Abstract
While pulmonary hypertension (PH) has traditionally not been considered as a disease that is directly linked to or, potentially, even caused by inflammation, a rapidly growing body of evidence has demonstrated the accumulation of a variety of inflammatory and immune cells in PH lungs, in and around the wall of remodeled pulmonary resistance vessels and in the vicinity of plexiform lesions, respectively. Concomitantly, abundant production and release of various inflammatory mediators has been documented in both PH patients and experimental models of PH. While these findings unequivocally demonstrate an inflammatory component in PH, they have fueled an intense and presently ongoing debate as to the nature of this inflammatory aspect: is it a mere bystander of or response to the actual disease process, or is it a pathomechanistic contributor or potentially even a trigger of endothelial injury, smooth muscle hypertrophy and hyperplasia, and the resulting lung vascular remodeling? In this review, we will discuss the present evidence for an inflammatory component in PH disease with a specific focus on the potential role of the endothelium in this scenario and highlight future avenues of experimental investigation which may lead to novel therapeutic interventions.
Keywords: pulmonary hypertension, inflammation, endothelium, adhesion molecules
Inflammatory cells and mediators in pulmonary hypertension
Specific subclasses of pulmonary arterial hypertension (PAH) have traditionally been linked to inflammation and immunity due to the inflammatory or infectious nature of their underlying or associated disease. Prototypic examples are PAH forms related to connective tissue diseases such as systemic sclerosis or lupus erythematosus,1 but also PAH as a consequence of HIV infection or related to other viral etiologies.2 The implication of inflammation and immunity in pulmonary hypertension (PH), however, is much older, and seems to reach far beyond these most striking associations.
Already in 1878, when Paul Ehrlich identified the mast cell, he reported that these cells were most abundant in “brown induration of the lung,” i.e. in hemosiderosis, which we nowadays would classify as type II PH, i.e. PH following left heart disease.3 Subsequent clinical studies confirmed the accumulation of mast cells in lungs of patients with idiopathic PAH (iPAH)4–7 or “secondary” PAH (which nowadays would be considered associated PAH)6 or in patients with PH owing to mitral stenosis and left heart disease.8 Measurements of mast cell densities in lungs of native highlanders revealed that mast cell numbers were only increased in subjects with considerable muscularization of their pulmonary circulation, indicating a functional role for mast cells in lung vascular remodeling.9 These clinical findings were paralleled by reports of similar mast cell accumulations in experimental models of PH, notably in chronic hypoxic rats10,11 as well as calves, pigs, and sheep,12 in the rat monocrotaline model of PH,4,13,14 in rat models of left heart disease,13 or in a combined model of monocrotaline and left-to-right shunt.15
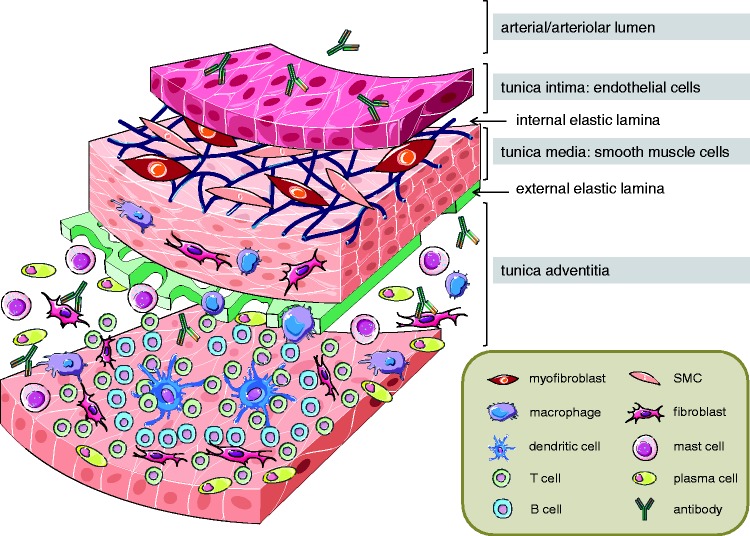
More recently, the recognition of immune cell abundance and infiltration in lung vascular lesions and remodeled vessels has expanded profusely (Fig. 1): innate immune cells such as macrophages and monocytes are characteristically detected in patients with iPAH,7,16 in PH secondary to congenital cardiac shunts17 and in murine and rat lungs in response to chronic hypoxia or monocrotaline.18,19 Vascular lesions and the adventitial space of remodeled blood vessels in iPAH patients and monocrotaline-induced experimental PH are also infiltrated by immature dendritic cells.7,20 In addition to infiltrating innate immune cells, the adaptive immune response has recently become implicated in PH based on the fact that perivascular accumulations of B cells have not only been documented in PAH associated with connective tissue disorders or HIV infection,21,22 but also in plexiform lesions of patients with iPAH,16 as well as in experimental PH.23 Work by the groups of Perros et al.24 and Colvin et al.25 as well as from our own laboratory26 has identified the formation of characteristic tertiary lymphoid tissue comprising B- and T-cell areas with high endothelial venules and dendritic cells in the vicinity of remodeled pulmonary arteri(ol)es and bronchi(oles) in iPAH patients as well as animal models of PH.
Fig. 1.
Infiltration of inflammatory and immune cells into the wall and perivascular space of pulmonary arteries in PH. A schematic cross-section shows a pulmonary artery with its different layers (intima, media, and adventitia) and the characteristic accumulation of inflammatory and immune cells, namely macrophages, dendritic cells, T cells, B cells, mast cells, and plasma cells, as well as the infiltration of fibroblasts and myofibroblasts and the production of (auto-)antibodies in PH disease.
This infiltration of innate and adaptive immune cells is associated with—and likely orchestrated by—the activation of several key transcription factors such as the nuclear factor of activated T cells (NFAT)27 and STAT328 contributing to the release of numerous cytokines. Patients with iPAH or associated PAH exhibit higher circulating levels and/or pulmonary expression of tumor necrosis factor (TNF),29,30 interleukins (IL) including IL-1β,31,32 IL-4,32,33 IL-6,31,32 IL-8,32 IL-10,32 IL-12p70,32 and IL-13,33,34 and a series of chemokines including fractalkine (CX3CL1),35 RANTES (CCL5),36 monocyte chemotactic protein-1 (MCP-1; CCL2),37,38 and interferon γ-induced protein 10 (IP-10; CXCL10).39 Of the characteristic cytokines, IL-6 seems of particular relevance in the context of PH as suggested by experimental studies and biomarker screenings: in PAH patients, plasma IL-6 levels predict five-year survival with a higher prognostic accuracy as functional or hemodynamic parameters.32 Similarly, circulating IL-6 in PAH patients contribute to the development of co-morbidities in PAH such as coronary artery diseases through the activation of the Bromodomain-containing protein 4 (BRD4),40,41 while increased TNF contributes to right ventricular failure through a miR-208/myocyte enhancer factor 2 (MEF2)-dependent mechanism.42 The importance of circulating cytokines especially IL-6 is reinforced by the fact that lung-specific overexpression of IL-6 in mice suffices to cause elevated right ventricular systolic pressures and right ventricular hypertrophy with corresponding pulmonary vascular changes.43 Conversely, IL-6-deficient mice develop less PH, right ventricular hypertrophy, and lung vascular remodeling in chronic hypoxia compared to their corresponding IL-6+/+ wild type (WT).44
In addition to classic inflammatory cytokines, immune responses in PH may also be triggered or aggravated by other pro-inflammatory mediators such as leukotrienes (LTs) or complement factors. Recent studies suggest an important role for LTB4 in the context of immune dysregulation in PAH.45 LTs are lipid mediators derived from arachidonic acid. Following activation of 5-lipoxygenase (5-LO) by 5-LO-activating protein (FLAP), arachidonic acid is converted to LTA4, an unstable epoxide which is subsequently further metabolized by either LTA4 hydrolase or LTC4 synthase to LTB4 or LTC4, respectively. In rats exposed to chronic hypoxia, expression levels of 5-LO and FLAP are increased in alveolar macrophages and endothelial cells,46 and in athymic rats treated with the VEGF receptor antagonist SU5416, which develop lethal PH even under normoxic conditions, LTB4 levels were found to be elevated in bronchoalveolar lavage fluid and serum.47 Analogously in PAH patients, alveolar macrophages expressing 5-LO and FLAP are more frequently clustered in the vicinity of remodeled blood vessels, while endothelial cells in plexiform and concentric lesion similarly express both enzymes.48 Pharmacological antagonization of 5-LO and FLAP by MK-866 was shown to prevent hypoxic pulmonary vasoconstriction and development of chronic hypoxia-induced PH, thus establishing a functional role of LTs in PH.46
LTB4 exerts its chemoattractant and pro-inflammatory effects predominantly via its high affinity receptor BLT1 which is widely expressed on immune cells including granulocytes, T cells, and dendritic cells.49 In vitro, LTB4 induces apoptosis of pulmonary artery endothelial cells via a signaling pathway that involves spingosine kinase 1 and endothelial nitric oxide synthase, and stimulates proliferation of human pulmonary artery smooth muscle cells, which is effectively blocked by the BLT-1 inhibitor U75302.47 In addition, LTB4 may further promote PH by stimulating proliferation, migration, and differentiation of pulmonary arterial adventitial fibroblasts.50 In vivo, inhibition of LTB4 formation by the LTA4 hydrolase inhibitor Ubenimex rescued PH rats from death. In PAH patients, LTA4 hydrolase expression was shown to be increased in CD68+ macrophages clustered around occluded vascular lumens of plexiform lesions, and circulating levels of LTB4 were found to be elevated in PAH patients with connective tissue disease though not in iPAH.47 From these studies, LTB4 has emerged as an important inflammatory signal and, hence, also potential drug target in PH. In respect to the latter, the recent completion of the LIBERTY trial, a phase 2 study on the effectiveness of the LTA4 hydrolase inhibitor Ubenimex in patients with PAH (LIBERTY:NCT02664558), is expected to provide key insight into the therapeutic potential of this pathway.
