Abstract
PURPOSE:
To assess the cost-effectiveness of a ceramide-infused skin barrier (CIB) versus other skin barriers (standard of care) among patients who have undergone ostomy creation.
DESIGN:
Cost-effectiveness analysis, based on a decision-analytic model that was estimated using data from the ADVOCATE (A Study Determining Variances in Ostomy Skin Conditions And The Economic Impact) trial, which investigated stoma-related healthcare costs over 12 weeks among patients who recently underwent fecal ostomy, and from other sources.
SUBJECTS AND SETTING:
Analysis was based on a hypothetical cohort of 1000 patients who recently underwent fecal ostomy; over a 1-year period, 500 patients were assumed to use CIB and 500 were assumed to use standard of care.
METHODS:
We adapted a previous economic model to estimate expected 1-year costs and outcomes among persons with a new ostomy assumed to use CIB versus standard of care. Outcomes of interest included peristomal skin complications (PSCs) (up to 2 during the 1-year period of interest) and quality-adjusted life days (QALDs); QALDs vary from 1, indicating a day of perfect health to 0, indicating a day with the lowest possible health (deceased). Subjects were assigned QALDs on a daily basis, with the value of the QALD on any given day based on whether the patient was experiencing a PSC. Costs included those related to skin barriers, ostomy accessories, and care of PSCs. The incremental cost-effectiveness of CIB versus standard of care was estimated as the incremental cost per PSC averted and QALD gained, respectively; net monetary benefit of CIB was also estimated. All analyses were run using the perspective of an Australian payer.
RESULTS:
On a per-patient basis, use of CIB was expected over a 1-year period to result in 0.16 fewer PSCs, an additional 0.35 QALDs, and a savings of A$180 (Australian dollars, US $137) in healthcare costs all versus standard of care. Management with CIB provided a net monetary benefit (calculated as the product of maximum willingness to pay for 1 QALD times additional QALDs with CIB less the incremental cost of CIB) of A$228 (US $174). Probabilistic sensitivity analysis was also completed; it revealed that 97% of model runs resulted in fewer expected PSCs with CIB; 92% of these runs resulted in lower expected costs with CIB.
CONCLUSIONS:
Findings suggest that the CIB is a cost-effective skin barrier for persons living with an ostomy.
Keywords: Cost-effectiveness, Costs and cost analysis, Economic evaluation, Economics, Ostomy, Postoperative complications, Surgical stomas
INTRODUCTION
Despite advances in stoma surgery and ostomy care, up to 75% of patients experience postoperative complications.1,2 Stomal and peristomal complications are attributable to various causes including moisture-associated skin damage with exposure to stomal effluent, mechanical trauma to the peristomal skin or hair follicles (eg, stripping injury and pressure injuries) due to the ostomy system or ostomy accessories, immunologic responses (hypersensitivity or allergic), and infections.2–5 One study estimated that peristomal skin complications (PSCs) account for 40% of all visits to ostomy care nurses.6 Over an approximate 7-week treatment period, a panel of 11 experienced stoma care nurses estimated the costs of treatment of PSCs to range from €23.10 (US $26.80) for mild cases to €141.20 (US $163.79) for severe cases (all assume typical care rendered in France and are in 2011 Euros).7 Martins and colleagues8 estimated the cost of these complications in the United Kingdom (all assumed to last 7 weeks) to range from ₤106.29 (US $139.24) for mild PSCs caused by mechanical trauma to ₤618.69 (US $810.48) for severe, disease-related PSCs. Given their prevalence and associated cost, ostomy patient instruction should include strategies to prevent PSCs and maintain peristomal skin health.
A novel barrier (CeraPlus) with Remois technology (Alcare Co Ltd, Sumida-ku, Tokyo, Japan) is currently marketed by Hollister, Inc (Libertyville, Illinois). This ceramide-infused barrier (CIB) is designed to maintain adhesive properties and features a formulation to help maintain healthy peristomal skin, decrease transepidermal water loss from damaged/eroded skin, and help protect the skin's natural moisture barrier.9–11 The ADVOCATE (A Study Determining Variances in Ostomy Skin Conditions And The Economic Impact) trial, a double-blind randomized controlled study, enrolled 153 adult subjects who were randomly allocated to use the CIB versus an alternative, currently marketed, barrier (New Image FlexWear or New Image Flextend, Hollister, Incorporated) as a control.12 The primary outcome was ostomy-related healthcare costs (ie, costs related to “typical” ostomy products such as skin barriers and select ostomy accessories plus costs related to PSC care). Subjects wore the control or experimental pouches over a 12-week period, and patients who experienced PSCs were followed up over an additional 4-week “resolution period” during which all PSC-related costs were captured. Analysis revealed that the mean total healthcare costs during follow-up were A$36.46 (US $27.89) less among patients randomized to the ceramide-infused barrier (n = 79) versus the control barrier (n = 74) (A$223.73 vs A$260.19; US $171.14 vs US $199.03; P = .017). The cost differences were primarily due to differences in ostomy accessory use. In addition, 55.4% of subjects allocated to the CIB experienced PSCs versus 40.5% of control subjects (P = .069).
Findings from the ADVOCATE trial were limited to a 12-week follow-up period, and the cost-effectiveness of the CIB versus other barriers over a longer period is not known. To address this limitation, we developed a decision-analytic model to assess the cost-effectiveness of the CIB versus other skin barriers within a hypothetical cohort of 1000 Australians with fecal ostomies over a 360-day period (approximately 1 year); analyses were undertaken within the context of the Australian healthcare system and differences were analyzed using Australian dollars (A$).
