Multiple myeloma (MM) is characterized by secretion of monoclonal (M) protein from clonal plasma cells.1 In most cases, intact immunoglobulins (Igs) consisting of heavy and light chains are secreted, whereas in about 20% of cases only free light chains (FLCs) are secreted (‘light chain only, LCO, MM’).1 MM is also characterized by a number of cytogenetic abnormalities (CAs) categorized into primary (IgH translocations and hyperdiploid trisomies) and secondary (other CAs) events. CAs may be used for MM risk stratification in routine clinical practice.2–4 One study showed that cases with IgH translocations had higher FLC levels and abnormal FLC ratio.5 Recently, prognostic significance was shown for isotype matched Ig ratios (e.g. IgGκ/IgGλ).6–8
So far, the possible impact of CAs on Ig and FLC subtypes and their levels has been poorly studied in MM. The hypothesis is that CAs are associated with the involved Ig isotypes in MM. We carried out a systematic analysis of Ig and FLC subtypes and their serum levels in relation with common CAs using data on 523 patients recruited in the German-Speaking Myeloma Multicenter Group (GMMG)-MM5 trial.9,10 For purpose of replication, an independent cohort of 325 cases from the GMMG-HD4 trial was obtained.11,12
Blood samples were collected prior to treatment initiation in the above trials, co-ordinated by University Clinic Heidelberg. Patients’ characteristics are summarized in Online Supplementary Table S1.9,10 Methods used to measure Ig concentrations and subtypes are described elsewhere13 and in the Online Supplementary Appendix. CAs were identified for available samples using fluorescence in situ hybridization (FISH) techniques.11 To assign translocation positivity, 10% or more of the tested affected cells had to demonstrate positive FISH test. All statistical analyses were performed using R software (version 3.2.3). χ2 test of independence and Fisher exact test were used to test equality of proportions of CA positive cases. Multivariable linear regression models were fitted to test the relationship between clinical variables and CAs. Covariates included in the models were International Staging System (ISS), sex, light chain type, bone marrow cell count and secondary CAs.
Collection of patients’ samples and associated clinical information within both clinical trials was approved by the ethical review board of Heidelberg University, in accordance with the Declaration of Helsinki.
Table 1 depicts the proportion of CA positive cases in three major MM isotypes. In both cohorts, the most frequent CA was hyperdiploidy (57%). Proportions of three CAs varied significantly depending upon MM type. t(4;14) was significantly higher in IgA MM compared with IgG MM (23% vs. 6%; P=1.1×10−5) and LCO MM (23% vs. 7%; P=0.02). Hyperdiploidy occurred more frequently in IgG MM and IgA MM compared with LCO MM (66% vs. 32%, P=1.3×10−6 and 53% vs. 32%, P=0.03, respectively). t(11;14) was most common in LCO MM compared with IgG MM (41% vs. 19%; P=6.0×10−4) and IgA MM (41% vs. 14%; P=4.2×10−4). In the GMMG-HD4 cohort, we replicated association of t(4;14) with IgA MM compared to IgG MM (28% vs. 10%; P=3.3×10−3) and association of hyperdiploidy with IgG MM compared to LCO MM (66% vs. 35%; P=6.6×10−4). t(14;16) did not influence MM isotype in either cohort. We further analyzed the association between CAs and MM isotypes by considering possible combinations of heavy-light chains. Similar patterns of differences in the proportions were observed across heavy-light chain matched isotypes, as noticed above, for three CAs. Further, isotype-matched pairwise comparisons showed significant differences in proportions of three CAs for the IgGκ and IgGλ pair (Online Supplementary Table S2). In the GMMG-HD4 cohort, directions of changes remained but the case numbers were small. No such differences were observed in the isotype-matched pairwise comparisons for IgA and LCO MM (data not shown). These results indicate that the above noticed association between CAs and Ig isotypes is not influenced by the light chain type.
Table 1.
Proportion of positivity for cytogenetic abnormalities (CAs) across multiple myeloma (MM) isotypes, IgG, IgA and LCO.
