Abstract
Background:
To determine the prevalence and nature of potential drug–drug interactions (DDIs) with direct oral anticoagulants (DOACs) in elderly hospitalized patients.
Methods:
This was a retrospective observational study. Inclusion criteria were: aged over 65 years; taking apixaban, rivaroxaban or dabigatran; and admitted to the Repatriation General Hospital between April 2014 and July 2015. A list of clinically relevant ‘perpetrator’ drugs was compiled from product information, the Australian Medicines Handbook, the Australian National Prescribing Service resources, and local health network guidelines. The prevalence and nature of potential DDIs with DOACs was determined by comparing inpatient drug charts with the list of perpetrator drugs.
Results:
There were 122 patients in the study with a mean age of 82 years. Most patients had nonvalvular atrial fibrillation and were taking DOACs to prevent thrombotic stroke (83%). Overall, 45 patients (37%) had a total of 54 potential DDIs. Thirty-five patients had potential pharmacodynamic DDIs with antidepressants, nonsteroidal anti-inflammatory drugs and antiplatelets (35/122, 29%). Nineteen patients had potential pharmacokinetic DDIs (19/122, 16%). Of these, 68% (13/19) were taking drugs that increase DOAC plasma concentrations (amiodarone, erythromycin, diltiazem or verapamil) and 32% (6/19) were taking drugs that decrease DOAC plasma concentrations (carbamazepine, primidone or phenytoin). There were no cases of patients taking contraindicated interacting drugs.
Discussion:
Potential DDIs with DOACs in elderly hospital inpatients are relatively common, particularly interactions that may increase the risk of bleeding. The risk–benefit ratio of DOACs in elderly patients on polypharmacy should always be carefully considered.
Keywords: bleeding, direct oral anticoagulants, DOACs, drug–drug interactions, selective serotonin reuptake inhibitors
Introduction
The rapid increase in direct oral anticoagulant (DOAC) use has raised concern in clinical practice about safety in patients who were not well represented in the randomized controlled trials (RCTs). In particular, the risk of bleeding in patients with chronic kidney disease, those with multiple comorbidities, the elderly, the frail, and in patients taking polypharmacy (defined as at least five concomitant drugs).1 Polypharmacy is a well known risk factor for adverse drug reactions (ADRs) that result from drug–drug interactions (DDIs).2 There are two major types of DDIs. Pharmacokinetic DDIs (PK-DDIs) occur when the concentration of the ‘victim’ drug is altered by the introduction of a ‘perpetrator’, altering how much and for how long the victim is present at the active site, and pharmacodynamic DDIs (PD-DDIs) occur when interacting drugs have either additive or opposing pharmacological effects.3 Drug interactions with DOACs may arise via pharmacokinetic or pharmacodynamic mechanisms. The pharmacokinetics of DOACs is dependent to varying degrees on gastrointestinal and hepatic P-glycoprotein (P-gp) and cytochrome P4503A (CYP3A), the activities of which can be altered significantly by commonly used drugs.4 The pharmacodynamics of DOACs can be enhanced by several drug classes, including other anticoagulants, antiplatelets, nonsteroidal anti-inflammatory drugs (NSAIDs) and the selective serotonin and selective noradrenaline reuptake inhibitors (SSRIs/SNRIs).5–7 Table 1 summarizes the important pharmacological properties of the DOACs currently available in Australia.
Table 1.
