Abstract
Background:
The role of desmopressin (DDAVP) to prevent or treat rapid serum sodium concentration ([Na]s) correction during hyponatremia management remains unclear.
Objective:
To assess DDAVP use during the first 48 hours of severe, hypovolemic hyponatremia management. The primary study hypothesis was that the use of DDAVP would slow the rate of [Na]s correction compared with those not receiving DDAVP.
Design:
A retrospective, observational, comparison study.
Setting:
A single, Canadian, tertiary center.
Patients:
All admitted patients referred to the nephrology service for severe, hypovolemic hyponatremia ([Na]s < 125 mmol/L) over a 12-month period from November 2015.
Measurements:
The primary outcomes measure was the [Na]s after medical management for 48 hours. The length of hospital stay was also measured.
Methods:
Patients were grouped based on whether they received DDAVP during the first 48 hours of treatment, and [Na]s correction was compared between groups using linear regression. An exploratory, multivariable, linear regression model was used to adjust for diabetes status, active malignancy, intensive care unit (ICU) admission, and hypertonic saline administration.
Results:
Twenty-eight patients were identified, with baseline mean [Na]s of 112.7 ± 6.6 mmol/L versus 117 ± 4.3mmol/L (P = .06) in those receiving (n = 16) and not receiving DDAVP (n = 12), respectively. The DDAVP group had a more rapid [Na]s correction on the first day compared with those not receiving DDAVP, 7.7 ± 3.8 mmol/L/d versus 5.1 ± 2.0 mmol/L/d (P = .04). On the second day, there was a similar rate of [Na]s correction between groups: 1.3 ± 4.3 mmol/L/d versus 2.6 ± 3.2 mmol/L/d (P = .39), respectively. Overall, there was no difference in [Na]s correction after 48 hours between those who received DDAVP and those who did not: 121.7 ± 7.5 mmol/L versus 124.8 ± 5.7 mmol/L (P = .24). Patients who had experienced an overcorrection were successfully treated with DDAVP (n = 5), so that no patient had an ongoing overcorrection by 48 hours.
Limitations:
The limited sample size and lack of randomization preclude definitive conclusion on the additional benefit of DDAVP to standard care.
Conclusion:
DDAVP appears to be safe and effective in the management of severe, hypovolemic hyponatremia, associated with similar [Na]s correction to those who did not receive DDAVP after 48 hours, despite an initial more rapid correction. A randomized trial should examine what benefit DDAVP confers in addition to standard care in the management of severe, hypovolemic hyponatremia.
Keywords: hyponatremia, desmopressin, antidiuretic hormone, DDAVP, overcorrection
Abrégé
Contexte:
Dans le contexte du traitement de l’hyponatrémie, le rôle de la desmopressine (DDAVP) pour prévenir et contrer la correction rapide de la concentration de sodium sérique ([Na] s) demeure nébuleux.
Objectif de l’étude:
Notre objectif était d’étudier l’effet de l’administration de DDAVP au cours des 48 premières heures du traitement de l’hyponatrémie hypovolémique grave. L’hypothèse principale de l’étude était que l’administration de DDAVP ralentit le rythme de correction de la [Na] s chez les patients traités en comparaison des parents non traités.
Cadre et type d’étude:
Il s’agit d’une étude comparative observationnelle, menée de façon rétrospective dans un centre de soins tertiaires canadien.
Patients:
Ont été inclus tous les patients admis et ayant été dirigés vers l’unité de néphrologie en raison d’une hyponatrémie hypovolémique grave ([Na] s inférieure à 125 mmol/l) sur une période de douze mois à partir de novembre 2015.
Mesures:
La [Na] s après les 48 premières heures de traitement constituait la mesure principale. On a également noté la durée de l’hospitalisation des patients.
Méthodologie:
Les patients ont été regroupés selon qu’ils avaient reçu ou non de la DDAVP dans les 48 premières heures de traitement, et la comparaison de la correction de la [Na] s entre les groupes a été analysée par régression linéaire. Un modèle de régression linéaire multivariée et exploratoire a été utilisé pour ajuster les résultats en tenant compte de la présence de diabète, d’une affection maligne active, d’une admission à l’unité des soins intensifs et d’une administration de solution saline hypertonique.
