Abstract
Objectives
We aimed to develop a simple protocol for deriving insulin-producing cells (IPCs) from adipose-derived mesenchymal stem cells (ADSCs). We established a 2-step creation method and an acceleration strategy with a histone deacetylase inhibitor that promoted a pro–endocrine pancreatic lineage.
Methods
We seeded ADSCs in 96-well dishes and cultured in Dulbecco's modified Eagle's medium/F12 medium containing 1% fetal bovine serum, 1% B27 supplement, 1% N2 supplement, 50-ng/mL human activin A, and 10-nM exendin-4 for step 1 of differentiation (7 days). Then 10-mM nicotinamide and 50-ng/mL human hepatocyte growth factor, with or without 1 mM histone deacetylase inhibitor, were added for step 2 of differentiation (14 days). After the 2-step differentiation was complete, cell morphology, immunohistochemistry, messenger RNA expression, and function were investigated.
Results
Our new differentiation protocol with the histone deacetylase inhibitor significantly accelerated IPC differentiation compared with the conventional protocol without the histone deacetylase inhibitor (median, 21.6 vs 38.8 days; P < 0.05). It also improved the islet morphology score (P < 0.05) and the glucose stimulation index (3.1).
Conclusions
By applying our new and easy 2-step protocol using a histone deacetylase inhibitor, ADSCs may be an effective cell source for differentiation of IPCs.
Key Words: insulin-producing cell (IPC), adipose-derived mesenchymal stem cell (ADSC), valproic acid (VA), differentiation protocol, regenerative medicine
Islet transplantation (ITx) is one of the therapeutic options for type-1 diabetes mellitus and is now used clinically in the United States and Canada.1 However, ITx has some urgent issues that need to be addressed before wide-spread clinical use, including the requirement of a large islet yield2 and severe donor shortages in some countries, such as Japan.3 Islet shipping or changes of the allocation system may help to solve these issues; however, ethical and governmental controversies still remain. Therefore, development of a new insulin-producing cell source is required.
To solve these issues, several options have been considered and we have focused on stem cells for creating insulin-producing cells (IPCs), especially adipose-derived mesenchymal stem cells (ADSCs), as a new cell source.4,5 The reason we selected this cell type is that ADSCs can be obtained from the patient's own fat tissue with only a mildly invasive procedure and auto-ADSC transplantation has fewer ethical problems compared with the use of induced pluripotent stem or embryonic stem cells. Furthermore, induced pluripotent stem cells can have DNA damage that causes transformation and carcinogenesis.6 Indeed, ADSCs have already been clinically applied for repair of damage after surgery for breast cancer and regeneration of cardiovascular cells after acute myocardial infarction.7,8 Moreover, some reports indicate that ADSCs show superior differentiation into various cell types,9 leading us to select this cell source for our study.
We have also investigated the role of histone deacetylases (HDACs) in cancer10 and in transplantation.11 Briefly HDACs, in conjunction with histone acetyltransferases, control the level of acetylation on lysine residues in histones. Treatment of cells with an HDAC inhibitor (HDACi) such as trichostatin A or suberoylanilide hydroxamic acid leads to hyperacetylation of histones, resulting in a more open chromatin architecture and increased access for transcription factors.12 Histone deacetylases inhibition regulates gene expression as well as the functions of more than 50 transcription factors and nonhistone proteins,13 and we have used HDAC inhibition to accelerate cancer sphere formation. Furthermore, HDAC inhibition is reported to be strong driver of pancreatic cell lineage progenitors.14 Therefore, we investigated the use of an HDACi (valproic acid) for acceleration of IPC formation. Here, we established a new 2-step method for IPC production and an acceleration strategy with an HDACi that promotes the pro–endocrine pancreatic lineage.