Considering that complement factors C3 and C4a have been identified as biomarkers of iPAH,51,52 it is surprising that the functional role of the complement system as a critical regulator of innate and adaptive immune responses in PH has thus far scarcely been addressed. In what is to our knowledge the only mechanistic study so far, Bauer et al. were able to demonstrate increased deposition of the C3 degradation product C3d in the pulmonary vessel wall in a murine PH model of chronic hypoxia and in human iPAH patients.53 More importantly, C3-deficient mice developed less PH with no increase in pulmonary IL-6 or tissue factor, less P-selectin on platelets, and less pulmonary intercellular adhesion molecule 1 (ICAM-1) expression during chronic hypoxia compared to WT mice, suggesting that C3 plays an important role in the pathophysiology of PH, potentially by promoting inflammatory cell interaction and recruitment via adhesion molecules such as P-selectin and ICAM-1. Since various novel therapies targeting the complement system are presently tested in clinical trials,54,55 the functional role of the complement system in PH and its therapeutic exploitation pose intriguing topics for future research.
The functional relevance of immune responses in the initiation and/or progression of PH has recently become evident, in that pharmacological inhibition, depletion, or genetic deficiency in specific cell subsets such as mast cells4,13,15 or B cells26,56 has been shown to confer protection from the development of PH in a variety of animal models. Furthermore, IL-6 has been identified as a critical link between mast cells and B cells and, hence, between the innate and adaptive immune system in that mast cell-derived IL-6 promotes the formation of tertiary lymphoid tissue in PH lungs26 in line with previous data demonstrating that mast cells synthesize and release IL-6.57,58 Originally identified as a B cell stimulatory factor that induces differentiation into antibody-producing plasma cells,59 IL-6 production has also been linked to increased Ig secretion and production of autoantibodies.60 A similar and potentially parallel pathway linking mast cells to B cells may act via another member of the IL-6 family, the pleiotropic cytokine oncostatin M.61 Like IL-6, OSM is also secreted by mast cells62 and upregulated in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and scleroderma or in plasma of PAH patients.63,64 Endotracheal administration of an adenoviral vector expressing mouse oncostatin M promotes B cell activation and formation of tertiary lymphoid tissue independent of IL-6,65 suggesting that mast cells may regulate adaptive (auto-)immunity and formation of tertiary lymphoid tissue via different pathways in PH.
Importantly, the emerging role of mast cells, B cells, and other inflammatory cells in PH is not a purely theoretical concept but of tremendous translational potential and, as such, has already reached the clinical setting.66 Mast cell stabilizers have been shown to reduce inflammation and increase exhaled NO in PAH patients in a small pilot study,67 while a clinical phase II trial on the effect of the B cell depleting anti-CD20 antibody rituximab for the treatment of systemic sclerosis-associated PAH is currently underway (NCT01086540). Care should, however, be taken with respect to existing immunosuppressive drugs, as poor safety profiles and potentials for drug–drug interactions with current PH therapies may present dangerous pitfalls for their implementations in clinics.68
While mast cells and B cells promote the development of PH, CD4+CD25+FoxP3+ regulatory T cells (T regs), a subpopulation of T cells which maintains tolerance to self-antigens and downregulates autoimmune disease, seem to attenuate experimental PH. Athymic mice or rats lacking T cells characteristically develop more severe PH in response to monocrotaline or SU5416 compared to euthymic rodents.69,70 The beneficial effect of T regs in PH was particularly evident in a study in athymic rats in that the development of lung vascular remodeling and PH in response to SU5416 was attenuated in animals that had been reconstituted with CD4+CD25+ T regs.71 This may at first seem contradictory, as B cells are considered to maintain72 and expand73–76 T regs. Conversely however, a series of animal studies on autoimmune diseases show that depletion of B cells causes activation and proliferation of T regs which are associated with marked improvement of histological or functional parameters of disease severity.77–79 The clinical relevance of this scenario is highlighted by the fact that B cell depletion with rituximab restores T reg numbers in peripheral blood of patients with immune thrombocytopenia (ITP), an effect that is particularly evident in therapy responders.80 Conversely, B cells may thus potentially promote inflammatory (auto-)immune responses by inhibiting T regs.
The differential involvement of innate and adaptive immune cells, the temporal sequence of their infiltration, their mutual crosstalk, and interdependency are exciting topics of ongoing and future research in PH of which the interaction between mast cells and B cells via cytokines of the IL-6 family as outlined above may only serve as prototypical example. At present, our insight into the individual mechanisms that trigger adaptive versus innate immune responses in PH is rudimentary, as is our understanding regarding their differential potential as therapeutic targets in PH. While an exhaustive discussion of this topic is beyond the scope of this manuscript, the dissection of innate and adaptive immune responses in PH clearly deserves deeper exploration in terms of experimental studies and state-of-the-art reviews.
