Abstract
Objectives
Extended-release (ER) carbidopa-levodopa (CD-LD) (IPX066/RYTARY/NUMIENT) produces improvements in “off” time, “on” time without troublesome dyskinesia, and Unified Parkinson Disease Rating Scale scores compared with immediate-release (IR) CD-LD or IR CD-LD plus entacapone (CLE). Post hoc analyses of 2 ER CD-LD phase 3 trials evaluated whether the efficacy and safety of ER CD-LD relative to the respective active comparators were altered by concomitant medications (dopaminergic agonists, monoamine oxidase B [MAO-B] inhibitors, or amantadine).
Methods
ADVANCE-PD (n = 393) assessed safety and efficacy of ER CD-LD versus IR CD-LD. ASCEND-PD (n = 91) evaluated ER CD-LD versus CLE. In both studies, IR- and CLE-experienced patients underwent a 6-week, open-label dose-conversion period to ER CD-LD prior to randomization. For analysis, the randomized population was divided into 3 subgroups: dopaminergic agonists, rasagiline or selegiline, and amantadine. For each subgroup, changes from baseline in PD diary measures (“off” time and “on” time with and without troublesome dyskinesia), Unified Parkinson Disease Rating Scale Parts II + III scores, and adverse events were analyzed, comparing ER CD-LD with the active comparator.
Results and Conclusions
Concomitant dopaminergic agonist or MAO-B inhibitor use did not diminish the efficacy (improvement in “off” time and “on” time without troublesome dyskinesia) of ER CD-LD compared with IR CD-LD or CLE, whereas the improvement with concomitant amantadine failed to reach significance. Safety and tolerability were similar among the subgroups, and ER CD-LD did not increase troublesome dyskinesia. For patients on oral LD regimens and taking a dopaminergic agonist, and/or a MAO-B inhibitor, changing from an IR to an ER CD-LD formulation provides approximately an additional hour of “good” on time.
Key Words: amantadine, dopaminergic agonist, extended release, levodopa, monoamine oxidase inhibitor
Oral levodopa (LD) administered in combination with a peripherally active decarboxylase inhibitor such as carbidopa (CD) is the most widely used therapy for the symptomatic treatment of Parkinson disease (PD).1 However, prolonged use of LD and/or progression of the disease is often associated with the development of motor fluctuations, including end-of-dose “wearing off” and dyskinesia. Mechanisms leading to the development of the various patterns of motor fluctuations are incompletely understood,2 although there is evidence suggesting that pulsatile stimulation of dopamine receptors in the basal ganglia is involved.3,4 Several pharmacological strategies have been used to achieve more sustained plasma LD concentrations to manage, and possibly avoid, motor fluctuations associated with oral LD. These include the use of extended-release (ER) LD formulations and inhibitors of catechol-O-methyltransferase (COMT) and monoamine oxidase B (MAO-B).5,6
In advanced PD, available adjunctive therapies can reduce “off” time associated with LD therapy, which in some studies exceeds 6 hours per day. In placebo-controlled studies, dopaminergic agonists7–13 and inhibitors of MAO-B14–16 have been shown to produce clinically meaningful reductions in “off” time as measured by patient home diaries and improved scores in the activities of daily living (Part II) and motor examination scores (Part III) of the Unified Parkinson Disease Rating Scale (UPDRS). Amantadine, a nonspecific glutamate N-methyl-d-aspartate receptor antagonist, reduces the severity and duration of LD-induced dyskinesia.4,17–19 A Cochrane Collaboration meta-analysis of efficacy and safety studies investigated adjunct oral treatments for PD patients with motor complications who were on stable immediate-release (IR) oral LD regimens and found that dopaminergic agonists were more effective at controlling parkinsonian symptoms than catechol-O-methyltransferase inhibitors or MAO-B inhibitors. The overall incidence of side effects was similar among the 3 groups.20 Current treatment guidelines reflect this conclusion.6,21
Extended-release CD-LD (IPX066, RYTARY, NUMIENT; Impax Laboratories, Inc, Hayward, Calif) is an oral CD-LD formulation that combines IR and ER components with the goal of more sustained plasma LD concentrations. Extended-release CD-LD pharmacokinetic studies have shown that LD plasma concentrations rise to a typical therapeutic range within 1 hour after oral dosing and are maintained for 4 to 6 hours.22,23 The safety and efficacy of ER CD-LD in patients with advanced PD have been evaluated in 2 multicenter, randomized, phase 3 trials (ADVANCE-PD24 and ASCEND-PD25). In each of these trials, patients treated with ER CD-LD had less “off” time and more “on” time without troublesome dyskinesia and had improved UPDRS Parts II + III scores compared with patients given IR CD-LD24 or IR CD-LD plus entacapone (CLE).25 Because a significant proportion of these advanced PD patients were receiving adjunctive PD therapies, we performed a post hoc analysis of data from these trials to evaluate if the efficacy and safety of ER CD-LD relative to the respective active comparators were affected by concomitant medications.
METHODS
Original Trial Designs
The study designs and protocols of the trials from which the current data were obtained have been published in detail.24,25 ADVANCE-PD and ASCEND-PD were phase 3, multinational, randomized, double-blind, double-dummy trials; ADVANCE-PD was a parallel-group study to assess the safety and efficacy of ER CD-LD versus IR CD-LD, whereas ASCEND-PD was a crossover study to evaluate ER CD-LD versus CLE. The primary clinical end point in both studies was “off” time as a percentage of waking hours. Both studies were performed in accordance with the Declaration of Helsinki. All sites received institutional review board approval, and each patient provided written informed consent prior to participation.
Safety was evaluated in all patients who received at least 1 dose of any study medication. Treatment-emergent adverse events (AEs) and serious AEs were recorded throughout the study.
