Abstract
The prevalence of the metabolic syndrome among adults from the French LEA childhood acute leukemia survivors’ cohort was prospectively evaluated considering the type of anti-leukemic treatment received, and compared with that of controls. The metabolic profile of these patients was compared with that of controls. A total of 3203 patients from a French volunteer cohort were age- and sex-matched 3:1 to 1025 leukemia survivors (in both cohorts, mean age: 24.4 years; females: 51%). Metabolic syndrome was defined according to the National Cholesterol Education Program’s Adult Treatment Panel III criteria. Metabolic syndrome was found in 10.3% of patients (mean follow-up duration: 16.3±0.2 years) and 4.5% of controls, (OR=2.49; P<0.001). Patients transplanted with total body irradiation presented the highest risk (OR=6.26; P<0.001); the other treatment groups also showed a higher risk than controls, including patients treated with chemotherapy only. Odd Ratios were 1.68 (P=0.005) after chemotherapy only, 2.32 (P=0.002) after chemotherapy and cranial irradiation, and 2.18 (P=0.057) in patients transplanted without irradiation. Total body irradiation recipients with metabolic syndrome displayed a unique profile compared with controls: smaller waist circumference (91 vs. 99.6 cm; P=0.01), and increased triglyceride levels (3.99 vs. 1.5 mmol/L; P<0.001), fasting glucose levels (6.2 vs. 5.6 mmol/L; P=0.049), and systolic blood pressure (137.9 vs. 132.8 mmHg; P=0.005). By contrast, cranial irradiation recipients with metabolic syndrome had a larger waist circumference (109 vs. 99.6 cm; P=0.007) than controls. Regardless of the anti-leukemic treatment, metabolic syndrome risk was higher among childhood leukemia survivors. Its presentation differed depending on the treatment type, thus suggesting a divergent pathophysiology. This study is registered at clinicaltrials.gov identifier: 01756599.
Introduction
The number of long-term survivors of childhood acute leukemia (AL) has dramatically increased over the past decades. Improvements in treatment and supportive care have resulted in 5-year survival rates exceeding 85% in children with acute lymphoblastic leukemia (ALL)1–3 and approximately 55–60% among children with acute myeloid leukemia (AML).4–6 Therefore, the long-term chronic health condition of such patients has become a major public health concern.7 Several authors have shown that long-term survivors of childhood cancer are at risk of metabolic syndrome, a well-known marker of cardiovascular morbidity and mortality.8–12
Overall, previous studies in this field showed that long-term survivors of childhood AL are at risk of developing a metabolic syndrome, as compared with controls, but several issues need to be addressed. Firstly, our team and others have previously shown that hematopoietic stem cell transplantation (HSCT) and cranial irradiation11,13–18 are clearly associated with a higher risk of metabolic syndrome, while the risk of metabolic syndrome for patients who received chemotherapy only has not been specifically assessed in large comparative studies.
Secondly, prevalence of the metabolic syndrome varies greatly from one country to another.19–21 Many metabolic syndrome studies were carried out in the USA,11,17,22 where metabolic syndrome occurs more frequently among the general population than in France19,23–25 or other European countries. Consequently, extrapolating US-based results to understand metabolic syndrome in a non-US country may not be appropriate. Furthermore, the incidence of metabolic syndrome increases with age and is influenced by sex, country of origin, socio-economic status24 and academic level.26 Thus, the real risk of developing metabolic syndrome among survivors of childhood AL cannot be accurately evaluated without considering all these parameters.
This study is based on data from the LEA (French acronym for “leukemia in children and adolescents”) cohort, a French prospective multicenter cohort designed to evaluate the long-term health status of childhood AL survivors.
The primary objective of the present study was to evaluate the prevalence of metabolic syndrome and its components in adults from the LEA cohort, and to compare it with that found in controls from a French volunteer cohort. More than 1000 patients from the LEA cohort (60% of them treated with chemotherapy only), were compared with age- and sex-matched controls from the Investigation and Clinical Prevention (IPC) cohort, in which volunteer patients were recruited from one of the largest preventive healthcare centers in France.
We also aimed to determine the potential effects of different therapeutic modalities used during leukemia treatment on metabolic syndrome occurrence, and to assess the risk of metabolic syndrome among patients who received only chemotherapy [without HSCT and without central nervous system (CNS) irradiation]. Lastly, we aimed to investigate whether the metabolic syndrome profile (i.e. the cluster of components that constitute metabolic syndrome) varied between the IPC cohort and the LEA cohort, which would suggest divergent mechanisms concerning metabolic syndrome development between these two groups.
Methods
This study is based on a comparison between the LEA cohort and a control group from the IPC cohort.
The LEA group
The LEA program was implemented in 2004 to prospectively evaluate the long-term health status, Quality of Life and socioeconomic status of childhood AL survivors enrolled in treatment programs from 1980 to the present, in 16 cancer centers in France. The details of the program have been previously described.27,28 Data regarding different long-term complications were collected during specific medical visits at pre-defined dates (Online Supplementary Appendix). Since 2007, assessment of metabolic syndrome has been systematically proposed to all adults participating in the LEA program. The study was approved by the French National Program for Clinical Research, the National Cancer Institute, and by the review boards of the institutions involved. All patients provided written informed consent for participation in the study.
The inclusion criteria for the current study were: 1) participation in the LEA program between 2007 and 2014; 2) older than 18 years of age at last LEA evaluation; and 3) at least one complete evaluation for metabolic syndrome.
Comparison cohort: IPC group
The IPC centers are dedicated to the evaluation of the general health status of French patients living in the Paris area (France). These medical centers, funded by the French National Social Security, offer a free medical examination every five years to working and retired employees and their families. During each examination, patients benefit from a medical check-up including medical examination and biological tests. A self-administered questionnaire provides information concerning higher education, medical history, current health status and medication. During these medical check-ups all patients were screened for metabolic syndrome. Selected controls were age- (2-year categories) and sex-matched 3:1 to the LEA patients.