Résultats:
Un total de 28 patients a été retenu pour l’étude. La [Na] s initiale moyenne était de 112,7 ± 6,6 mmol/l pour les patients ayant reçu de la DDAVP (n = 16) contre 117 ± 4,3 mmol/l pour les douze patients du groupe non traité (P = 0,06). Dans le groupe traité, la correction de la [Na] s a été plus rapide au cours de la première journée comparativement à celle mesurée chez les patients non traités (7,7 ± 3,8 mmol/l/jour contre 5,1 ± 2,0 mmol/l/jour; P = 0,04). Le taux de correction de la [Na] s s’est avéré similaire dans les deux groupes au cours de la deuxième journée de suivi, avec des valeurs de 1,3 ± 4,3 mmol/l/jour pour le groupe ayant reçu de la DDAVP, et de 2,6 ± 3,2 mmol/l/jour pour le groupe non traité. Dans l’ensemble, aucune différence de correction de la [Na] s n’a été observée entre les deux groupes après 48 heures de suivi. Les valeurs se situaient alors à 121,7 ± 7,5 mmol/l pour les patients ayant reçu de la DDAVP et à 124,8 ± 5,7 mmol/l pour les patients non traités (P = 0,24). Cinq patients ont expérimenté une surcorrection et ont dû être traités à la DDAVP; le traitement a bien fonctionné, de sorte qu’aucun patient ne présentait de surcorrection après 48 heures.
Limites de l’étude:
La taille restreinte de l’échantillon et l’absence de répartition aléatoire des cas nous empêchent de tirer une conclusion définitive quant à l’avantage supplémentaire apporté par l’ajout de la DDAVP aux procédures de soins courantes.
Conclusion:
La DDAVP semble efficace et sécuritaire pour traiter l’hyponatrémie hypovolémique grave. Elle est associée à une correction similaire à celle mesurée après 48 heures chez les patients non traités, quoiqu’elle entraîne une correction initiale de la [Na] s plus rapide. Nous sommes d’avis qu’un essai clinique à répartition aléatoire devrait être mené pour étudier les bienfaits offerts par l’ajout de la DDAVP aux procédures de soins courantes contre l’hyponatrémie hypovolémique grave.
What was known before
The role of DDAVP in the management of severe hyponatremia is unclear, and what benefit it may infer in addition to standard management is unknown. There are no current existing data on the use of DDAVP in a cohort of patients with severe hyponatremia that is attributable to a hypovolemic etiology.
What this adds
This study identifies a practice pattern of administration of intravenous DDAVP to more severe cases of hypovolemic hyponatremia, those with a more rapidly correcting serum sodium concentration [Na]s in the initial phase of management and those who have overcorrected the [Na]s. DDAVP use appears to be safe and is associated with a similar degree of [Na]s correction after 48 hours to those patients who did not receive DDAVP.
Introduction
Severe hyponatremia, defined as a serum sodium concentration ([Na]s) <125 mmol/L or hyponatremia with acute neurological symptoms, is associated with significant morbidity and mortality, particularly in the setting of preexisting cardiac, renal, or liver disease.1-3 Based on the chronicity of onset, clinical volume assessment, and other biochemistry, an appropriate treatment plan can be devised. For patients with true hypovolemic hyponatremia, this will usually involve administration of isotonic intravenous fluid to correct hypovolemia, with restriction of free water intake and frequent observation of the trend in the [Na]s correction. Measurement of the hourly urine volume and the tonicity of urine allows identification of an excessive aquaresis that may occur when the hypovolemia-mediated pituitary hormone 8-arginine vasopressin (ADH) response has been abolished. It is often an unanticipated aquaresis, rather than the volume of isotonic fluid infused, that will result in erroneous predictions of the rate of rise of [Na]s, leading to overcorrection.4
Desmopressin acetate (DDAVP), a synthetic analogue of the ADH, can control aquaresis through its action on the V2-receptors in the renal collecting system. There is limited evidence in the literature supporting its use in hyponatremia management, and current clinical guidelines do not advocate its use.5,6 Observational studies reporting on the use of intravenous DDAVP in hyponatremia are limited to case reports and small, uncontrolled case series with heterogeneous study participants in terms of hyponatremia etiology.7-13 DDAVP is prescribed for a number of specific indications such as central diabetes insipidus, bleeding disorders, and enuresis.14-16 While its efficacy has been demonstrated in these conditions, DDAVP-induced severe hyponatremia, with fatal outcomes in some cases, has been reported.17,18 Therefore, practitioners who wish to use DDAVP in the management of hyponatremia must become familiar with its parenteral administration to prevent drug safety concerns.