Study Participants
Eligible patients had idiopathic PD (Hoehn and Yahr stage ≤4 in the “on” state), with motor fluctuations (≥2.5 hours of “off” time/d) despite taking equal to or greater than 400 mg LD/d in 4 doses or more per day, and a Mini-Mental State Examination score of 26 or greater. At enrollment, patients had at least 4 weeks of unchanged treatment with either an LD IR formulation24 or CLE25 at a dosing frequency of 4 or more times per day. Concomitant use of dopaminergic agonists, MAO-B inhibitors, amantadine, or anticholinergic drugs was permitted if doses were stable for at least 4 weeks prior to study entry. Key exclusion criteria included atypical or secondary parkinsonism, history of lack of response to LD, prior neurosurgical treatment for PD, severe dyskinesia, active psychosis (or treatment with antipsychotic medications), or prior participation in an ER CD-LD study.
Analysis
Efficacy
In each study, the randomized patient population was divided into 3 subgroups based on the use of concomitant medications at study entry: a dopaminergic agonist group, a MAO-B inhibitor group (rasagiline and selegiline), and an amantadine group. Patients could be receiving more than 1 of these adjunctive medications concurrently, and these patients were included in more than 1 subgroup for analysis. For each subgroup, the changes from baseline in PD diary measures (“off” time and “on” time with and without troublesome dyskinesia) and UPDRS sum of Parts II (activities of daily living) and III (motor examination) scores in the “on” state were analyzed, comparing ER CD-LD with the respective active comparator.
Safety Measures
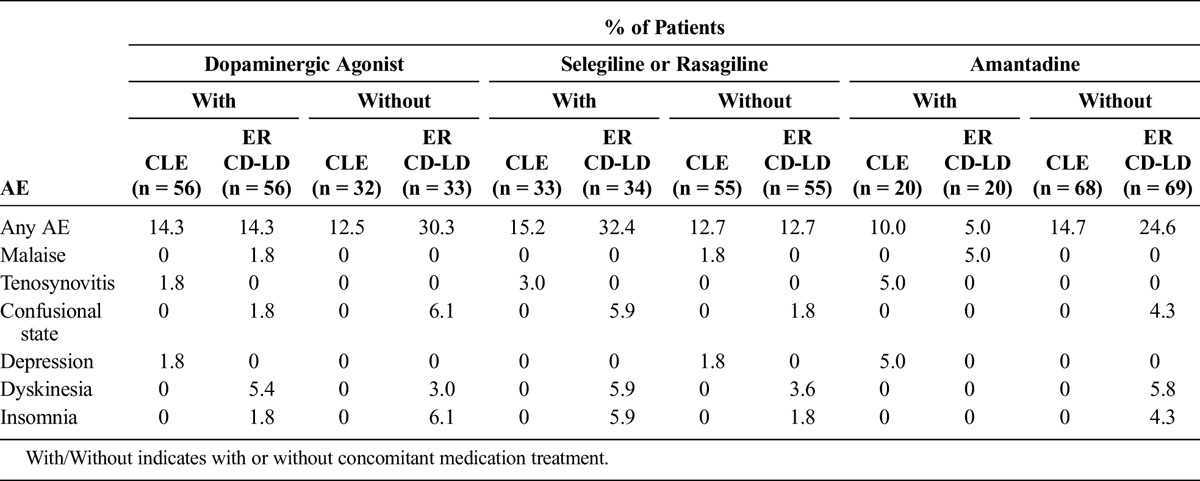
Adverse event rates for the overall studies are reported in the primary articles for each study.24,25 Adverse events reported by 4% or more of patients during the double-blind portions of each study are reported for each treatment group within each concomitant medication subgroup.
Statistical Analyses
Post hoc analyses of the changes from baseline in PD diary measures and UPDRS Parts II and III scores at end of study were performed using a 2-way analysis of variance with subgroup variables of concomitant medication (with or without) and study treatment (ER CD-LD vs IR CD-LD or ER CD-LD vs CLE). Because of the exploratory nature of these analyses, no corrections or adjustments were made for multiple comparisons.
RESULTS
Patient Demographics
Patient demographics are summarized in Table 1. Totals of 393 and 91 patients were randomized in ADVANCE-PD and ASCEND-PD, respectively. Age, duration of PD, and duration of LD treatment were similar between or within studies according to concomitant medication use (Table 1). Of the patients randomized, at least 50% in both studies received a concomitant dopaminergic agonist. Fewer than 25% of patients took amantadine in either trial. Fewer than 25% of patients took selegiline or rasagiline in ADVANCE-PD, whereas 35% of patients took either one of these in ASCEND-PD.
TABLE 1.