Autoimmunity in pulmonary hypertension
The recognition of B cell activation and tertiary lymphoid tissue formation has recently fueled the intriguing hypothesis of a relevant autoimmune component in the pathogenesis and/or pathophysiology of PH. This view is supported by genomic analyses which identified a distinct RNA expression profile in peripheral blood B cells, indicative of their activation in patients with iPAH compared to healthy controls.81 Notably, while differentiation of B cells to antibody-producing plasma cells was originally considered to be restricted to lymph nodes,82 the recent recognition of functional ectopic lymphoid tissues adjacent to remodeled vessels in lungs of patients with iPAH clearly demonstrates that B cell differentiation and subsequent antibody production can occur and may be regulated at the local level in PH lungs.83 Many of these ectopic lymphoid tissues form germinal centers where somatic hypermutation and class-switching occur, thereby providing an optimal environment for the generation of pathogenic autoantibodies.83 Formation of autoantibodies has long been recognized as a key factor that is assumed to account for the high prevalence of PAH in patients with connective tissue diseases (CTDs) such as systemic sclerosis84,85 or Sjögren's syndrome86 where antibody deposits have been found localized in the pulmonary artery walls.87,88 However, the presence of tertiary lymphoid tissue in patients with iPAH as well as in various animal models of hypoxia- or monocrotaline-induced PH suggests a much broader relevance of autoimmunity in the pathogenesis of PAH. Along these lines, increased levels of various circulating autoantibodies have been detected in patients with non-CTD PAH: It is estimated that 10–15% of iPAH patients are positive for antiphospholipid antibodies,89 30–40% express antinuclear antibodies,90,91 40% anti-fibroblast antibodies,92 62% anti-endothelial cell antibodies,93,94 and up to 93% anti-fibrillin-1 antibodies.95 In recent collaborative work, we furthermore identified the prevalence of circulating agonistic autoantibodies against endothelin receptor type A (ETAR) and the angiotensin receptor type-1 (AT1R), which not only predicted the development of PAH and PAH-associated mortality in patients with systemic sclerosis, but were also present in 11% and 21% of patients with iPAH, respectively.96 The functional relevance of these autoantibodies was recently elegantly demonstrated, in that passive transfer of either autoantibody-rich plasma or purified immunoglobulin (Ig) G from rats with monocrotaline-induced PH was sufficient to induce the de novo formation of lung ectopic lymphoid tissue and the development of PH in naïve rats.97 Notably, increased production of autoantibodies does not seem to be restricted to PAH patients or the monocrotaline model of PH, as increased levels of circulating immunoglobulin G were similarly detected in a rat model of PH with left heart disease,26 in which moreover immunoglobulin-encoding genes were found to be the most mast cell-dependent regulated genes in lung tissue.26 While these findings point to an overarching relevance of pathogenic autoantibodies in the development of PH that is not restricted to CTDs, the mechanisms that drive this acquired autoimmunity remain unclear.
Autoimmunity emerges when the fragile balance between self-recognition and protection from non-self-pathogens is lost.98 It has been estimated that 50–75% of newly produced human B cells are autoreactive and must be eliminated by tolerance mechanisms.99 This B cell tolerance is established at multiple checkpoints throughout B cell development, both in the bone marrow and in the periphery by mechanisms such as receptor editing, clonal deletion, and anergy, which serve to eliminate autoreactive B cells.100–102 The actual development of autoimmunity is driven via the activation of distinct pro-inflammatory signaling pathways, which have accordingly emerged as potential novel therapeutic targets for individual autoimmune diseases, including transcription factors such as STAT4,103 cytokines such as interleukin-17,104 or alarmins such as high mobility group box 1 (HMGB1).105–107 HMGB1 gains particular relevance as a potential link between inflammation/autoimmunity and pulmonary vascular disease through its recent implication in both clinical and preclinical PAH. HMGB1 is abundant both in serum and in vascular lesions of patients with iPAH,108,109 and the increase in circulating HMGB1 correlates with mean pulmonary artery pressure.108 Evidence for a functional role of HMGB1 in PAH comes from rodent models of chronic hypoxia and monocrotaline, respectively, where treatment with anti-HMGB1 antibodies attenuated the development of PH108,110 as did a non-specific inhibitor of HMGB1, glycyrrhizin.111 Notably, the fact that actively secreted HMGB1 is primarily derived from macrophages112 provides an intriguing link to a series of recent publications which attribute a key role for macrophages in the initiation of PAH.113–117 Indeed, remodeled vessels in lung samples of patients with iPAH are often surrounded by HMGB1-positive cells in the adventitia109 where perivascular macrophages accumulate in PAH.118 Of particular relevance in the context of autoimmunity, extracellular HMGB1 in isolation or in complex with DNA promotes the proliferation and activation of autoreactive B cells.119–121 Consistent with this view, autoantibody production was recently found to correlate with HMGB1 serum levels in patients with systemic lupus erythematosus.122 Conversely, blockade of extracellular HMGB1 suppresses xenoreactive B cell responses, autoantibody production, and delays acute vascular rejection following heterotopic heart xenotranplantation.123 Additionally, HMGB1, as a part of DNA-anti-DNA immune complexes, can interact with the receptor for advanced glycation endproducts (RAGE) on the surface of plasmacytoid dendritic cells and B cells leading to TLR9-dependent interferon-α release and activation of autoreactive B cells.124 The latter aspect may be of particular relevance in the context of PH as RAGE activation is increased in PAH patients and systemic vascular diseases.125,126 Thus, HMGB1 emerges as a potent trigger and promoter of autoimmunity via both direct and indirect effects on B cells and as a trigger or promoter of vascular remodeling in PH; yet although it is tempting to postulate a functional link between these two effects a direct causal interrelationship remains to be shown.
The endothelium in inflammation and immunity
What remains obscure and has not been addressed in either preclinical or clinical studies so far is the origin of these inflammatory and immune cells in PH, and the mechanisms that trigger and regulate their recruitment to the lung parenchyma and into the vicinity of the remodeling pulmonary blood vessels. At later stages of the disease, some of these cells may expand locally within the tissue and the newly formed lymphoid tissues, yet it seems fair to assume that at least initially the inflammatory cells infiltrate the lung largely from the circulating blood. This notion is supported, for example, by flow cytometric analyses in chronic hypoxic mice demonstrating the mobilization of cells positive for the mast cell (and stem cell) marker c-kit from the bone marrow into the circulation and their subsequent accumulation in remodeled pulmonary artery vessel walls.127,128 In neonatal rats and calves, development of chronic pulmonary hypertension required the recruitment of a monocyte/macrophage precursor from the circulating blood into the pulmonary perivascular space.129 Similarly, flow cytometric analyses in various organs of rats with monocrotaline-or SU5416/hypoxia-induced PH revealed an increase in B cells abundance in virtually all organs including lung, lymph nodes, spleen, and circulating blood with the notable exception of bone marrow.26
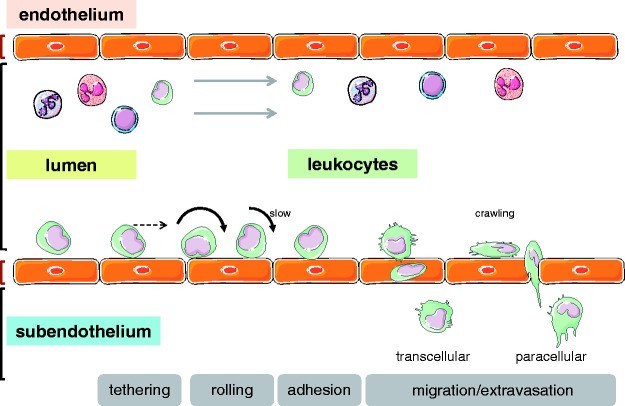
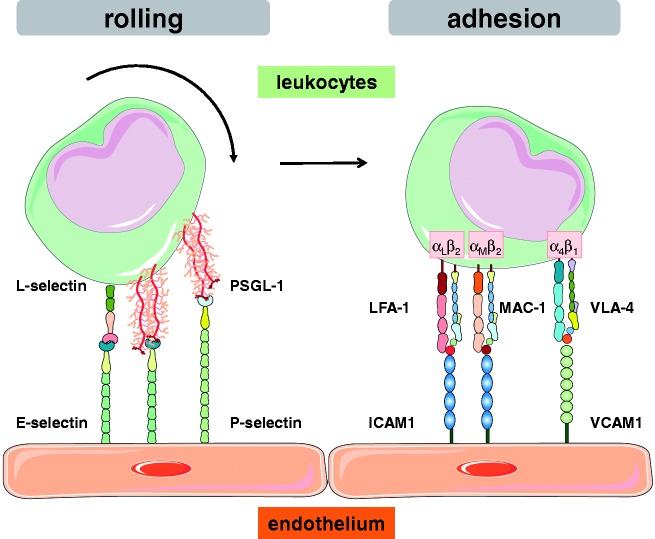
It remains unclear whether the inflammatory and immune cells that migrate into the lungs in PH are recruited directly from the pulmonary arteries or arterioles, from the vasa vasorum and the perivascular capillary network,130 or from the lymphatic vasculature. That notwithstanding, the recruitment will have to occur along the lines of the classic leukocyte adhesion and migration cascade that has been extensively studied and described in the past (Fig. 2). In brief, in order for circulating leukocytes to leave the blood stream and enter the perivascular space and the lung parenchyma, they first need to interact, adhere, and ultimately migrate through the vascular (or, alternatively, lymphatic) endothelium in a tightly controlled sequence of events.131,132 This cascade comprises initial steps of tethering and rolling, followed by firm arrest and occasional crawling along the vessel wall until the inflammatory cell exits the vessel by ways of paracellular or, occasionally, transcellular migration. The sequential steps of this adhesion cascade are mediated by the interaction of adhesion molecules expressed on the vascular endothelium with their respective counterligands on circulating leukocytes or platelets that may in turn form aggregates with leukocytes.133 In what has become known as the classic paradigm of the leukocyte adhesion cascade, leukocytes first start to role via the interaction of leukocytic L-selectin and P-selectin glycoprotein ligand-1 (PSGL-1) with P- and E-selectin expressed on the vascular endothelium, and may subsequently adhere via CD11a/CD18 (lymphocyte function-associated antigen 1, LFA-1) or CD11b/CD18 (macrophage-1 antigen, Mac-1) interacting with ICAM-1, or integrin α4β1 (very late antigen-4, VLA-4) interacting with vascular cell adhesion protein 1 (VCAM-1) (Fig. 3). This general concept applies not only for neutrophils, but equally for migrating mast cells,134,135 B cells,136 and mononuclear cells.137 While the exact molecular players that orchestrate this sequence will vary between different vascular beds and leukocyte subsets, it is a fundamental paradigm that each of these steps is mediated by the intimate interaction of adhesion molecules expressed on the infiltrating leukocyte on the one, and the vessel outlining endothelium on the other hand.137
Fig. 2.