The aim of this study was to compare the rate of [Na]s correction in patients with severe, hypovolemic hyponatremia who received DDAVP with patients who did not receive DDAVP, which has not previously been reported. The primary study hypothesis was that DDAVP would significantly slow the rate of [Na]s correction achieved after the initial 48 hours.
Methods
Patient Selection, Data Collection, and Outcomes Measures
All inpatient nephrology referrals for hyponatremia during a 12-month period from November 2015 were retrospectively identified from an electronic record. Patients must have been referred within 24 hours of diagnosis of hyponatremia. A minimum of 24 hours of follow-up after referral was required. To obtain a homogenous cohort in terms of hyponatremia etiology, we selected cases of severe, hypovolemic hyponatremia. Inclusion criteria were [Na]s <125 mmol/L at referral, serum osmolality <275 mOsm/kg, urine sodium <30 mmol/L, and urine osmolality >100 mOsm/kg. Patients with signs of extracellular fluid (ECF) compartment overload were excluded. Patients with a history of chronic hyponatremia were excluded. The administration of intravenous DDAVP and the indication for DDAVP were recorded, including the number of doses and cumulative dose. The use of hypertonic 3% saline infusion was recorded. The initial [Na]s at the time of diagnosis was denoted as [Na]s0, the [Na]s after 24 hours was denoted as [Na]s24, and the [Na]s after 48 hours was denoted as [Na]s48. These results were taken as the closest laboratory result within 2 hours of the observation point if an exact time-point was not available. If no data point was available within 2 hours of the observation point, then a time-averaged estimate of the [Na]s was calculated from the closest available [Na]s straddling the relevant time-point.
Patient demographic and clinical data were gathered from an electronic medical record, including age, gender, intensive care unit (ICU) admission, diabetes status, presence of peripheral vascular disease, coronary artery disease, cerebrovascular disease, an active malignant cancer diagnosis, use of a thiazide diuretic and selective serotonin reuptake inhibitor, and the presence of acute kidney injury (AKI) or chronic kidney disease (CKD). AKI was defined by the Kidney Disease: Improving Global Outcomes (KDGIO) criteria and CKD as a Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 on 2 consecutive readings at least 3 months apart.19,20 The Acute Physiology and Chronic Health Evaluation II (APACHE-II) score was calculated for patients requiring ICU admission.21 The chronicity of the hyponatremia and whether there was any abnormal neurology at presentation were recorded.
The primary outcome measures were the serial 24-hour and overall 48-hour rate of [Na]s correction. For patients who were acutely hospitalized with hyponatremia as the primary or co-primary diagnosis, the hospital length of stay from admission and survival to discharge were assessed. The primary and secondary outcome measures were compared between patients who received DDAVP and those who did not. Overcorrection in [Na]s was defined as a rise of >10 mmol/L in a 24-hour period and/or >18 mmol/L in a 48-hour period.
Statistical Analysis
Baseline demographic and clinical data were compared between those who received DDAVP and those who did not. Categorical data were presented as proportions and compared using the Fisher exact test. Continuous data were presented as means (±standard deviation) and compared using the Student t test. Unadjusted linear regression was used to examine any difference in the rate of change of [Na]s over the first 48 hours of management and any significant association of length of hospital stay with DDAVP use. As an exploratory analysis, due to the small sample size, a multivariable linear regression was also performed, with adjustment for diabetes, active malignancy, ICU admission, and hypertonic saline administration. A chi-square test was used to assess the association of DDAVP use with survival to discharge. An alpha level of 0.05 was statistically significant for all tests. The study was approved by the local research ethics board.
Results
Patient Characteristics
There were 139 patients referred for hyponatremia in the study period. Of these, 111 cases were excluded for the syndrome of inappropriate ADH secretion (n = 52), ECF expansion (n = 21), delayed referral (n = 5), not meeting the inclusion biochemical criteria (n = 21), known chronic hyponatremia (n = 2), follow-up duration <24 hours (n = 1), or being incorrectly identified as having hyponatremia (n = 2). The remaining 28 eligible patients were grouped into those who received DDAVP (n = 16) and those who did not (n = 12). Baseline data for eligible patients were compared by DDAVP exposure (Table 1). Those who received DDAVP had a clinically significant lower [Na]s0 (112.7 ± 6.6 mmol/L vs 117 ± 4.3 mmol/L, P = .06), more symptomatic hyponatremia (62.5% vs 33.3%, P = .25), a higher ICU admission rate (68.7% vs 33.3%, P = .12), and more AKI (31.3% vs 8.3%, P = .19) compared with those who did not receive DDAVP. For ICU admissions, the APACHE-II scores were similar between those who received DDAVP and those who did not (15.6 ± 4.9 vs 12.8 ± 6.1, P = .36). There was a higher proportion of active malignancy cases in the group who did not receive DDAVP (66.6% vs 31.3%, P = .05).
