Abstract
Background:
Healthcare-acquired hypernatremia (serum sodium >145 mEq/dL) is common among critically ill and other hospitalized patients and is usually treated with hypotonic fluid and/or diuretics to correct a “free water deficit.” However, many hypernatremic patients are eu- or hypervolemic, and an evolving body of literature emphasizes the importance of rapidly returning critically ill patients to a neutral fluid balance after resuscitation.
Objective:
We searched for any randomized- or observational-controlled studies evaluating the impact of active interventions intended to correct hypernatremia to eunatremia on any outcome in volume-resuscitated patients with shock and/or sepsis.
Data sources:
We performed a systematic literature search with studies identified by searching MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, ClinicalTrials.gov, Index-Catalogue of the Library of the Surgeon General’s Office, DARE (Database of Reviews of Effects), and CINAHL and scanning reference lists of relevant articles with abstracts published in English.
Data synthesis:
We found no randomized- or observational-controlled trials measuring the impact of active correction of hypernatremia on any outcome in resuscitated patients.
Conclusion:
Recommendations for active correction of hypernatremia in resuscitated patients with sepsis or shock are unsupported by clinical research acceptable by modern evidence standards.
Keywords: Hypernatremia, sodium, fluid therapy, water–electrolyte imbalances, critical illness, shock
Rationale
Hypernatremia is associated with increased incidences of morbidity and mortality across heterogeneous populations, including intensive care units (ICUs), general wards, and outpatient settings.1–9 While less than 10% of patients are hypernatremic upon admission (“community-acquired” hypernatremia), up to 26% of medical ICU patients and 10% of surgical ICU patients become hypernatremic during their stay in the ICU,10,11 including as many as half of critically ill patients with sepsis.12 Hospital-acquired hypernatremia (HAH), specifically hypernatremia that develops in the ICU, is associated with increased risk of death with reported crude in-hospital mortality rates ranging from 14% to 48%.10 Morbid outcome associations include prolonged hospital and ICU stay as well as need for dialysis/renal replacement therapy.13 Similar findings have been reported in the pre- and postsurgical setting.14–16 Some authors have suggested that incident hypernatremia be used as a quality metric of appropriate care.17–20
Hypervolemic hypernatremia after resuscitation is now common in ICUs and on medical wards accounting for about half of HAH cases in some reports.21–24 One study showed that nearly half of ICU patients with sepsis developed hypernatremia.12 Published literature generally recommends a similar therapeutic strategy to treat community-acquired hypernatremia (CAH) and HAH: correcting a free water deficit with solute-poor/solute-free fluid. Some sources recommend diuretics along with free water replacement in hypervolemic HAH out of a belief that excess iatrogenic sodium loading may contribute to the hypernatremia.1,5–8 Fluids administered for this purpose are “discretionary fluids” and contribute positively to the patient’s net fluid balance.25
Accumulating evidence suggests that a positive fluid balance after the initial resuscitation phase worsens outcomes in critically ill patients.26–28 This creates a clinical dilemma regarding how best to therapeutically approach volume status in hypernatremic resuscitated patients. Because expert recommendations appear generally to assume a hypovolemic hypernatremic state (as commonly occurs in CAH) and because we were personally unaware of any controlled randomized or observational cohort studies designed to measure the impact of any therapeutic action intended to correct HAH on clinically relevant outcomes among otherwise-similar patients or in animal models, we undertook a structured literature search to identify any studies providing evidence assessing therapeutic interventions to correct hypernatremia in resuscitated adult patients with sepsis or other forms of shock.
Objectives
We searched for any randomized- or observational-controlled trials evaluating the impact of active correction of hypernatremia to eunatremia on any outcomes in adult patients with shock or sepsis that have been adequately resuscitated as assessed by the treating or the research team.
Eligibility criteria
Studies were identified by searching electronic databases (K.S.) and scanning the reference lists of relevant articles (D.E.S. and J.W.Q.). No date limits were applied to the search, but we restricted studies to English language due to time and resource constraints (funding for and availability of translation services). The search was adapted from some of the search domains used in the searches constructed for the NICE guidelines on Intravenous fluid therapy in adults in hospital as appropriate. The search was constructed by the librarian and search terms were reviewed and agreed upon by authors prior to application within the databases.