Patient Demographics, and Baseline ER CD-LD Dosing Characteristics
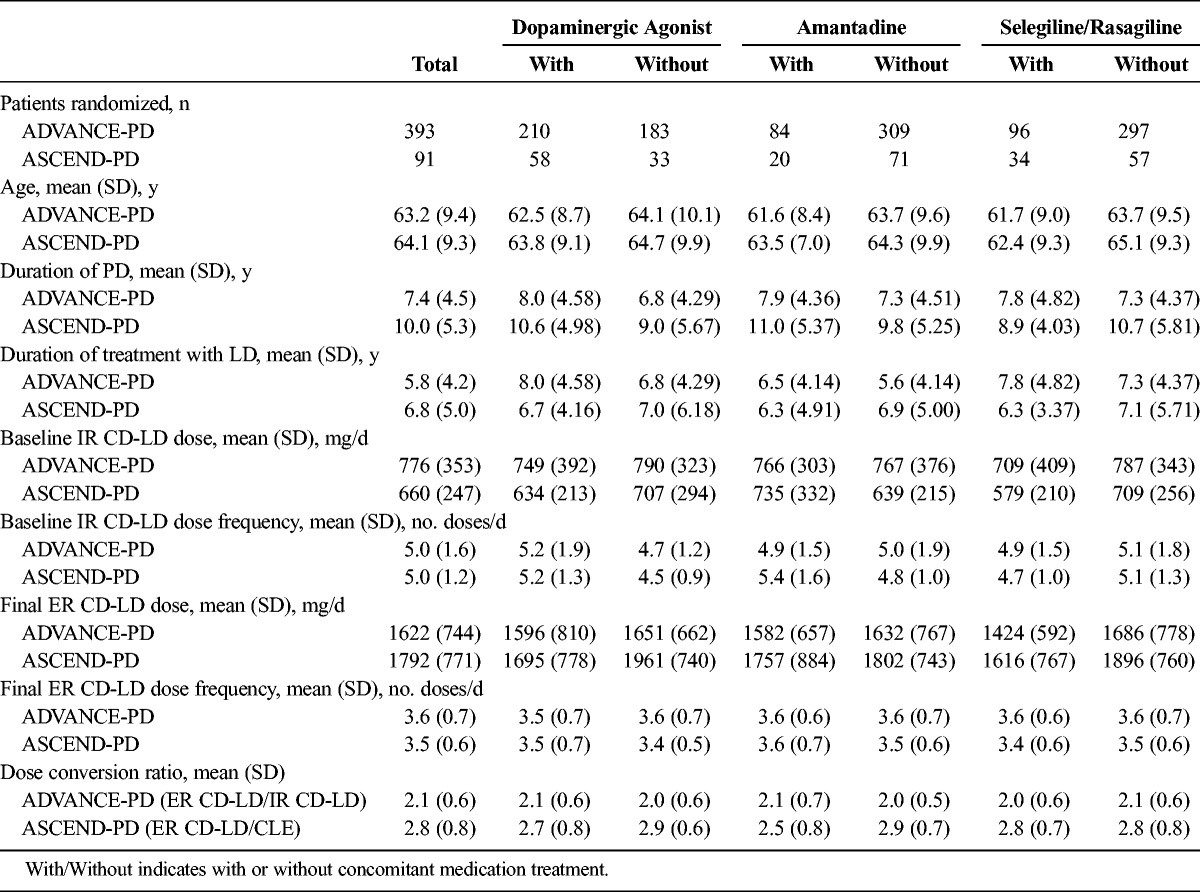
Overall, there were more terminations in the ER CD-LD group (7.5% overall) when compared with those taking IR CD-LD (5.2% overall) and when taking a concomitant medication of the same class. Most of this difference could be accounted for by greater numbers of subjects voluntarily withdrawing from the study rather than by other factors. The number withdrawing because of AEs was similar between subgroups in ADVANCE-PD, 9 patients each for both ER CD-LD and IR CD-LD (1.5% overall for ER CD-LD vs 1.6% for IR CD-LD). The numbers of discontinuations were similar between subgroups when there was no concomitant medication. There were only 4 early discontinuations in the ASCEND-PD study overall (all in the ER CD-LD subgroup), so no pattern could be discerned.
Levodopa doses given at baseline and after dose conversion were, in general, similar among subgroups. The mean daily dose (mg LD/d) conversion ratio of ER CD-LD to IR CD-LD was 2.0 to 2.1, depending on the concomitant medication subgroup, and for ER CD-LD to CLE, it was 2.5 to 2.9. The dosing frequencies were 3.6 and 3.5 doses/d for the ER CD-LD formulation compared with the 5 doses/d for the IR CD-LD and CLE formulations, respectively (Table 1).
Baseline PD Diary Measures
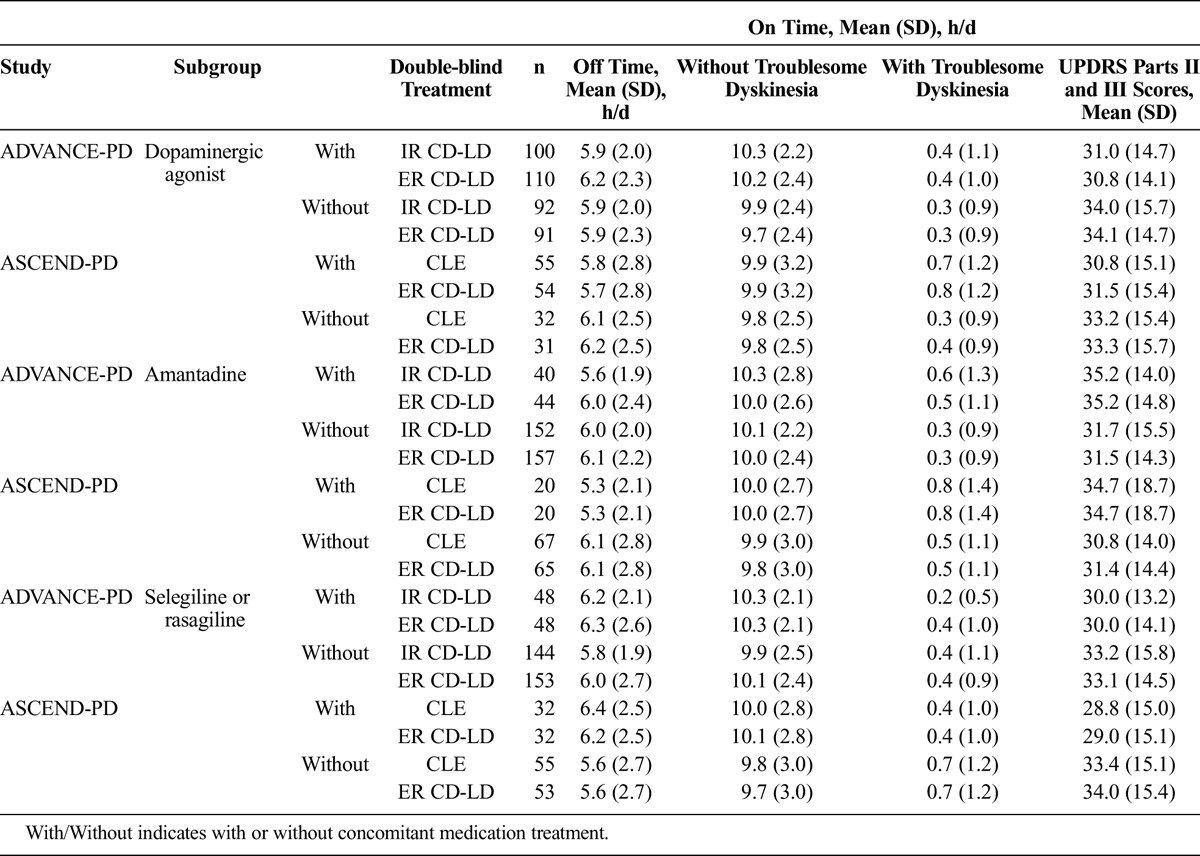
Parkinson disease diary measures at baseline are summarized in Table 2. In both studies, the ranges at baseline of mean daily “off” time, “on” time without troublesome dyskinesia, and “on” time with troublesome dyskinesia were 5.6 to 6.4 h/d, 9.7 to 10.3 h/d, and 0.2 to 0.8 h/d, respectively. There were no differences between baseline values for patients randomized to ER CD-LD versus IR CD-LD or ER CD-LD versus CLE with or without any of the concomitant medication subgroups.