Schematic representation of the classic leukocyte adhesion cascade. Following initial tethering at the endothelial cell surface, leukocytes start to roll and ultimately may firmly arrest on the vessel surface to finally migrate into the subendothelial space, typically via a paracellular but occasionally also via a transcellular route.
Fig. 3.
Schematic representation of central adhesion molecules mediating rolling and firm adhesion of circulating leukocytes on the vascular endothelium. Rolling is mediated by interaction of endothelial E- and P-selectin with L-selectin and P-selectin glycoprotein ligand-1 (PSGL-1) on the surface of the leukocyte, while firm adhesion involves interaction of endothelial intercellular adhesion molecule (ICAM)-1 with αLβ2 (lymphocyte function-associated antigen 1 [LFA-1]) or αMβ2 (macrophage-1 antigen [MAC-1]) integrin, or interaction of vascular cell adhesion molecule 1 (VCAM-1; CD106) with α4β1 integrin (very late antigen 4 [VLA-4]) on the leukocyte.
With that in mind it must come as a surprise that so far no clinical or preclinical studies have to our knowledge addressed the role of specific adhesion molecules in PH, either by pharmacological or antibody blockade or by genetic deletion strategies. There is, however, an abundance of studies that have reported elevated levels of circulating soluble adhesion molecules in PH patients. As such, marked increases in the plasma concentration of soluble P-selectin have been detected in PAH patients with either idiopathic138,139 or non-idiopathic disease.138 Similarly, patients with PH due to left heart disease have been found to exhibit higher circulating levels of soluble P-selectin, although this finding did not reach significance,138 and P-selectin expression on platelets as an indicator of their activation is increased in patients with chronic thromboembolic pulmonary hypertension (CTEPH).140 In patients with congenital heart disease (CHD), P-selectin genotype polymorphism of -825T/C differs significantly between patients with and without PH, suggesting a potential contributory role of the P-selectin genotype in the development of PH in this patient population.141 Circulating levels of soluble P-selectin did, however, not correlate with World Health Organization functional class (FC) or transplant-free survival in a recent biomarker study in 65 PAH patients,142 and were not associated with changes in 6-min walk distance or FC in response to PAH therapy by trepostinil.143
For soluble E-selectin, elevated levels have been reported in iPAH patients compared to healthy controls.144 Likewise, soluble intercellular adhesion molecule-1 (ICAM-1) levels in serum are significantly higher in children with PAH secondary to CHD compared to children with CHD without associated PAH, or in a healthy control group.145,146 ICAM-1 levels are also increased in iPAH patients and patients with PAH associated with connective tissue disease (PAH-CTD), whereas circulating levels of soluble vascular cell adhesion protein 1 (VCAM-1) were elevated in PAH-CTD only.147 Conversely, soluble ICAM-1 levels decreased when young patients with CHD and PH were treated with the phosphodiesterase 5 inhibitor sildenafil.148 Interestingly, ICAM-1 expression on pulmonary arterial but not microvascular endothelial cells can be increased when endothelial cells are exposed to microparticles isolated from PH rats.149 In line with their proposed role as disseminators of inflammatory signaling in the lung,150 circulating microparticles may thus specifically promote inflammatory responses in the pulmonary arterial compartment in PAH.
In sickle cell patients, circulating levels of soluble vascular cell adhesion protein 1 (VCAM-1) show the most consistent correlation with PAH, while ICAM-1, E- and P-selectin show correlations and/or linearity in some but not all studies.151,152 In systemic sclerosis, circulating levels of soluble P-selectin, ICAM-1, VCAM-1 and platelet endothelial cell adhesion molecule-1 (PECAM-1) were found markedly elevated. While this effect was seemingly independent from the presence or absence of PAH, treatment of PAH with the endothelin-1 receptor antagonist bosentan significantly reduced the levels of all four adhesion molecules by up to 80%.153 Notably, since PECAM-1 acts as important endothelial shear sensor, increased cleavage of PECAM-1 from the endothelial surface may not only result in elevated levels of circulating soluble PECAM-1, but also impair microvascular adaptation to shear and thus, promote the development of occlusive vascular lesions in PAH.154
In addition to analyses of circulating soluble adhesion molecules, a few studies have addressed the expression of endothelial adhesion molecules in human tissue samples or primary endothelial cells, respectively. As such, Vengethasamy et al. showed that pulmonary microvascular endothelial cells isolated from lungs of transplanted PAH patients show an elevated ICAM-1 expression in BMPR2 mutation carriers compared to patients without mutations.155 Consistent with this finding, pulmonary microvascular endothelial cells from BMPR2 mutation carriers showed an enhanced adhesiveness for monocytes in response to inflammatory mediators, suggesting that BMPR2 mutations could increase the susceptibility to inflammatory cell recruitment in PAH.155 In line with these data, Le Hiress et al. detected an increased expression of the adhesion molecules ICAM-1, VCAM-1, and E-selectin on the endothelium of pulmonary arteries in human iPAH compared to controls that was associated with a higher number of peripheral blood mononuclear cells adhering to the endothelium.156
Taken together, these studies at large demonstrate an association of increased levels of circulating or endothelial adhesion molecules with PH, whereby the individual adhesion molecules may vary based on severity, time course, or underlying cause/class of the disease. Expectedly, adhesion molecule expression correlates with leukocyte interaction with the pulmonary endothelium in PH; importantly, however, experiments on the functional role of adhesion molecules in PH in vivo are to our knowledge lacking as of now.
The potential triggers of endothelial inflammation and adhesion molecule expression in PH are likely manifold, and would exceed the purpose of this review. It must suffice to point out that, for example, endothelial expression of P-selectin is stimulated by most of the classic triggers of PH in animal models, including monocrotaline,157 hydrostatic stress,158 or hypoxia159,160 (modelling clinical PH of groups 2, 3, and—arguably in case of MCT—group 1, respectively). Similarly, environmental stresses, endothelial dysfunction, or injury will inevitably upregulate endothelial adhesion molecules and, thus, facilitate inflammatory cell infiltration into the vessel wall and perivascular space as well as trigger formation and release of pro-inflammatory cytokines. Finally, it is tempting to speculate that DNA damage, mosaic chromosomal abnormalities, and microsatellite instabilities as previously detected in pulmonary arterial endothelial cell cultures from PAH lungs161–164 may facilitate expression of adhesion molecules and inflammatory cell recruitment, yet this concept remains to be experimentally tested.