Outcome measurements
Metabolic syndrome was defined according to the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATPIII) revised in 200523 as the combination of at least three of the following criteria: 1) increased waist circumference (≥102 cm in men and ≥88 cm in women); 2) increased blood pressure (systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg) or treatment for hypertension; 3) reduced HDL-cholesterol [<40 mg/dL (1.03 mmol/L) in men, <50 mg/dl (1.3 mmol/l) in women]; 4) elevated fasting glucose levels (≥5.5 mmol/L) or treatment for hyperglycemia; and 5) increased triglycerides (≥1.7 mmol/L) or treatment for hypertriglyceridemia. For further details, see the Online Supplementary Appendix.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and Intercooled Stata 9.0 for Windows. Qualitative data are expressed as percentages. Quantitative data are shown as mean±Standard Error of Mean (SEM). χ2 and Fischer exact tests were used to compare qualitative variables. Quantitative variables were compared using the Student test or the Mann-Whitney test. Logistical regression, adjusted for sex and age, was used to evaluate the probability of developing metabolic syndrome in the LEA subgroups and the IPC group. Odds Ratios (ORs) were estimated with 95% confidence interval (CI). P<0.05 was considered significant.
Results
Characteristics of the LEA cohort
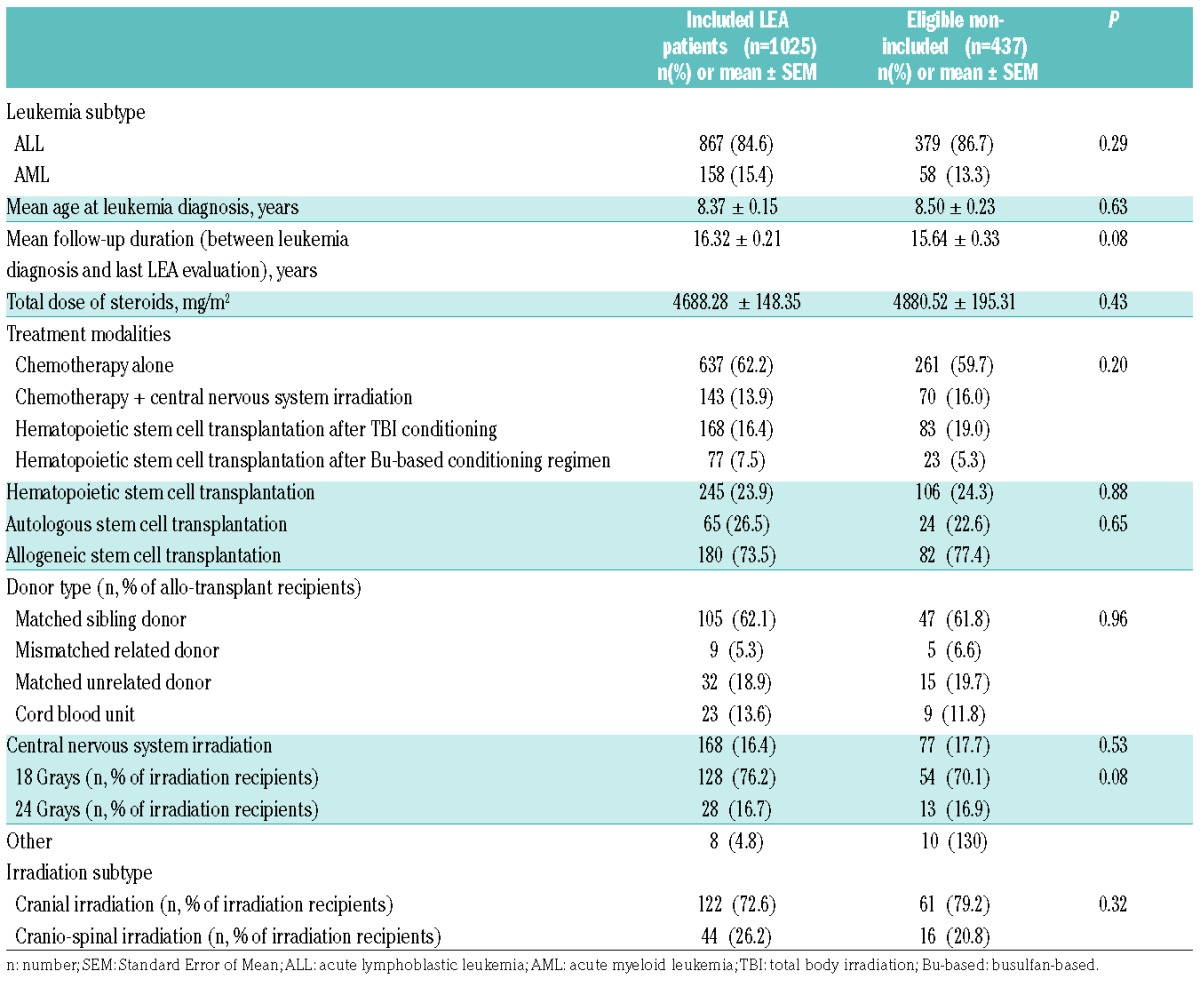
Among the 3188 participants in the LEA cohort between 2007 and 2014, 1462 were eligible (aged >18 years at last evaluation) of whom 1025 had a complete evaluation of the metabolic syndrome; all 1025 were included in the present study (for details, see the flow chart in Online Supplementary Figure S1). Characteristics of the LEA patients are summarized in Table 1, which also includes a comparison between included and eligible but not included patients. No significant difference was noted between included and eligible but not included patients in terms of AL subtype, age at diagnosis, and AL treatment modalities. Among the 1025 included patients, 524 (51.1%) were females; 867 patients (84.6%) had had ALL and 15.4% AML. The mean follow-up duration from leukemia diagnosis to last metabolic syndrome evaluation was 16.32±0.21 years. Patients were treated according to the protocols in use at the time of AL diagnosis, depending on leukemia subtype (AML or ALL) (i.e. FRALLE, EORTC, LAME or ELAM). Most of the included patients received chemotherapy only (n=637, 62.2%), while 143 patients (13.9%) were treated with chemotherapy and CNS irradiation. Overall, 245 patients (23.9%) received HSCT after a conditioning regimen with (n=168, 68.6%) or without (n=77, 31.4%) TBI. Finally, 180 (73.5%) of the 245 transplanted patients received allogeneic stem cell transplantation.
Table 1.
Leukemia subtypes and treatment regimen in patients in the Leukemia in Children and Adolescents (LEA) cohort. Comparison between included and eligible but non-included patients.