TABLE 2.
Baseline PD Diary Measures and UPDRS Parts II and III Scores by Subgroup in ADVANCE-PD and ASCEND-PD
Effects of Concomitant Dopaminergic Agonists
The decrease from baseline to end of double-blind treatment in “off” time was significantly greater in patients given ER CD-LD versus IR CD-LD (Fig. 1A) or ER CD-LD versus CLE (Fig. 1D) with or without concomitant dopaminergic agonist administration. The improvement in “on” time without troublesome dyskinesia was significantly greater with ER CD-LD versus IR CD-LD (Fig. 1B; ADVANCE-PD) and with ER CD-LD versus CLE (Fig. 1E; ASCEND-PD) in patients receiving a concomitant dopaminergic agonist. The increase in “on” time without troublesome dyskinesia with ER CD-LD in patients not taking a concomitant dopaminergic agonist was significantly greater only versus IR CD-LD (Fig. 1B). Relative to each study comparator, ER CD-LD did not significantly worsen “on” time with troublesome dyskinesia with or without a concomitant dopaminergic agonist (Figs. 1C, F).
FIGURE 1.
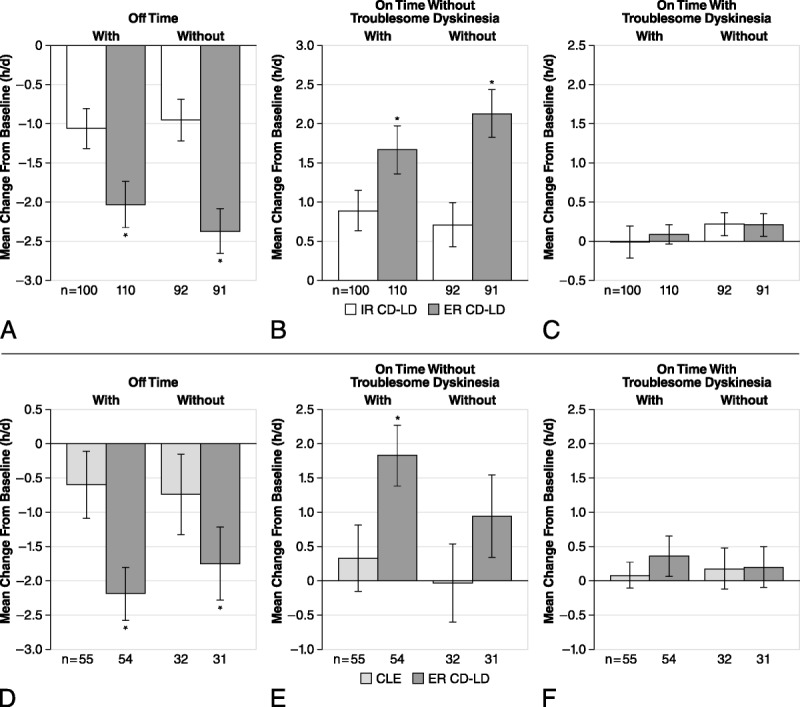
Effect of concomitant use of a dopaminergic agonist with ER CD-LD versus IR CD-LD (A–C) and ER CD-LD versus CLE (D–F) on PD diary measures. Changes from baseline to end of double-blind treatment were assessed for “off” time (A, D), “on” time without troublesome dyskinesia (B, E), and “on” time with troublesome dyskinesia (C, F). *P < 0.05 versus IR CD-LD within each subgroup. Error bars represent SEM.
Effect of Concomitant Selegiline or Rasagiline
Extended-release CD-LD produced significantly greater improvements in “off” time (Figs. 2A, D) and in “on” time without troublesome dyskinesia (Figs. 2B, E) versus IR CD-LD or CLE in patients with and without concomitant selegiline or rasagiline. There was no significant worsening of “on” time with troublesome dyskinesia with or without concomitant selegiline or rasagiline use in either study (Figs. 2C, F).
FIGURE 2.
