug Administration [Internet]. FDA alerts healthcare professionals about clinical trials with zydelig (idelalisib) in combination with the other cancer medicines. Drug Safety and Availability; Updated 2016 Mar 14 [cited 2017 Jan 9]. Available from: http://www.fda.gov/Drugs/Drugsafety/ucm490618.htm.
- 31.Chen Q, Jain N, Ayer T, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol. 2017;35(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.