METHODS
The cost-utility model used in this study compared anticipated outcomes and costs among 2 cohorts of ostomy patients; the first group used the CIB and the second used other commercially available barriers (standard of care, SoC). We assumed that SoC barriers represented any number of barriers from multiple manufacturers and were selected following discussion between patients and their care providers. The model included various ostomy accessories used during typical ostomy care and during the management of PSCs. The time horizon for our analysis was 360 days. The model allows for the possibility of 2 PSCs in the year (ie, one during weeks 1-12 and the other during weeks 13-52) and differing levels of PSC severity. Health-related quality of life was assessed via quality-adjusted life days (QALDs), accounting for the differential impact on quality of life of varying severity levels of PSCs. The model also included both deterministic and probabilistic sensitivity analyses (DSA and PSA, respectively). In the DSA various inputs were varied one at a time in order to identify the factor(s) to which the model was most (or least) sensitive. In the PSA, a large number of input values were varied simultaneously, allowing an examination of the sensitivity of results to the overall level of uncertainty in model inputs. A schematic of the model is presented in Figure 1.
Figure 1.
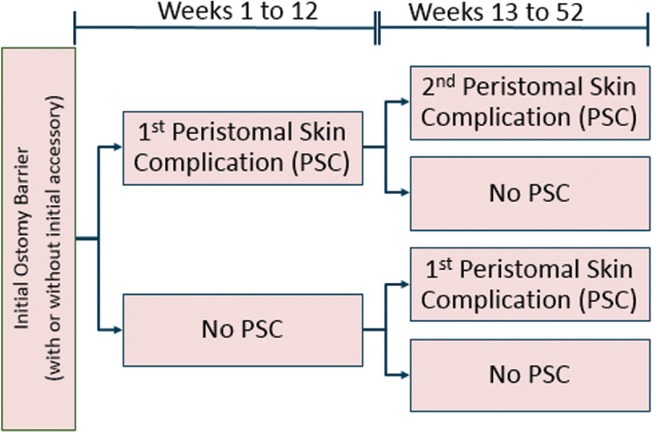
Model diagram. PSC indicates peristomal skin complication.
The model compared expected costs associated with an uncomplicated ostomy, increased use of pouching supplies and accessories during PSCs, other healthcare services required to treat and manage PSCs, and daily use of accessories subsequent to PSC resolution in persons using a CIB or an SoC pouching system. The difference in expected healthcare costs between these 2 scenarios is expressed as the incremental cost (barrier and PSC management). Similar calculations were used to estimate total expected QALDs and the incremental differences between use of the CIB versus SoC. Key assumptions of this model are summarized in Table 1.
TABLE 1. Key Assumptions Used to Construct Cost-effectiveness Model.
| Assumption | Description |
|---|---|
| 1 | Patients can experience up to 2 PSCs during the 1-y time horizon |
| 2 | PSCs occur either on d 42 (the middle of the first 12-wk period) or on d 224 (the midpoint of wk 12-52 of the 1-y time horizon) |
| 3 | Only patients who experience a PSC on d 42 can experience a second PSC during the 1-y time horizon |
| 4 | 65% of initial PSCs occur on d 42; the remaining 35% of initial PSCs occur on d 224 (ie, the model assumes that patients have a higher likelihood of PSC development relatively early following stoma creation) |
| 5 | Increases in use of PSC-related pouching supplies and accessories last from PSC onset until PSC resolution |
| 6 | Time to PSC resolution is dependent on its severity |
| 7 | Patients who experience a PSC can add accessories to their daily regimen following PSC resolution if they are not already using accessories at PSC onset |
| 8 | Costs of accessories added after PSC resolution are incurred from the date of resolution until the end of the model time horizon |
| 9 | Irrespective of PSC occurrence, switching from CIB to SoC or vice versa is not allowed |
Abbreviations: CIB, ceramide-infused skin barrier; PSC, peristomal skin complication; SoC, standard of care.
Cost-Utility Model
Hypothetical participants entered the model having undergone fecal stoma surgery within the past 12 weeks, and they were discharged home using either a CIB pouching system or a SoC system. Given that the percentage of 1- and 2-piece users in Australia is approximately 50%, the distribution of subjects in ADVOCATE (in which only 2-piece products were used) between CIB and SoC was also applied to 1-piece users (Table 2). Pouching supply use was based on information collected during the ADVOCATE trial, which is described in greater detail elsewhere.12 The costs of pouching supplies for SoC were based on publicly available information from the Australian healthcare system13; we assumed that the costs of pouching supplies for CIB were equal to those of SoC, since in Australia barriers in each category (ie, 1- and 2-piece systems) are priced at parity.
TABLE 2. Use and Cost of Pouching Supplies.
| Name | Patients Using the Pouching Supply, n (%)a | Component | Average Changes per Week per Patient, n | Package Cost, A$ | Units/Package |
|---|---|---|---|---|---|
| SoC | |||||
| One-piece | |||||
| Flat open (drainable) | 88 (17.5) | System | 7 | 146.40 | 30 |
| Convex open (drainable) | 163 (32.5) | System | 7 | 65.80 | 10 |
| Two-piece | |||||
| Flat | 88 (17.5) | Baseplate | 3.2 | 23.71 | 5 |
| Pouch | 3.2 | 36.60 | 10 | ||
| Convex | 163 (32.5) | Baseplate | 3.2 | 29.20 | 5 |
| Pouch | 3.2 | 36.30 | 10 | ||
| Ceramide-infused skin barrier | |||||
| One-piece | |||||
| Flat open | 83 (16.5) | System | 7 | 146.40 | 30 |
| Convex open | 168 (33.5) | System | 7 | 65.80 | 10 |
| Two-piece | |||||
| Flat | 83 (16.5) | Baseplate | 2.9 | 23.71 | 5 |
| Pouch | 2.9 | 36.60 | 10 | ||
| Convex | 168 (33.5) | Baseplate | 3.3 | 29.20 | 5 |
| Pouch | 3.3 | 36.30 | 10 | ||
Abbreviation: SoC, standard of care.
aNumbers and percentages are based off of a hypothetical cohort of 500 patients
The model we used also accounted for accessory use (eg, flat rings, ostomy belts, and adhesive remover spray) by means of an “average accessory use profile,” percentages of patients assumed to use each accessory, and the corresponding number of units and cost per package. Accessory use, shown in Table 3, was assumed to depend on the number of times patients change their pouching supplies.
