Short abstract
Aims
Insular cortex is a brain region critical for processing of the sensation. Purinergic receptors are involved in the formation of chronic pain. The aim of the present study was to explore the role and mechanism of P2X3 receptors (P2X3Rs) in insular cortex in chronic visceral pain.
Methods
Chronic visceral pain in adult rats was induced by neonatal maternal deprivation and measured by detecting the threshold of colorectal distension. Western blotting, immunofluorescence, and real-time quantitative polymerase chain reaction techniques were used to detect the expression and distribution of P2X3Rs. Synaptic transmission in insular cortex was recorded in brain slices by patch clamp techniques.
Results
Expression of P2X3Rs both at mRNA and protein levels in right hemisphere of insular cortex was significantly increased in neonatal maternal deprivation rats. In addition, P2X3Rs were expressed with NeuN or synaptophysin but not with glial fibrillary acidic protein and CD11b. The co-localization of P2X3Rs with NeuN or synaptophysin was greatly enhanced in right hemisphere of insular cortex in neonatal maternal deprivation rats. Furthermore, neonatal maternal deprivation markedly increased both the frequency and amplitude of miniature excitatory postsynaptic current in right hemisphere of insular cortex. Incubation of A347091 significantly decreased the frequency of spontaneous excitatory postsynaptic current and miniature excitatory postsynaptic current of insular cortex neurons of neonatal maternal deprivation rats. Incubation of P2X3Rs agonists α,β-mATP remarkably increased the frequency of spontaneous excitatory postsynaptic current and miniature excitatory postsynaptic current of the right hemisphere of insular cortex neurons of healthy control rats. Importantly, injection of A317491 significantly enhanced the colorectal distension threshold of neonatal maternal deprivation rats, while injection of α,β-mATP into right but not left insular cortex markedly decreased the colorectal distension threshold in healthy control rats.
Conclusions
Overall, our data provide integrated pharmacological, biochemical, and functional evidence demonstrating that P2X3Rs are physically and functionally interconnected at the presynaptic level to control synaptic activities in the right insular cortex, thus contributing to visceral pain of neonatal maternal deprivation rats.
Keywords: Visceral pain, P2X3 receptors, synaptic transmission, neonatal maternal deprivation, insular cortex
Introduction
Merging evidence suggests that adenosine triphosphate (ATP) functions as a neurotransmitter or neuromodulator in the mammalian brain, where it activates several different types of ionotropic and G protein-coupled ATP receptors.1–3 Using spinal cord slice preparations and patch-clamp techniques in lamina II and V regions, Nakatsuka et al.4 reported that P2X receptor subtypes differentially modulate glutamate release from primary sensory terminals innervating different receptive fields. P2X7 receptors in the spinal dorsal horn were significantly upregulated in a rat model of bone cancer pain.5 Besides, P2X7 receptors in glia cells also involved in long-term potentiation at synapse between primary afferents and spinal dorsal horn neurons.6 Recent studies have been more focusing on the roles and synaptic mechanisms at the spinal dorsal horn level. However, the roles of specific ATP receptors in synaptic plasticity of the insular cortex (IC) have not been fully established under chronic pain conditions.
IC is a brain region critical for processing of the sense of taste, the emotion, and perception of innocuous warm and cold.7,8 IC also responds to visceral and nociceptive stimulation and plays an important role in processing of pain, including modulation of affective component and sensory component of pain.9,10 Painful stimulation could activate the IC, and direct stimulation of the IC can evoke painful reaction.11 All these changes are believed to result from long-lasting changes in the function and structure of synapses in IC area. Our recent report that neonatal maternal deprivation (NMD) enhances synaptic transmission by sensitization of P2X7Rs in right IC suggests an alteration of synapse plasticity in IC.12 Studies from clinic perspective also provided several lines of evidence to strongly support an involvement of IC in chronic visceral hypersensitivity of patients with irritable bowel syndrome (IBS). Using a task-dependent functional magnetic resonance imaging technique to investigate the brain activity, two groups showed that hypersensitive patients with IBS had greater activation of insula and reduced deactivation in pregenual anterior cingulate cortex during noxious rectal distensions, compared to controls and normosensitive patients with IBS.13,14 In addition, they also demonstrated that during the uncued condition contrasted to the cued safe condition, IBS subjects (compared to healthy control subjects) showed greater brain activations in the affective (amygdala, anterior insula) and attentional (middle frontal gyrus) regions.15 Together, these data indicate a role of IC in the process of visceral hypersensitivity. However, the precise molecular mechanisms underlying the activation of IC remain largely unknown.