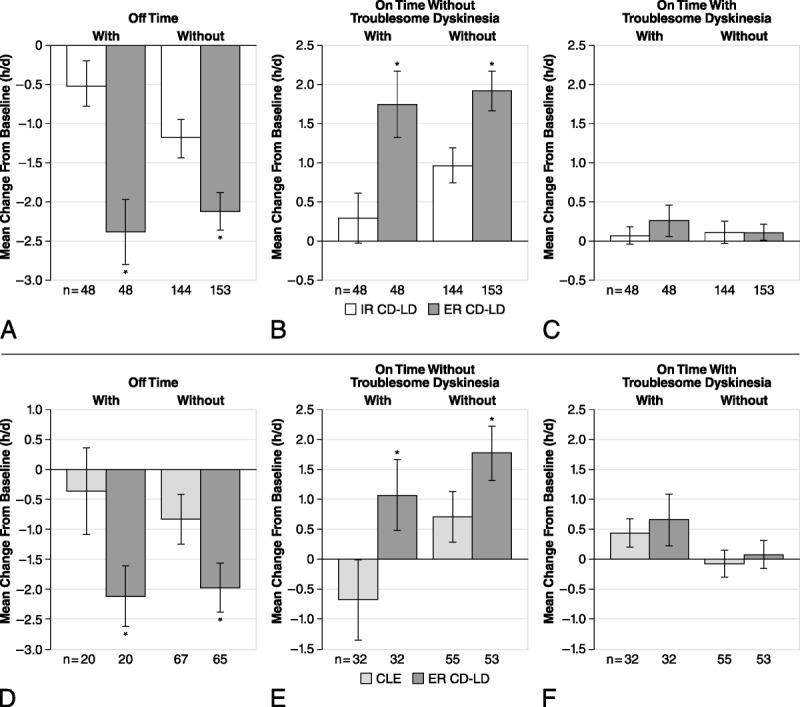
Effect of concomitant use of selegiline or rasagiline with ER CD-LD versus IR CD-LD (A–C) and ER CD-LD versus CLE (D–F) on PD diary measures. Changes from baseline to end of double-blind treatment were assessed for “off” time (A, D), “on” time without troublesome dyskinesia (B, E), and “on” time with troublesome dyskinesia (C, F). *P < 0.05 versus IR CD-LD within each subgroup. Error bars represent SEM.
Effect of Concomitant Amantadine
Extended-release CD-LD caused significantly greater improvements in “off” time (Figs. 3A, D) and “on” time without troublesome dyskinesia (Figs. 3B, E) versus IR CD-LD or CLE only in patients not receiving concomitant amantadine treatment. There was no significant change in “on” time with troublesome dyskinesia with ER CD-LD, IR CD-LD, or CLE with or without concomitant amantadine (Figs. 3C, F).
FIGURE 3.
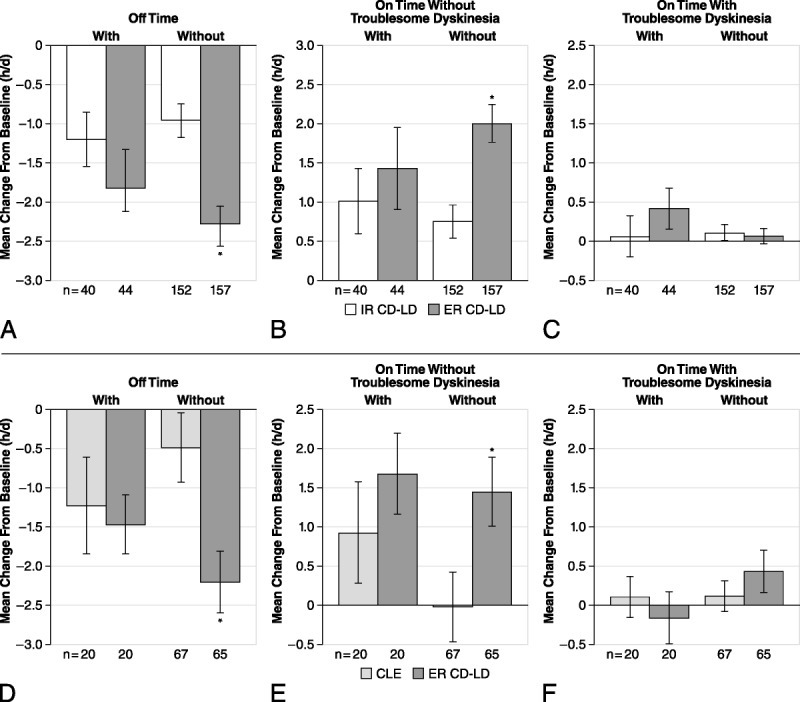
Effect of concomitant use of amantadine with ER CD-LD versus IR CD-LD (A–C) and ER CD-LD versus CLE (D–F) on PD diary measures. Changes from baseline to end of double-blind treatment were assessed for “off” time (A, D), “on” time without troublesome dyskinesia (B, E), and “on” time with troublesome dyskinesia (C, F). *P < 0.05 versus IR CD-LD within each subgroup. Error bars represent SEM.
Effect of Concomitant Medications on UPDRS Parts II and III Scores
Decreases (improvements) in UPDRS Parts II and III scores were significantly greater with ER CD-LD versus IR CD-LD and with ER CD-LD versus CLE in patients not taking a concomitant dopaminergic agonist (Figs. 4A, D), selegiline or rasagiline (Figs. 4B, E), or amantadine (Figs. 4C, F). Significantly greater improvements in UPDRS Parts II and III scores were observed with ER CD-LD versus IR CD-LD in those patients taking a dopaminergic agonist (Fig. 4A), but not in patients taking the other concomitant medications. Relative to CLE treatment, ER CD-LD significantly improved UPDRS Parts II and III scores only in those without concomitant medication.
FIGURE 4.