Table 1.
Baseline Patient Demographic and Clinical Characteristics of Patients Referred With Severe, Hypovolemic, Hypo-osmolar Hyponatremia, Stratified by DDAVP Use.
| DDAVP (n = 16) | No DDAVP (n = 12) | P value | |
|---|---|---|---|
| Mean age (years) | 63.75 (±17.8) | 65.4 (±9.2) | .77 |
| Female gender (%) | 8 (50) | 6 (50) | 1.0 |
| Initial serum sodium (mmol/L) | 112.7 (6.6) | 117 (4.3) | .06 |
| Symptomatic hyponatremia (%) | 10 (62.5) | 4 (33.3) | .25 |
| Onset of hyponatremia >48 h (%) | 15 (93.8) | 12 (100) | 1.0 |
| ICU admission (%) | 11 (68.7) | 4 (33.3) | .12 |
| APACHE-II scorea | 15.6 (4.9) | 12.8 (6.1) | .36 |
| Diabetes (%) | 5 (31.3) | 2 (16.6) | .66 |
| CAD (%) | 2 (12.5) | 2 (16.6) | 1.0 |
| PVD (%) | 1 (6.3) | 1 (8.3) | 1.0 |
| Thiazide diuretic (%) | 3 (18.8) | 0 | .23 |
| Active malignancy (%) | 5 (31.3) | 9 (66.6) | .05 |
| AKI (%) | 5 (31.3) | 1 (8.3) | .19 |
| CKD (%) | 1 (6.3) | 1 (8.3) | 1.0 |
| Hypertonic saline use | 2 (12.5) | 3 (25) | .62 |
Note. Continuous data are expressed as mean, with standard deviation in parenthesis. DDAVP = desmopressin; ICU = intensive care unit; APACHE-II = Acute Physiology and Chronic Health Evaluation II; CAD = coronary artery disease; PVD = peripheral vascular disease; AKI = acute kidney injury; CKD = chronic kidney disease.
Only calculated for patients admitted to ICU.
Trend in [Na]s
Serial [Na]s during the first 48-hour period of management are displayed in Figure 1. The mean [Na]s correction on the first day after diagnosis was higher in the DDAVP group than in the comparison group: 7.7 ± 3.8 mmol/L/d versus 5.1 ± 2.0 mmol/L/d, respectively (P = .04). The mean [Na]s correction in the second day after diagnosis was then similar between the groups: 1.3 ± 4.3 mmol/L/d versus 2.6 ± 3.2 mmol/L/d, respectively (P = .39). The [Na]s24 and [Na]s48 were similar between the DDAVP group and the comparison group: 120.4 ± 6.9 mmol/L versus 122.2 ± 4.9mmol/L (P = .43) and 121.7 ± 7.5 mmol/L versus 124.8 ± 5.7 mmol/L (P = .24), respectively. Within the DDAVP group, the [Na]s correction rate declined from 7.7 ± 3.8 mmol/L/d on the first day to 1.3 ± 4.3 mmol/L/d on the second day (P = .001). Within the group comparison group, a similar magnitude of decline in the rate of [Na]s correction from the first day to the second day was not evident: 5.1 ± 2.0 mmol/L/d versus 2.6 ± 3.2 mmol/L/d (P = .07), respectively (Figure 2). Unadjusted linear regression analysis demonstrated that [Na]s correction on the first day tended to be more rapid in those who received DDAVP compared with those who did not (r2 = 0.14, P = .04), but that the rate of correction became similar on the second day (r2 = 0.03, P = .39). The rate of [Na]s correction was similar in the overall study period in both groups (r2 = 0.02, P = .44). These results did not change significantly following the exploratory adjusted regression analysis (Table 2).
Figure 1.