Summary
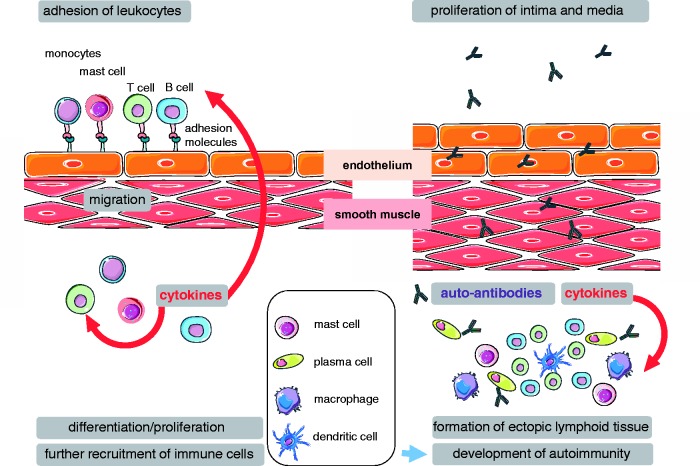
Intriguingly, the recognition of an inflammatory and autoimmune component in PH sheds new light on the pivotal role of the endothelium in this disease that extends beyond classic concepts of endothelial damage or dysfunction, endothelial apoptosis and proliferation, or anti-endothelial antibodies,26,165 but points to a key role of the endothelium and its adhesion molecules as first line in the regulation of inflammatory and immune cell recruitment and infiltration (Fig. 4). While respective mechanistic studies seem long overdue, premature enthusiasm for potential translational benefits should be cautioned. Some 25 years ago, therapeutic targeting of adhesion molecules for the treatment or prevention of inflammatory disorders such as sepsis or the adult respiratory distress syndrome (ARDS) had already received a huge surge of attention both from the scientific community and industry; yet, to this date no approved therapy based on these concepts has materialized. That notwithstanding, a better understanding of the how, where, and when of immune cell adhesion and recruitment in PH may provide important novel insights into the mechanisms of disease, and—potentially—identify new therapeutic targets.
Fig. 4.
Schematic summary of the proposed role of the endothelium and its adhesion molecules in PH. Endothelial adhesion and subsequent emigration of monocytes, mast cells, T cells, and B cells is an essential step for inflammatory and immune cell recruitment and infiltration. Following migration into the vascular wall and adventitial space, emigrated cells will further promote perivascular abundance of immune cells by perivascular differentiation and proliferation as well as by additional recruitment of immune cells via the release of pro-inflammatory and chemotactic mediators such as cytokines, alarmins, or leukotrienes. Perivascular accumulations of T cells, B cells, plasma cells, and dendritic cells organize into ectopic lymphoid tissue, which acts as source of autoantibodies and immune-cell derived mediators such as cytokines driving intimal and medial proliferation and, thus, vascular remodeling in PH.
2017 Grover Conference Series
This review article is part of the 2017 Grover Conference Series. The American Thoracic Society and the conference organizing committee gratefully acknowledge the educational grants provided for the support of this conference by Actelion Pharmaceuticals US, Inc., Gilead Sciences, Inc., and United Therapeutics Corporation. Additionally, the American Thoracic Society is grateful for the support of the Grover Conference by the American Heart Association, the Cardiovascular Medical Research and Education Fund, and the National Institutes of Health.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Arata Tabuchi http://orcid.org/0000-0003-3543-176X
References
- 1.Johnson SR, Granton JT. Pulmonary hypertension in systemic sclerosis and systemic lupus erythematosus. Eur Respir Rev 2011; 20: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigna JJ, Nansseu JR, Um LN, et al. Prevalence and incidence of pulmonary hypertension among HIV-infected people in Africa: a systematic review and meta-analysis. BMJ Open 2016; 6: e011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrlich P. Beiträge zur Kenntnis der granulierten Bindegewebszellen und der eosinophilen Leukozyten. Arch Anat Physiol 1879; 3: 166–169. [Google Scholar]
- 4.Dahal BK, Kosanovic D, Kaulen C, et al. Involvement of mast cells in monocrotaline-induced pulmonary hypertension in rats. Respir Res 2011; 12: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath D, Yacoub M. Lung mast cells in plexogenic pulmonary arteriopathy. J Clin Pathol 1991; 44: 1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitani Y, Ueda M, Maruyama K, et al. Mast cell chymase in pulmonary hypertension. Thorax 1999; 54: 88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savai R, Pullamsetti SS, Kolbe J, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 897–908. [DOI] [PubMed] [Google Scholar]
- 8.Heath D, Trueman T, Sukonthamarn P. Pulmonary mast cells in mitral stenosis. Cardiovasc Res 1969; 3: 467–471. [DOI] [PubMed] [Google Scholar]
- 9.Heath D. Mast cells in the human lung at high altitude. Int J Biometeorol 1992; 36: 210–213. [DOI] [PubMed] [Google Scholar]
- 10.Kay JM, Waymire JC, Grover RF. Lung mast cell hyperplasia and pulmonary histamine-forming capacity in hypoxic rats. Am J Physiol 1974; 226: 178–184. [DOI] [PubMed] [Google Scholar]
- 11.Williams A, Heath D, Kay JM, et al. Lung mast cells in rats exposed to acute hypoxia, and chronic hypoxia with recovery. Thorax 1977; 32: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker A, McMurtry IF, Alexander AF, et al. Lung mast cell density and distribution in chronically hypoxic animals. J Appl Physiol 1977; 42: 174–178. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann J, Yin J, Kukucka M, et al. Mast cells promote lung vascular remodeling in pulmonary hypertension. Eur Respir J 2011; 37: 1400–1410. [DOI] [PubMed] [Google Scholar]
- 14.Kay JM, Gillund TD, Heath D. Mast cells in the lungs of rats fed on Crotalaria spectabilis seeds. Am J Pathol 1967; 51: 1031–1044. [PMC free article] [PubMed] [Google Scholar]
- 15.Bartelds B, van Loon RL, Mohaupt S, et al. Mast cell inhibition improves pulmonary vascular remodeling in pulmonary hypertension. Chest 2012; 141: 651–660. [DOI] [PubMed] [Google Scholar]
- 16.Tuder RM, Groves B, Badesch DB, et al. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994; 144: 275–285. [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto RF, Higuchi ML, Aiello VD. Decreased numbers of T-lymphocytes and predominance of recently recruited macrophages in the walls of peripheral pulmonary arteries from 26 patients with pulmonary hypertension secondary to congenital cardiac shunts. Cardiovasc Pathol 2004; 13: 268–275. [DOI] [PubMed] [Google Scholar]
- 18.Minamino T, Christou H, Hsieh CM, et al. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A 2001; 98: 8798–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyata M, Sakuma F, Yoshimura A, et al. Pulmonary hypertension in rats. 1. Role of bromodeoxyuridine-positive mononuclear cells and alveolar macrophages. Int Arch Allergy Immunol 1995; 108: 281–286. [DOI] [PubMed] [Google Scholar]
- 20.Perros F, Dorfmuller P, Souza R, et al. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J 2007; 29: 462–468. [DOI] [PubMed] [Google Scholar]
- 21.Cool CD, Kennedy D, Voelkel NF, et al. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol 1997; 28: 434–442. [DOI] [PubMed] [Google Scholar]
- 22.Voelkel NF, Tuder RM. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur Respir J 1995; 8: 2129–2138. [DOI] [PubMed] [Google Scholar]
- 23.Song Y, Coleman L, Shi J, et al. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol 2008; 295: H677–H690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perros F, Dorfmuller P, Montani D, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 185: 311–321. [DOI] [PubMed] [Google Scholar]
- 25.Colvin KL, Cripe PJ, Ivy DD, et al. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med 2013; 188: 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breitling S, Hui Z, Zabini D, et al. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2017; 312: L710–L721. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet S, Rochefort G, Sutendra G, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A 2007; 104: 11418–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulin R, Meloche J, Bonnet S. STAT3 signaling in pulmonary arterial hypertension. JAKSTAT 2012; 1: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 30.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 31.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 32.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 33.Daley E, Emson C, Guignabert C, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 2008; 205: 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecker M, Zaslona Z, Kwapiszewska G, et al. Dysregulation of the IL-13 receptor system: a novel pathomechanism in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 182: 805–818. [DOI] [PubMed] [Google Scholar]