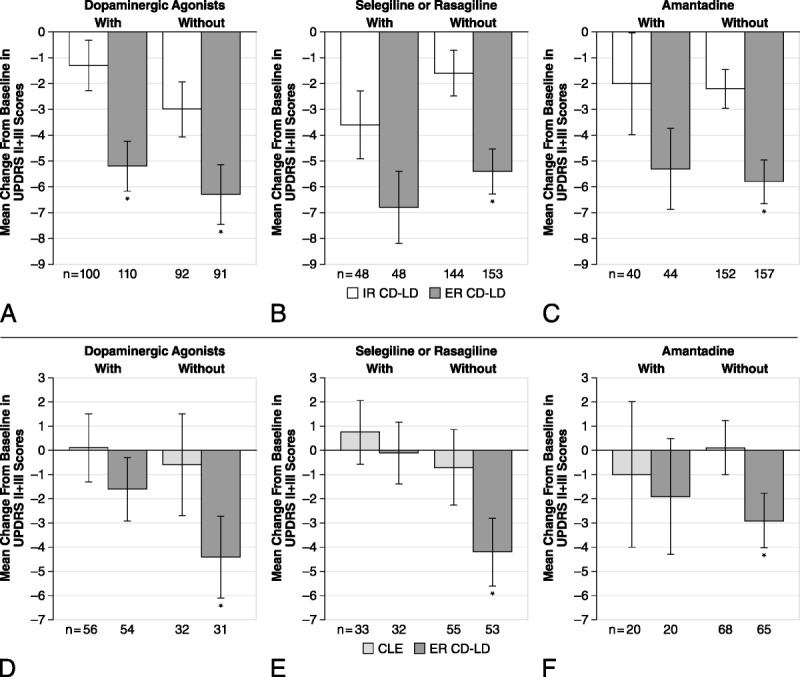
Effect of concomitant use of medications with ER CD-LD versus IR CD-LD (A–C) and ER CD-LD versus CLE (D–F) on UPDRS Parts II and III scores in the “on” state. Changes from baseline to end of double-blind treatment were assessed with or without a concomitant dopaminergic agonist (A, D), selegiline or rasagiline (B, E), and amantadine (C, F). *P < 0.05 versus IR CD-LD within each subgroup. Error bars represent SEM. MAO, monoamine oxidase.
Safety
Summaries of safety data are shown in Table 3 (ADVANCE-PD) and Table 4 (ASCEND-PD).
TABLE 3.
Adverse Events Reported in 4% or More of Patients in Any Subgroup During the Double-blind Treatment Period in ADVANCE-PD
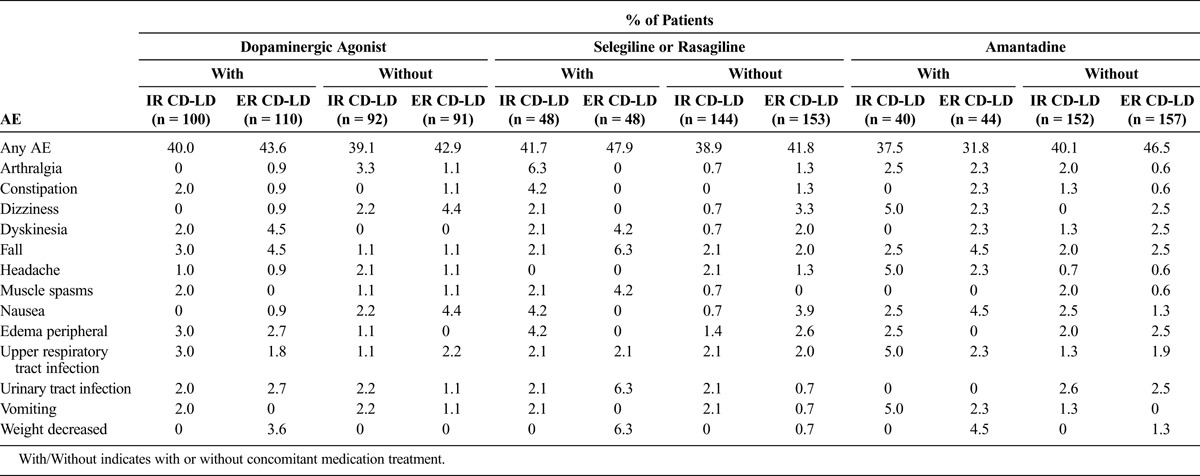
TABLE 4.
Adverse Events Reported in 4% or More of Patients in Any Subgroup During the Double-blind Treatment Period in ASCEND-PD
The overall percentage of AEs for those patients taking concomitant medications was similar for ER LD-CD and IR CD-LD groups in ADVANCE-PD and for ER LD-CD and CLE in ASCEND-PD.
For those taking concomitant medications, there were higher incidences of dyskinesia and falls in the ER CD-LD group versus the IR CD-LD group, even though the overall numbers were low. The dyskinesia rate was higher for the ER CD-LD group versus the CLE group only for those patients taking dopaminergic agonists or selegiline or rasagiline. Adverse events of weight decrease occurred in the ER CD-LD with concomitant medication groups, and these were absent in the equivalent IR CD-LD groups.
Overall serious AEs have been reported in previous publications.24,25 In ADVANCE-PD, during the double-blind treatment period, 11 patients (5%) in the ER CD-LD group reported 13 serious AEs. In the IR CD-LD group, there were 5 patients (3%) who experienced 8 serious AEs. In ASCEND-PD, only 1 serious AE (sciatica) occurred during double-blind treatment in an ER CD-LD patient who was also taking a dopaminergic agonist and a MAO inhibitor.25 The low number of serious AEs and absence of any clear pattern did not allow for meaningful comparisons to be made between subgroups. For any subgroup, only a single occurrence of each AE type was observed.
DISCUSSION
Relative to both comparator therapies, treatment with ER CD-LD was associated with improvement in the key patient diary measures (“off” and “on” time without troublesome dyskinesia), regardless of whether the patient was taking a dopaminergic agonist or a MAO-B inhibitor. Numerical improvements by ER CD-LD versus each comparator were observed in patients treated with concomitant amantadine, but these were not statistically significant in either study. Relative to each comparator and with all the concomitant medication subgroups, ER CD-LD was not associated with worsening of troublesome dyskinesia. Results of the UPDRS Parts II and III showed similar trends to findings from the PD diary in favor of ER CD-LD. Extended-release CD-LD treatment consistently improved scores compared with IR CD-LD and CLE; however, the numerical improvement was not statistically significant in patients receiving a MAO-B inhibitor or amantadine.
The final dose ratio of preconversion and postconversion to ER CD-LD from IR CD-LD were all 2.0 to 2.1, whereas from CLE the ratio was 2.7 to 2.9 for the subgroups except for the amantadine group, where the ratio was 2.5.