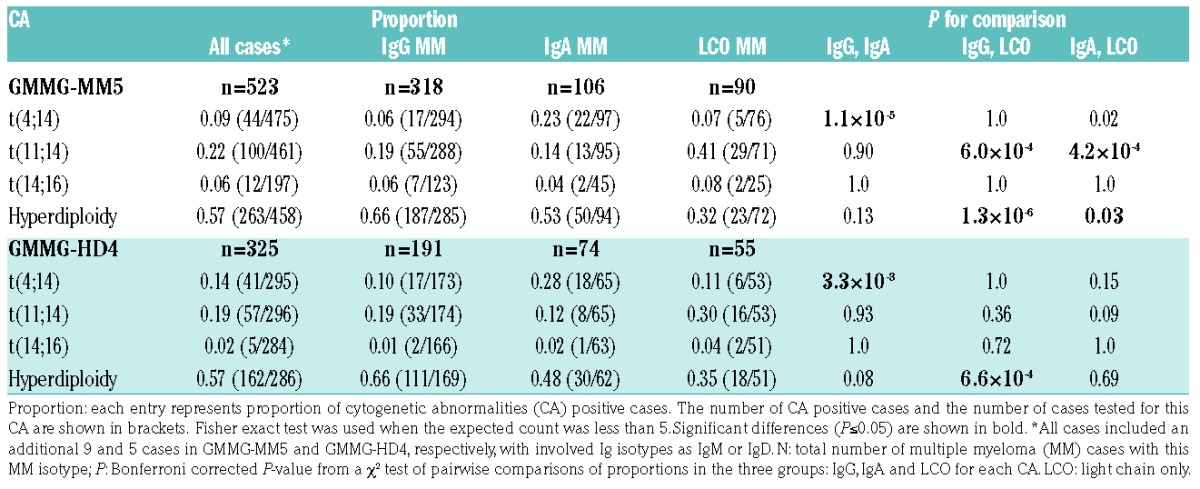
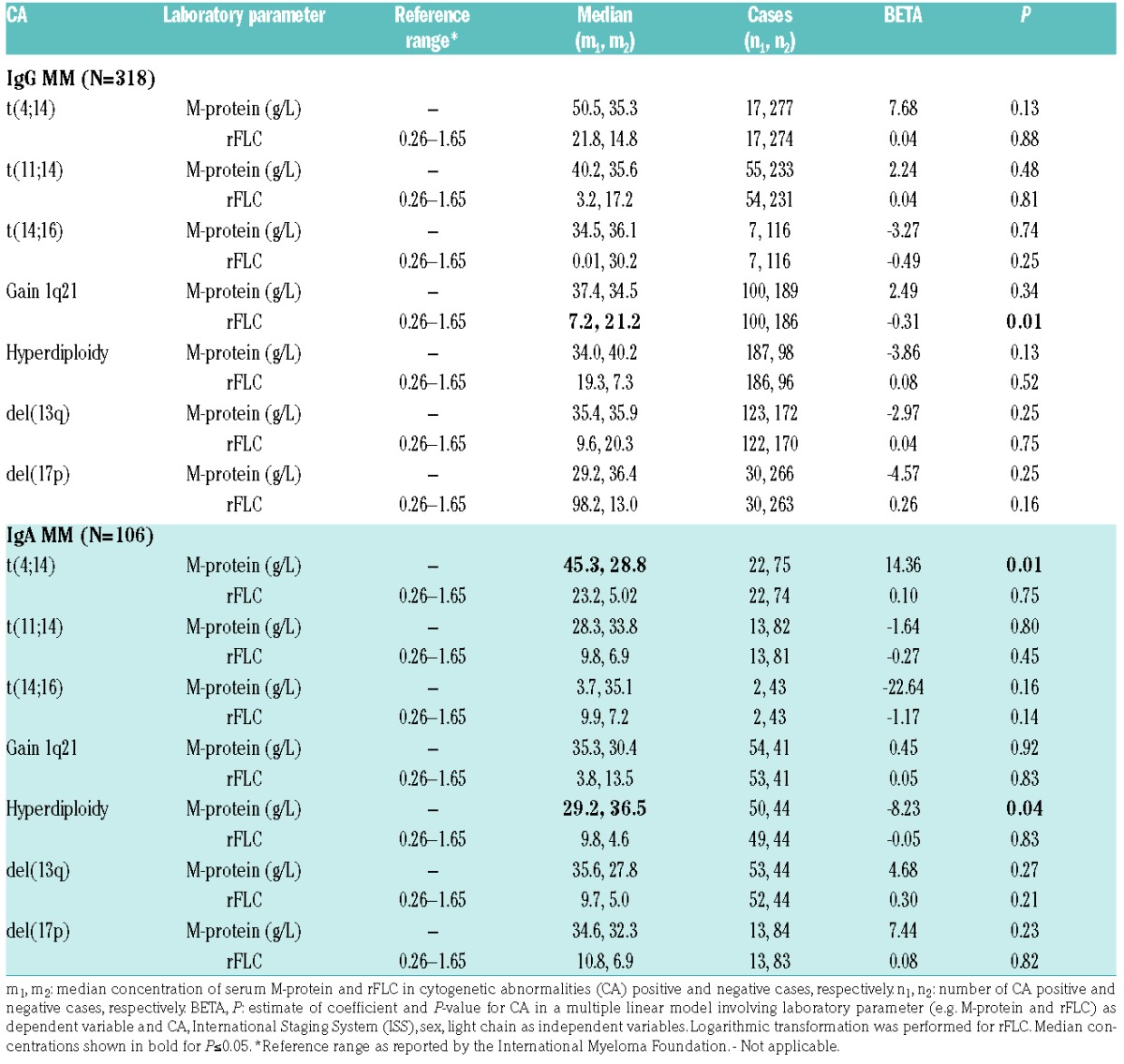
Table 2 shows median concentrations of serum M-protein and rFLC (κ/λ ratio) in CA positive and negative patients for IgG MM and IgA MM. M-protein levels were systematically increased in t(4;14) positive patients compared with t(4;14) negative patients for both IgG MM and IgA MM. Statistical significance was reached for IgA MM alone (P=0.01; β=14.36) after adjusting for the covariates (see above). This observation was further supported by a significant positive correlation between percentage of t(4;14) harboring plasma cells and M-protein level for IgA MM (Pearson correlation coefficient, r = 0.46; P=2.6×10−4). There was a consistent negative relationship, of borderline significance, between M-protein level and hyperdiploidy status for IgG MM and IgA MM (P=0.13; β=−3.86 and P=0.04; β=−8.23, respectively). Differences in M-protein level were smaller and insignificant for the remaining five CAs. The rFLC values were outside the reference range (0.26–1.65) for all cases and, with one exception, they were above the reference range, i.e. ratios shifted in favor of κ chains. Significant differences in rFLC were found for gain 1q21 (P=0.01; β=−0.31) in IgG MM but not in IgA MM (P=0.83; β=0.05).
Table 2.
Association between cytogenetic abnormalities (CA) and concentration of serum M-protein and rFLCs (κ/λ ratio) in IgG MM and IgA MM.
The median M-protein levels were significantly higher in IgG MM cases compared with IgA MM (36.0 g/L vs. 32.3 g/L; P=2.2×10−3) (Online Supplementary Table S3). Consistently, in the GMMG-HD4 cohort, the level in IgG MM was also higher compared with IgA MM (42.0 g/L vs. 35.8 g/L; P=4.0×10−4). No differences in M-protein levels were observed across the three IgH translocation types (P >0.05). For IgA MM, median M-protein