| Rivaroxaban | Apixaban | Dabigatran | |
|---|---|---|---|
| Indications | Nonvalvular AF Prevention/treatment of VTE Prevention of VTE after elective hip or knee replacement |
Nonvalvular AF Prevention/treatment of VTE Prevention of VTE after elective hip or knee replacement |
Nonvalvular AF Prevention/treatment of VTE Prevention of VTE after elective hip or knee replacement |
| Pharmacodynamics | Factor Xa inhibitors Selectively inhibit factor Xa, blocking thrombin production, conversion of fibrinogen to fibrin, and thrombus development |
Thrombin (IIa) inhibitor Reversibly inhibit both free and fibrin-bound thrombin, preventing conversion of fibrinogen to fibrin, preventing thrombus formation |
|
| Bioavailability (%) | ~66 to >80% (food) | ~50 | ~7 |
| Half life (h) | 7–11 | 8–15 | 12–14 |
| Mechanisms of clearance | Hepatic (65%) Renal (35%) |
Hepatic (75% ) Renal (25%) |
Renal (80%) Hepatic (20%) |
| Dosing recommendations | Once daily dosing Prevention of VTE after hip/knee replacement: 10 mg daily Acute VTE: 15 mg twice daily for 3 weeks then 20 mg daily Prevention of emboli in AF: 20 mg daily (15 mg daily if CrCl 30–49 ml/min) |
Twice daily dosing Prevention of VTE after hip/knee replacement: 2.5 mg twice daily Acute VTE: 10 mg twice daily for 7 days then 5 mg twice daily Prevention of subsequent VTE: 2.5 mg twice daily Prevention of emboli in AF: 5 mg twice daily (2.5 mg twice daily if at least two of the following: weight <60 kg, age >80 years, serum creatinine >133 μmol/liter) Contraindicated with CrCl <25 ml/min |
Twice daily dosing Prevention of VTE after hip/knee replacement: initial dose 110 mg then 220 mg once daily (150 mg daily if CrCl 30–50 ml/min). AF, acute VTE and subsequent VTE: 150 mg twice daily (>75 years or increased risk major bleeding, or CrCl 30–50 ml/min, 110 mg twice daily) Contraindicated if CrCl <30 ml/min |
AF, atrial fibrillation; CrCl, creatinine clearance; VTE, venous thromboembolism.
Despite numerous phase I studies characterizing the changes in DOAC pharmacokinetics with P-gp or CYP3A inhibitors and inducers, as described in the product information for each drug, the clinical significance of many DDIs with DOACs is still unclear. Two post hoc analyses of RCTs with apixaban (ARISTOTLE) and rivaroxaban (ROCKET-AF) reported no significant impact of interacting drugs on bleeding risk or thrombosis, but in these analyses strong P-gp or CYP3A inhibitors and inducers were excluded, and the impact of PD-DDIs was not assessed.4,5 In contrast, other post hoc analyses of concomitant antiplatelet use in DOAC RCTs showed increased risks of major bleeding, with hazard ratios (HRs) of 1.60 [95% confidence interval (CI) 1.42–1.82] for single antiplatelet use and 2.31 (95% CI 1.79–2.98) for dual antiplatelet use in RE-LY (dabigatran), and a HR of 1.32 (95% CI 1.21–1.43) for aspirin use in ROCKET-AF (rivaroxaban).8,9 Several recent studies of DOACs in ‘real-world’ clinical settings have shown similar efficacy and safety to the RCTs, but these were not designed to investigate interacting drugs.10–15 Some data are available on clinical outcomes of specific PK-DDIs with DOACs but the evidence is conflicting. For example, amiodarone has been associated with increased odds of bleeding in patients taking rivaroxaban,16 and patients who had major bleeds on rivaroxaban appeared twice as likely to be taking a P-gp inhibitor with or without a CYP3A inhibitor.17 This contrasts two post hoc analyses of the ROCKET-AF and ARISTOTLE trials that found no significant difference for any bleeding outcome in patients taking rivaroxaban or apixaban with amiodarone respectively.18,19 To add to the debate over clinical relevance, there are several case reports about bleeding on DOACs following the commencement of drugs that inhibit P-gp or CYP3A, including amiodarone,20–22 and there are also case reports of decreased efficacy on CYP3A inducers such as phenytoin.23–27
A surrogate marker to identify safety concerns with DDIs in clinical practice is the reporting of ‘potential DDIs’. This is the review of medication regimens to search for theoretical DDIs, based only on knowledge of underlying mechanisms, or known DDIs, based on previously established clinical importance. After collating this literature on DOACs, between 40% and 88% of patients in various clinical settings (general medical units, orthopaedic surgery units, primary care, tertiary care etc.) have at least one potential DDI with DOACs.28–34 For example, one study showed that nearly 80% of hospitalized patients on dabigatran had potential PK-DDIs,32 whereas another showed that concomitant use of dabigatran with P-gp inhibitors occurred in 45% of patients.33 Likewise, in a study of rivaroxaban after major orthopaedic surgery, there was a high prevalence of potential PD-DDIs, particularly with NSAIDs (52% of patients), although concomitant use of CYP3A or P-gp inhibitors or inducers was very low (<5% of patients).34 Despite these data, the proportion of potential DDIs that cause actual DDIs and harm to patients on DOACs is unknown.
Increasing adult age is associated with polypharmacy due to comorbidities and an increased prevalence of ADRs caused by DDIs.35 Elderly patients may also have several DDIs considered clinically irrelevant individually but when taken together can result in serious ADRs. Given the widespread use of DOACs in the elderly, and increasing efforts to capture ‘real-world’ data about their safety, the aim of this study was to determine the prevalence and nature of potential DDIs with DOACs in hospitalized patients aged over 65 years.