MATERIALS AND METHODS
Adipose-Derived Mesenchymal Stem Cell Preparation (Step 1)
The protocols for creating IPCs are based on D'Amour et al,15 and we modified that protocol into a 2-step differentiation protocol. We purchased ADSCs (RAWMD-01001) from Cyagen Biosciences Inc (Santa Clara, Calif), and the ADSCs were cultured according to the manufacturer's guidelines. After thawing using established procedures, cells were cultured until passages 5 and 6, then 5 × 105 cells were seeded into 12-well ultra-low attachment plates (Sigma-Aldrich Japan Co, LLC, Tokyo, Japan). For step 1, cells were cultured for 7 days in a differentiation cocktail of Dulbecco's modified Eagle's medium/F12 (Thermo Fisher Scientific Inc, Waltham, Mass), 1% fetal bovine serum, 1% B27 supplement (Thermo Fisher Scientific Inc), 1% N2 supplement (Thermo Fisher Scientific Inc), 50-ng/mL activin A (Sigma-Aldrich Japan), and 10-nM exendin-4 (Sigma-Aldrich Japan), then these cells were subjected to step 2 (IPC differentiation phase, Fig. 1).
FIGURE 1.
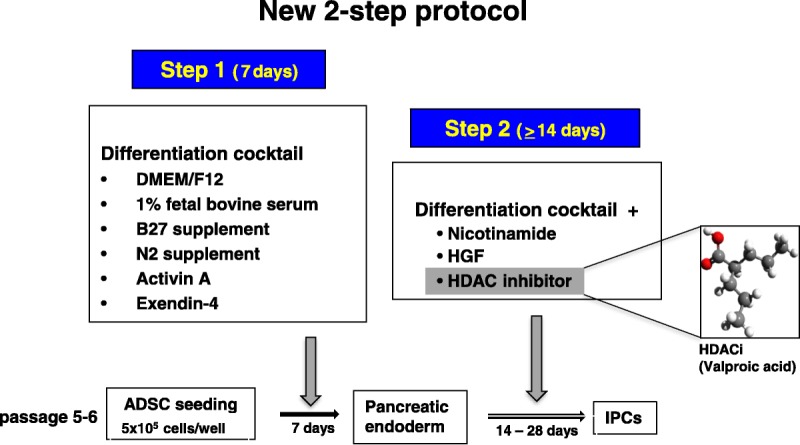
The scheme of the new 2-step protocol for generating IPCs from ADSCs.
Insulin-Producing Cell Differentiation (Step 2)
Briefly, in step 1 undifferentiated ADSC spheres were generated and in step 2, differentiated pancreatic endoderm was derived by culturing in the same differentiation medium as above, but with the addition of 50-ng/mL hepatocyte growth factor (HGF; Funakoshi Co, Ltd, Tokyo, Japan) and 10-mM nicotinamide (Sigma-Aldrich Japan), with or without HDACi (valproic acid; Fig. 1).
Cell Counts, Purity, and Viability
Cell number, purity, and viability were determined as described previously.2 Briefly, at least 3 samples of the generated cells were assessed for cell number and purity by staining with dithizone dye (Sigma-Aldrich Japan) dissolved in dimethyl sulfoxide (Burdick and Jackson, Morristown, NJ) and a propidium iodide (PI) staining kit (TAKARA Bio Inc, Tokyo, Japan), and assessment by fluorescence microscopy.
Morphological Analysis
We assessed morphological changes of IPCs using a morphological score according to islet status. Insulin-producing cells were scored for shape (flat vs spherical), border (irregular vs well-rounded), integrity (fragmented vs solid/compact), uniformity of stain (not uniform vs perfectly uniform), and diameter (all < 100 μm vs more than 10% > 200 μm), as previously described.16,17
Immunohistochemical Staining
The cells were pelleted by centrifugation and incubated with primary antibody against insulin (aa287-299, LS-B129; LifeSpan BioSciences, Inc, Seattle, Wash) at a dilution of 1:100 in phosphate-buffered saline for 1 hour at room temperature. Then cells were incubated with biotinylated secondary antibody, followed by a streptavidin-biotin-horseradish peroxidase complex. Finally, positive staining was visualized with diaminobenzidine and cell nuclei were counterstained with Mayer's hematoxylin.