levels in patients with any IgH translocations was more than double the median M-protein level in hyperdiploidy group (42.4 g/L vs. 19.0 g/L; P=1.1×10−4). The difference remained consistent, but smaller and of borderline significant in the GMMG-HD4 cohort (44.9 g/L vs. 39.1 g/L; P=0.06).
The individual FLCκ levels were outside the reference range for 80% of cases, and for 63% cases exceeded the upper limit (>19.4 mg/L) (Online Supplementary Table S4). FLCλ levels were outside the reference range for 63% cases, and for 31% cases surpassed the upper limit (>26.3 mg/L). Online Supplementary Table S4 shows median concentration of uninvolved Igs and FLCs by CA type for three MM isotypes. Practically all uninvolved IgG, IgA and IgM were below the reference values, indicating immunoparesis.14 On the contrary, FLCκ level was above and FLCλ level was within the reference values. For IgG MM, t(11;14) positive cases had suppressed IgA and IgM levels (P=0.03; β=−0.14 and P=0.02; β=−0.13, respectively). Differences in FLCλ levels were observed for gain 1q21 in IgG MM (P=0.01; β=0.20), thus explaining the above noticed differences in rFLC for this CA (Table 2). For IgA MM, IgG levels were significantly suppressed in cases positive for del(13q) or gain 1q21 (P=0.03; β=−0.11 and P=3.7×10−3; β=−0.15, respectively), but significantly elevated in hyperdiploidy cases (P=0.01; β=0.13). Cases with gain 1q21 had IgM levels suppressed (P=0.01; β=−0.19) while cases with del(13q) had FLCλ levels suppressed (P=0.04; β=−0.34). For LCO MM, gain 1q21 positive cases had all the Igs levels significantly suppressed. Hyperdiploidy cases had IgA levels elevated (P=0.04; β=0.21). Cases with t(11;14) had FLCk levels suppressed (P=0.02; β=−0.40) while cases with hyperdiploidy or del(17p) had elevated FLCk levels (P=0.05; β=0.36 and P=0.01; β=0.78, respectively).