Characteristics of the IPC group
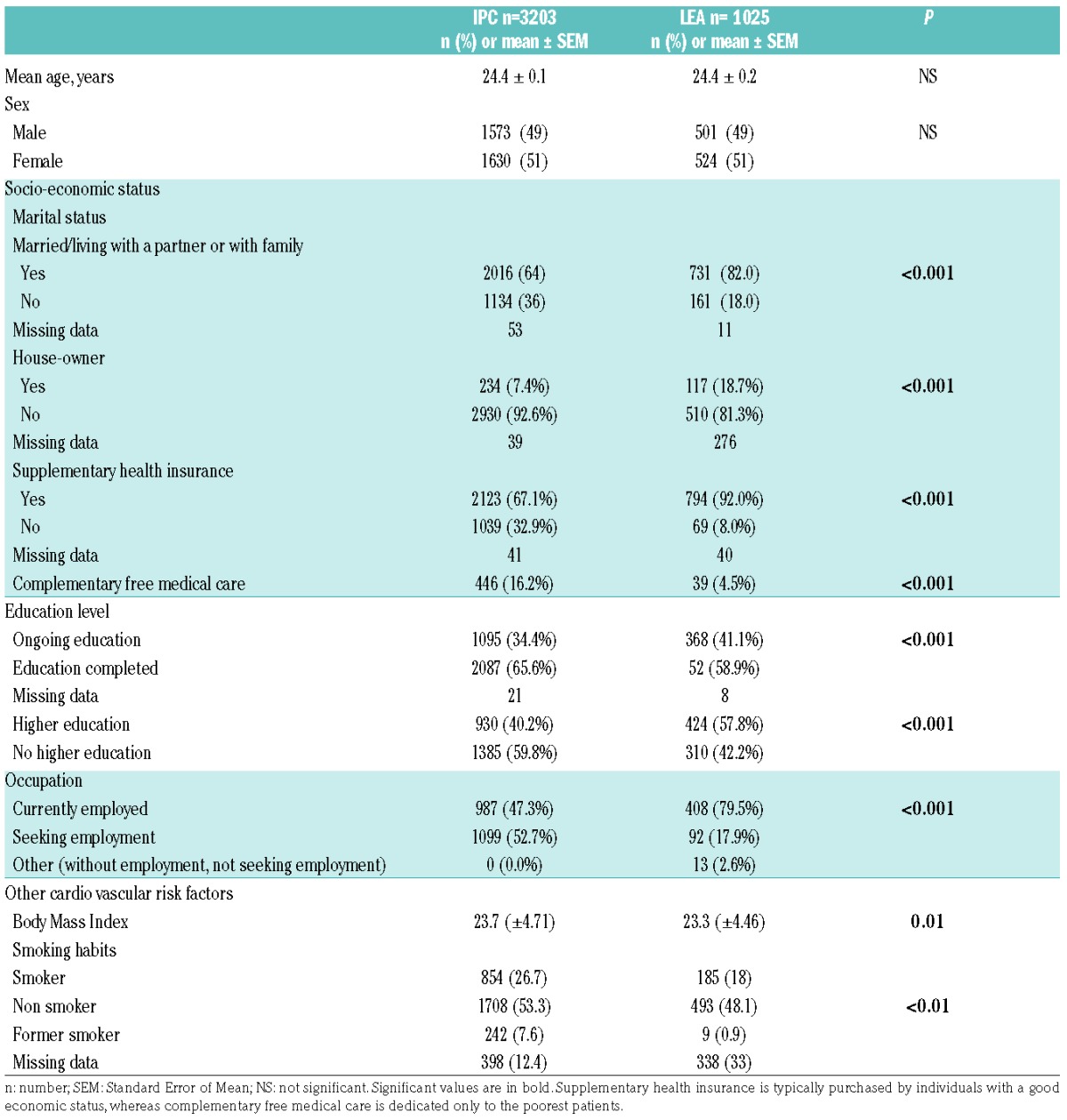
The control group included 3203 patients who were age- and sex-matched 3:1 to the LEA patients. Table 2 provides demographic characteristics and socio-economic data concerning the LEA and IPC patients. As expected, no difference in age at evaluation for metabolic syndrome was noted between the LEA and IPC groups (24.4±0.2 vs. 24.4± 0.1 years, respectively). Similarly, the sex ratio was the same in both groups. Notably, the LEA patients had an overall higher socio-economic status and higher education level compared to controls; for example, LEA patients were more frequently currently employed than controls (79.5% vs. 47.3%, respectively; P<0.001) and were more likely to have a higher level of education (57.8% vs. 40.2%, respectively; P<0.001). Furthermore, controls (IPC group) had a higher mean body mass index (BMI) than LEA patients (23.7±4.71 and 23.3±4.46 for IPC and LEA patients, respectively; P=0.01).
Table 2.
Characteristics of the Leukemia in Children and Adolescents (LEA) and the Investigation and Clinical Prevention) (IPC) groups. Comparison of age, sex, socio-economic status and education level.
Prevalence of metabolic syndrome: comparison between the LEA and the IPC groups
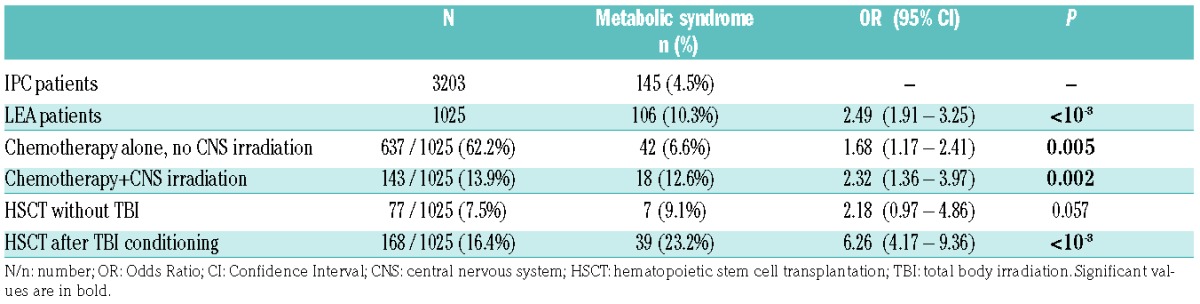
The metabolic syndrome prevalence was 10.3% (n=106/1025) in the LEA group and 4.5% (n=145/3203) in the IPC group (P<0.001), with an OR of 2.49 (95%CI: 1.91–3.25) (Table 3). The metabolic syndrome occurred in 9.7% of female LEA patients, whereas the syndrome occurred in 4% of control females (OR: 2.56, 95%CI: 1.75–3.74; P<0.001). Metabolic syndrome was observed in 11% of male LEA patients and 5% of male controls (OR: 2.33, 95%CI: 1.63–3.34; P<0.001).