Methods
This was a retrospective observational study of patient characteristics, clinical information, and drug charts in an electronic health record (the Enterprise Patient Information System, EPAS). Ethics approval was granted by the Southern Adelaide Clinical Human Research Ethics Committee (application number 324.15). All inpatients at the Repatriation General Hospital (RGH) in Adelaide who were prescribed a DOAC (apixaban, rivaroxaban or dabigatran) from April 2014 to July 2015 and were over 65 years of age were included. EPAS was searched to retrieve data on serum creatinine, height, weight, age, comorbidities and medications. Creatinine clearance was estimated using the optimized Cockcroft-Gault equation.36 Data were collated and entered in an Excel spreadsheet.
A list of drugs with the potential to cause clinically relevant DDIs with DOACs in Australia was compiled from various prescribing information resources (Table 2). The resources used were the Australian product information for each DOAC from the manufacturer;6,7,37 the Australian Medicines Handbook, which is the national drug formulary of Australia updated annually;38 the National Prescribing Service online resources, an Australian Government funded organization that provides evidence-based information to healthcare professionals and consumers;39 and the South Australian health guidelines on DAOC use, which were compiled by a senior clinical pharmacist in collaboration with medical consultants from relevant clinical units. Commercial DDI compendia were searched to check for any missed interacting drugs, but, because of the disparity between these compendia,40 they were not used as primary resources to generate the list. Drugs that cause at least fivefold increase in DOAC area under the plasma concentration–time curves (AUCs) are typically strong inhibitors of P-gp or CYP3A, and these drugs are all contraindicated interacting medications. Drugs that cause at least twofold but up to fivefold increases in DOAC AUC are typically moderate inhibitors of P-gp or CYP3A. Many of these drugs are not contraindicated, but prescribing advice is to use with caution. Drugs that are inducers of P-gp or CYP3A have highly variable effects on drug exposure due to time dependence and differences in study designs used for characterization, and no work has yet catalogued inducers according to changes in DAOC AUC. Interestingly, drug information resources are inconsistent with prescribing advice for DOACs in the presence of these inducers, with some resources stating that inducers are contraindicated,38 whereas others advise caution with an assessment of overall thrombotic risk.39 Medications that interact with DOACs through pharmacodynamic mechanisms are also considered to be used with caution, the exception being other anticoagulants which are contraindicated. When considering drug classes [e.g. human immunodeficiency virus (HIV) protease inhibitors, NSAIDs, SSRIs/SNRIs, anticoagulants, antiplatelets and proton pump inhibitors (PPIs)], all drugs in the class were considered equal as potential perpetrators of DDIs with DOACs.
Table 2.
| Dabigatran | Rivaroxaban and apixaban |
|---|---|
| Pharmacokinetic interactions | |
| Increase DOAC AUC > fivefold | |
|
Strong P-gp inhibitors
*
Itraconazole, ketoconazole, cyclosporine, dronedarone, tacrolimus |
Strong CYP3A and P-gp inhibitors
*
Itraconazole, ketoconazole, posaconazole, voriconazole, HIV protease inhibitors |
| Increase DOAC AUC ⩾ twofold but ⩽ fivefold | |
|
Moderate P-gp inhibitors
$
Amiodarone, clarithromycin, erythromycin, HIV protease inhibitors, quinidine, ticagrelor, verapamil† |
Moderate CYP3A and P-gp inhibitors
$
Amiodarone, cyclosporine, clarithromycin, diltiazem, dronedarone, erythromycin, fluconazole, quinidine, tacrolimus, verapamil |
| Decrease DOAC AUC with variable magnitude | |
|
P-gp or CYP3A inducers
‡
Phenytoin, carbamazepine, phenobarbitone, phenytoin, rifampicin, St John’s Wort | |
| Pharmacodynamic interactions | |
| Aspirin, NSAIDs, clopidogrel, ticagrelor, prasugrel, SSRIs/SNRIs, anticoagulants* | |
Please note that this table was compiled from various prescribing information sources relevant for Australian clinical practice including, but not exclusively, product information (see Methods). The guidance’s ‘contraindicated’, ‘use with caution’ and ‘combination not recommended’ were taken from the product information for DOACs in Australia but these designations may vary among geographical locations.
Contraindicated.
Use with caution.
Contraindicated if started simultaneously with dabigatran.