Information sources
The search was initially constructed, tested, and conducted in MEDLINE (PubMed, 1946 to present) and adapted for Embase (1947 to present), Cochrane Central Register of Controlled Trials (1991 to present), Cochrane Database of Systematic Reviews (2005 to present), DARE (Database of Reviews of Effects, 1991–2015), ClinicalTrials.gov (2000 to present), and CINAHL (1937 to present), with the index terms being mapped to non-MEDLINE equivalents, as necessary. Additionally, the Index-Catalogue of the Library of the Surgeon General’s Office was searched without date limits. The search was applied until 11 February 2017.
Search strategy
1. Dysnatremias search domain
A. “Hyponatremia” [MeSH]
B. “Hypernatremia” [MeSH]
C. hyponatremia[tiab] OR hypernatremia[tiab] OR dysnatremia[tiab] OR hyponatraemia[tiab] OR hypernatraemia[tiab] OR dysnatraemia[tiab] OR hypernatremias[tiab] OR hyponatremias[tiab] OR dysnatremias[tiab] OR hypernatraemias[tiab] OR hyponatraemias[tiab] OR dysnatraemias[tiab] OR “sodium imbalance”[tiab] OR “sodium imbalances”[tiab]
D. 1A OR 1B OR 1C
2. Fluid therapy search domain
A. “time factors”[mh]
B. (rapid[tiab] OR fast[tiab] OR slow[tiab] OR slowness[tiab] OR slowly[tiab]) AND
C. (infuse[tiab] OR infuses[tiab] OR infusion[tiab] OR infusions[tiab] OR administration[tiab] OR administrate[tiab] OR administrates[tiab] OR administrations[tiab] OR fluid[tiab] OR fluids[tiab] OR volume[tiab])
D. 2B AND 2C
E. (small[tiab] OR large[tiab] OR high[tiab] OR low[tiab])
F. (infuse[tiab] OR infuses[tiab] OR infusion[tiab] OR infusions[tiab] OR administration[tiab] OR administrate[tiab] OR administrates[tiab] OR administrations[tiab] OR fluid[tiab] OR fluids[tiab] OR volume[tiab])
G. 2E AND 2F
H. (restrict[tiab] OR restriction[tiab] OR restrictive[tiab] OR restrictions[tiab] OR restrictively[tiab] OR conservative[tiab] OR conservatively[tiab] OR liberal[tiab] OR liberally[tiab])
I. (fluid[tiab] OR fluids[tiab] OR regime[tiab] OR regimes[tiab] OR regimen[tiab] OR regimens[tiab] OR regimentation[tiab] OR regimentations[tiab] OR protocol[tiab] OR protocols[tiab] OR intake[tiab] OR intakes[tiab])
J. 2H AND 2I
K. (timing[tiab] OR delayed[tiab] OR intermediate[tiab] OR early[tiab] OR selective[tiab] OR rapid[tiab] OR immediate[tiab] OR immediately[tiab])
L. (fluid[tiab] OR fluids[tiab] OR therapy[tiab] OR therapies[tiab] OR intravenous[tiab] OR intravenously[tiab] OR iv[tiab])
M. 2K AND 2L
N. (fluid[tiab] OR fluids[tiab])
O. bolus[tiab])
P. 2N AND 2O
Q. 2A OR 2D OR 2G OR 2J OR 2M OR 2P
3. Shock, hypovolemia, and sepsis search domain
A. shock[mh] OR “Hypovolemia”[MeSH:noexp] OR hypotension[MeSH:noexp] OR dehydration[MeSH:noexp] OR “fluid therapy”[majr]
B. (fluid[tiab] OR fluids[tiab] OR volume[tiab] OR volumes[tiab] OR volumetric[tiab])