Table 3.
Metabolic syndrome prevalence among Leukemia in Children and Adolescents (LEA) patients according to treatment modality. Comparison with the Investigation and Clinical Prevention) (IPC) group (sex- and age-matched controls).
Since blood pressure is the variable the most affected by fluctuations and might be overestimated in some cases, we analyzed the metabolic syndrome occurrence excluding the hypertension criteria: 46 LEA patients (4.5%) had a metabolic syndrome versus 66 controls (2.1%). The difference was still statistically significant (P<0.001).
We analyzed the cumulative incidence of the metabolic syndrome among LEA patients over time: 7.86% (95%CI: 5.99–10.29) at 25 years, and 14.42% (95%CI: 11.22–18.43) at 30 years (Online Supplementary Appendix).
Prevalence of the metabolic syndrome components (considered separately) also differed between the LEA and IPC patients: LEA patients were more likely than IPC subjects to have a larger waist circumference [17% vs. 10.6%, respectively, OR=1.79 (95%CI: 1.43–2.23); P<0.001], elevated triglycerides [14% vs. 4.5%, respectively, OR=3.59 (95%CI: 2.8–4.6); P<0.001] and high blood pressure [33.9% vs. 22.8%, respectively, OR=1.81 (95%CI: 1.53–2.14); P<0.001]. By contrast, the prevalence of elevated fasting glucose and low HDL-cholesterol levels were not elevated in the LEA group.
The highest prevalence of metabolic syndrome was found in patients who received HSCT (prevalence: 18.8%, OR: 4.87, 95%CI: 3.4–6.99; P<0.001). TBI before HSCT was associated with the highest prevalence of metabolic syndrome (23.2%) as well as the highest relative risk of developing metabolic syndrome (OR=6.26, 95%CI: 4.17–9.36; P<0.001). Notably, women who received HSCT after TBI were at particularly high risk of developing metabolic syndrome compared with females from the control group [OR=9.25 (95%CI: 5.33–16.1); P<0.001]. Male patients receiving TBI, compared with males from the IPC cohort, were also at higher risk of metabolic syndrome [OR=4.13 (95%CI: 2.26–7.56); P<0.001]. Transplanted patients without TBI had also a higher risk of metabolic syndrome, even if it did not reach the significance threshold (OR=2.18, 95%CI: 0.97–4.86; P=0.057). We also found that patients who underwent CNS irradiation were more likely to develop metabolic syndrome compared with controls [OR= 2.32 (95%CI: 1.36–3.97); P=0.002].
Interestingly, patients who received chemotherapy only were also more likely to develop metabolic syndrome than controls [OR= 1.68 (95%CI: 1.17–2.41); P=0.005] (Table 3).
Age at diagnosis or at transplantation did not impact the metabolic syndrome risk among the LEA patients.
Metabolic syndrome profile: impact of treatment modalities
We also aimed to determine whether the metabolic profile among patients who had a metabolic syndrome was different between LEA patients and controls. This is the reason why in this study we selected only patients with a metabolic syndrome from the LEA and IPC cohorts (Figures 1 and 2).
Figure 1.
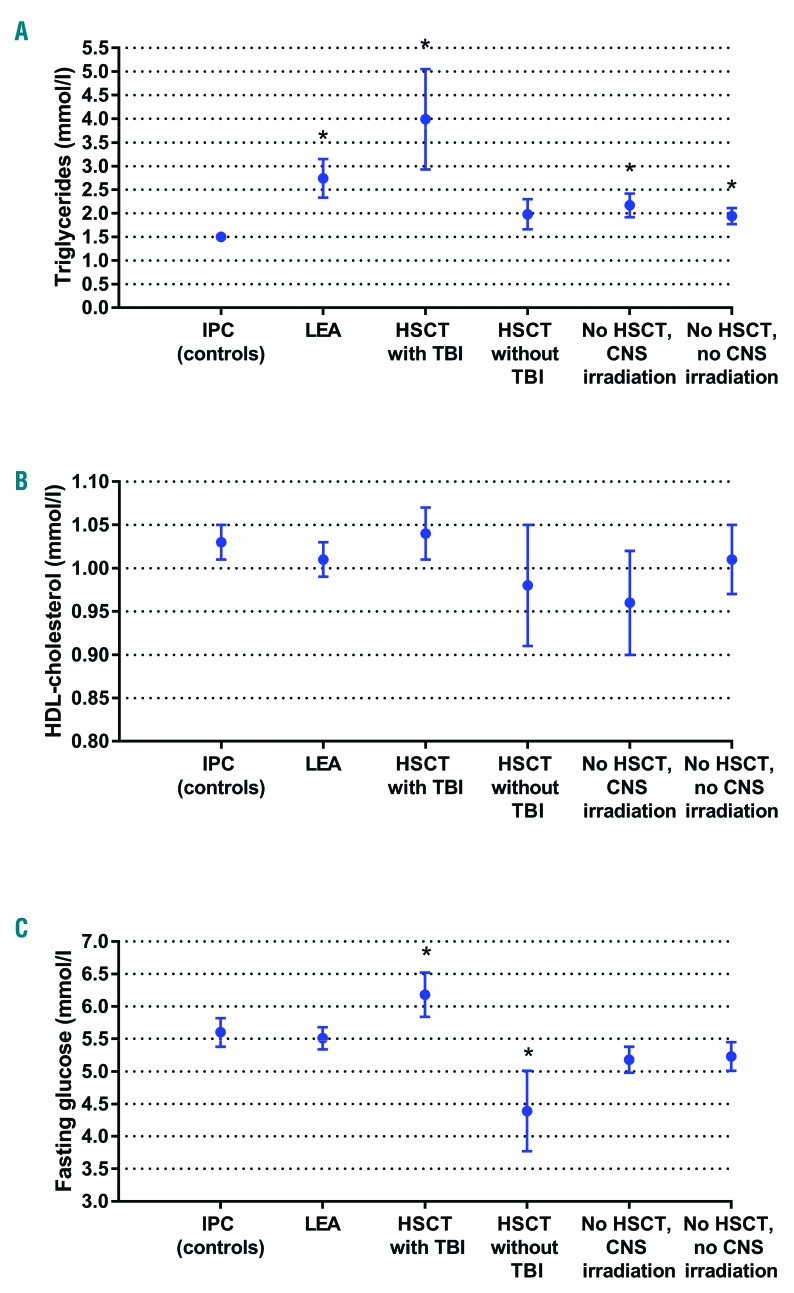
Biological markers. Biological markers of metabolic syndrome (triglycerides, HDL-cholesterol and fasting glucose levels) among Leukemia in Childhood and Adolescents (LEA) cohort patients displaying a metabolic syndrome (n=106) according to treatment modality: hematopoietic stem cell transplantation (HSCT) with total body irradiation (TBI): n=39; HSCT without TBI: n=7; no HSCT with central nervous system (CNS) irradiation: n=18; no HSCT/no CNS irradiation: n=42. LEA patients were compared with Investigation and Clinical Prevention (IPC) group patients (controls) with metabolic syndrome (n=145), adjusted according to sex and age. Results are expressed as mean±Standard Error of Mean (SEM). (A) Triglyceride levels. (B) HDL cholesterol levels. (C) Fasting glucose levels.