TABLE 3. Accessory Use.
| Patients Using the Accessory, % | Average Cost per Patient Using Accessory/wk, A$b | |||||||
|---|---|---|---|---|---|---|---|---|
| Medical Resource | Package Cost, A$ | Units/Package | Units Used/wka | Unit Cost, A$ | SoC | CIB | SoC | CIB |
| Flat ring | 45.30 | 10 | 5.1 | 4.53 | 47 | 33 | 10.86 | 7.60 |
| Paste | 11.28 | 20 | 5.1 | 0.56 | 39 | 28 | 1.12 | 0.80 |
| Ostomy belt | 5.98 | 90 | 5.1 | 0.07 | 31 | 19 | 0.11 | 0.06 |
| Adhesive remover spray | 10.23 | 50 | 5.1 | 0.20 | 29 | 35 | 0.30 | 0.36 |
| Skin film wipes | 14.50 | 50 | 5.1 | 0.29 | 25 | 24 | 0.37 | 0.35 |
| Ostomy powder | 8.70 | 50 | 5.1 | 0.17 | 20 | 17 | 0.18 | 0.15 |
Abbreviations: CIB, ceramide-infused skin barrier; SoC, standard of care.
aCalculated as the average of the number of ostomy system changes per week, weighted by the proportions receiving 1- and 2-piece systems, respectively (see Table 2 for the proportions and changes).
bCalculated by multiplying units used per week times unit cost times the percentage of patients using the accessory. Average daily cost per patient for SoC is calculated based on the average number of daily pouching supply changes calculated from the baseplate change frequency summarized in Table 2.
Incidence of Peristomal Complications
The annual probability of PSCs for CIB and SoC was based on information from the ADVOCATE trial, as was the severity distribution of the initial PSC. Because only 2 patients experienced 2 PSCs in ADVOCATE (both allocated to the SoC group), the trial could not be used to estimate the probability of a second PSC. In the absence of this information, we kept the 5% absolute difference in risk observed in ADVOCATE consistent and assumed that 15% of SoC patients and 10% of CIB patients would experience a second PSC. We also assumed, irrespective of barrier used, that use of additional accessories following an initial PSC would reduce the risk of a second PSC by 50%. All baseline values related to PSC incidence and the accompanying severity distribution are presented in Table 4, and were taken from the ADVOCATE trial unless otherwise noted.
TABLE 4. Incidence and Severity of PSCsa.
| Parameter | SoC | CIB |
|---|---|---|
| Annual probability of PSC | 55% (41/74) | 41% (32/79) |
| Proportion of patients who experience a second PSC, among patients who already experienced a PSC, without addition of accessory | 15% | 10% |
| Reduction in the incidence of a second PSC with the addition of an accessoryb | 50% | 50% |
| Probability first PSC is mild | 69% (28/41) | 72% (23/32) |
| Probability first PSC is moderate | 29% (12/41) | 25% (8/32) |
| Probability first PSC is severe | 2% (1/41) | 3% (1/32) |
| Probability second PSC is mild, given first PSC is mild | 0% (0/1) | 33%c |
| Probability second PSC is moderate, given first PSC is mild | 100% (1/1) | 33%c |
| Probability second PSC is severe, given first PSC is mild | 0% (0/1) | 34%c |
| Probability second PSC is mild, given first PSC is moderate | 0% (0/1) | 33%c |
| Probability second PSC is moderate, given first PSC is moderate | 100% (1/1) | 33%c |
| Probability second PSC is severe, given first PSC is moderate | 0% (0/1) | 34%c |
| Probability second PSC is mild, given first PSC is severe | 33%c | 33%c |
| Probability second PSC is moderate, given first PSC is severe | 33%c | 33%c |
| Probability second PSC is severe, given first PSC is severe | 34%c | 34%c |
Abbreviations: CIB, ceramide-infused skin barrier; PSC, peristomal skin complication; SoC, standard of care.
aNumerators and denominators are in parentheses.
bADVOCATE Study did not provide input on this parameter. Both ceramide-infused skin barrier and SoC values were assumed to be 50% to be conservative.
cNo values were observed in ADVOCATE; these values are assumptions.
Findings from the ADVOCATE trial indicated that the average time to PSC resolution was 18 days for mild cases (defined as a Discoloration, Erosion, Tissue overgrowth [DET] instrument score <4), 33 days for moderate cases (defined as a DET score >4 and <7), and 33 days for severe cases (defined as a DET score ≥7). The time to PSC resolution was assumed to be equal in both arms.
Cost of treatment of PSC included additional pouching supplies and healthcare services until projected resolution. Information on use of pouching supplies during PSCs is set forth in Table 5, including use of 1- and 2-piece systems. For 2-piece systems, use of baseplates during PSCs was allowed to vary at a level potentially different from that of 1-piece pouches. Estimates are provided by PSC severity and by barrier received (ie, CIB or SoC). Information on use of healthcare services, including additional visits to a healthcare provider such as an ostomy nurse or physician, and ostomy accessories such as an ostomy belt or powder is summarized in Table 6. As with pouching supplies, this information is categorized based on PSC severity and barrier received. Data from the ADVOCATE trial were used to inform estimates of pouching supplies and healthcare services used during a PSC (while ADVOCATE did not evaluate 1-piece pouching systems, were assumed that these barriers would perform similarly from their 2-piece counterparts). Based on usage patterns in Australia and supplies provided by the Australian healthcare system, CIB and SoC barriers were both assumed to be changed once daily. Costs for medical resources were taken from the Stoma Appliance Scheme Schedule (2017).13 Quality-adjusted life days represent participants' daily quality of life; a value of 1 refers to 1 day in perfect health, and a value of 0 refers to worst possible health (deceased). Patients were assigned 1 of 4 possible QALD values daily based on no PSC (subsequently referred to as an uncomplicated ostomy), mild PSC, moderate PSC, or severe PSC (Table 7). For example, given a QALD value of 0.754 for an uncomplicated ostomy (no PSC), a QALD value for mild PSC of 0.697, and a duration of mild PSC of 18 days, a patient who experiences 1 mild PSC would accumulate a total of 274.18 QALDs over a 1-year period (ie, [365 – 18] × 0.754 + [18 × 0.697]). Similarly, given the assumed value associated with a day spent with no PSC, the maximum number of QALDs a patient could accumulate over a 1-year period is 275.21 (ie, 365 × 0.754). We acknowledge that estimates from the ADVOCATE trial were influenced by the effects of surgery and postsurgical recovery such as impaired social activities, reduced satisfaction with life, feelings of social isolation, and need of emotional support.14 We further acknowledge that health-related quality of life may also be impacted by nonsurgical factors, such as changes in work life or relationship with a partner/spouse.15 Because our focus was the decrement specific to experiencing PSCs, utility decrements were based on a separate, substantially larger study, the Pouch Impact Assessment by Hollister, which included 3123 patients with ostomy.16 In this study, the sum of QALDs during days spent with or without a PSC was calculated to derive the total expected QALDs per patient over the 360-day period.