Combination not recommended.
AUC, area under the plasma concentration-time curve; CYP3A, cytochrome P4503A; DDI, drug–drug interaction; DOAC, direct oral anticoagulant; NSAID, nonsteroidal anti-inflammatory drug; P-gp, P-glycoprotein; SNRI, selective noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Drugs that were prescribed, dispensed and administered during the hospital admissions of patients taking DOACs were included in the analysis, except for stat (one-off) doses. Thus, potential interacting drugs had to be administered to patients on multiple occasions. These drugs were cross checked with the drugs in Table 2 to identify potential DDIs with DOACs and then categorized by the type and mechanism of the interaction. To compare the prevalence of potential DDIs with previous studies, two separate overall analyses were conducted, one including PPIs (omeprazole, esomeprazole, pantoprazole, lansoprazole and rabeprazole) and one excluding PPIs (i.e. only the drugs listed in Table 2). The rationale for this is that PPIs were previously considered as perpetrators of PK-DDIs with dabigatran.5 Data were analysed by simple statistics and expressed as percentages.
Results
Twenty-five individual drugs and five drug classes (HIV protease inhibitors, NSAIDs, SSRIs/SNRIs, antiplatelets and anticoagulants) were identified as potential perpetrators of DDIs with DOACs that are relevant for Australian clinical practice. Table 2 shows the list of interacting drugs, DDI type and mechanism, and the estimated changes in DAOC exposure for PK-DDIs.
The characteristics of patients in the study are summarized in Table 3. There were 122 patients with a mean age of 82 years (48.4% men and 51.6% women). Forty-nine (40%) patients were taking rivaroxaban, 50 (41%) were taking apixaban and 23 (19%) were taking dabigatran. Most patients had nonvalvular atrial fibrillation and were on DOACs to prevent thrombotic stroke (83%). The mean creatinine clearance was 44 ml/min and the mean CHADSVasc score was 4.83, which translates to a thrombotic stroke risk of 5–6% per year.
Table 3.
Patient characteristics.
| All(n = 122) | Rivaroxaban (n = 49) | Apixaban(n = 50) | Dabigatran (n = 23) | |
|---|---|---|---|---|
| Mean age, years (range) | 83 (65–98) | 83 (66–98) | 84 (65–97) | 79 (67–91) |
| Women | 63 (52) | 26 (53) | 25 (50) | 12 (52) |
| Mean weight, kg (range) | 74 (40–165) | 70 (59–125) | 73 (40–165) | 82 (45–113) |
| Mean serum creatinine concentration, µmol/liter (range) | 88 (38–238) | 82 (38–125) | 92 (38–238) | 92 (51–160) |
| Mean creatinine clearance, ml/min (range) | 44 (19–91) | 45 (19–91) | 41 (19–88) | 50 (34–83) |
| Anticoagulation indication: | ||||
| AF | 101 (83%) | 33 (67%) | 47 (94%) | 21 (91%) |
| VTE treatment | 4 (3%) | 3 (6%) | 1 (2%) | 0 |
| VTE prophylaxis | 12 (10%) | 11 (22%) | 0 | 0 |
| AF and VTE treatment | 5 (4%) | 0 | 0 | 1 (4%) |
| CHADSVasc score (range) | 4.83 (2–8) | 4.43 (2–8) | 5.36 (2–8) | 4.57 (2–7) |
AF, atrial fibrillation; VTE, venous thromboembolism.
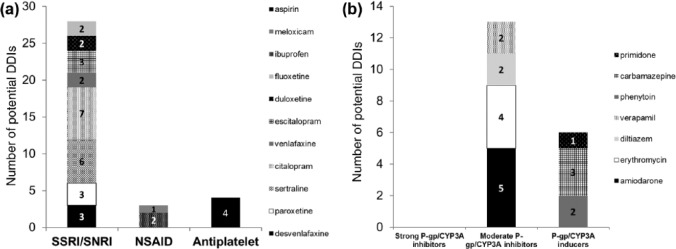
Overall, 45 patients (37%) had a total of 54 potential interactions. Thirty-five of the 122 patients had potential PD-DDIs (29%) and 19 patients had potential PK-DDIs (16%). Of the patients who had potential pharmacodynamic interactions, 80% (28/35) were taking SSRIs/SNRIs (desvenlafaxine, paroxetine, sertraline, citalopram, venlafaxine, escitalopram, duloxetine or fluoxetine), 8.6% (3/35) were taking NSAIDs (ibuprofen or meloxicam) and 11% (4/35) were taking aspirin [Figure 1(a)]. Of the patients who had potential pharmacokinetic interactions, 68% (13/19) were taking medications that increase DOAC plasma concentrations (amiodarone, erythromycin, diltiazem or verapamil) and 32% (6/19) were taking medications that decrease DOAC plasma concentrations (carbamazepine, primidone or phenytoin) [Figure 1(b)]. There were no cases of patients taking contraindicated interacting drugs. The rank order of prevalence of potential DDIs was rivaroxaban (88%) > dabigatran (52%) > apixaban 30%. When PPIs were included in the analysis, 18 patients had potential interactions with dabigatran (18/23, 78% of patients on dabigatran), to give an overall prevalence of patients with potential DDIs with DOACs of 42%.