Quantitative Reverse Transcription-Polymerase Chain Reaction for Messenger RNA Expression
We investigated the gene expression profile of the transcription factor NeuroD1 and the endocrine hormone insulin as we reported previously.18 Total RNA was extracted from cells using an RNeasy Mini Kit (Qiagen, Hilden, Germany), and complementary DNA was synthesized from 2.5 μg of total RNA by reverse transcription using a SuperScript RT kit (Promega, Madison, Wis), following the manufacturer's instructions. Then, quantitative reverse transcription—polymerase chain reaction was performed using the Applied Biosystems 7500 real-time polymerase chain reaction system, TaqMan Gene Expression Assays on demand, and a TaqMan Universal Master Mix (gene-specific TaqMan probes on a StepOne Plus; Applied Biosystems, Foster City, Calif). NeuroD1 (NM_019218) and Insulin1 (NM_019129) primers (Qiagen) were used. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for normalization. Expression levels of all genes were calculated as a ratio to glyceraldehyde-3-phosphate dehydrogenase. Amplification data were analyzed with an Applied Biosystems Prism 7500 Sequence Detection System version 1.3.1 (Thermo Fisher Scientific Inc).
Glucose-Stimulated Insulin Secretion
The glucose stimulation index (SI) of IPCs was calculated as previously described.19 First, IPCs were cultured in RPMI-1640 medium with 5-mM glucose for 1 hour and then in medium containing 45-mM glucose for 1 hour, before being cultured in medium containing 5-mM glucose for an additional 1 hour. The insulin concentration of the supernatant was analyzed by enzyme-linked immunosorbent assay (AKRIN-011H, Shibayagi, Japan), with a microplate reader at a wavelength of 450 nm. Total DNA was extracted to determine the cell count. Then SI was calculated by dividing the amount of insulin secretion from the high-glucose incubation by the insulin secretion from the low-glucose incubation. Three independent experiments were undergone for this calculation.
Statistics
Descriptive statistics were presented as mean (standard deviation), median with range for quantitative variables and number (percentages) for qualitative variables. Univariate analysis was performed by using 1-way analysis of variation, paired and unpaired t-tests, Scheffe's test, or the Log-rank test, as appropriate. P < 0.05 was considered statistically significant, and all P values reported were 2-sided. All analyses were performed with State Mate III version 3.14 (ATMS Co, LTD, Tokyo, Japan) for Windows.
RESULTS
The 2-Step Protocol Generated Cells With IPC Morphology
Morphological changes from ADSCs to IPCs (Fig. 2A) were examined at day 14 (Fig. 2B), at day 35 (Fig. 2C), and at day 90 (Fig. 2D). Islet-like cell formations were confirmed at around day 14 (Fig. 2B, not stained), and these cells strongly stained with dithizone at day 35 (Fig. 2E, [HPF]) and at day 90 (Fig. 1F: HPF). Then we compared morphology of IPCs cultured and differentiated with or without HDACi. Cell morphological scores were significantly higher when HDACi was added (P < 0.05, Fig. 2G).
FIGURE 2.