TABLE 5. Percentage Increase in Pouching Supply Use Until PSC Resolutiona.
| PSC Severity | Mild | Moderate | Severe | |||
|---|---|---|---|---|---|---|
| Pouching Supplies | SoC | CIB | SoC | CIB | SoC | CIB |
| Increase in the use of initial pouching supplies, 1-piece systems (until healing), % | 64 | 29 | 49 | 83 | 49 | 83 |
| Increase in the use of initial pouching supplies—baseplate, 2-piece systems (until healing), % | 64 | 29 | 49 | 83 | 49 | 83 |
| Increase in the use of initial pouching supplies—pouch, 2-piece systems (until healing) | 64 | 29 | 49 | 83 | 49 | 83 |
TABLE 6. Direct Medical Care Use by PSC Severity by Barrier Receiveda.
| Patients, % | ||||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| Medical Resource | SoC | CIB | SoC | CIB | SoC | CIB |
| STN | ||||||
| First visit | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Second visit | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Third visit | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Fourth visit | 14.3 | 8.7 | 16.7 | 50.0 | 0.0 | 0.0 |
| Physician (first visit) | 3.6 | 4.3 | 8.3 | 0.0 | 0.0 | 0.0 |
| Ostomy belt | 14.4 | 17.0 | 25.0 | 13.0 | 0.0 | 0.0 |
| Facility fee (x3)b | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Facility fee (x1)b | 14.0 | 8.7 | 16.7 | 50.0 | 0.0 | 0.0 |
| Adhesive spray | 21.0 | 13.0 | 42.0 | 25.0 | 100.0 | 0.0 |
| Adhesive remover | 32.1 | 43.5 | 25.0 | 12.5 | 0.0 | 0.0 |
| Topical antibiotic | 3.6 | 8.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ostomy powder | 43.0 | 35.0 | 58.0 | 50.0 | 0.0 | 100.0 |
| Film wipes | 36.0 | 13.0 | 50.0 | 38.0 | 0.0 | 100.0 |
Abbreviations: CIB, ceramide-infused skin barrier; SoC, standard of care; STN, stomal therapy nurse.
aValues are taken from the ADVOCATE study; percentages estimated based on subjects enrolled in ADVOCATE. A total of 28, 12, and 1 SoC patients experienced mild, moderate, and severe PSCs, respectively; corresponding values for ceramide-infused skin barrier were 23, 8, and 1, respectively.
bFacility fee (x3) is the facility fee associated with the first 3 STN visits. Facility fee (x1) is the facility fee associated with the fourth STN visit.
TABLE 7. QALDs for Health States.
| Health State/Event | SoC (SE) | Ceramide-Infused Skin Barrier (SE) |
|---|---|---|
| Uncomplicated ostomy | 0.754 (0.006) | 0.754 (0.006) |
| Decrement per PSC eventa | ||
| Mild PSC | −0.057 (0.004) | −0.057 (0.004) |
| Moderate PSC | −0.107 (0.008) | −0.107 (0.008) |
| Severe PSC | −0.165 (0.016) | −0.165 (0.016) |
Abbreviations: PSC, peristomal skin complication; QALD, quality adjust life days; SE, standard error; SoC, standard of care.
aTo estimate QALDs per PSC event, subtract the decrement associated with the relevant PSC event from the uncomplicated ostomy value (eg, the QALD for mild PSC is 0.697, or 0.754-0.057).
Data Analysis
Expected PSCs, costs (including those related to PSC-related care and ostomy-related care), and QALDs, respectively, were estimated for both CIB and SoC subjects. The incremental cost-effectiveness ratio of CIB versus SoC was reported as incremental cost per PSC avoided and number of QALD gained. The net monetary benefit was also calculated, using the formula (E ×λ) – C,17 where E is the expected benefit of CIB (in terms of the expected incremental gain in QALDs relative to SoC); λ, the willingness-to-pay threshold (assumed to be A$50,000/QALY, or A$136.89/QALD [ie, A$50,000/365.25]); and C is the anticipated incremental cost of CIB (vs SoC).
Deterministic and probabilistic sensitivity analyses were performed. For both analyses, we varied the following parameters: annual incidence of PSC with SoC and CIB; severity mix for first and second PSCs; days to PSC resolution; percentage of patients using accessories at model entry; QALD decrements by PSC severity; percentage increase in use of pouching supplies for 1- and 2-piece system baseplates and 2-piece system pouches; percentage of patients using each type of medical care at PSC onset; and percentage increase in accessory use after a PSC. In the probabilistic sensitivity analysis, the increase in accessory supplies was capped at 240% (ie, a scenario representing more than a twofold increase in accessory use relative to base case estimates), to exclude unrealistically high accessory supply usage during PSC. In the deterministic sensitivity analysis, each parameter was varied one at a time, with the base case values changed to the respective upper and lower 95% confidence interval bounds. For the probabilistic sensitivity analyses, we ran the model 2000 times, each time simultaneously replacing parameter estimates for any included variable with an alternative value derived by sampling from its underlying distribution.