Figure 1.
Potential pharmacodynamic drug–drug interactions (DDIs) (a) and pharmacokinetic DDIs (b) with direct oral anticoagulants (DOACs). CYP3A, cytochrome P4503A; NSAID, nonsteroidal anti-inflammatory drug; P-gp, P-glycoprotein; SNRI, selective noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
There were eight patients in the study who had more than one potential interaction with a DOAC (6.6%). Four of these patients had the combination of a PD-DDI and a PK-DDI with an inhibitor of P-gp or CYP3A. One patient was taking rivaroxaban with ibuprofen (NSAID) and citalopram (SSRI), one was taking apixaban with amiodarone (P-gp/CYP3A inhibitor) and phenytoin (P-gp/CYP3A inducer) and two patients had combinations of potential PD-DDIs and PK-DDIs, with apixaban–duloxetine (SNRI) and carbamazepine (P-gp/CYP3A inducer) in one, and ibuprofen and phenytoin in the other.
Discussion
This is the first study to investigate potential DDIs with DOACs exclusively in elderly hospitalized patients. The mean age was high (>82 years) and well above the exclusion cutoff of 65 years. Thirty-seven percent of patients had potential interactions, which is just below the lower limit of the range collated from other studies with DOACs in clinical settings (40–88%).28–34 Twice as many patients had potential PD-DDIs with DOACs compared with PK-DDIs, driven predominantly by concomitant use of SSRIs/SNRIs [Figure 1(a, b)].
The lower prevalence of patients with potential DDIs with DOACs in our study compared with previous work could be for several reasons. First, prescribers may be becoming more familiar with DOAC interactions as clinical experience with their use increases. Second, there may be heightened awareness of DDIs in the study population, the elderly, who are well known to have increased susceptibility to ADRs. Third, apixaban, rivaroxaban and dabigatran were studied here, whereas most of the comparator studies included only dabigatran.28–34 The prevalence difference could be explained because PPIs were classified as interacting drugs in several of the previous dabigatran studies, in which up to 64% of patients were taking dabigatran and PPIs together.32 The rationale for this classification is that the bioavailability of dabigatran is dependent on an acidic gastric environment, and pantoprazole decreased dabigatran absorption by 30% in a phase I healthy volunteer study and by an average of 12.5% in RE-LY.7 We also found very high concurrent use of dabigatran and PPIs (78% of patients). Indeed, when PPIs were included as perpetrators, this increased the overall prevalence of patients with potential interactions to 42%, consistent with the range of previous work (40–88%).28–34 However, the consensus now is that interactions between dabigatran and PPIs are not clinically important.5 Therefore, PPIs were excluded from the list of clinically relevant perpetrators (Table 2) and the final prevalence calculations. Fourth, other variations in perpetrator lists could result in prevalence differences, a known problem when comparing commercial DDI compendia.40 Fifth, the clinical setting and the types of cases can influence DDI risk. For example, acute medical units have a high patient turnover and wide patient demographic, and studies there would capture more patients taking contraindicated interacting drugs such as azole antifungals and HIV protease inhibitors. Finally, the availability of clinical pharmacology or clinical pharmacy support could also influence the likelihood of interacting drugs being coprescribed. This study was conducted at the RGH where clinical pharmacists attend all ward rounds and for each patient determine the medication history and conduct a full medication review.