Figure 2.
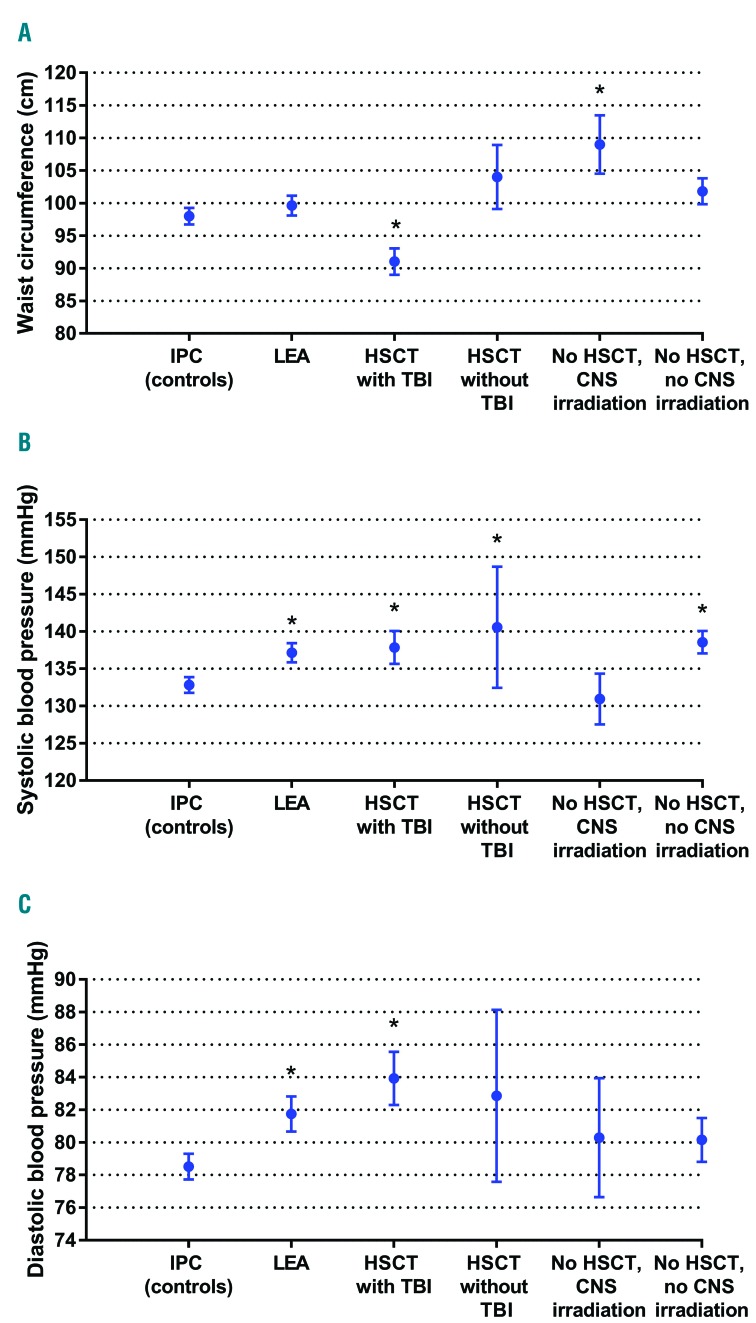
Clinical markers. Clinical markers (blood pressure and waist circumference) of metabolic syndrome among Leukemia in Childhood and Adolescents (LEA) cohort patients who show a metabolic syndrome (n=106) according to treatment modality: hematopoietic stem cell transplantation (HSCT) with total body irradiation (TBI): n=39; HSCT without TBI: n=7; no HSCT with central nervous system (CNS) irradiation: n=18; no HSCT/no CNS irradiation: n=42. LEA patients with metabolic syndrome were compared with Investigation and Clinical Prevention (IPC) group patients (controls) with metabolic syndrome (n=145). Results are expressed as mean±Standard Error of Mean (SEM). (A) Waist circumference. (B) Systolic blood pressure. (C) Diastolic blood pressure. *Significant difference,
When assessing the metabolic syndrome patients in both groups (n=106 in the LEA group, n=145 in the control group), metabolic profile differed between the two groups of patients. In the LEA group with metabolic syndrome, patients had significantly higher levels of triglycerides [mean triglyceride level: 2.74±0.4 mmol/L (LEA patients) vs. 1.5±0.1 mmol/L (IPC patients); P=0.001] and elevated systolic and diastolic blood pressure (mean systolic blood pressure: 137.1±1.3 (LEA) and 132.8±1.1 mmHg (IPC); P=0.005; mean diastolic blood pressure: 81.7±1.1 (LEA) vs. 78.5±0.8 mmHg (IPC); P=0.01 (Figures 1 and 2).