We also ran 2 scenario analyses: 1 in which severity distribution of PSCs associated with CIB use was assumed to be equal to those of SoC, and 1 in which all parameters for CIB save those related to incidence and severity of PSCs were set equal to ostomy management using an SoC pouching system. As noted earlier, all analyses were conducted from the perspective of the Australian healthcare system; expected costs are presented as both Australian dollars (A$) and US dollars (US$). Because the model was based on living with an ostomy for 360 days, we did not discount either outcomes or costs. This decision was based on guidelines for development of this type of economic model.18,19
RESULTS
In the cohort of 500 hypothetical new ostomy patients created for this model, use of CIB over a 360-day period is expected to decrease ostomy- and PSC-related costs by A$90,038 (US $69,329), increase (all versus SoC) QALDs by 174, and reduce the number of PSCs by 82 (Table 8). On a per-patient basis, CIB was expected to result in healthcare cost savings of A$180 (US $139), 0.35 additional QALDs, and 0.16 fewer PSCs. Given the expected reduction in healthcare costs and increase in QALDs, CIB yielded better outcomes at lower cost when compared to SoC. The corresponding net monetary benefit to the Australian healthcare system associated with use of CIB versus SoC was A$227.91 (US $175.49) (i.e. [a willingness-to-pay threshold of A$136.89/QALD × 0.35 additional QALDs] – the incremental savings of CIB of A$180). Based on our findings and assuming approximately 40,000 Australians living with a fecal ostomy,20 a switch of 25% to CIB could result in 1643 fewer PSCs and a cost saving of A$1,800,762 (US $1,386,587) over a 360-day period.
TABLE 8. Basecase Results.
| Aggregate Cohort (n = 1000) | Per-Patient Basis | |||||
|---|---|---|---|---|---|---|
| Outcome | CIB (n = 500) | SoC (n = 500) | Δ | CIB | SoC | Δ |
| Number of PSCs | 215 | 297 | −82 | 0.595 | 0.431 | −0.164 |
| Healthcare costs, A$ | ||||||
| Usual ostomy care | ||||||
| One-piece systems | 541,710.00 | 538,650.00 | 3,060 | 1,083.42 | 1,077.30 | 6.120 |
| Two-piece systems—baseplate | 224,361.44 | 224,463.09 | −102 | 448.72 | 448.93 | −0.203 |
| Two-piece systems—pouches | 147,855.09 | 149,348.57 | −1,493 | 295.71 | 298.70 | −3 |
| Accessories | 175,239.09 | 219,526.92 | −44,288 | 350.48 | 439.05 | −88.576 |
| Total usual ostomy care | 1,089,166 | 1,131,989 | −42,823 | 2,178.33 | 2,263.98 | −85.65 |
| PSC-related | ||||||
| Pouching supplies | ||||||
| One-piece systems | 8,736 | 13,759 | −5,022 | 17.47 | 27.52 | −10.04 |
| Two-piece systems—baseplate | 3,685 | 5,816 | −2,131 | 7.37 | 11.63 | −4.26 |
| Two-piece systems—pouches | 2,090 | 3,205 | −1,115 | 4.18 | 6.41 | −2.23 |
| Total pouching supplies | 14,512 | 22,780 | −8,268 | 29.02 | 45.56 | −16.54 |
| Accessories | ||||||
| Flat ring | 719 | 27,718 | −26,999 | 1.44 | 55.44 | −54.00 |
| Paste | 15,011 | 2,864 | −12,147 | 30.02 | 5.73 | 24.29 |
| Ostomy belt | 1,586 | 268 | −1,318 | 3.17 | 0.54 | 2.64 |
| Adhesive remover spray | 699 | 772 | −74 | 1.40 | 1.54 | −0.15 |
| Skin film wipes | 127 | 944 | −817 | 0.25 | 1.89 | −1.63 |
| Ostomy powder | 297 | 453 | −156 | 0.59 | 0.91 | −0.31 |
| Total accessories | 18,438 | 33,019 | −14,580 | 36.88 | 66.04 | −29.16 |
| Direct medical care | ||||||
| Physician (first visit) | 555.51 | 1,308.56 | −753.05 | 1.11 | 2.62 | −1.51 |
| STN | 27,287.06 | 37,229.70 | −9,942.64 | 54.57 | 74.46 | −19.89 |
| Ostomy belt | 196.01 | 315.90 | −119.89 | 0.39 | 0.63 | −0.24 |
| Facility feea | 27,458.68 | 37,441.07 | −9,982.39 | 54.92 | 74.88 | −19.96 |
| Adhesive spray | 1,187.01 | 3,149.28 | −1,962.27 | 2.37 | 6.30 | −3.92 |
| Adhesive remover | 741.90 | 884.26 | −142.36 | 1.48 | 1.77 | −0.28 |
| Topical antibiotic | 81.06 | 41.70 | 39.36 | 0.16 | 0.08 | 0.08 |
| Ostomy powder | 781.67 | 1,224.71 | −443.04 | 1.56 | 2.45 | −0.89 |
| Film wipes | 766.50 | 1,826.86 | −1,060.36 | 1.53 | 3.65 | −2.12 |
| Total direct medical care | 59,055 | 83,422 | −24,367 | 118.11 | 166.84 | −48.73 |
| Total PSC-related | 92,005 | 139,221 | −47,215 | 184 | 278 | −94 |
| Total healthcare costs, A$ | 1,181,171 | 1,271,209 | −90,038 | 2,362.34 | 2,542.42 | −180.08 |
| QALDs | 135,363 | 135,189 | 174 | 270.73 | 270.38 | 0.35 |
Abbreviations: CIB, ceramide-infused skin barrier; PSC, peristomal skin complication; STN, stomal therapy nurse; QALD, quality-adjusted life day; SoC, standard of care.
aFacility fee is the facility fee associated with the all STN visits.