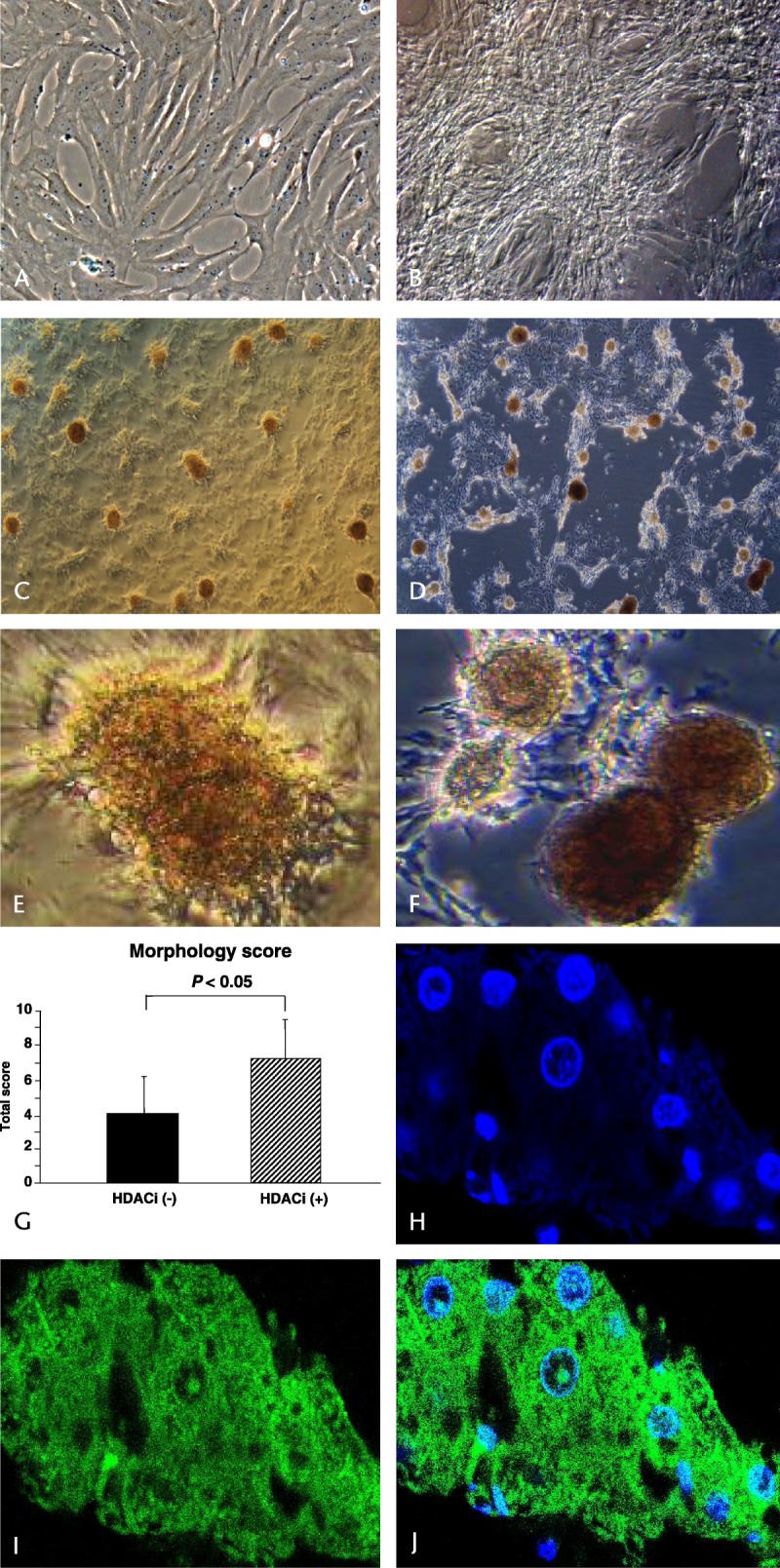
Morphological changes from ADSCs to IPCs. A, ADSC, not differentiated; B, at 14 days; C, at 35 days; D, at 90 days of differentiation; E, dithizone staining at day 35, original magnification × 400, high power field; F, dithizone staining at day 90, original magnification × 400, high power field. G, cell morphological scores were significantly higher when we added HDACi (unpaired t-test, P < 0.05). H–J, the differentiated IPCs with this 2-step protocol using HDACi retained good cell viability and function, as shown by nuclear, cytoplasmic (insulin) staining and the marge image, respectively.
Immunohistochemical Staining Showed that Viable IPCs Secreted Insulin
Insulin-producing cells differentiated with this 2-step protocol including HDACi showed good cell viability, as determined by nuclear staining (Fig. 2H). Moreover, their cytoplasm was well stained by an anti-insulin antibody (Figs. 2I, J).