Patients with metabolic syndrome from the LEA group who received TBI had a particular metabolic profile: in spite of a smaller mean abdominal circumference [91±2 (LEA) vs. 99.6±1.5 cm (IPC); P=0.01], these patients displayed higher mean triglyceride levels (3.99±1.06 vs. 1.5±0.07 mmol/L, respectively; P<0.001), higher mean blood pressure (systolic blood pressure: 137.9±2.2 mmHg vs. 132.8±1.1, respectively; P=0.005) and higher mean fasting glucose levels (6.2±0.3 vs. 5.6±0.1 mmol/L, respectively; P=0.049) than patients with metabolic syndrome from the control group. By contrast, metabolic syndrome patients from the LEA group who received CNS irradiation without TBI also displayed higher triglyceride levels (mean: 2.17±0.25 mmol/L vs. 1.5±0.07, respectively; P=0.002) but had a larger waist circumference (109±4.5 cm vs. 99.6±1.5 cm, respectively; P=0.007) compared with metabolic syndrome patients in the control group.
Metabolic syndrome patients who received HSCT without TBI in the LEA cohort showed higher systolic blood pressure levels (mean: 140.6±8.1 mmHg vs. 132.8±1.1 mmHg, respectively; P=0.039) but lower fasting glucose levels (mean: 4.4±0.6 mmol/L vs. 5.6±0.1 mmol/L; P=0.049) compared with metabolic syndrome controls. Lastly, LEA patients with metabolic syndrome who were treated with chemotherapy only displayed higher triglyceride levels (1.94±0.17 vs. 1.5±0.07 mmol/L, respectively; P=0.008) and higher systolic blood pressure (138.6±1.5 vs. 132.8±1.1; P=0.02) compared with metabolic syndrome controls.
Discussion
Here we report on one of the largest comparative studies on metabolic syndrome prevalence among adults treated for AL during childhood or adolescence. We found that patients from the LEA cohort were at greater risk of developing metabolic syndrome (OR=2.49, 95%CI: 1.91–3.25) compared to controls, regardless of the treatment they received.
Furthermore, the risk was significantly higher for patients treated exclusively with chemotherapy compared to controls (OR= 1.68, 95%CI: 1.17–2.41; P=0.005), a fact which has never been shown before, even though it had been suspected. The results presented in previous meta-analyses or comparative studies have so far remained unconfirmed, probably due to the relatively low number of patients receiving chemotherapy only11,29–31 or the lack of proper controls.32,33 The Saint Jude study published by Nottage et al.11 reported a higher risk of metabolic syndrome in 784 AL survivor patients as compared to controls, but 64.6% of them had received cranial irradiation. In the subgroup of patients treated exclusively with chemotherapy, no significant increase in the risk of developing metabolic syndrome could be shown, probably due to the small number of patients who received chemotherapy only (n=277). By contrast, the LEA patients included in this study were mainly treated with chemotherapy only (n=637, 62.2%). So, we demonstrate here that, even in the case of treatment with chemotherapy only, the metabolic syndrome risk is higher among long-term AL survivors than in the control population. This is important since nowadays, the majority of children treated for an AL will receive only chemotherapy, without any irradiation or HSCT. Several mechanisms have been discussed to explain how metabolic syndrome develops after chemotherapy treatment. Alkylating agents are known to induce mitochondrial dysfunction and endothelial cytotoxicity, which can lead to insulin resistance, steatosis and hypertension. Anthracyclins and antimetabolites can also cause mitochondrial and endothelial dysfunction. Steroids can induce hyperglycemia and dyslipidemia. Vinca alkaloids induce endothelial toxicity and can cause hyperglycemia by inhibition of GLUT2/4 vesicle translocation.34 Lastly, iron overload, a frequent complication after multiple transfusions in patients treated for acute leukemia, could also increase the risk for metabolic syndrome through hepatic toxicity. As a consequence, the notable metabolic syndrome risk in patients treated exclusively with chemotherapy, who represent the majority of future AL survivors, should be taken into careful consideration throughout their long-term follow up.
This high risk among LEA patients is striking given that the LEA patients were found to benefit from more favorable socio-economic conditions (e.g. higher level of education, higher rates of employment) than IPC patients. Indeed, it is well known that lower socio-economic status is associated with a higher risk of metabolic syndrome.24,35 As the control group (IPC population) was characterized by particularly unfavorable social and economic conditions (unemployed: 52.7%, no higher education: 59.8%), the relative risk of metabolic syndrome in the LEA group may be underestimated. Due to these unfavorable socioeconomic conditions, the IPC group is probably at higher risk of metabolic syndrome than the general French population, which makes the risk described in the LEA patients even higher. Moreover, LEA patients had a lower mean BMI, and were less frequently smokers than controls, which could contribute to their relative risk of developing a metabolic syndrome being underestimated.
Few studies on metabolic syndrome in AL survivors consider socio-economic factors,36 whereas studies about metabolic syndrome in the general population have shown this to be very important. Given the impact of socio-economic status on the development of the metabolic syndrome, further studies are needed to investigate a potential correlation between lifestyle (e.g. sedentary behavior), eating habits, and the occurrence of metabolic syndrome in AL survivors.
Our findings concerning metabolic syndrome prevalence in the LEA cohort (10.3%) is difficult to compare with studies from other countries, as metabolic syndrome prevalence in the general population varies from one country to another. Furthermore, metabolic syndrome prevalence is lower in France than in many other industrialized countries.25,37 Previous reports of metabolic syndrome prevalence among AL survivors range from 4.2% to 49%,11,16,32,38–40 with prevalence depending mainly on the treatment type (chemotherapy, HSCT or CNS irradiation), follow-up duration and metabolic syndrome definition. In several studies, HSCT was associated with a high prevalence of metabolic syndrome, ranging from 31% to 49%.16,39,40 The study by Nottage et al. reported a higher metabolic syndrome prevalence among non-transplanted patients (33.6%) than that found in our study, and this can partly be explained by the number of patients who received CNS irradiation (507 of 784 patients) and the older age of the patients.11
The risk of developing metabolic syndrome was even higher among patients who received CNS irradiation (OR: 2.32, 95%CI: 1.36–3.97; P=0.002), which confirms the results of previous studies.15,17,38 Interestingly, those patients had a much larger waist circumference compared with the IPC group. This highlights the impact of CNS irradiation on the development of obesity, an observation which has been previously described11,41,42 and debated.43,44
The highest risk of metabolic syndrome was observed among transplanted patients (prevalence: 18.8%, OR: 4.87, 95%CI: 3.4–6.99; P<0.001), especially those who received TBI (OR: 6.26, 95%CI: 4.17–9.36; P<0.001). The deleterious impact of TBI on the development of the metabolic syndrome has previously been reported by our group13 and others;18,32 here, these data are confirmed with a larger number of patients. Apart from TBI, so far additional risk factors for metabolic syndrome in HSCT patients have not been clearly documented: some authors have suggested that graft-versus-host disease would increase this risk,18 but this remains controversial.