Sensitivity Analyses
Results of the deterministic sensitivity analyses suggested that use of CIB in lieu of SoC would lower healthcare costs under multiple potential scenarios within the model we created. For example, increasing the proportion of SoC patients on relatively low-cost pouching supplies to 27% resulted in expected cost savings with CIB of A$106 (US $82); reducing the proportion of SoC patients on these supplies resulted in expected cost savings with CIB of A$241 (US $186, Figure 2). Among the clinical parameters, the most influential one on the cost savings was the probability of experiencing a PSC with the use of SoC. Reducing this parameter value to 44% from the base case value of 55% reduced the cost savings to A$125 (US $96.70). Estimates of expected incremental cost savings were most sensitive to the percentage of patients using the CIB or SoC and the proportion using flat ring accessories (the most expensive accessory).
Figure 2.
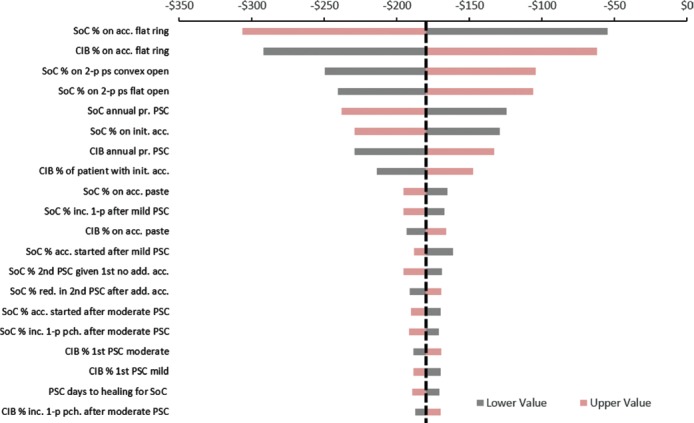
Expected incremental cost of ceramide-infused skin barrier versus SoC in deterministic sensitivity analyses. The vertical dotted black line represents incremental per patient cost savings associated with use of ceramide-infused skin barrier in the base case (A$180). Negative estimates indicate expected cost savings associated with use of ceramide-infused skin barrier instead of SoC. acc indicates accessories; add, addition; bp, baseplate; CIB, ceramide-infused skin barrier; inc, increase; pce, piece; pr, probability; ps, pouching supplies; SoC, standard of care; sys, system.
In the probabilistic sensitivity analyses, we completed 2000 runs, each using different values for the various model inputs. Each run was plotted in Figure 3 based on its expected incremental benefit (ie, expected QALDs gained with CIB – expected QALDs gained with SoC) and the expected incremental cost. Results of these analyses indicated that use of CIB was expected to result in fewer PSCs in 97% of simulations, lower healthcare costs in 92% of simulations, reduce both PSCs and healthcare costs in 90% of simulations, and increased QALDs in 61% of simulations (Figure 3). Given a willingness-to-pay threshold of A$136.89/QALD (US $105.41/QALD), represented by the diagonal line (typically used in these diagrams to indicate the threshold below which an intervention is considered cost-effective) as seen in Figure 3, CIB was preferred over SoC in approximately 92.2% of the simulations. In more than half of these simulations (55.3%), CIB was expected to yield equal or greater QALDs at equal or lower cost. In contrast, CIB was expected to yield greater QALDs at higher cost in 3.75% of simulations. In 33.5% of simulations, CIB was expected to yield fewer QALDs at lower cost; however, the additional QALDs expected with SoC exceeded the willingness-to-pay ratio.
Figure 3.
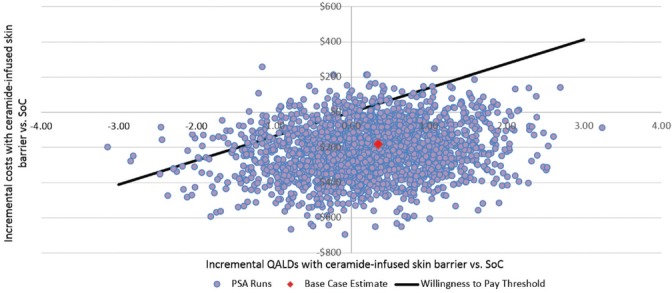
Incremental costs and QALDs associated with use of ceramide-infused skin barrier versus SoC in probabilistic sensitivity analyses. PSA indicates probabilistic sensitivity analysis; QALD, quality-adjusted life day; SoC, standard of care. Note: Each point on the figure is the result of one of a total of 2000 runs of the model, each implemented with a different set of input values. Each point represents the incremental benefit (estimated as total expected QALDs with CIB – total expected QALDs with SoC) and incremental cost (estimated as total expected cost with CIB – total expected cost with SoC) associated with use of CIB versus SoC. Results are expressed on a per-patient basis.
Scenario Analyses
We completed 2 scenario analyses; one assumed the same severity distributions of PSCs for CIB and SoC, and the other varied the likelihood of a PSC and its severity based on findings from the ADVOCATE trial.2 In the scenario where we assumed PSC severity distributions were equal, CIB use was expected to create a cost reduction of A$88,083 (US $67,824). The anticipated cost reduction on a per-patient basis was A$176 (US $136) and the expected QALD scores were 158 and 0.32, respectively. In the scenario where the only differences between CIB and SoC were in incidence and severity of PSCs, CIB resulted in expected per-patient cost savings of A$76 (US $59) and a QALD gain of 0.34. In summary, both scenarios favored use of the CIB.
DISCUSSION
Results from our model suggest that use of a CIB among persons with a new fecal ostomy is expected to result in lower costs, results in fewer PSCs, and modestly increased QALDs. These findings were consistent across various sensitivity analyses, as well as 2 relatively conservative scenarios that assumed a parity across treatment arms not observed in ADVOCATE.12 Assuming that 25% of the approximate 40,000 Australians living with fecal ostomy used the CIB, we project a 1-year cost saving to the Australian healthcare system of approximately A$1,800,762 (US $1,386,587). We also project that persons with an ostomy who switch to the CIB would experience 1643 fewer PSCs.