Insulin and NeuroD Messenger RNA Expression by IPCs
The messenger RNA (mRNA) expression of NeuroD significantly increased when ADSCs were differentiated with HDACi compared with cells without HDACi or the normal control (Fig. 3A), as did that of the insulin gene INS1 (Fig. 3B).
FIGURE 3.
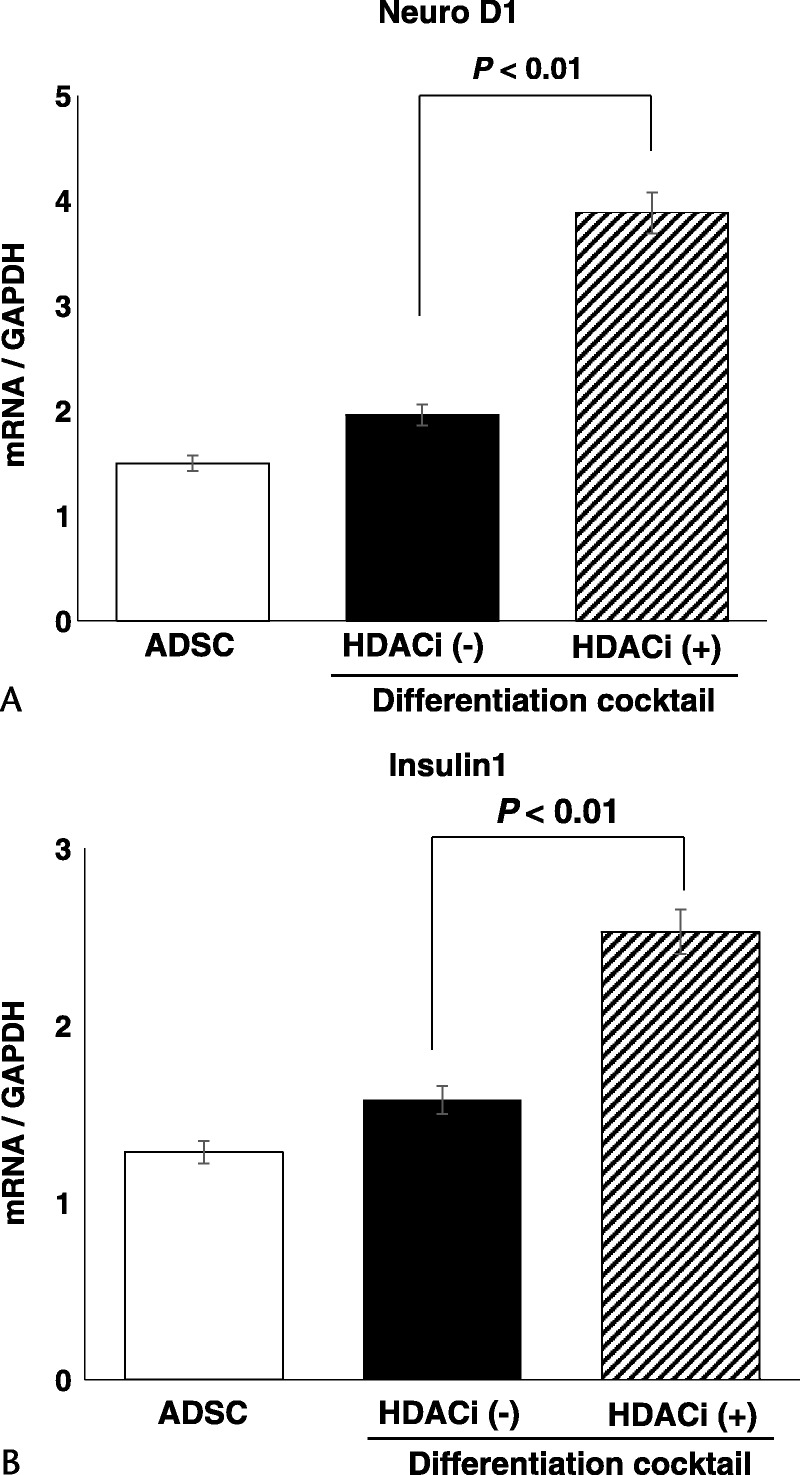
The gene expression profile of the transcription factor NeuroD1 and the endocrine hormone insulin. A, mRNA expression of NeuroD in controls and differentiated ADSCs without and with HDACi at step 2 of differentiation. B, mRNA expression of INS1 compared with controls and differentiated ADSCs without and with HDACi at step 2 of differentiation.