Studies have shown that dopaminergic agonists26–28 and MAO-B inhibitors29–31 are effective as monotherapy for the control of motor symptoms. However, their effectiveness in patients with advanced PD has been examined only as adjunct therapies to LD. These drug classes can improve UPDRS Parts II and III scores and reduce the amount of “off” time.7–16,32 Thus, it is conceivable that differences in the efficacy of ER CD-LD versus IR CD-LD or CLE found in the ADVANCE-PD and ASCEND-PD trials may have been lessened in those patients who received concomitant dopaminergic agonist or MAO-B inhibitor therapy. Our analysis shows the opposite; namely, improvements in PD diary measures afforded by ER CD-LD versus IR CD-LD or CLE were not diminished by the concomitant administration of these classes of medication. Differences in UPDRS Parts II and III scores were statistically significant only in the ADVANCE-PD trial in patients receiving a concomitant dopaminergic agonist. Explanations for this observation may be related to the smaller overall change in UPDRS scores in the ASCEND-PD trial compared with improvements in ADVANCE-PD (due possibly to the shorter treatment duration). In addition, the between-treatment-groups differences in UPDRS II + III were relatively small compared with those observed in the patient diary measures, so when the study was subdivided, it should not be surprising that the statistical significance of some of the subgroup differences had declined.
Amantadine is commonly prescribed as a mild antiparkinsonian medication or as an adjunctive therapy to treat tremor or LD-induced dyskinesia,6,33 and it has also been shown to reduce “off” time.34 Concomitant use of amantadine with ER CD-LD produced a numerical decrease in “off” time and an increase in “on” time without troublesome dyskinesia compared with IR CD-LD, but this difference was not significant (P = 0.5 and P = 0.65, respectively). This may be due to inherent differences in those patients who require amantadine treatment for their dyskinesia such that it affects their LD response or may reflect the smaller sample size of the subgroups compared with the dopaminergic agonist subgroups.
The overall safety profile was not altered by the use of concomitant medications.24,25 Dyskinesia is a well documented adverse effect of all dopaminergic therapies.8,9,11,32 In the present study, there was an increased incidence of dyskinesia (reported as an AE) with ER CD-LD for those taking concomitant dopaminergic agonists and selegiline or rasagiline compared with prior regimens of IR CD-LD and CLE. However, the overall reporting rates for dyskinesia were low and did not translate into a significant increase in “on” time with troublesome dyskinesia (per patient diaries) for either of these concomitant therapies.
A limitation of the present analysis is that some of the patients may have received more than 1 class of concomitant therapy. Thus, the effects observed by one class may not be independent of the effects caused by the others and may be the result of interactions between the different classes. However, limiting patient selection to those given only 1 of the 3 classes used in the present analysis may have yielded sample sizes too low to allow for conclusions to be drawn.
In conclusion, the use of a concomitant dopaminergic agonist or MAO-B inhibitor did not diminish the efficacy of ER CD-LD compared with IR CD-LD or CLE, whereas the improvement observed with concomitant amantadine failed to reach statistical significance. Treatment with ER CD-LD also did not increase troublesome dyskinesia compared with IR CD-LD or CLE for any of the studied subgroups. Safety and tolerability were similar among the patient subgroups.
Footnotes
These studies were funded by Impax Laboratories, Inc. Medical writing support was provided by Gerard D'Angelo, PhD, and Robin Smith, PhD, of The Curry Rockefeller Group, LLC, supported by Impax Laboratories, Inc.
P.A.L. and L.V.M. have served as consultants and have been investigators in clinical trials sponsored by Impax Laboratories, Inc. P.A.L. has also served as a consultant or advisor for Britannia, Acorda, Concit, Dexcel, Depomed, Insightec, Intec, Ipsen, Merck, Merz, NeuroDerm, Noven, Parkinson Study Group, Pfizer, ProStrakan, Teva, and US WorldMeds and has received speaker honoraria from the International Parkinson's Disease and Movement Disorders Society, Lundbeck, US WorldMeds, and the World Parkinson Congress. He is compensated for services as editor-in-chief of Clinical Neuropharmacology and serves without compensation on the editorial boards of Journal of Neural Transmission, Translational Neurodegeneration, and Journal of Parkinson's Disease. The Parkinson's Disease and Movement Disorders Program that P.A.L. directs has received clinical research grant support (for conducting clinical trial and other research) from Acorda, Adamas, Biotie, Great Lakes Neurotechnologies, Kyowa, The Michael J. Fox Foundation for Parkinson's Research, Pharma 2B, and US WorldMeds. L.V.M. received compensation from Medtronic, St Jude Medical, Britannia, US WorldMeds, and Cynapsus for consulting services. He has also received financial support for research activities from Medtronic, Boston Scientific, Adamas, Osmotica, Pfizer, US WorldMeds, National Institutes of Health, and Michael J Fox Foundation, in a role as principal investigator for research studies. R.R., S. Khanna, S. Kell, and S.G. are employees of Impax Laboratories, Inc, and hold Impax stock and/or stock options.