The primary function of an ostomy barrier is to prevent water loss through the epidermis, and to prevent stoma effluent from permeating into the skin and initiating an irritative or immune response. Compromise of the skin barrier of an ostomy pouching system changes the lipid composition of the peristomal skin.23–25 The model we created extrapolates results of the ADVOCATE trial over a 360-day period in an attempt to demonstrate potential benefits and associated costs. The majority of simulations (1849 out of 2000 or 92.4%) we completed resulted in lower expected costs and fewer PSCs in persons using the CIB (1947 out of 2000 runs, or 97.3%). In comparison, fewer simulations (1214 out of 2000 or 60.7%) resulted in increased QALDs. These seemingly discrepant findings are a result of allowing both the incidence and severity of PSCs to vary simultaneously, which resulted in scenarios where CIB use was associated with fewer but more severe and/or moderate PSCs relative to SoC skin barrier use. When the probabilistic sensitivity analyses was run with a fixed PSC severity distribution, 98% of simulations (1960 out of 2000 runs) resulted in increased QALDs with use of the CIB. Accordingly, while CIB was preferred in 92% of the simulations (1840/2000 runs), only 59% of runs indicated that CIB was expected to provide equal or greater QALDs at a lower cost or at a higher cost that did not exceed the willingness-to-pay ratio of A$136.89/QALD (US$105.41/QALD). Because QALD is the benefit used in estimating the incremental cost-effectiveness ratio, it is not surprising that CIB was deemed cost-effective in approximately 59% of the runs. While there is evidence to suggest that the current willingness-to-pay threshold we used may be lower than that reflected in reality,26 it is unlikely that an increased threshold would increase the proportion of simulations in which CIB would be deemed cost-effective (Figure 2). In the other 33.15% of the 2000 simulations in which CIB use emerged as cost-effective, it was expected to yield fewer QALDs as compared to SoC; however, the incremental cost associated with SoC in those scenarios exceeded the willingness-to-pay threshold. We note that if the measure of cost-effectiveness was defined based on averted PSCs (vs increased QALDs), CIB would likely be preferred in more than 90.3% of simulations, although a definitive conclusion is difficult without knowledge of the Australian government's willingness-to-pay threshold to avert a PSC.
Limitations
Our study has several limitations. As with all economic models, several assumptions were required to develop our model. We assumed that patients could experience no more than 2 PSCs within the time horizon, and that those PSCs would occur at the midpoints of weeks 1 to 12 and weeks 13 to 52. However, the timing of these events may differ in clinical practice. Jonkers and colleagues27 observed that 82% of consecutive patients (n = 100) who underwent ostomy surgery experienced complications within the subsequent year, with “skin irritation” being the most common (55% of the cohort, 67% of the subgroup who experienced complications). In their study of 180 persons with ostomies, Persson and colleagues28 noted that complications were observed as early as 2 weeks following surgery. Persson's group also reported a total of 303 stoma complications over 2 years (an average of 1.68 complications per patient); 94% of these complications occurred during the first year following surgery. Accordingly, we may have underestimated the risk of PSCs; both in terms of the initial risk of having a PSC and the risk of subsequent events. In addition, our model also did not differentiate between ostomy types (eg, colostomy and ileostomy), surgical approach, and other factors likely to influence the incidence of PSCs.
We also assumed that the cost of CIB was equal to that of SoC to be consistent with the Australian healthcare system that prices all barriers in the same category at parity. While this rendered our analyses consistent with current reimbursement practices in Australia, it has a large impact on findings, as reductions in the costs of PSCs are not offset by increased cost of ostomy supplies. Therefore, the impact of any subsequent price modifications to ostomy products (either CIB or other skin barriers) on the expected cost-effectiveness of CIB would require separate analysis. While the ADVOCATE trial did not assess 1-piece pouching systems, we included them in our assessment in an effort to better reflect “real-world” use of ostomy systems. Evidence concerning the performance of 1- and 2-piece systems is absent, and caution is warranted in interpretation of our findings with respect to the performance of 1- versus 2-piece pouching systems.
We assumed that PSCs were of fixed duration. While this simplified the model, it also placed “caps” on the cost and health-state burden associated with these PSCs. As other studies have reported longer resolution times ranging from 7 weeks7,8 to more than 3 months,29 we may have underestimated the cumulative costs of PSCs, and consequently, the expected cost-effectiveness of regular use of CIB relative to SoC pouching systems.
In addition, parameter estimations used in our model were based in large part on findings from the ADVOCATE trial. While enormously helpful in providing estimates of the incidence of PSCs and costs thereof, the trial is limited in terms of the overall number of enrollees and the duration of follow-up. Accordingly, we were required to make several assumptions concerning events that occurred in weeks 13 to 52. While we believe them reasonable, analyses would be more robust if these assumptions were replaced by additional “real-world” data that reflect the experience of new ostomates over the period of interest.
Finally, we assumed that decisions to use specific healthcare resources could only be made at certain times during the 1-year period of interest. For example, we assumed that patients would not switch from a CIB to another (SoC) pouching system or vice versa regardless of whether they experienced a PSC. Similarly, we assumed that the only time accessory use would change was following a PSC, and that once changed, it would remain at those levels until the end of the time horizon. These assumptions may have increased estimates of expected total healthcare costs of patients who experienced PSCs. However, the degree to which patients in clinical practice use accessories as prophylaxis for subsequent PSCs is unknown.
CONCLUSIONS
Based on the model presented in this study, we found that use of a CIB pouching system resulted in a per-patient healthcare cost savings of A$180 (US $138.60), along with a gain of 0.35 QALDs and 0.16 fewer PSCs. Findings were consistent across a number of sensitivity analyses, and more than 90% of simulations projected lower costs and fewer PSCs with use of the ceramide-infused barrier. Our analyses therefore suggest that the CIB is a cost-effective choice of skin barrier for persons with a new fecal ostomy when used over an extended period of time.