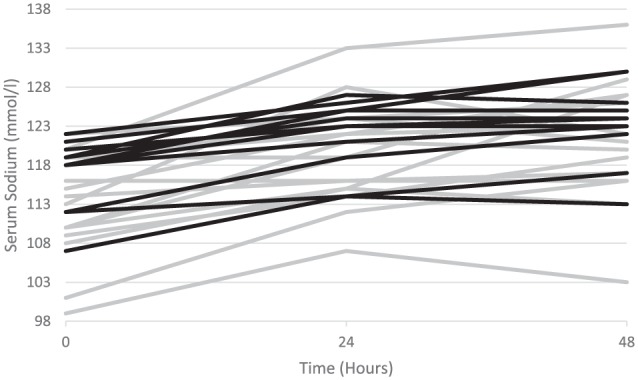
Serial serum sodium levels in patients who received DDAVP (gray) and those who did not receive DDAVP (black) at baseline, 24 hours after initiation of treatment, and 48 hours after initiation of treatment.
Note. DDAVP = desmopressin.
Figure 2.
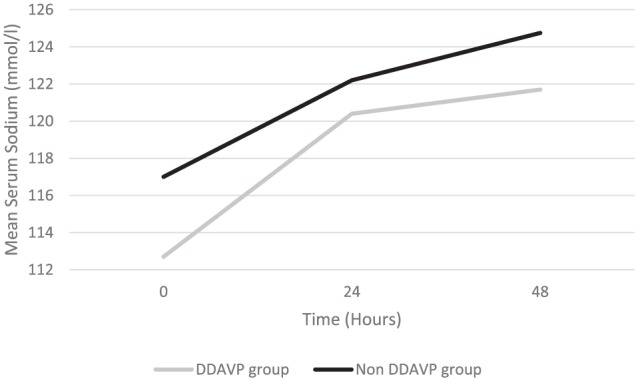
Comparison of the trend in correction of the mean serum sodium concentration over 48 hours of treatment between patients who received DDAVP and those who did not receive DDAVP.
Note. DDAVP = desmopressin.
Table 2.
Univariate and Multivariate Linear Regression Models Showing the Association Between the Serum Sodium Correction Over Time and the Use of DDAVP.
| Mean [Na]s correction (mmol/L/d) |
Unadjusted |
Adjusteda |
||||
|---|---|---|---|---|---|---|
| DDAVP | No DDAVP | R 2 | P value | R 2 | P value | |
| First day | 7.7 ± 3.8 | 5.1 ± 2.0 | 0.14 | .04 | 0.37 | .05 |
| Second day | 1.3 ± 4.3 | 2.6 ± 3.2 | 0.03 | .39 | 0.11 | .73 |
| Overall 48-h period (per day) | 4.5 ± 2.5 | 3.9 ± 1.4 | 0.02 | .44 | 0.09 | .81 |
Note. Mean values are presented with standard deviation. [Na]s = serum sodium concentration; DDAVP = desmopressin; ICU = intensive care unit.
Adjusted for diabetes status, ICU admission, active malignancy, and administration of 3% hypertonic saline.
DDAVP Dosing
A single dose of DDAVP was administered to 43.8% (n = 7) of patients in the DDAVP group. The remainder received either 2 doses (n = 5), 3 doses (n = 1), 5 doses (n = 1), or 6 doses (n = 2). The mean cumulative dose per patient was 9.0 ± 7.0 µg. The [Na]s48 for patients who received a single dose of DDAVP was similar to those who received multiple doses: 123.8 ± 5.8 mmol/L versus 120 ± 8.5 mmol/L, P = .32. By univariate linear regression analysis, there was no significant association between the [Na]s48 and the cumulative dose of DDAVP administered (r2 = 0.0002, P = .95). Also, those patients who received multiple doses did not appear to have a higher likelihood of having a lowering in [Na]s during the second day after diagnosis (odds ratio [OR] = 1.24, 95% confidence interval [CI]: 0.14-10.7, P = .83) compared with those who received a single dose.
DDAVP Treatment Approach
For patients who received DDAVP (n = 16), the indications were “prophylactic” to avoid too rapid [Na]s correction at the outset of treatment (n = 5), “reactive” to avoid [Na]s overcorrection during treatment (n = 9) or, last, “rescue” use to reverse an overcorrection in hyponatremia (n = 2), done in conjunction with the infusion of hypotonic solution in both cases (5% dextrose in water). Administration of DDAVP was not associated with a higher likelihood of having a lowering in [Na]s during either the second day compared with those not receiving DDAVP: 31.3% versus 16.6% (P = .66), respectively. For those patients who did experience a lowering of [Na]s, the mean magnitude of the lowering tended to be greater in those who received DDAVP compared with those who did not: 3.2 ± 1.9 mmol/L/d versus 1.0 ± 0mmol/L/d, P = .66.