REFERENCES
- 1.LeWitt PA. Levodopa for the treatment of Parkinson's disease. N Engl J Med 2008;359(23):2468–2476. [DOI] [PubMed] [Google Scholar]
- 2.LeWitt PA. Levodopa therapy for Parkinson's disease: pharmacokinetics and pharmacodynamics. Mov Disord 2015;30(1):64–72. [DOI] [PubMed] [Google Scholar]
- 3.Calabresi P, Di Filippo M, Ghiglieri V, et al. Molecular mechanisms underlying levodopa-induced dyskinesia. Mov Disord 2008;23(Suppl 3):S570–S579. [DOI] [PubMed] [Google Scholar]
- 4.Metman LV, Konitsiotis S, Chase TN. Pathophysiology of motor response complications in Parkinson's disease: hypotheses on the why, where, and what. Mov Disord 2000;15(1):3–8. [DOI] [PubMed] [Google Scholar]
- 5.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311(16):1670–1683. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson's disease. Eur J Neurol 2013;20(1):5–15. [DOI] [PubMed] [Google Scholar]
- 7.Guttman M. Double-blind comparison of pramipexole and bromocriptine treatment with placebo in advanced Parkinson's disease. International Pramipexole-Bromocriptine Study Group. Neurology 1997;49(4):1060–1065. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman A, Olanow CW, Sethi K, et al. A multicenter trial of ropinirole as adjunct treatment for Parkinson's disease. Ropinirole Study Group. Neurology 1998;51(4):1057–1062. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman A, Ranhosky A, Korts D. Clinical evaluation of pramipexole in advanced Parkinson's disease: results of a double-blind, placebo-controlled, parallel-group study. Neurology 1997;49(1):162–168. [DOI] [PubMed] [Google Scholar]
- 10.Rascol O, Lees AJ, Senard JM, et al. A placebo-controlled study of ropinirole, a new D2 agonist, in the treatment of motor fluctuations of l-DOPA–treated parkinsonian patients. Adv Neurol 1996;69:531–534. [PubMed] [Google Scholar]
- 11.Moller JC, Oertel WH, Koster J, et al. Long-term efficacy and safety of pramipexole in advanced Parkinson's disease: results from a European multicenter trial. Mov Disord 2005;20(5):602–610. [DOI] [PubMed] [Google Scholar]
- 12.Barone P, Lamb J, Ellis A, et al. Sumanirole versus placebo or ropinirole for the adjunctive treatment of patients with advanced Parkinson's disease. Mov Disord 2007;22(4):483–489. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno Y, Abe T, Hasegawa K, et al. Ropinirole is effective on motor function when used as an adjunct to levodopa in Parkinson's disease: STRONG study. Mov Disord 2007;22(13):1860–1865. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol 2005;62(2):241–248. [DOI] [PubMed] [Google Scholar]
- 15.Rascol O, Brooks DJ, Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet 2005;365(9463):947–954. [DOI] [PubMed] [Google Scholar]
- 16.Waters CH, Sethi KD, Hauser RA, et al. Zydis selegiline reduces off time in Parkinson's disease patients with motor fluctuations: a 3-month, randomized, placebo-controlled study. Mov Disord 2004;19(4):426–432. [DOI] [PubMed] [Google Scholar]
- 17.Luginger E, Wenning GK, Bosch S, et al. Beneficial effects of amantadine on l-dopa-induced dyskinesias in Parkinson's disease. Mov Disord 2000;15(5):873–878. [DOI] [PubMed] [Google Scholar]
- 18.Metman LV, Del Dotto P, LePoole K, et al. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol 1999;56(11):1383–1386. [DOI] [PubMed] [Google Scholar]
- 19.Verhagen Metman L, Del Dotto P, van den Munckhof P, et al. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease. Neurology 1998;50(5):1323–1326. [DOI] [PubMed] [Google Scholar]
- 20.Stowe R, Ives N, Clarke CE, et al. Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Parkinson s disease patients with motor complications. Cochrane Database Syst Rev 2010(7):CD007166. [DOI] [PubMed] [Google Scholar]
- 21.Pahwa R, Factor SA, Lyons KE, et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66(7):983–995. [DOI] [PubMed] [Google Scholar]
- 22.Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson's disease. Mov Disord 2011;26(12):2246–2252. [DOI] [PubMed] [Google Scholar]
- 23.Yao HM, Hsu A, Gupta S, et al. Clinical pharmacokinetics of IPX066: evaluation of dose proportionality and effect of food in healthy volunteers. Clin Neuropharmacol 2016;39(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauser RA, Hsu A, Kell S, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol 2013;12(4):346–356. [DOI] [PubMed] [Google Scholar]
- 25.Stocchi F, Hsu A, Khanna S, et al. Comparison of IPX066 with carbidopa-levodopa plus entacapone in advanced PD patients. Parkinsonism Relat Disord 2014;20(12):1335–1340. [DOI] [PubMed] [Google Scholar]
- 26.Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 2004;61(7):1044–1053. [DOI] [PubMed] [Google Scholar]
- 27.Kieburtz K. Twice-daily, low-dose pramipexole in early Parkinson's disease: a randomized, placebo-controlled trial. Mov Disord 2011;26(1):37–44. [DOI] [PubMed] [Google Scholar]
- 28.Rascol O, Dubois B, Caldas AC, et al. Early piribedil monotherapy of Parkinson's disease: a planned seven-month report of the REGAIN study. Mov Disord 2006;21(12):2110–2115. [DOI] [PubMed] [Google Scholar]
- 29.Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med 2009;361(13):1268–1278. [DOI] [PubMed] [Google Scholar]
- 30.Parkinson Study Group. A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Arch Neurol 2004;61(4):561–566. [DOI] [PubMed] [Google Scholar]
- 31.Stern MB, Marek KL, Friedman J, et al. Double-blind, randomized, controlled trial of rasagiline as monotherapy in early Parkinson's disease patients. Mov Disord 2004;19(8):916–923. [DOI] [PubMed] [Google Scholar]
- 32.Olanow CW, Fahn S, Muenter M, et al. A multicenter double-blind placebo-controlled trial of pergolide as an adjunct to Sinemet in Parkinson's disease. Mov Disord 1994;9(1):40–47. [DOI] [PubMed] [Google Scholar]
- 33.Schaeffer E, Pilotto A, Berg D. Pharmacological strategies for the management of levodopa-induced dyskinesia in patients with Parkinson's disease. CNS Drugs 2014;28(12):1155–1184. [DOI] [PubMed] [Google Scholar]
- 34.Pahwa R, Tanner CM, Hauser RA, et al. ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID study): a randomized clinical trial. JAMA Neurol 2017;74(8):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]