The 2-Step Protocol With HDACi Accelerated the Differentiation of IPCs
We defined the completion of differentiation as the time when cells were strongly stained with Dithizone. Differentiation completion (differentiation culture duration) was compared between IPCs cultured and differentiated with or without HDACi. The duration was significantly shortened when HDACi was added to the culture medium compared with no HDACi (median, 21.6 vs 38.8 days; P < 0.05; Fig. 4).
FIGURE 4.
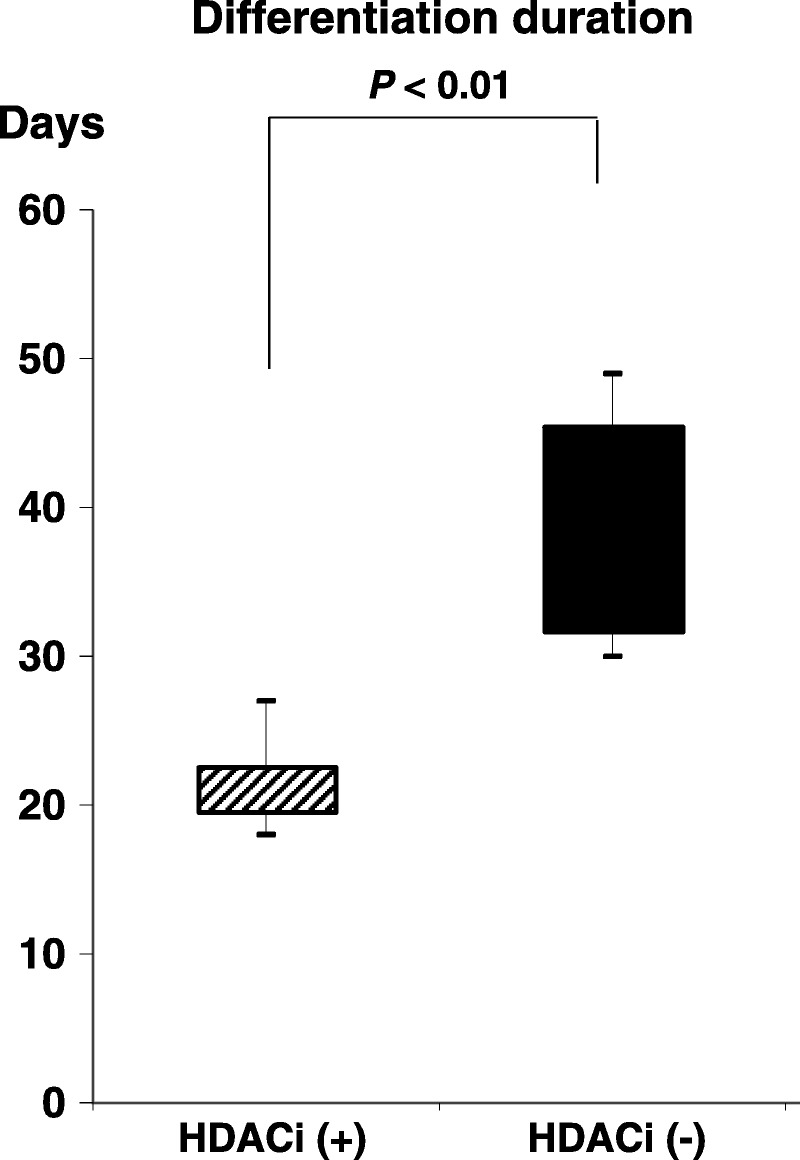
Differentiation completion time. The total duration of differentiation from ADSCs to mature IPCs was significantly shortened when HDACi was added to the culture medium (median, 21.6 vs 38.8 days; unpaired t-test, P < 0.05).