Several studies, including ours, have also shown an association between metabolic syndrome and growth hormone deficiency,14,18,33,45 particularly in patients who received CNS irradiation or TBI. However, the precise mechanism by which growth hormone deficiency could induce a metabolic syndrome remains unclear.
We also aimed to determine whether the metabolic profile among patients who had a metabolic syndrome was different between LEA patients and controls. This is why we only selected patients with a metabolic syndrome from among LEA and IPC patients in order to study this metabolic profile (Figures 1 and 2). Interestingly, we found that, compared with metabolic syndrome controls, patients with metabolic syndrome who received HSCT after TBI had a specific metabolic profile: they had more elevated triglycerides and fasting glucose levels, as well as higher blood pressure. This suggests that these patients develop a metabolic syndrome with more severe features than that of controls, which could lead to higher rates of cardiovascular morbidity, as suggested for the general population.46
Patients who developed metabolic syndrome after TBI had a smaller waist circumference than IPC patients with metabolic syndrome. Altogether, these results suggest that obesity is not a key factor after TBI, in contrast to the general population. Different hypotheses can be made concerning the pathophysiology in those patients. Irradiation of the pancreas during TBI can induce diabetes47 and therefore metabolic syndrome. Furthermore, some authors have found that, in patients treated with TBI, insulin resistance was not associated with obesity but rather with abnormal fat mass repartition.48,49 Modification of adipose tissue metabolism has been recognized as a fundamental mechanism behind metabolic syndrome development.50 Therefore, TBI exposure may induce adipose tissue abnormalities, as suggested by animal models,51 and contribute to the development of metabolic syndrome. Our previous studies indicated that high-dose corticosteroids do not have an impact on metabolic syndrome,11,15 and, therefore, this hypothesis remains controversial.11,15
Patients with metabolic syndrome who received only chemotherapy displayed higher systolic blood pressure and increased triglyceride levels compared with metabolic syndrome controls, thus suggesting a more severe form of metabolic syndrome.
The waist circumference of patients who received CNS irradiation was markedly larger than that of the controls. Obesity caused by CNS irradiation, as previously described,41 is probably linked to the metabolic syndrome development. As the hypothalamus exerts central neuroendocrine functions that control hunger and satiety, hypothalamic irradiation could lead to modifications in food intake and energy balance.
One limitation of our study involved the fact that some important metabolic syndrome factors such as physical activity, eating habits and lifestyle have not been documented in our population. However, those factors are known to be very important in the metabolic syndrome genesis. A reduction in physical activity in LEA patients, as well as unhealthy eating habits could worsen the risk of developing metabolic syndrome. Another bias is linked to the control population, which includes only people from the Ile de France region, whereas LEA patients are recruited from throughout France. Some regional differences in the metabolic syndrome prevalence could be found, which might make it difficult to extrapolate the results from the IPC population to the general French population.
In summary, our study suggests that metabolic syndrome may develop through different mechanisms, depending on the treatments received. In the present study, we used age-matched controls, which enabled us to accurately evaluate metabolic syndrome risk in this young AL survivor population (mean age: 24 years), regardless of the fact that metabolic syndrome prevalence among individuals in this young age range is poorly described. This prevalence will probably increase with age, as is the case for the general population.23
In conclusion, this study reveals an increased risk of metabolic syndrome among adult survivors of AL, regardless of the treatment they received. Moreover, metabolic syndrome seems to be more severe in the LEA patients than in the control group. The highest risk is observed in patients who received TBI, a group that displays a specific metabolic profile, but all patients treated for childhood AL should be considered at risk of metabolic syndrome, regardless of the treatment they received, even in the case of chemotherapy only. We hypothesize that if early detection of metabolic syndrome is followed by changes in lifestyle (e.g. improved eating habits, more physical activity), it will help to prevent cardiovascular events in this at-risk population.36 We are currently planning controlled intervention studies in order to explore such an approach.
Supplementary Material
Acknowledgments
The authors would like to thank the LEA study group (Supplemental Data), for data collection, as well as the patients and their family.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/4/645
Funding
The study was funded by the French National Clinical Research Program, the French National Cancer Institute (InCA), the “Laurette Fugain” association, the French National Research Agency (ANR), the “Ligue Contre le Cancer” association, Cancéropôle PACA, the Regional Council PACA and the French Institute for Public Health Research (IRESP).