C. (restoration[tiab] OR restore[tiab] OR restores[tiab] OR restorations[tiab] OR resuscitation[tiab] OR resuscitations[tiab] OR resuscitate[tiab] OR resuscitative[tiab] OR replace[tiab] OR replaces[tiab] OR replacement[tiab] OR replacements[tiab] OR replacing[tiab] OR deplete[tiab] OR depletes[tiab] OR depleting[tiab] OR depletion[tiab] OR depletions[tiab] OR deficiency[tiab] OR deficient[tiab] OR deficiencies[tiab])
D. 3B AND 3C
E. (hypotension[tiab] OR hypotensive[tiab] OR hypotensions[tiab])
F. (resuscitation[tiab] OR resuscitations[tiab] OR resuscitate[tiab] OR resuscitative[tiab])
G. 3E AND 3F
H. (shock[tiab] OR resuscitation[tiab] OR resuscitations[tiab] OR resuscitate[tiab] OR resuscitative[tiab] OR hypotention[tiab] OR hypotensive[tiab] OR hypotensions[tiab] OR dehydrate[tiab] OR dehydration[tiab] OR dehydrates[tiab] OR dehydrations[tiab] OR dehydrating[tiab])
I. (fluid[tiab] OR fluids[tiab])
J. 3H AND 3I
K. (hypovolemia[tiab] OR hypovolaemia[tiab] OR hypovolemic[tiab] OR hypovolaemic[tiab] OR hypovolemics[tiab] OR hypovolaemics[tiab] OR “sepsis syndrome”[tiab] OR “sepsis syndromes”[tiab] OR “circulatory failure”[tiab] OR “circulatory failures”[tiab])
L. (circulatory[tiab] OR hemodynamic[tiab] OR haemodynamic[tiab])
M. (failure[tiab] OR failures[tiab] OR insufficiency[tiab] OR insufficiencies[tiab] OR insufficient[tiab] OR abnormality[tiab] OR abnormalities[tiab] OR instability[tiab] OR instabilities[tiab])
N. 3L AND 3M
O. (shock[tiab] OR resuscitation[tiab] OR resuscitations[tiab] OR resuscitate[tiab] OR resuscitative[tiab] OR hypotension[tiab] OR hypotensive[tiab] OR hypotensions[tiab] OR dehydrate[tiab] OR dehydration[tiab] OR dehydrates[tiab] OR dehydrations[tiab] OR dehydrating[tiab])
P. 3A OR 3D OR 3G OR 3J OR 3K OR 3N OR 30
4. Adult filter
A. Child[mh]
B. child[tiab] OR children[tiab] OR infant[tiab] OR infants[tiab] OR neonate[tiab] OR neonates[tiab] OR neonatal[tiab] OR newborn[tiab] OR newborns[tiab] OR boy[tiab] OR boys[tiab] OR girl[tiab] OR girls[tiab] OR adolescent[tiab] OR adolescents[tiab]
C. 4A OR 4B
D. Adult[mh]
E. adult[tiab] OR adults[tiab]
F. 4D OR 4F
G. 4C NOT 4F
5. Humans filter
A. Animals[MeSH]
B. Humans[MeSH]
C. 5A NOT 5B
6. Final search combination
((1D AND (2Q OR 3P)) NOT 4G) NOT 5C
Study records
Data management
After deduplication in RefWorks, citations were exported to Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) in a spreadsheet format. This file was then duplicated and given to both reviewers (J.W.Q. and D.E.S.) to work independently and to avoid potential bias.
Selection process
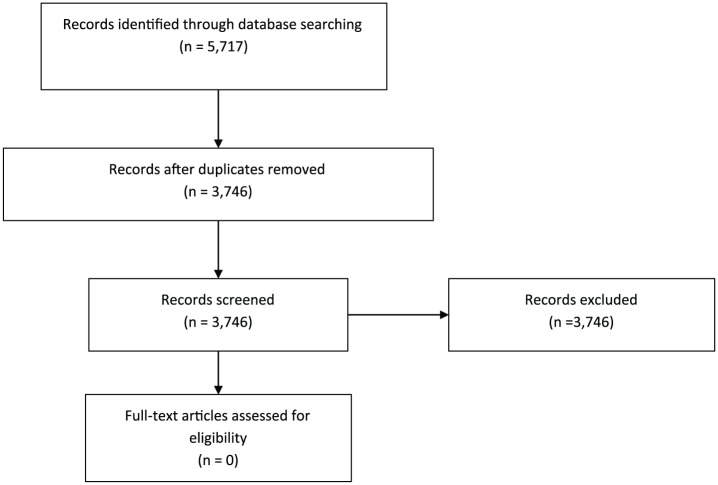
After deduplication, 3746 article abstracts were identified and individually reviewed to determine whether any controlled trials supporting correction to eunatremia in the formerly described search criteria were present. As the spreadsheet had been duplicated, the reviewers were not aware of the other’s review. There was no disagreement in the final conclusion, so no consensus was needed for resolution of inclusion decisions (Figure 1).
Figure 1.
PRISMA flow diagram of systematic review.