In the present study, we tested the hypothesis that P2X3Rs in IC are sensitized after NMD thus contributing to the visceral pain in adult rats. We showed that NMD significantly enhanced P2X3Rs expression in IC and that inhibition of P2X3Rs signaling not only suppressed the synaptic activity but also attenuated visceral pain responses. These findings might provide novel evidence for the involvement of insular abnormalities in the pathophysiology and potential targets for the treatment for chronic visceral pain in patients with IBS.
Materials and methods
Induction of chronic visceral hyperalgesia
Experiments were performed on male Sprague–Dawley rats. Care and handling of these rats were approved by the Institutional Animal Care and Use Committee of the Soochow University and were in accordance with the guidelines of the International Association for the Study of Pain. Chronic visceral hyperalgesia was induced by NMD and assessed by colorectal distension (CRD) threshold as described previously.12,16 Experiments were performed in NMD rats at age of 6 to 7 weeks. The age-matched healthy male rats were used as control (CON).
Drug administration
For behavioral experiments, α,β-mATP (P2X3Rs agonist) or A317491 (potent P2X3Rs antagonist) dissolved in normal saline (NS) was directly injected into the right or left IC of rats, as described previously in literature.12 The drug concentrations used in the present study were based on our preliminary study and reports from other groups.17,18
Western blotting
The process of Western blotting was performed according to the protocols described in our previous reports.19 In brief, the total protein was exacted from IC of rats by ultrasonic cracker in lysate and fractionated on polypropylene electrophoresis (Bio-Rad, Hercules, CA). Proteins were transferred to polyvinylidene difluoride membranes for 2 h at 200 mA. The polyvinylidene difluoride membranes were immersed in the 5% fat-free milk for 2 h and then incubated with anti-P2X3Rs primary antibody (1:200, Alomone lab) or anti-GAPDH antibody (1:1000, Hangzhou Goodhere Biotechnology) at 4°C overnight in Tris-buffered saline containing 1% milk. Band density was measured using ImageJ software. P2X3Rs expression was normalized to GAPDH.
Path clamp recordings on brain slices
Rats of both control and NMD group (100–130 g, 6–7 weeks) were anesthetized with 4% chloral hydrate. The brain was rapidly removed and embedded with 1.6% high strength agarose (Type I-B, Sigma, USA). Transverse brain slices of the IC (400 μm) were cut using standard methods20,21 in oxygenated (95% O2, 5% CO2) solution (in mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 12 NAC, and 25 glucose, around 32°C. Ten minutes after cutting, the brain slices were transferred into oxygenated holding solution (in mM): 94 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 2 MgSO4, 2 CaCl2, 12 NAC, and 25 glucose, at room temperature (RT). After recovery for at least 30 min, slices were transferred to recording chamber with artificial cerebrospinal fluid (in mM): 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 24 NaHCO3, 5 HEPES, 12.5 glucose, 2 MgSO4, and 2 CaCl2.
The recording was performed on the arena of BX51WI microscope (Olympus) equipped with infrared differential interference contrast optics for visualizing whole cell patch-clamp. The pipette liquor for recording excitatory postsynaptic current (EPSC) contained (in mM): 133 K-gluconate, 8 NaCl, 0.6 EGTA, 10 HEPES, 2 Mg-ATP, and 0.3 Na-GTP. EPSC was recorded from IC with a Digidata 1440A interface, MultiClamp 700B amplifier, and pClamp10 software (Axon Instruments). The cell type was distinguished under current clamp mode according to the electrophysiological peculiarity described by Washburn and Moises22 in reflect to intracellular injection of a depolarizing current (100–300 pA, step 50 pA, duration 1000 ms). The membrane potential was held at −70 mV for EPSC recording. The data recorded in excitatory neurons were used in further analysis of EPSC. The extracellular solution containing tetrodotoxin (1 μM) was used to record miniature EPSC (mEPSC) of IC neurons. α,β-mATP and A317491 purchased from Sigma (USA) were freshly diluted in artificial cerebrospinal fluid before used in the electrophysiological experiments.
Histology and immunofluorescence studies
Animals were intracardially perfused with NS solution, followed by 4% paraformaldehyde. The brain was allowed to postfix by paraformaldehyde overnight and followed gradient dehydration by 10 to 30% sucrose solution; 14 μm frozen sections contained IC area were used in immunofluorescence study as described previously in literature.12 Briefly, sections were bathed by phosphate-buffered saline for three times and then blockade with 7% donkey serum at RT for 1 h. After that, the sections were simultaneously incubated with primary antibodies (anti-P2X3Rs, 1:100, Alomone lab; anti-GFAP, 1:300, Cell Signaling Technology; anti-CD11b, 1:100, Bio-Rad; anti-NeuN, 1:50, Merk Millpore; anti-synaptophysin, 1:100, Abcam) for overnight at 4°C and then incubated with secondary antibodies with Alexa Fluor 488 (1:100) and 555 (1:500, Life Technologies Inc.) for 2 h at RT. Negative controls were performed without the primary antibody.