References
- 1.Silverman LB, Stevenson KE, O’Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000). Leukemia. 2010;24(2):320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373(16):1541–1552. [DOI] [PubMed] [Google Scholar]
- 4.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J Clin Oncol. 2015;33(27):2949–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creutzig U, Zimmermann M, Bourquin JP, et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 2013;122(1):37–43. [DOI] [PubMed] [Google Scholar]
- 6.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia S, Armenian SH, Armstrong GT, et al. Collaborative Research in Childhood Cancer Survivorship: The Current Landscape. J Clin Oncol. 2015;33(27):3055–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–1778. [DOI] [PubMed] [Google Scholar]
- 10.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. [DOI] [PubMed] [Google Scholar]
- 11.Nottage KA, Ness KK, Li C, Srivastava D, Robison LL, Hudson MM. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia - From the St. Jude Lifetime Cohort. Br J Haematol. 2014;165(3):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eschwege E. The dysmetabolic syndrome, insulin resistance and increased cardiovascular (CV) morbidity and mortality in type 2 diabetes: aetiological factors in the development of CV complications. Diabetes Metab. 2003;29(4 Pt 2):6S19–27. [DOI] [PubMed] [Google Scholar]
- 13.Oudin C, Simeoni MC, Sirvent N, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood. 2011;117(17):4442–4448. [DOI] [PubMed] [Google Scholar]
- 14.Oudin C, Auquier P, Bertrand Y, et al. Metabolic syndrome in adults who received hematopoietic stem cell transplantation for acute childhood leukemia: an LEA study. Bone Marrow Transplant. 2015;50(11):1438–1444. [DOI] [PubMed] [Google Scholar]
- 15.Saultier P, Auquier P, Bertrand Y, et al. Metabolic syndrome in long-term survivors of childhood acute leukemia treated without hematopoietic stem cell transplantation: an L.E.A. study. Haematologica. 2016;101(12):1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27(22):3698–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman DN, Hilden P, Moskowitz CS, et al. Cardiovascular Risk Factors in Survivors of Childhood Hematopoietic Cell Transplantation Treated with Total Body Irradiation: A Longitudinal Analysis. Biol Blood Marrow Transplant. 2017;23(3):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- 20.Procopiou M, Philippe J. The metabolic syndrome and type 2 diabetes: epidemiological figures and country specificities. Cerebrovasc Dis. 2005;20(Suppl 1):2–8. [DOI] [PubMed] [Google Scholar]
- 21.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33(2):351–375. [DOI] [PubMed] [Google Scholar]
- 22.Oeffinger KC. Are survivors of acute lymphoblastic leukemia (ALL) at increased risk of cardiovascular disease? Pediatr Blood Cancer. 2008;50(2 Suppl):462–467. [DOI] [PubMed] [Google Scholar]
- 23.Pannier B, Thomas F, Eschwege E, et al. Cardiovascular risk markers associated with the metabolic syndrome in a large French population: the “SYMFONIE” study. Diabetes Metab. 2006;32(5 Pt 1):467–474. [DOI] [PubMed] [Google Scholar]
- 24.Vernay M, Salanave B, de Peretti C, et al. Metabolic syndrome and socioeconomic status in France: The French Nutrition and Health Survey (ENNS, 2006–2007). Int J Public Health. 2013;58(6):855–864. [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 26.Scuteri A, Vuga M, Najjar SS, et al. Education eclipses ethnicity in predicting the development of the metabolic syndrome in different ethnic groups in midlife: the Study of Women’s Health Across the Nation (SWAN). Diabet Med. 2008;25(12):1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berbis J, Michel G, Baruchel A, et al. Cohort Profile: the French childhood cancer survivor study for leukaemia (LEA Cohort). Int J Epidemiol. 2015;44(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel G, Bordigoni P, Simeoni MC, et al. Health status and quality of life in long-term survivors of childhood leukaemia: the impact of haematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40(9):897–904. [DOI] [PubMed] [Google Scholar]
- 29.Karakurt H, Sarper N, Kilic SC, Gelen SA, Zengin E. Screening survivors of childhood acute lymphoblastic leukemia for obesity, metabolic syndrome, and insulin resistance. Pediatr Hematol Oncol. 2012;29(6):551–561. [DOI] [PubMed] [Google Scholar]
- 30.Follin C, Thilen U, Ahren B, Erfurth EM. Improvement in cardiac systolic function and reduced prevalence of metabolic syndrome after two years of growth hormone (GH) treatment in GH-deficient adult survivors of childhood-onset acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2006;91(5):1872–1875. [DOI] [PubMed] [Google Scholar]
- 31.Blijdorp K, van Waas M, van der Lely AJ, Pieters R, van den Heuvel-Eibrink M, Neggers S. Endocrine sequelae and metabolic syndrome in adult long-term survivors of childhood acute myeloid leukemia. Leuk Res. 2013;37(4):367–371. [DOI] [PubMed] [Google Scholar]
- 32.Chow EJ, Simmons JH, Roth CL, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biol Blood Marrow Transplant. 2010;16(12):1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107(6):1303–1312. [DOI] [PubMed] [Google Scholar]
- 34.Rosen GP, Nguyen HT, Shaibi GQ. Metabolic syndrome in pediatric cancer survivors: a mechanistic review. Pediatr Blood Cancer. 2013;60(12):1922–1928. [DOI] [PubMed] [Google Scholar]
- 35.Vernay M, Malon A, Oleko A, et al. Association of socioeconomic status with overall overweight and central obesity in men and women: the French Nutrition and Health Survey 2006. BMC Public Health. 2009;9:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith WA, Li C, Nottage KA, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. 2014;120(17):2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavrila D, Salmeron D, Egea-Caparros JM, et al. Prevalence of metabolic syndrome in Murcia Region, a southern European Mediterranean area with low cardiovascular risk and high obesity. BMC Public Health. 2011;11:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faienza MF, Delvecchio M, Giordano P, et al. Metabolic syndrome in childhood leukemia survivors: a meta-analysis. Endocrine. 2015;49(2):353–360. [DOI] [PubMed] [Google Scholar]
- 39.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(9):797–804. [DOI] [PubMed] [Google Scholar]
- 40.Paris C, Yates L, Lama P, Zepeda AJ, Gutierrez D, Palma J. Evaluation of metabolic syndrome after hematopoietic stem cell transplantation in children and adolescents. Pediatr Blood Cancer. 2012;59(2):306–310. [DOI] [PubMed] [Google Scholar]
- 41.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(28):4639–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(7):1359–1365. [DOI] [PubMed] [Google Scholar]
- 43.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110(10):2313–2320. [DOI] [PubMed] [Google Scholar]
- 44.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25(10):1183–1189. [DOI] [PubMed] [Google Scholar]
- 45.Link K, Moell C, Garwicz S, et al. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 2004;89(10):5003–5012. [DOI] [PubMed] [Google Scholar]
- 46.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58(12):2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Vathaire F, El-Fayech C, Ben Ayed FF, et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. Lancet Oncol. 2012;13(10):1002–1010. [DOI] [PubMed] [Google Scholar]
- 48.Frisk P, Rossner SM, Norgren S, Arvidson J, Gustafsson J. Glucose metabolism and body composition in young adults treated with TBI during childhood. Bone Marrow Transplant. 2011;46(10):1303–1308. [DOI] [PubMed] [Google Scholar]
- 49.Wei C, Thyagiarajan MS, Hunt LP, Shield JP, Stevens MC, Crowne EC. Reduced insulin sensitivity in childhood survivors of haematopoietic stem cell transplantation is associated with lipodystropic and sarcopenic phenotypes. Pediatr Blood Cancer. 2015;62(11):1992–1999. [DOI] [PubMed] [Google Scholar]
- 50.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome–an allostatic perspective. Biochim Biophys Acta. 2010;1801(3):338–349. [DOI] [PubMed] [Google Scholar]
- 51.Poglio S, Galvani S, Bour S, et al. Adipose tissue sensitivity to radiation exposure. Am J Pathol. 2009;174(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.