Data collection process
No randomized or observational-controlled studies were identified that assessed the impact of any active therapeutic maneuver intended to correct serum sodium concentrations on any outcome in resuscitated adult patients with resolving sepsis or other shock. Less exhaustive supplementary searches failed to identify similar supporting literature among pediatric, general hospitalized in-patient, or animal populations. We identified two small randomized studies evaluating the effectiveness of diuretics in manipulating sodium concentrations in critically ill patients, but these studies targeted a normonatremic population and were not intended to assess clinically important outcomes.29,30
Discussion
Our systematic review did not identify a single controlled study evaluating the impact of any active measure intended to correct hypernatremia in resuscitated patients with sepsis or other forms of shock on any clinically relevant endpoints. Although not the primary focus of our search, we also failed to identify any study demonstrating that free water or hypotonic fluid administration effectively reduces serum sodium concentrations in this setting.
The association between hypernatremia and increased morbidity and mortality is well established in critically ill patients.1,5–8 However, evidence to establish the extent to which this association reflects causal mediation versus confounding is limited. Recent analyses suggest that existing prediction formulas cannot accurately predict changes in serum sodium in hyponatremic31 or hypernatremic32 in-patients and that hypernatremia in critically ill patients is not adequately explained by excess sodium administration or a water deficit.33 One reasonable alternative hypothesis is that hypernatremia is primarily a marker of severe illness in resuscitated critically ill patients rather than a direct cause of poor outcomes. The recent observation that fluctuations in serum sodium concentrations even within a normal range carries increased risk of death34 suggests that absolute serum sodium concentration may be less important than the loss of homeostatic regulation that results in hypernatremia and supports such a hypothesis.
Accumulating evidence challenges the traditional equilibration model of sodium balance whose assumptions underlie accepted treatment strategies for hypernatremia.35 Skin, skeletal muscle, bone, and cartilage are known to act as reservoirs of sodium.35–38 These reservoirs do not always equilibrate with water and are not controlled by the kidney39 and may be more rapidly mobilized than previously recognized.40 Additional evidence indicates that sodium is intimately tied to immune function and regulation, including inflammation and infection41,42 leading some authors to theorize that a hypertonic environment may serve as a host defense.42 The interplay of these systems during metabolically stressful events provides a mechanism by which sepsis and other forms of shock might result in an “intrinsic” form of hypernatremia independent of volume status.
Given the demonstrated morbidity associated with hypervolemia in critically ill patients,27,43–45 the established benefits of “deresuscitation” in such patients,43,46 and the absence of evidence of improved outcomes with volume administration to treat hypernatremia in resuscitated patients, we believe that a cautiously “salt-tolerant/fluid-restricted” therapeutic strategy is at least as well supported by existing research as is the dominant “salt-intolerant/fluid-liberal” paradigm.
There are limitations to our research. Our search required at least the search terms and the abstract be available in English. In addition, we may have overlooked gray literature on the topic such as doctoral dissertations or conference proceedings that were not published as supplements to medical journals.
Conclusion
We did not identify any randomized or observational-controlled trials evaluating the use of active measures to correct hypernatremia in resuscitated patients recovering from critical illness. It is apparent that the existing body of scientific knowledge pertaining to sodium metabolism and homeostasis in critically ill patients is distressingly sparse and is inadequate to meaningfully inform therapeutic decisions. Additional animal and clinical data are needed to empirically establish (1) the mechanisms driving hypernatremia in critically ill patients, (2) appropriate indications for therapeutic correction of hypernatremia following resuscitation, and (3) effective therapeutic strategies for improving clinically important outcomes in hypernatremic resuscitated critically ill patients.