The Generated IPCs Showed a Good Glucose SI
The insulin concentration in the supernatant of the IPCs at day 28 significantly increased when these cells were incubated in “low” glucose medium (5 mM, P < 0.05) and when moved to “high” glucose medium (45 mM, P < 0.01). The final glucose SI was 3.2 (Fig. 5).
FIGURE 5.
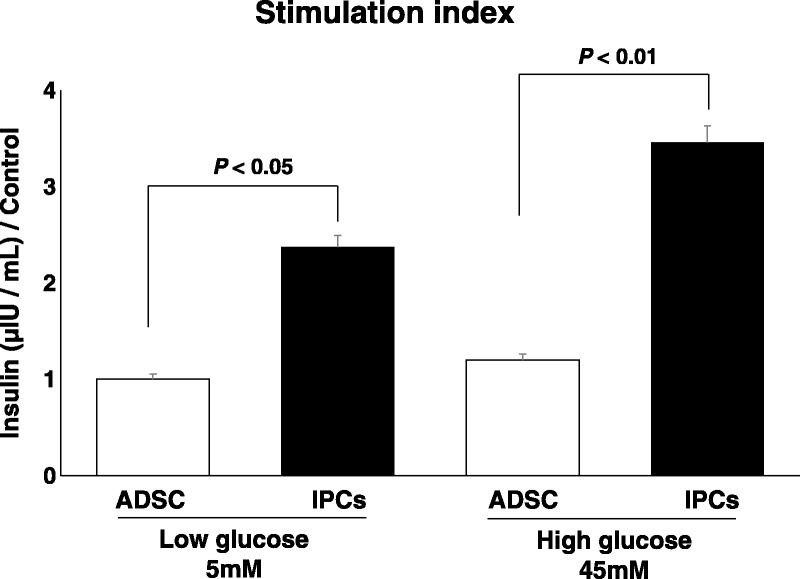
Glucose SI. The insulin concentration in the supernatant of IPCs at day 28 significantly increased when these cells were put in “low” glucose medium (5 mM; paired t-test, P < 0.05) and when moved into “high” glucose medium (45 mM; paired t-test, P < 0.01).
DISCUSSION
Islet transplantation (ITx) is a useful option for treatment of type-1 diabetes mellitus, which was refined by Shapiro et al20; however, these authors also noted that ITx has limitations. One of them is the low rate of insulin independence after a single ITx; in one study, after 5 years, only 9% of patients had achieved insulin-free status.21 Thus, repeated ITx was recommended to increase the possibility of achieving insulin-free status. However, although enough cadaveric donors are available for multidonor–one-recipient ITx in North America or some countries in Europe, it is very difficult to find enough cadaveric donors for ITx in counties such as Japan, even though the Japanese law regarding transplantation dramatically changed in 2007. Thus, transplant surgeons in such countries have struggled to find a new cell source. Consequently, we focused on mesenchymal stem cells that can be used for autotransplantation.
Among mesenchymal stem cells, we focused on ADSCs because they have some advantages as a cell source, such as significant potential as stem cells, better yield compared with bone marrow, less invasive procurement, and no ethical issues for autotransplantation when clinical application is considered. However, even though ADSCs have these advantages, some urgent issues need to be solved before clinical application. The differentiation protocol is very complex; culture duration exceeds 30 days, and the cell expansion rate and actual cell function as IPCs are extremely poor. Thus, we developed a new 2-step differentiation protocol for generating IPCs that solves some of the issues regarding differentiation duration and poor functional ability. However, some issues remain to be resolved, including IPC function and long term fate in vivo after transplantation. We have already commenced in vivo research to address these issues (data not shown).