- 35.Balabanian K, Foussat A, Dorfmuller P, et al. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 36.Dorfmuller P, Zarka V, Durand-Gasselin I, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 534–539. [DOI] [PubMed] [Google Scholar]
- 37.Itoh T, Nagaya N, Ishibashi-Ueda H, et al. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology 2006; 11: 158–163. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez O, Marcos E, Perros F, et al. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2007; 176: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 39.Heresi GA, Aytekin M, Newman J, et al. CXC-chemokine ligand 10 in idiopathic pulmonary arterial hypertension: marker of improved survival. Lung 2010; 188: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meloche J, Lampron MC, Nadeau V, et al. Implication of inflammation and epigenetic readers in coronary artery remodeling in patients with pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2017; 37: 1513–1523. [DOI] [PubMed] [Google Scholar]
- 41.Meloche J, Potus F, Vaillancourt M, et al. Bromodomain-containing protein 4: The epigenetic origin of pulmonary arterial hypertension. Circ Res 2015; 117: 525–535. [DOI] [PubMed] [Google Scholar]
- 42.Paulin R, Sutendra G, Gurtu V, et al. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res 2015; 116: 56–69. [DOI] [PubMed] [Google Scholar]
- 43.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savale L, Tu L, Rideau D, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 2009; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian W, Jiang X, Sung YK, et al. Leukotrienes in pulmonary arterial hypertension. Immunol Res 2014; 58: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voelkel NF, Tuder RM, Wade K, et al. Inhibition of 5-lipoxygenase-activating protein (FLAP) reduces pulmonary vascular reactivity and pulmonary hypertension in hypoxic rats. J Clin Invest 1996; 97: 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian W, Jiang X, Tamosiuniene R, et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 2013; 5: 200ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright L, Tuder RM, Wang J, et al. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am J Respir Crit Care Med 1998; 157: 219–229. [DOI] [PubMed] [Google Scholar]
- 49.Yokomizo T. Two distinct leukotriene B4 receptors, BLT1 and BLT2. J Biochem 2015; 157: 65–71. [DOI] [PubMed] [Google Scholar]
- 50.Qian J, Tian W, Jiang X, et al. Leukotriene B4 activates pulmonary artery adventitial fibroblasts in pulmonary hypertension. Hypertension 2015; 66: 1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdul-Salam VB, Paul GA, Ali JO, et al. Identification of plasma protein biomarkers associated with idiopathic pulmonary arterial hypertension. Proteomics 2006; 6: 2286–2294. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Zhang Y, Li N, et al. Potential diagnostic biomarkers in serum of idiopathic pulmonary arterial hypertension. Respir Med 2009; 103: 1801–1806. [DOI] [PubMed] [Google Scholar]
- 53.Bauer EM, Zheng H, Comhair S, et al. Complement C3 deficiency attenuates chronic hypoxia-induced pulmonary hypertension in mice. PLoS One 2011; 6: e28578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawksworth OA, Li XX, Coulthard LG, et al. New concepts on the therapeutic control of complement anaphylatoxin receptors. Mol Immunol 2017; 89: 36–43. [DOI] [PubMed] [Google Scholar]
- 55.Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov 2015; 14: 857–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuno S, Farkas L, Al HA, et al. Severe pulmonary arterial hypertension induced by SU5416 and ovalbumin immunization. Am J Respir Cell Mol Biol 2012; 47: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sayed BA, Christy A, Quirion MR, et al. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol 2008; 26: 705–739. [DOI] [PubMed] [Google Scholar]
- 58.Theoharides TC, Kempuraj D, Tagen M, et al. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev 2007; 217: 65–78. [DOI] [PubMed] [Google Scholar]
- 59.Kishimoto T, Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol 1988; 6: 485–512. [DOI] [PubMed] [Google Scholar]
- 60.Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol 1992; 62: S60–S65. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka M, Miyajima A. Oncostatin M, a multifunctional cytokine. Rev Physiol Biochem Pharmacol 2003; 149: 39–52. [DOI] [PubMed] [Google Scholar]
- 62.Salamon P, Shoham NG, Puxeddu I, et al. Human mast cells release oncostatin M on contact with activated T cells: possible biologic relevance. J Allergy Clin Immunol 2008; 121: 448–455. [DOI] [PubMed] [Google Scholar]
- 63.Mozaffarian A, Brewer AW, Trueblood ES, et al. Mechanisms of oncostatin M-induced pulmonary inflammation and fibrosis. J Immunol 2008; 181: 7243–7253. [DOI] [PubMed] [Google Scholar]
- 64.Weldy C, Bea F, Wijelath E, et al. Oncostatin M is elevated in the plasma of patients with pulmonary arterial hypertension, but not in patients with ischemic heart disease or dilated cardiomyopathy: Insight into mechanisms in vitro. Circulation 2011; 124: A13449. [Google Scholar]
- 65.Botelho FM, Rangel-Moreno J, Fritz D, et al. Pulmonary expression of oncostatin M (OSM) promotes inducible BALT formation independently of IL-6, despite a role for IL-6 in OSM-driven pulmonary inflammation. J Immunol 2013; 191: 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonnet S, Provencher S, Guignabert C, et al. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med 2017; 195: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farha S, Sharp J, Asosingh K, et al. Mast cell number, phenotype, and function in human pulmonary arterial hypertension. Pulm Circ 2012; 2: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meloche J, Renard S, Provencher S, et al. Anti-inflammatory and immunosuppressive agents in PAH. Handb Exp Pharmacol 2013; 218: 437–476. [DOI] [PubMed] [Google Scholar]
- 69.Miyata M, Sakuma F, Ito M, et al. Athymic nude rats develop severe pulmonary hypertension following monocrotaline administration. Int Arch Allergy Immunol 2000; 121: 246–252. [DOI] [PubMed] [Google Scholar]
- 70.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, et al. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 2007; 175: 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tamosiuniene R, Tian W, Dhillon G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 2011; 109: 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ray A, Basu S, Williams CB, et al. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol 2012; 188: 3188–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen LC, Delgado JC, Jensen PE, et al. Direct expansion of human allospecific FoxP3+CD4+ regulatory T cells with allogeneic B cells for therapeutic application. J Immunol 2009; 183: 4094–4102. [DOI] [PubMed] [Google Scholar]
- 74.Reichardt P, Dornbach B, Rong S, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood 2007; 110: 1519–1529. [DOI] [PubMed] [Google Scholar]
- 75.Shah S, Qiao L. Resting B cells expand a CD4+CD25+Foxp3+ Treg population via TGF-b3. Eur J Immunol 2008; 38: 2488–2498. [DOI] [PubMed] [Google Scholar]
- 76.Zheng J, Liu Y, Lau YL, et al. CD40-activated B cells are more potent than immature dendritic cells to induce and expand CD4+ regulatory T cells. Cell Mol Immunol 2010; 7: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoehlig K, Shen P, Lampropoulou V, et al. Activation of CD4+Foxp3+ regulatory T cells proceeds normally in the absence of B cells during EAE. Eur J Immunol 2012; 42: 1164–1173. [DOI] [PubMed] [Google Scholar]
- 78.Marino E, Villanueva J, Walters S, et al. CD4+CD25+ T-cells control autoimmunity in the absence of B-cells. Diabetes 2009; 58: 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu S, Ellis JS, Dunn R, et al. Transient depletion of B cells in young mice results in activation of regulatory T cells that inhibit development of autoimmune disease in adults. Int Immunol 2012; 24: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stasi R, Cooper N, Del PG, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 2008; 112: 1147–1150. [DOI] [PubMed] [Google Scholar]