Acknowledgments
D.E.S. is the guarantor of the protocol. J.W.Q., D.E.S., and K.S. drafted the manuscript. J.W.Q., D.E.S., and K.S. contributed to the development of the selection criteria and data extraction criteria. K.S. developed the search strategy for the protocol. In the event of protocol amendments, the date of each amendment will be accompanied by a description of the change and the rationale.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Joseph W Quinn  https://orcid.org/0000-0002-5134-9670
https://orcid.org/0000-0002-5134-9670
Kerry Sewell  https://orcid.org/0000-0002-0405-3789
https://orcid.org/0000-0002-0405-3789
References
- 1. Eizadi-Mood N, Sabzghabaee AM, Hosseini H, et al. Is admission serum sodium concentration a clinical predictor for the outcome of therapy in critically ill poisoned patients? Med Arch 2015; 69(4): 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lantigua H, Ortega-Gutierrez S, Michael Schmidt J, et al. Subarachnoid hemorrhage: who dies, and why? Crit Care 2015; 19(1): 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Güçyetmez B, Ayyildiz AC, Ogan A, et al. Dysnatremia on intensive care unit admission is a stronger risk factor when associated with organ dysfunction. Minerva Anestesiol 2014; 80(10): 1096–1104. [PubMed] [Google Scholar]
- 4. Sun T, Wu Q, Kan Q, et al. The influence of hypernatremia on mortality in intensive care unit patients: a meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26(4): 228–232. [DOI] [PubMed] [Google Scholar]
- 5. Toor MR, Singla A, DeVita MV, et al. Characteristics, therapies, and factors influencing outcomes of hospitalized hypernatremic geriatric patients. Int Urol Nephrol 2014; 46(8): 1589–1594. [DOI] [PubMed] [Google Scholar]
- 6. Wu CJ, Li CS. The impact of iatrogenic hypernatremia on the prognosis of critical patient. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2009; 21(8): 474–477. [PubMed] [Google Scholar]
- 7. Vand De, Louw A, Shaffer C, Schaefer E. Early intensive care unit-acquired hypernatremia in severe sepsis patients receiving 0.9% saline fluid resuscitation. Acta Anaesthesiol Scand 2014; 58(8): 1007–1014. [DOI] [PubMed] [Google Scholar]
- 8. Funk GC, Lindner G, Druml W, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med 2010; 36(2): 304–311. [DOI] [PubMed] [Google Scholar]
- 9. Crivellin C, Cagnin A, Manara R, et al. Risk factors for central pontine and extrapontine myelinolysis after liver transplantation: a single-center study. Transplantation 2015; 99(6): 1257–1264. [DOI] [PubMed] [Google Scholar]
- 10. Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care 2013; 28(2): 216.e11–220. [DOI] [PubMed] [Google Scholar]
- 11. Hu B, Han Q, Mengke N, et al. Prognostic value of ICU-acquired hypernatremia in patients with neurological dysfunction. Medicine 2016; 95(35): e3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ni HB, Hu XX, Huang XF, et al. Risk factors and outcomes in patients with hypernatremia and sepsis. Am J Med Sci 201; 351(6): 601–605. [DOI] [PubMed] [Google Scholar]
- 13. Mendes RS, Soares M, Valente C, et al. Predialysis hypernatremia is a prognostic marker in acute kidney injury in need of renal replacement therapy. J Crit Care 2015; 30(5): 982–987. [DOI] [PubMed] [Google Scholar]
- 14. Wu Y, Ren J, Wang G, et al. Serum sodium: a reliable and validated predictor for mortality in enteric fistula patients complicated with sepsis. J Invest Surg 2015; 28(3): 131–139. [DOI] [PubMed] [Google Scholar]
- 15. Lindner G, Funk GC, Lassnigg A, et al. Intensive care-acquired hypernatremia after major cardiothoracic surgery is associated with increased mortality. Intensive Care Med 2013; 36(10): 1718–1723. [DOI] [PubMed] [Google Scholar]
- 16. Cecconi M, Hochrieser H, Chew M, et al. Preoperative abnormalities in serum sodium concentrations are associated with higher in-hospital mortality in patients undergoing major surgery. Br J Anaesth 2016; 116(1): 63–69. [DOI] [PubMed] [Google Scholar]
- 17. Wolff A, Stuckler D, McKee M. Are patients admitted to hospitals from care homes dehydrated? A retrospective analysis of hypernatraemia and in-hospital mortality. J R Soc Med 2015; 108(7): 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah MK, Workeneh B, Taffet GE. Hypernatremia in the geriatric population. Clin Interv Aging 2014; 19(9): 1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polderman KH, Schreuder WO, Strack van Schijndel RJ, et al. Hypernatremia in the intensive care unit: an indicator of quality of care? Crit Care Med 1999; 27(6): 1105–1108. [DOI] [PubMed] [Google Scholar]