The trend in [Na]s for patients who received DDAVP either prophylactically or in a reactive manner are displayed in Figure 3. Administration of DDAVP in a prophylactic manner, compared with a reactive manner, was not associated with baseline factors such as mean [Na]s0 (P = .75), diabetes status (P = 1.0), ICU admission (P = .58), the presence of AKI (P = 1.0), or symptomatic hyponatremia (P = 1.0). The respective groups received a similar mean number of doses and mean cumulative dose: 2.4 ± 2.1 doses versus 2.4 ± 1.9 doses (P = .96) and 9.6 ± 8.3 µg versus 9.3 ± 7.4 µg (P = .2). Patients who received DDAVP prophylactically had a trend toward a lower mean [Na]s24 than those who received DDAVP in a reactive manner, 116.8 ± 6.7 versus 120.7 ± 6.2, P = .28, respectively, with a modest and similar [Na]s correction in both groups in the second day after diagnosis, 1.4 ± 6.3 mmol/L/d versus 0.66 ± 3.4 mmol/L/d (P = .77), respectively. Overall in the study period, prophylactic DDAVP was associated with a trend toward a lower mean [Na]s correction compared with those who received reactive DDAVP: 5.4 ± 6.8 mmol/L versus 9.8 ± 2.7 mmol/L, respectively (r2 = 0.2, P = .1).
Figure 3.
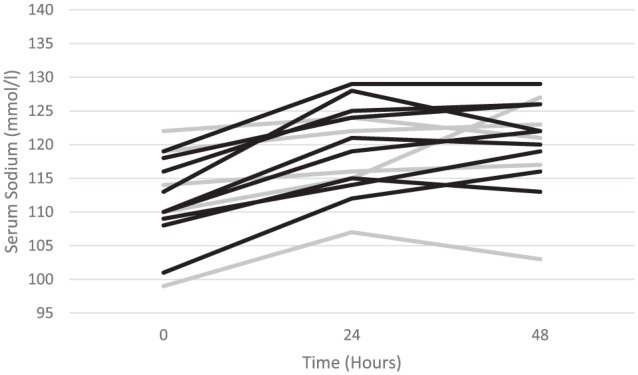
Serial serum sodium concentration in those receiving DDAVP in a prophylactic manner (gray) or in a reactive manner to prevent an anticipated overcorrection in serum sodium once treatment was underway (black).
Note. DDAVP = desmopressin.
[Na]s Overcorrection
There were no episodes of [Na]s overcorrection in patients who did not receive DDAVP. In the DDAVP group, an overcorrection occurred on the first day in 5 patients, with DDAVP being administered in 2 of these cases only after the overcorrection had occurred. For these 5 patients, the rate of [Na]s correction fell significantly from the first day to the second day, 11.4 ± 3.0 mmol/L/d versus 1.0 ± 4.5 mmol/L/d, P = .03, respectively. All patients with an initial overcorrection were within the recommended level for correction by 48 hours. No cases of seizure, osmotic demyelination, or other de novo neurological sequelae were diagnosed in either group during treatment.
Secondary Outcomes
There was a longer mean length of hospital stay in the DDAVP group compared with those not receiving DDAVP: 17.8 ± 21.9 days versus 10.3 ± 6.3 days (r2 = 0.04, P = .28). The mean length of hospital stay for patients who received DDAVP prophylactically tended to be shorter than in those who received DDAVP in a reactive manner: 11.4 ± 6.3 days versus 17.6 ± 22.3 days (P = .58). Survival to hospital discharge was similar in both the DDAVP group and the comparison group: 86.6% versus 90.9% (χ2 = 0.12, P = .73), respectively.
Discussion
This study is the first to compare the clinical characteristics and outcomes of contemporaneous patients managed in a single center for severe hypovolemic hyponatremia based on DDAVP exposure. DDAVP was effective in controlling the [Na]s correction in patients with severe hypovolemic hyponatremia. Patients who received DDAVP had a significantly more rapid trajectory of [Na]s correction on the first day after diagnosis, which was the likely reason for DDAVP administration. The [Na]s correction on the second day was effectively slowed, achieving a similar overall magnitude of correction as the comparison group. All patients who had experienced an overcorrection on the first day after diagnosis were successfully treated with DDAVP, which significantly lowered the rate of [Na]s correction so that no patient had an overcorrection at 48 hours. There was a trend toward a longer length of hospital stay for the group who received DDAVP, which can likely be attributed to increased medical complexity in those patients consistent with their lower baseline [Na]s and higher proportion of ICU admissions, diabetes, and AKI. Within the DDAVP-exposed patients, those treated in a prophylactic manner tended to have a shorter length of hospital stay than those treated in a reactive manner.