There were fewer patients in the study with potential PK-DDIs compared with potential PD-DDIs, 16% versus 29% respectively. This was also reported in patients taking rivaroxaban after major orthopaedic surgery, when NSAIDs were coprescribed in 54% but the prevalence of potential PK-DDIs was only 4.6%.34 Other studies have shown similar frequencies of PD-DDIs, particularly due to coprescription of aspirin (47–60%).30,33,41 The comparably low prevalence of PD-DDIs in our study (29%) was largely due to minimal antiplatelet use (3.3%), possibly because prescribers were reluctant to use an antiplatelet and anticoagulant combination in elderly inpatients. Interestingly, potential interactions between DOACs and SSRIs/SNRIs were common, occurring in about a quarter of inpatients (28/122). Many prescribers may not be aware of the bleeding risks of SSRIs/SNRIs, especially when used in combination with antiplatelets or anticoagulants.42–45 One cohort study found that in patients with atrial fibrillation taking warfarin, bleeding rates were higher during periods of SSRI use compared with periods when they were not taken (2.32 per 100 person-years versus 1.35 per 100 person-years, p ⩽ 0.001).44 There are also some data about the risks of bleeding with coadministration of DOACs and SSRI/SNRIs. An analysis of the RE-LY trial showed an increased risk of bleeding when SSRIs were used in combination with dabigatran, but detail about the magnitude of this risk has not been published.7 The product information for apixaban suggests using the combination of apixaban and SSRIs/SNRIs with caution, presumably based on first principles since a reference to primary literature is not given.6 Taken together, these data represent relatively weak clinical evidence to support the SSRI/SNRI-DOAC interactions and further work is required to address the question of clinical significance in sufficient detail. Therefore, it is important to note that the high prevalence of DOAC and SSRI/SNRI combinations reported here in the elderly (28/122) represents potential rather than actual DDIs, and that withholding therapies based on this finding alone may be inappropriate.
No patients in the study took contraindicated interacting medications with DOACs. This is pleasing considering that Candel and colleagues28 and Trujillano and colleagues31 found 8.6% and 8.2% of patients in their respective studies took contraindicated drugs. Importantly, there were eight patients in the study who had greater than one potential DDI. How such interactions play out in clinical practice is complex and difficult to predict. The most concerning drug combinations give an increased risk of bleeding: concurrent PD-DDI and PK-DDI involving a P-gp or CYP3A inhibitor, since increases in DAOC concentration could augment already enhanced pharmacodynamics (this occurred in four patients); and concurrent use of multiple drugs with additive pharmacodynamic properties, for example one patient was on rivaroxaban, ibuprofen and citalopram.
This study was not designed to measure clinical events. It is therefore not possible to determine the proportion of potential DDIs with DOACs that subsequently contributed to any patient harm. This is a universal limitation of studies reporting potential DDIs. However, an important attribute of this work is the quick and cheap identification of prescribing patterns that could negatively impact drug safety. Indeed, our results were used to educate local prescribers, highlighting the high mean age of patients taking DOACs at the hospital and the frequently seen DOAC and SSRI/SNRI combinations.
In conclusion, potential DDIs with DOACs in elderly hospitalized patients are relatively common, occurring in about one third of patients. Most of these potential interactions may increase the risk of bleeding, either by additive pharmacological effects, by increasing DOAC exposure, or by a combination of both. The risk–benefit ratio of DOACs in elderly patients on polypharmacy should always be carefully considered.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Heather L. Forbes, Department of Pharmacy, Repatriation General Hospital, Daw Park, South Australia, 5041, Australia
Thomas M. Polasek, Department of Clinical Pharmacology, Flinders University School of Medicine, Bedford Park, South Australia 5042, and d3 Medicine, a Certara company, Parkville, Victoria, 3052 Australia.
References
- 1. Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30: 949–958. [DOI] [PubMed] [Google Scholar]
- 2. Guthrie B, Makubate B, Hernandez-Santiago V, et al. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med 2015; 13: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Snyder BD, Polasek TM, Doogue MP. Drug interactions: principles and practice. Aust Prescr 2012; 35: 85–88. [Google Scholar]
- 4. Polasek TM, Lin FP, Miners Jo, et al. Perpetrators of pharmacokinetic drug-drug interactions arising from altered cytochrome P450 activity: a criteria-based assessment. Br J Clin Pharmacol 2011; 5: 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellwig T, Gulseth M. Pharmacokinetic and pharmacodynamic drug interactions with new oral anticoagulants. Ann Pharmacother 2013; 47(11): 1478–1487. [DOI] [PubMed] [Google Scholar]
- 6. MIMS. Eliquis, https://www-mimsonline-com-au.salus.idm.oclc.org/Search/FullPI.aspx?ModuleName=Product%20Info&searchKeyword=eliquis&PreviousPage=~/Search/QuickSearch.aspx&SearchType=&ID=90400001_2 (2015, accessed 3 July 2016).