- 81.Ulrich S, Taraseviciene-Stewart L, Huber LC, et al. Peripheral blood B lymphocytes derived from patients with idiopathic pulmonary arterial hypertension express a different RNA pattern compared with healthy controls: a cross sectional study. Respir Res 2008; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hofmann J, Greter M, Du PL, et al. B-cells need a proper house, whereas T-cells are happy in a cave: the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol 2010; 31: 144–153. [DOI] [PubMed] [Google Scholar]
- 83.Perros F, Dorfmuller P, Montani D, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 185: 311–321. [DOI] [PubMed] [Google Scholar]
- 84.Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144: 1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest 2010; 138: 1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobak S, Kalkan S, Kirilmaz B, et al. Pulmonary arterial hypertension in patients with primary Sjogren's syndrome. Autoimmune Dis 2014; 2014: 710401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakagawa N, Osanai S, Ide H, et al. Severe pulmonary hypertension associated with primary Sjogren's syndrome. Intern Med 2003; 42: 1248–1252. [DOI] [PubMed] [Google Scholar]
- 88.Quismorio FP, Jr, Sharma O, Koss M, et al. Immunopathologic and clinical studies in pulmonary hypertension associated with systemic lupus erythematosus. Semin Arthritis Rheum 1984; 13: 349–359. [DOI] [PubMed] [Google Scholar]
- 89.Karmochkine M, Cacoub P, Dorent R, et al. High prevalence of antiphospholipid antibodies in precapillary pulmonary hypertension. J Rheumatol 1996; 23: 286–290. [PubMed] [Google Scholar]
- 90.Rich S, Kieras K, Hart K, et al. Antinuclear antibodies in primary pulmonary hypertension. J Am Coll Cardiol 1986; 8: 1307–1311. [DOI] [PubMed] [Google Scholar]
- 91.Yanai-Landau H, Amital H, Bar-Dayan Y, et al. Autoimmune aspects of primary pulmonary hypertension. Pathobiology 1995; 63: 71–75. [DOI] [PubMed] [Google Scholar]
- 92.Tamby MC, Humbert M, Guilpain P, et al. Antibodies to fibroblasts in idiopathic and scleroderma-associated pulmonary hypertension. Eur Respir J 2006; 28: 799–807. [DOI] [PubMed] [Google Scholar]
- 93.Arends SJ, Damoiseaux J, Duijvestijn A, et al. Prevalence of anti-endothelial cell antibodies in idiopathic pulmonary arterial hypertension. Eur Respir J 2010; 35: 923–925. [DOI] [PubMed] [Google Scholar]
- 94.Tamby MC, Chanseaud Y, Humbert M, et al. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax 2005; 60: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morse JH, Antohi S, Kasturi K, et al. Fine specificity of anti-fibrillin-1 autoantibodies in primary pulmonary hypertension syndrome. Scand J Immunol 2000; 51: 607–611. [DOI] [PubMed] [Google Scholar]
- 96.Becker MO, Kill A, Kutsche M, et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med 2014; 190: 808–817. [DOI] [PubMed] [Google Scholar]
- 97.Colvin KL, Cripe PJ, Ivy DD, et al. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med 2013; 188: 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.La Cava A. Putting together the autoimmunity puzzle. J Clin Invest 2015; 125: 2184–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science 2003; 301: 1374–1377. [DOI] [PubMed] [Google Scholar]
- 100.Casellas R, Shih TA, Kleinewietfeld M, et al. Contribution of receptor editing to the antibody repertoire. Science 2001; 291: 1541–1544. [DOI] [PubMed] [Google Scholar]
- 101.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol 2004; 5: 645–650. [DOI] [PubMed] [Google Scholar]
- 102.Hippen KL, Schram BR, Tze LE, et al. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J Immunol 2005; 175: 909–916. [DOI] [PubMed] [Google Scholar]
- 103.Liang Y, Pan HF, Ye DQ. Therapeutic potential of STAT4 in autoimmunity. Expert Opin Ther Targets 2014; 18: 945–960. [DOI] [PubMed] [Google Scholar]
- 104.Ebihara S, Date F, Dong Y, et al. Interleukin-17 is a critical target for the treatment of ankylosing enthesitis and psoriasis-like dermatitis in mice. Autoimmunity 2015; 48: 259–266. [DOI] [PubMed] [Google Scholar]
- 105.Cully M. Connective tissue diseases: HMGB1 helps elicit anti-dsDNA antibody production in SLE. Nat Rev Rheumatol 2013; 9: 321. [DOI] [PubMed] [Google Scholar]
- 106.Nogueira-Machado JA, de Oliveira Volpe CM. HMGB-1 as a target for inflammation controlling. Recent Pat Endocr Metab Immune Drug Discov 2012; 6: 201–209. [DOI] [PubMed] [Google Scholar]
- 107.Pan HF, Wu GC, Li WP, et al. High Mobility Group Box 1: a potential therapeutic target for systemic lupus erythematosus. Mol Biol Rep 2010; 37: 1191–1195. [DOI] [PubMed] [Google Scholar]
- 108.Bauer EM, Shapiro R, Zheng H, et al. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med 2012; 18: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zabini D, Crnkovic S, Xu H, et al. High-mobility group box-1 induces vascular remodelling processes via c-Jun activation. J Cell Mol Med 2015; 19: 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sadamura-Takenaka Y, Ito T, Noma S, et al. HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS One 2014; 9: e102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang PS, Kim DH, Lee YJ, et al. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir Res 2014; 15: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Palmblad K, Schierbeck H, Sundberg E, et al. High systemic levels of the cytokine-inducing HMGB1 isoform secreted in severe macrophage activation syndrome. Mol Med 2014; 20: 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.El Kasmi KC, Pugliese SC, Riddle SR, et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 2014; 193: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sawada H, Saito T, Nickel NP, et al. Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J Exp Med 2014; 211: 263–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian W, Jiang X, Tamosiuniene R, et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 2013; 5: 200ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xi X, Liu S, Shi H, et al. Serum-glucocorticoid regulated kinase 1 regulates macrophage recruitment and activation contributing to monocrotaline-induced pulmonary arterial hypertension. Cardiovasc Toxicol 2014; 14: 368–378. [DOI] [PubMed] [Google Scholar]
- 117.Vergadi E, Chang MS, Lee C, et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 2011; 123: 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Savai R, Pullamsetti SS, Kolbe J, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 897–908. [DOI] [PubMed] [Google Scholar]
- 119.Avalos AM, Kiefer K, Tian J, et al. RAGE-independent autoreactive B cell activation in response to chromatin and HMGB1/DNA immune complexes. Autoimmunity 2010; 43: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McDonnell M, Liang Y, Noronha A, et al. Systemic Toll-like receptor ligands modify B-cell responses in human inflammatory bowel disease. Inflamm Bowel Dis 2011; 17: 298–307. [DOI] [PubMed] [Google Scholar]
- 121.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007; 8: 487–496. [DOI] [PubMed] [Google Scholar]
- 122.Wen Z, Xu L, Chen X, et al. Autoantibody induction by DNA-containing immune complexes requires HMGB1 with the TLR2/microRNA-155 pathway. J Immunol 2013; 190: 5411–5422. [DOI] [PubMed] [Google Scholar]
- 123.Li JH, Zhao B, Zhu XH, et al. Blockade of extracellular HMGB1 suppresses xenoreactive B cell responses and delays acute vascular xenogeneic rejection. Am J Transplant 2015; 15: 2062–2074. [DOI] [PubMed] [Google Scholar]
- 124.Urbonaviciute V, Voll RE. High-mobility group box 1 represents a potential marker of disease activity and novel therapeutic target in systemic lupus erythematosus. J Intern Med 2011; 270: 309–318. [DOI] [PubMed] [Google Scholar]