- 20. Oude Lansink-Hartgring A, Hessels L, Weigel J, et al. Long-term changes in dysnatremia incidence in the ICU: a shift from hyponatremia to hypernatremia. Ann Intensive Care 2016; 6(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarahian S, Pouria MM, Ing TS, et al. Hypervolemic hypernatremia is the most common type of hypernatremia in the intensive care unit. Int Urol Nephrol 2015; 47(11): 1817–1821. [DOI] [PubMed] [Google Scholar]
- 22. Felizardo Lopes I, Dezelée S, Brault D, et al. Prevalence, risk factors and prognosis of hypernatraemia during hospitalisation in internal medicine. Neth J Med 2015; 73(10): 448–454. [PubMed] [Google Scholar]
- 23. Kahn T. Hypernatremia without water depletion. Clin Nephrol 2011; 76(2): 130–135. [DOI] [PubMed] [Google Scholar]
- 24. Liamis G, Filippatos TD, Elisaf MS. Evaluation and treatment of hypernatremia: a practical guide for physicians. Postgrad Med 2016; 128(3): 299–306. [DOI] [PubMed] [Google Scholar]
- 25. Bashir MU, Tawil A, Mani VR, et al. Hidden obligatory fluid intake in critical care patients. J Intensive Care Med 2017; 32(3): 223–227. [DOI] [PubMed] [Google Scholar]
- 26. Alsous F, Khamiees M, DeGirolamo A, et al. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest 2000; 117(6): 1749–1754. [DOI] [PubMed] [Google Scholar]
- 27. Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39(2): 259–265. [DOI] [PubMed] [Google Scholar]
- 28. Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34(2): 344–353. [DOI] [PubMed] [Google Scholar]
- 29. Bihari S, Holt AW, Prakash S, et al. Addition of indapamide to frusemide increases natriuresis and creatinine clearance, but not diuresis, in fluid overloaded ICU patients. J Crit Care 2016; 33: 200–206. [DOI] [PubMed] [Google Scholar]
- 30. Apte Y, Bellomo R, Warrillow S, et al. Pilot randomised double-blind controlled trial of high-dose spironolactone in critically ill patients receiving a frusemide infusion. Crit Care Resusc 2008; 10(4): 306–311. [PubMed] [Google Scholar]
- 31. Hanna RM, Yang WT, Lopez EA, et al. The utility and accuracy of four equations in predicting sodium levels in dysnatremic patients. Clin Kidney J 2016; 9(4): 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindner G, Schwarz C, Kneidinger N, et al. Can we really predict the change in serum sodium levels? An analysis of currently proposed formulae in hypernatraemic patients. Nephrol Dial Transplant 2008; 23(11): 3501–3508. [DOI] [PubMed] [Google Scholar]
- 33. Van IJzendoorn MCO, Buter H, Kingma WP, et al. The development of intensive care unit acquired hypernatremia is not explained by sodium overload or water deficit: a retrospective cohort study on water balance and sodium handling. Crit Care Res Pract 2016; 2016: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sakr Y, Rother S, Ferreira AM, et al. Fluctuations in serum sodium level are associated with an increased risk of death in surgical ICU patients. Crit Care Med 2013; 41(1): 133–142. [DOI] [PubMed] [Google Scholar]
- 35. Titze J. A different view on sodium balance. Curr Opin Nephrol Hypertens 2015; 24(1): 14–20. [DOI] [PubMed] [Google Scholar]
- 36. Oh YS, Appel LJ, Galis ZS, et al. NHLBI Working Group report on salt in human health and sickness. Hypertension 2016; 68: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sterns RH. Disorders of plasma sodium-causes, consequences, and correction. N Engl J Med 2015; 372: 55–65. [DOI] [PubMed] [Google Scholar]
- 38. Kurtz I, Nguyen MK. Evolving concepts in the quantitative analysis of the determinants of the plasma water sodium concentration and the pathophysiology and treatment of the dysnatremias. Kidney Int 2005; 68(5): 1982–1993. [DOI] [PubMed] [Google Scholar]
- 39. Hofmeister LH, Perisic S, Titze J. Tissue sodium storage: evidence for kidney-like extrarenal countercurrent systems? Pflugers Arch 2015; 467(3): 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hew-Butler T, Stuempfle KJ, Hoffman MD. Bone: an acute buffer of plasma sodium during exhaustive exercise? Horm Metab Res 2013; 45(10): 697–700. [DOI] [PubMed] [Google Scholar]
- 41. Schatz V, Neubert P, Schröder A, et al. Elementary immunology: Na+ as a regulator of immunity. Pediatr Nephrol 2017; 32(2): 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jantsch J, Schatz V, Friedrich D, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 2015; 21(3): 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 2014; 46(5): 361–380. [DOI] [PubMed] [Google Scholar]
- 44. Mitchell KH, Carlbom D, Caldwell E, et al. Volume overload: prevalence, risk factors, and functional outcome in survivors of septic shock. Ann Am Thorac Soc 2015; 12(12): 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salahuddin N, Sammani M, Hamdan A, et al. Fluid overload is an independent risk factor for acute kidney injury in critically Ill patients: results of a cohort study. BMC Nephrol 2017; 18: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med 2017; 43(2): 155–170. [DOI] [PubMed] [Google Scholar]