Few previous studies have focused on DDAVP use in hyponatremia treatment. Perianayagam et al described the effective use of DDAVP in reversal of established [Na]s overcorrection or to prevent an anticipated [Na]s overcorrection in 20 patients who received DDAVP over a 6-year period in one center, with all but one patient being able to avoid an overcorrection at 48 hours.11 Rafat et al successfully corrected an excessive initial [Na]s correction rate in 20 ICU-admitted patients by using intravenous DDAVP, with a lowering of [Na]s in 11 patients, and reduced [Na]s variability.12 Although informative, these studies are small and uncontrolled, and cannot draw conclusions on how DDAVP compares with standard management. Our results demonstrate that DDAVP use appeared to have been appropriately directed to those at risk of overcorrection, or those who had already overcorrected, and the overall results achieved within 48 hours were then similar to the comparison group.
Sood et al have described a novel approach to severe hyponatremia management, using a regimen of regular intravenous DDAVP administration every 6 to 8 hours, while infusing hypertonic saline based on the patient’s weight.13 The case series of 25 patients demonstrated reliable and consistent [Na]s correction over 48 hours, without any overcorrections or episodes of lowering of [Na]s. Unfortunately, there was no comparison group to demonstrate whether this approach was superior to more standard practice. The rationale was to preempt the abolition of the intrinsic ADH stimulus that occurs during successful treatment, so that the exogenous DDAVP will then counteract any unpredictable aquaresis that could lead to an overcorrection in [Na]s. Although the results appear impressive, this approach could be viewed as nonphysiological, first administering DDAVP to patients already in a high ADH state at presentation, and second, ignoring one of the fundamental principle in treatment of hypo-osmolar hyponatremia that the patient must achieve a net negative in electrolyte-poor water to achieve a lasting [Na]s correction. Also, prolonged DDAVP administration could compound or worsen hyponatremia if there is not simultaneous close monitoring and restriction of free water intake.
The optimal strategy for DDAVP use to prevent rapid sodium correction in severe hyponatremia remains unknown.22 Our data, acknowledging the limitations of the small sample size, did not suggest any significant baseline differences in those who received either a prophylactic or a reactive approach, and it is probable that the decision was at the discretion of the treating physician. There was a nonsignificant trend toward a lower [Na]s48 correction in the prophylactic group compared with the reactive group. Interestingly, whether patients received a single DDAVP dose or multiple doses did not affect the [Na]s48, nor did the cumulative dose of DDAVP given. One possible explanation is that additional DDAVP was being administered at times when there was no excessive aquaresis. These additional doses may have been a precautionary measure to prevent the emergence of aquaresis, for example, overnight when on-call physicians may not have been familiar with DDAVP administration or if the clinical environment precluded reliable hourly urine output monitoring (eg, a patient in the emergency department), making real-time dosing of DDAVP impractical. It could also suggest that a single dose of DDAVP is all that is required in this setting.
Strengths of the current study include that presence of a contemporaneous comparison group who did not receive DDAVP and the homogenous etiology of hyponatremia in both groups. The study is limited primarily by its sample size and study design. First, patients were not randomized to receive DDAVP or not, in addition to there not being a standardized care plan for the approach to monitoring and treating cases of hypovolemic hyponatremia (which is not unique to this center). Second, owing to treatment bias in the prescription of DDAVP, there were clinically significant differences in the patient group who received DDAVP, such as a higher proportion of ICU admissions, a higher rate of AKI, and more patients with diabetes. In relation to ICU admission, it should be borne in mind that many patients with severe hyponatremia are often admitted to ICU as a precautionary step to allow close observation, and not because they are requiring hemodynamic or ventilatory support. Hence, an ICU admission in this case is not necessarily the usual surrogate for extreme acute illness.
Conclusion
The use of DDAVP in the management of severe, hypovolemic hyponatremia appears to be safe and effective when compared with similar patients who do not receive DDAVP, with no evidence of a delay in [Na]s correction, prolonged hospital length of stay, or reduced survival to hospital discharge. DDAVP appears to have a useful role in managing hyponatremia where the rate of correction is more rapid than desired, or in those who have already overcorrected. Randomized controlled trials are warranted to investigate the role of DDAVP in the management of severe, hypovolemic hyponatremia. Such trials should focus on identifying whether there is additional benefit to be gained by 1 or more doses of DDAVP compared with standard practice and, if so, whether a prophylactic, reactive, or rescue approach to DDAVP administration is optimal.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval was granted by the Sunnybrook Healthcare Sciences Centre Research Ethics Board.