- 7. MIMS. Pradaxa, https://www.mimsonline.com.au/Search/AbbrPI.aspx?ModuleName=ProductInfosearchKeyword=pradaxaPreviousPage=~/Search/QuickSearch.aspxSearchType=ID=82340001_2 (2015, accessed 3 July 2016).
- 8. Jaspers Focks J, Brouwer MA, Wojdyla DM, et al. Polypharmacy and effects of apixaban versus warfarin in patients with atrial fibrillation: post hoc analysis of the ARISTOTLE trial. BMJ 2016; 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piccini JP, Hellkamp AS, Washam JB, et al. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation 2016; 133: 352–360. [DOI] [PubMed] [Google Scholar]
- 10. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 2013; 127: 634–640. [DOI] [PubMed] [Google Scholar]
- 11. Shah R, Hellkamp A, Lokhnygina Y, et al. Use of concomitant aspirin in patients with atrial fibrillation: findings from the ROCKET AF trial. Am Heart J 2016; 179:77–86. [DOI] [PubMed] [Google Scholar]
- 12. Romanelli RJ, Nolting L, Dolginsky M, et al. Dabigatran versus warfarin for atrial fibrillation in real-world clinical practice: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2016; 9: 126–134. [DOI] [PubMed] [Google Scholar]
- 13. Baron-Esquivias G, Fernandez-Aviles F, Atienza F, et al. Efficacy and safety of rivaroxaban in real-life patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2015; 13: 341–353. [DOI] [PubMed] [Google Scholar]
- 14. Ageno W, Mantovani LG, Haas S, et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): an international, prospective, non-interventional study. Lancet Haematol 2016; 3: e12–e21. [DOI] [PubMed] [Google Scholar]
- 15. Camm AJ, Amarenco P, Haas S, et al. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J 2016; 37: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howell D, Hoch E, Shulman EH, et al. Interaction between amiodarone and rivaroxaban and the risk of major bleeding. Heart Rhythm 2016; 13: S512. [Google Scholar]
- 17. Liang D, Peak J, Lumbert K, et al. Major bleed risk in patients on novel oral anticoagulants with PGP and/or CYP450 3A4 inhibitors. In: 45th Critical Care Congress of the Society of Critical Care Medicine, Orlando, FL, USA, 20–24 February 2016, paper no 630, p.159 Chicago: Critical Care Medicine. [Google Scholar]
- 18. Flaker G, Lopez RD, Hylek E, et al. Amiodarone, anticoagulation, and clinical events in patients with atrial fibrillation: insights from the ARISTOTLE trial. J Am Coll Cardiol 2014; 64: 1541–1550. [DOI] [PubMed] [Google Scholar]
- 19. Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Use and outcomes of antiarrhythmic therapy in patients with atrial fibrillation receiving oral anticoagulation: results from the ROCKET AF trial. Heart Rhythm 2014; 11: 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fountzilas C, George J, Levine R. Dabigatran overdose secondary to acute kidney injury and amiodarone use. N Z Med J 2013; 126: 110–112. [PubMed] [Google Scholar]
- 21. Fralick M, Juurlink DN, Marras T. Bleeding associated with coadministration of rivaroxaban and clarithromycin. CMAJ 2016; 188: 669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wannhoff A, Weiss KH, Schemmer P, et al. Increased levels of rivaroxaban in patients after liver transplantation treated with cyclosporine A. Transplantation 2014; 98: e12–e13. [DOI] [PubMed] [Google Scholar]
- 23. Hager N, Bolt J, Albers L, et al. Development of left atrial thrombus after coadministration of dabigatran etexilate and phenytoin. Can J Cardiol 2017; 33: 554.e13–554.e14. [DOI] [PubMed] [Google Scholar]
- 24. Risselada AJ, Visser MJ, van Roon E. Pulmonary embolism due to interaction between rivaroxaban and carbamazepine. Ned Tijdschr Geneeskd 2013; 157: A6568. [PubMed] [Google Scholar]
- 25. Serra W, Li Calzi M, Coruzzi P. Left atrial appendage thrombosis during therapy with rivaroxaban in elective cardioversion for permanent atrial fibrillation. Clin Pract 2015; 5: 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stöllberger C, Finsterer J. Prolonged anticoagulant activity of rivaroxaban in a polymorbid elderly female with non-convulsive epileptic state. Heart Lung 2014; 43: 262–263. [DOI] [PubMed] [Google Scholar]