Footnotes
Funding for this research was provided by Hollister Incorporated.
Gary Inglese and George Skountrianos are employees of Hollister Incorporated. Ariel Berger and Mustafa Oguz are employees of Evidera Inc, which provides consulting and other research services to pharmaceutical, device, government, and nongovernment organizations. Evidera Inc received funding from Hollister Incorporated in connection with conducting this study and with developing this article. Tonny Karlsmark is an employee of the University of Copenhagen and did not receive any funding for the conduct of this study or for the development of this article.
REFERENCES
- 1.Sheetz KH, Waits SA, Krell RW, et al. Complication rates of ostomy surgery are high and vary significantly between hospitals. Dis Colon Rectum. 2014;57:632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colwell JC, McNichol L, Boarini J. North America Wound, Ostomy, and Continence and Enterostomal Therapy Nurses Current Ostomy Care Practice Related to Peristomal Skin Issues. J Wound Ostomy Continence Nurs. 2017;44(3):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvey B, Beck DE. Peristomal dermatology. Clin Colon Rectal Surg. 2008;21(01):041–044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nybaek H, Jemec G. Skin problems in stoma patients. J Eur Acad Dermatol Venereol. 2010;24(3):249–257. [DOI] [PubMed] [Google Scholar]
- 5.Doctor K, Colibaseanu DT. Peristomal skin complications: causes, effects, and treatments. Chronic Wound Care Manage Res. 2017;4:1–6. [Google Scholar]
- 6.Jemec GB. Peristomal skin problems account for more than one in three visits to ostomy nurses. Br J Dermatol. 2008;159:1211–1212. [DOI] [PubMed] [Google Scholar]
- 7.Meisner S, Lehur PA, Moran B, et al. Peristomal skin complications are common, expensive, and difficult to manage: a population based cost modeling study. PLoS One. 2012;7(5):e37813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins L, Tavernelli K, Sansom W, et al. Strategies to reduce treatment costs of peristomal skin complications. Gastrointestinal Nurs. 2012;10:24–32. [DOI] [PubMed] [Google Scholar]
- 9.Hollister. The CeraPlus Skin Barrier Product Evaluation Clinicians' Feedback. http://www.hollister.com/∼/media/files/pdfs–for–download/ostomy–clinical–evaluations–and–case–studies–pdfs/ceraplus-product-evaluation-113015.pdf?la=en. Accessed November 8, 2017.
- 10.Hollister. CeraPlus Skin Barrier. http://www.hollister.com/en/ceraplus. Published 2017. Accessed November 8, 2017.
- 11.Ostomy Wound Management. Innovations that work! Ostomy Wound Manage. 2015;61(7). http://www.o-wm.com/article/innovations-work-0. Accessed November 8, 2017. [Google Scholar]
- 12.Colwell JC, Pittman J, Raizman R, Salvadalena G. A Randomized Controlled Trial Determining Variances in Ostomy Skin Conditions and the Economic Impact (ADVOCATE). J Wound Ostomy Continence Nurs. 2018;45(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Australian Department of Health. Stoma Appliance Scheme—Schedule. http://www.health.gov.au/internet/main/publishing.nsf/Content/AB1C0973EEA98E14CA257BF0001E01C4/$File/sas-schedule-1-july-2017-full.pdf. Published 2017.
- 14.Nichols TR. Social connectivity in those 24 months or less postsurgery. J Wound Ostomy Continence Nurs. 2011;38(1):63–68. [DOI] [PubMed] [Google Scholar]
- 15.Nichols TR, Riemer M. The impact of stabilizing forces on postsurgical recovery in ostomy patients. J Wound Ostomy Continence Nurs. 2008;35(3):316–320. [DOI] [PubMed] [Google Scholar]
- 16.Nichols TR. Pouching System Impact Assessment. Data on File. Libertyville, IL: Hollister Incorporated; 2013. [Google Scholar]
- 17.Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analyses. Med Decis Making. 1998;18:S68–S80. [DOI] [PubMed] [Google Scholar]
- 18.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):231–250. [DOI] [PubMed] [Google Scholar]
- 19.Torgerson DJ, Raftery J. Economic notes. Discounting. BMJ. 1999;319(7214):914–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Australian Department of Health Website. Stoma Appliance Scheme. http://www.health.gov.au/stoma. Accessed September 10, 2017.
- 21.Michaels AS, Chandrasekaran SK, Shaw JE. Drug permeation through human skin: theory and in vitro experimental measurement. AIChE J. 1975;21(5):985–996. [Google Scholar]
- 22.Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 80(1, suppl):44s–49s. [PubMed] [Google Scholar]
- 23.Agner T. Susceptibility of atopic dermatitis patients to irritant dermatitis caused by sodium lauryl sulphate. Acta Derm Venereol. 1991;7(4):296–300. [PubMed] [Google Scholar]
- 24.Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130(10):2511–2514. [DOI] [PubMed] [Google Scholar]
- 25.Janssens M, van Smeden J, Gooris GS, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 53(12):2755-–2766.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 27.Jonkers HAF, Draaisma WA, Roskott AM, et al. Early complications after stoma formation: A prospective cohort study in 100 patients with 1-year follow-up. Int J Colorectal Dis. 2012;27:1095–1099. [DOI] [PubMed] [Google Scholar]
- 28.Persson E, Berndtsson I, Carlsson E, et al. Stoma-related complications and stoma size–a 2-year follow-up. Colorectal Disease. 2010;12:971–976. [DOI] [PubMed] [Google Scholar]
- 29.Herlufsen P, Olsen AG, Carlsen B, et al. Study of peristomal skin disorders in patients with permanent stomas. Br J Nurs. 2006;15:854–862. [DOI] [PubMed] [Google Scholar]