- 125.Meloche J, Courchesne A, Barrier M, et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc 2013; 2: e005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meloche J, Paulin R, Courboulin A, et al. RAGE-dependent activation of the oncoprotein Pim1 plays a critical role in systemic vascular remodeling processes. Arterioscler Thromb Vasc Biol 2011; 31: 2114–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Crossno JT, Jr, Garat CV, Reusch JE, et al. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 2007; 292: L885–L897. [DOI] [PubMed] [Google Scholar]
- 128.Davie NJ, Crossno JT, Jr, Frid MG, et al. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 2004; 286: L668–L678. [DOI] [PubMed] [Google Scholar]
- 129.Frid MG, Brunetti JA, Burke DL, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 2006; 168: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pabst R, Tschernig T. Perivascular capillaries in the lung: an important but neglected vascular bed in immune reactions?. J Allergy Clin Immunol 2002; 110: 209–214. [DOI] [PubMed] [Google Scholar]
- 131.Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7: 678–689. [DOI] [PubMed] [Google Scholar]
- 132.Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol 2014; 184: 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuebler WM. Selectins revisited: the emerging role of platelets in inflammatory lung disease. J Clin Invest 2006; 116: 3106–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sriramarao P, Anderson W, Wolitzky BA, et al. Mouse bone marrow-derived mast cells roll on P-selectin under conditions of flow in vivo. Lab Invest 1996; 74: 634–643. [PubMed] [Google Scholar]
- 135.Steegmaier M, Blanks JE, Borges E, et al. P-selectin glycoprotein ligand-1 mediates rolling of mouse bone marrow-derived mast cells on P-selectin but not efficiently on E-selectin. Eur J Immunol 1997; 27: 1339–1345. [DOI] [PubMed] [Google Scholar]
- 136.Li X, Abdi K, Rawn J, et al. LFA-1 and L-selectin regulation of recirculating lymphocyte tethering and rolling on lung microvascular endothelium. Am J Respir Cell Mol Biol 1996; 14: 398–406. [DOI] [PubMed] [Google Scholar]
- 137.Schmidt EP, Kuebler WM, Lee WL, et al. Adhesion molecules: master controllers of the circulatory system. Compr Physiol 2016; 6: 945–973. [DOI] [PubMed] [Google Scholar]
- 138.Sakamaki F, Kyotani S, Nagaya N, et al. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation 2000; 102: 2720–2725. [DOI] [PubMed] [Google Scholar]
- 139.Semenov AV, Kogan-Ponomarev MI, Ruda MI, et al. [Soluble P-selectin - a marker of platelet activation and vessel wall injury: increase of soluble P-selectin in plasma of patients with myocardial infarction, massive atherosclerosis and primary pulmonary hypertension]. Ter Arkh 2000; 72: 15–20. [PubMed] [Google Scholar]
- 140.Yaoita N, Shirakawa R, Fukumoto Y, et al. Platelets are highly activated in patients of chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol 2014; 34: 2486–2494. [DOI] [PubMed] [Google Scholar]
- 141.Li XF, Song CH, Sheng HZ, et al. P-selectin gene polymorphism associates with pulmonary hypertension in congenital heart disease. Int J Clin Exp Pathol 2015; 8: 7189–7195. [PMC free article] [PubMed] [Google Scholar]
- 142.Al-Naamani N, Palevsky HI, Lederer DJ, et al. Prognostic significance of biomarkers in pulmonary arterial hypertension. Ann Am Thorac Soc 2016; 13: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Richter MJ, Schermuly R, Seeger W, et al. Relevance of angiopoietin-2 and soluble P-selectin levels in patients with pulmonary arterial hypertension receiving combination therapy with oral treprostinil: a FREEDOM-C2 biomarker substudy. Pulm Circ 2016; 6: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lapa M, Acencio MM, Farias AQ, et al. Selectins and platelet-derived growth factor (PDGF) in schistosomiasis-associated pulmonary hypertension. Lung 2014; 192: 981–986. [DOI] [PubMed] [Google Scholar]
- 145.Oguz MM, Oguz AD, Sanli C, et al. Serum levels of soluble ICAM-1 in children with pulmonary artery hypertension. Tex Heart Inst J 2014; 41: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sungprem K, Khongphatthanayothin A, Kiettisanpipop P, et al. Serum level of soluble intercellular adhesion molecule-1 correlates with pulmonary arterial pressure in children with congenital heart disease. Pediatr Cardiol 2009; 30: 472–476. [DOI] [PubMed] [Google Scholar]
- 147.Calvier L, Legchenko E, Grimm L, et al. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart 2016; 102: 390–396. [DOI] [PubMed] [Google Scholar]
- 148.Zorzanelli L, Maeda N, Clave M, et al. Relation of cytokine profile to clinical and hemodynamic features in young patients with congenital heart disease and pulmonary hypertension. Am J Cardiol 2017; 119: 119–125. [DOI] [PubMed] [Google Scholar]
- 149.Blair LA, Haven AK, Bauer NN. Circulating microparticles in severe pulmonary arterial hypertension increase intercellular adhesion molecule-1 expression selectively in pulmonary artery endothelium. Respir Res 2016; 17: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012; 303: L364–L381. [DOI] [PubMed] [Google Scholar]
- 151.Kato GJ, Martyr S, Blackwelder WC, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol 2005; 130: 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klings ES, Anton BD, Rosenman D, et al. Pulmonary arterial hypertension and left-sided heart disease in sickle cell disease: clinical characteristics and association with soluble adhesion molecule expression. Am J Hematol 2008; 83: 547–553. [DOI] [PubMed] [Google Scholar]
- 153.Iannone F, Riccardi MT, Guiducci S, et al. Bosentan regulates the expression of adhesion molecules on circulating T cells and serum soluble adhesion molecules in systemic sclerosis-associated pulmonary arterial hypertension. Ann Rheum Dis 2008; 67: 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Szulcek R, Happe CM, Rol N, et al. Delayed microvascular shear adaptation in pulmonary arterial hypertension. role of platelet endothelial cell adhesion molecule-1 cleavage. Am J Respir Crit Care Med 2016; 193: 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vengethasamy L, Hautefort A, Tielemans B, et al. BMPRII influences the response of pulmonary microvascular endothelial cells to inflammatory mediators. Pflugers Arch 2016; 468: 1969–1983. [DOI] [PubMed] [Google Scholar]
- 156.Le Hiress M, Tu L, Ricard N, et al. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension. role of the macrophage migration inhibitory factor/CD74 complex. Am J Respir Crit Care Med 2015; 192: 983–997. [DOI] [PubMed] [Google Scholar]
- 157.Hironaka E, Hongo M, Sakai A, et al. Serotonin receptor antagonist inhibits monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Cardiovasc Res 2003; 60: 692–699. [DOI] [PubMed] [Google Scholar]
- 158.Kuebler WM, Ying X, Singh B, et al. Pressure is proinflammatory in lung venular capillaries. J Clin Invest 1999; 104: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kiefmann R, Rifkind JM, Nagababu E, et al. Red blood cells induce hypoxic lung inflammation. Blood 2008; 111: 5205–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sarada S, Himadri P, Mishra C, et al. Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema. Exp Biol Med (Maywood) 2008; 233: 1088–1098. [DOI] [PubMed] [Google Scholar]
- 161.Aldred MA, Comhair SA, Varella-Garcia M, et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 182: 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen PI, Cao A, Miyagawa K, et al. Amphetamines promote mitochondrial dysfunction and DNA damage in pulmonary hypertension. JCI Insight 2017; 2: e90427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ranchoux B, Meloche J, Paulin R, et al. DNA damage and pulmonary hypertension. Int J Mol Sci 2016; 17: E990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yeager ME, Halley GR, Golpon HA, et al. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 2001; 88: E2–E11. [DOI] [PubMed] [Google Scholar]
- 165.Tamby MC, Chanseaud Y, Humbert M, et al. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax 2005; 60: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]