- 27. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy 2016; 36: e5–e7. [DOI] [PubMed] [Google Scholar]
- 28. Candel MG, Sanz EU, Ruiz AT, et al. Potential interactions in patients treated with dabigatran, prevalence and therapeutic approach. In: 21st Congress of the EAHP, Vienna, Austria, 16–18 March 2016, paper no. PS-042, p.230 London: European Journal of Hospital Pharmacy. [Google Scholar]
- 29. Carter AA, Leblanc K, Woods A, et al. Utilization of dabigatran for atrial fibrillation at 3 tertiary care centres. Can J Hosp Pharm 2015; 68: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chin PK, Vella-Brincat JW, Walker SL, et al. Dosing of dabigatran etexilate in relation to renal function and drug interactions at a tertiary hospital. Intern Med J 2013; 43: 778–783. [DOI] [PubMed] [Google Scholar]
- 31. Trujillano Ruiz A, Urbieta Sanz E, Caballero Requejo C, et al. Drug interactions of the new oral anticoagulants. In: 42nd ESCP Symposium on Clinical Pharmacy: Implementation of Pharmacy Practice, Prague, Czech Republic, 16–18 October 2013, paper no. CP-PC20, p.1279 Netherlands: International Journal of Clinical Pharmacy. [Google Scholar]
- 32. Sidman E, Probst LA, Darko W, et al. Evaluation of dabigatran utilization and risk among hospitalized patients. Ann Pharmacother 2014; 48: 349–353. [DOI] [PubMed] [Google Scholar]
- 33. Armbruster AL, Buehler KS, Min SH, et al. Evaluation of dabigatran for appropriateness of use and bleeding events in a community hospital setting. Am Health Drug Benefits 2014; 7: 376–384. [PMC free article] [PubMed] [Google Scholar]
- 34. Kreutz R, Haas S, Holberg G, et al. Rivaroxaban compared with standard thromboprophylaxis after major orthopaedic surgery: co-medication interactions. Br J Clin Pharmacol 2016; 81(4): 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maher RL, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 2014; 1: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 37. MIMS. Xarelto, https://www-mimsonline-com-au.salus.idm.oclc.org/Search/FullPI.aspx?ModuleName=Product%20Info&searchKeyword=xarelto&PreviousPage=~/Search/QuickSearch.aspx&SearchType=&ID=81970001_2 (2015, accessed 3 July 2016)
- 38. Australian Medicines Handbook. Blood and Electrolytes [Internet]. Adelaide: Australian Medicines Handbook Pty Ltd, https://amhonline.amh.net.au/chapters/chap-07?menu=banner (2016, accessed 3 July 2016). [Google Scholar]
- 39. NPS Medicine Wise. Anticoagulants: interactions with dabigatran, rivaroxaban and apixaban, http://www.nps.org.au/medicines/heart-blood-and-blood-vessels/anti-clotting-medicines/for-individuals/anticoagulant-medicines/for-health-professionals/decision-tools/newer-anticoagulant-drug-interactions (2013, accessed 3 July 2016).
- 40. Sullivan K, Alborn J, Wigle PR, et al. Variations in dabigatran, rivaroxaban and warfarin drug interaction inclusion and severity level classifications among selected drug compendia. In: Annual Meeting of the American College of Clinical Pharmacy, Hollywood, FL, USA, 21–24 October 2012, paper no. 345, p.280 Massachusetts: Pharmacotherapy. [Google Scholar]
- 41. Lam J, Bress AP, Nutescu EA, et al. Evaluation of dabigatran prescribing practices at University of Illinois Medical Center. In: Annual Meeting of the American College of Clinical Pharmacy, Hollywood, FL, USA, 21–24 December 2012, paper no. 399, p.295 Massachusetts: Pharmacotherapy. [Google Scholar]
- 42. Hackam DG, Mrkobrada M. Selective serotonin reuptake inhibitors and brain hemorrhage: a meta-analysis. Neurology 2012; 79: 1862–1865. [DOI] [PubMed] [Google Scholar]
- 43. Labos C, Dasgupta K, Nedjar H, et al. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ 2011; 183: 1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quinn GR, Singer DE, Chang Y, et al. Effect of selective serotonin reuptake inhibitors on bleeding risk in patients with atrial fibrillation taking warfarin. Am J Cardiol 2014; 114: 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schelleman H, Brensinger CM, Bilker WB, et al. Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case-control study. PLoS One 2011; 6: e21447. [DOI] [PMC free article] [PubMed] [Google Scholar]