Consent for Publication: Consent for publication was obtained from all authors.
Availability of Data and Materials: Not available
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Holland-Bill L, Christiansen CF, Heide-Jørgensen U, et al. Hyponatremia and mortality risk: a Danish cohort study of 279 508 acutely hospitalized patients. Eur J Endocrinol. 2015;173(1):71-81. [DOI] [PubMed] [Google Scholar]
- 2. Whelan B, Bennett K, O’Riordan D, Silke B. Serum sodium as a risk factor for in-hospital mortality in acute unselected general medical patients. QJM. 2009;102:175-182. [DOI] [PubMed] [Google Scholar]
- 3. Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170:294-302. [DOI] [PubMed] [Google Scholar]
- 4. Tzamaloukas AH, Malhotra D, Rosen BH, Raj DS, Murata GH, Shapiro JI. Principles of management of severe hyponatremia. J Am Heart Assoc. 2013;2:e005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant. 2014;29(suppl 2):i1-i39. [DOI] [PubMed] [Google Scholar]
- 6. Nagler EV, Vanmassenhove J, van der Veer SN, et al. Diagnosis and treatment of hyponatremia: a systematic review of clinical practice guidelines and consensus statements. BMC Med. 2014;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldszmidt MA, Iliescu EA. DDAVP to prevent rapid correction in hyponatremia. Clin Nephrol. 2000;53(3):226-229. [PubMed] [Google Scholar]
- 8. Soupart A, Ngassa M, Decaux G. Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin Nephrol. 1999;51(6):383-386. [PubMed] [Google Scholar]
- 9. Tomlin SC, Williams R, Riley S. Preventing overcorrection of hyponatraemia with desmopressin. BMJ Case Rep. 2011;2011:bcr0720114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sterns RH, Hix JK, Silver S. Treating profound hyponatremia: a strategy for controlled correction. Am J Kidney Dis. 2010;56(4):774-779. [DOI] [PubMed] [Google Scholar]
- 11. Perianayagam A, Sterns RH, Silver SM, et al. DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol. 2008;3(2):331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rafat C, Schortgen F, Gaudry S, et al. Use of desmopressin acetate in severe hyponatremia in the intensive care unit. Clin J Am Soc Nephrol. 2014;9(2):229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sood L, Sterns RH, Hix JK, Silver SM, Chen L. Hypertonic saline and desmopressin: a simple strategy for safe correction of severe hyponatremia. Am J Kidney Dis. 2013;61(4):571-578. [DOI] [PubMed] [Google Scholar]
- 14. Cunnah D, Ross G, Besser GM. Management of cranial diabetes insipidus with oral desmopressin (DDAVP). Clin Endocrinol (Oxf). 1986;24(3):253-257. [DOI] [PubMed] [Google Scholar]
- 15. Federici AB. The use of desmopressin in von Willebrand disease: the experience of the first 30 years (1977-2007). Haemophilia. 2008;14(suppl 1):5-14. [DOI] [PubMed] [Google Scholar]
- 16. Vande Walle J, Rittig S, Bauer S, Eggert P, Marschall-Kehrel D, Tekgul S. Practical consensus guidelines for the management of enuresis. Eur J Pediatr. 2012;171:971-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Achinger SG, Arieff AI, Kalantar-Zadeh K, Ayus JC. Desmopressin acetate (DDAVP)-associated hyponatremia and brain damage: a case series. Nephrol Dial Transplant. 2014;29(12):2310-2315. [DOI] [PubMed] [Google Scholar]
- 18. Shindel A, Tobin G, Klutke C. Hyponatremia associated with desmopressin for the treatment of nocturnal polyuria. Urology. 2002;60(2):344. [DOI] [PubMed] [Google Scholar]
- 19. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl. 2012;2:1-138. [Google Scholar]
- 20. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl. 2013;3:1-150. [Google Scholar]
- 21. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. [PubMed] [Google Scholar]
- 22. MacMillan TE, Tang T, Cavalcanti RB. Desmopressin to prevent rapid sodium correction in severe hyponatremia: a systematic review. Am J Med. 2015;128(12):1362.e15-e24. [DOI] [PubMed] [Google Scholar]