In conclusion, ADSCs may be an effective cell source for generating functional IPCs through our new established 2-step protocol using an HDACi that promotes cells of the pancreatic lineage. This strategy may provide a breakthrough for clinical ITx in countries that are affected by severe donor shortage.
ACKNOWLEDGMENTS
We thank Ann Turnley, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Ikemoto T, Noguchi H, Shimoda M, et al. Islet cell transplantation for the treatment of type 1 diabetes in the USA. J Hepatobiliary Pancreat Surg. 2009;16:118–123. [DOI] [PubMed] [Google Scholar]
- 2.Ikemoto T, Noguchi H, Fujita Y, et al. New stepwise cooling system for short-term porcine islet preservation. Pancreas. 2010;39:960–963. [DOI] [PubMed] [Google Scholar]
- 3.Ikemoto T, Matsumoto S, Itoh T, et al. Assessment of islet quality following international shipping of more than 10,000 km. Cell Transplant. 2010;19:731–741. [DOI] [PubMed] [Google Scholar]
- 4.Yamada S, Shimada M, Utsunomiya T, et al. Trophic effect of adipose tissue-derived stem cells on porcine islet cells. J Surg Res. 2014;187:667–672. [DOI] [PubMed] [Google Scholar]
- 5.Saito Y, Shimada M, Utsunomiya T, et al. The protective effect of adipose-derived stem cells against liver injury by trophic molecules. J Surg Res. 2013;180:162–168. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno H. Adipose-derived stem and stromal cells for cell-based therapy: current status of preclinical studies and clinical trials. Curr Opin Mol Ther. 2010;12:442–449. [PubMed] [Google Scholar]
- 7.Shimizu T, Sekine H, Yamato M, et al. Cell sheet-based myocardial tissue engineering: new hope for damaged heart rescue. Curr Pharm Des. 2009;15:2807–2814. [DOI] [PubMed] [Google Scholar]
- 8.Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. [DOI] [PubMed] [Google Scholar]
- 9.Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–2674. [DOI] [PubMed] [Google Scholar]
- 10.Iwahashi S, Utsunomiya T, Imura S, et al. Effects of valproic acid in combination with S-1 on advanced pancreatobiliary tract cancers: clinical study phases I/II. Anticancer Res. 2014;34:5187–5191. [PubMed] [Google Scholar]
- 11.Sugimoto K, Itoh T, Takita M, et al. Improving allogeneic islet transplantation by suppressing Th17 and enhancing Treg with histone deacetylase inhibitors. Transpl Int. 2014;27:408–415. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin F, La Thangue NB. Histone deacetylase inhibitors open new doors in cancer therapy. Biochem Pharmacol. 2004;67:1139–1144. [DOI] [PubMed] [Google Scholar]
- 13.Lucas JL, Mirshahpanah P, Haas-Stapleton E, et al. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257:97–104. [DOI] [PubMed] [Google Scholar]
- 14.Haumaitre C, Lenoir O, Scharfmann R. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Mol Cell Biol. 2008;28:6373–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. [DOI] [PubMed] [Google Scholar]
- 16.Lakey JR, Warnock GL, Shapiro AM, et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999;8:285–292. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto S, Qualley SA, Goel S, et al. Effect of the two-layer (University of Wisconsin solution-perfluorochemical plus O2) method of pancreas preservation on human islet isolation, as assessed by the Edmonton Isolation Protocol. Transplantation. 2002;74:1414–1419. [DOI] [PubMed] [Google Scholar]
- 18.Wubetu GY, Utsunomiya T, Ishikawa D, et al. High STAT4 expression is a better prognostic indicator in patients with hepatocellular carcinoma after hepatectomy. Ann Surg Oncol. 2014;21(suppl 4):S721–S728. [DOI] [PubMed] [Google Scholar]
- 19.Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–195. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. [DOI] [PubMed] [Google Scholar]
- 21.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. [DOI] [PubMed] [Google Scholar]