One main finding of this study was that two CAs showed significant associations with the involved Ig isotypes in both cohorts, hyperdiploidy with IgG MM, and t(4;14) with IgA MM. An association was also found for t(11;14) with LCO MM but it did not reach statistical significance in the replication cohort (P=0.09), possibly due to a small sample size. In all these cases, CA positivity contributed to higher proportions of the indicated isotypes. What consequences the detected shifts might have remains speculative. t(4;14) positivity was not only related to the increased proportion of IgA isotype but also showed a significant increase in the M-protein level (45.3 g/L vs. 28.8 g/L) which was the highest measured median value for this isotype. Whether such high concentration might contribute to the poor prognosis in t(4;14) still has to be verified. Moreover, future studies should focus on comparing the clinical outcomes of non-IgA and IgA t(4;14) patients.
In a previous study, higher rFLC was found in patients with t(14;16) or del(13).5 We identified a significant suppression of rFLC in cases with gain 1q21 for IgG MM. M-protein levels in IgG MM were higher compared with those in IgA MM cases. This might be expected, as IgG is the principal isotype in the blood and extracellular fluid whereas IgA is the principal isotype in secretions of mucus epithelium of the intestinal and respiratory tracts. The observation that M-protein levels were higher in any IgH translocation group compared with the hyperdiploidy group can possibly be attributed to the active production of M-protein in actively proliferating plasma cells driven by the translocated cyclin D genes.15 Patients lacking hyperdiploidy had higher M-protein level compared with hyperdiploidy patients, although this difference was only statistically significant for IgA MM.
In summary, our study provides strong evidence for a complex modifying role of at least two CA types on immunophenotype. We also showed that serum Ig and FLC levels were influenced by several CA types. Therefore, our study sheds new light on the role of CA as an important component of MM pathogenesis, influencing immunoglobulin isotypes and the serum Ig and FLC levels.
Supplementary Material
Footnotes
Funding: this project was supported by the German Cancer Aid, the Harald Huppert Foundations, The German Federal Ministry of Education and Research (eMed, Cliommics 01ZX1309B) and the Multiple Myeloma Research Foundation.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: A report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manier S, Salem KZ, Park J, Landau DA, Getz G, Ghobrial IM. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2016;14(2):100–113. [DOI] [PubMed] [Google Scholar]
- 4.Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015;5(10):e365–e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Zhang L, Dispenzieri A, et al. Relationship between elevated immunoglobulin free light chain and the presence of IgH translocations in multiple myeloma. Leukemia. 2010;24(8):1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig H, Milosavljevic D, Berlanga O, et al. Suppression of the noninvolved pair of the myeloma isotype correlates with poor survival in newly diagnosed and relapsed/refractory patients with myeloma. Am J Hematol. 2016;91(3):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradwell A, Harding S, Fourrier N, et al. Prognostic utility of intact immunoglobulin Ig′κ/Ig′λ ratios in multiple myeloma patients. Leukemia. 2013;27(1):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michallet M, Chapuis-Cellier C, Dejoie T, et al. Heavy+light chain monitoring correlates with clinical outcome in multiple myeloma patients. Leukemia. 2017. June 30 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merz M, Salwender H, Haenel M, et al. Subcutaneous versus intravenous bortezomib in two different induction therapies for newly diagnosed multiple myeloma: An interim analysis from the prospective GMMG-MM5 trial. Haematologica. 2015;100(7):964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mai EK, Bertsch U, Dürig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015;29(8):1721–1729. [DOI] [PubMed] [Google Scholar]
- 11.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–948. [DOI] [PubMed] [Google Scholar]
- 12.Goldschmidt H, Lokhorst HM, Mai EK, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2017. July 4 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Bochtler T, Hegenbart U, Heiss C, et al. Evaluation of the serum-free light chain test in untreated patients with AL amyloidosis. Haematologica. 2008;93(3):459–462. [DOI] [PubMed] [Google Scholar]
- 14.González-Calle V, Cerdá S, Labrador J, et al. Recovery of polyclonal immunoglobulins one year after autologous stem cell transplantation as a long-term predictor marker of progression and survival in multiple myeloma. Haematologica. 2017;102(5):922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hose D, Rème T, Hielscher T, et al. Proliferation is a central independent prognostic factor and target for personalized and risk-adapted treatment in multiple myeloma. Haematologica. 2011;96(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