Real-time quantitative polymerase chain reaction
Total RNAs were extracted from IC of both hemispheres from control and NMD rats with TRIzol (Ambion, Shanghai, China). cDNA was synthesized from total RNA using a reverse transcription kit (Transgen Biotech, Beijing, China) following the supplier’s instructions. The sequences of the primer pairs for p2x3r were as follows: (F) 5′-TTGGGATCATCAACC GAGCC-3′ and (R) 5′-ATGACAAAGACAGAGGT GCCC-3′. The sequences of the primer pairs for gapdh (as an internal control) were as follows: (F) 5′-TGGA GTCTACTGGCGTCTT-3′ and (R) 5′-TGTCATATTT CTCGTGGTTCA-3′. Control reactions were performed without cDNA templates.
Data analyses
A fixed length of traces (4 min) of EPSCs was analyzed using MiniAnalysis program 6.0.3 (Synaptosoft). Before the comparison, all data were checked for normal distribution. Data were analyzed using paired sample t test, two-sample t test, Mann–Whitney test, or one-way repeated measures analysis of variance followed by Kruskal–Wallis test with Origin 8 (Origin Lab Inc., USA) and Prism 7 software (GraphPad Software, Inc, USA). All values were shown as mean ± standard error. p < 0.05 was considered statistically significant.
Results
P2X3Rs expression was upregulated in right IC of NMD rats
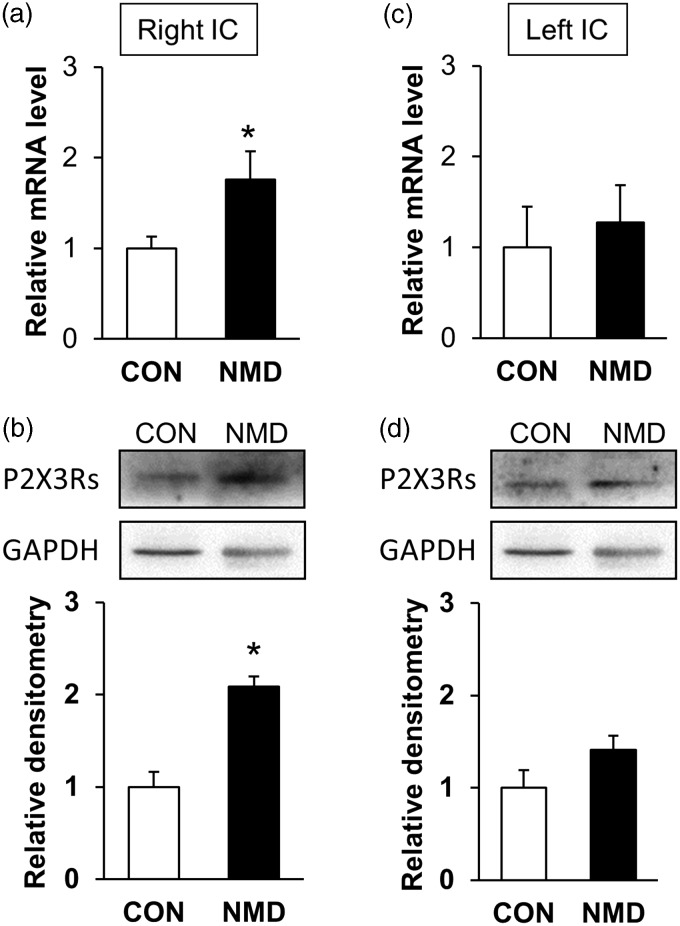
NMD significantly enhanced P2X3Rs expression at mRNA level in right hemisphere IC of NMD rats when compared with CON rats (Figure 1(a)). The relative value of P2X3Rs in right hemisphere IC was 1.76±0.31 in NMD rats (n = 4) and 1.00±0.13 in age-matched control rats (n = 4). However, the expression of P2X3Rs at mRNA level was not altered in left hemisphere IC of NMD rats (Figure 1(c)). The relative value of P2X3Rs in left hemisphere IC was 1.27±0.41 in NMD rats (n=4) and 1.00±0.45 in age-matched control rats (n=4). P2X3Rs expression at protein level was also examined at both hemisphere IC (Figure 1(b) and (d)). The relative value of P2X3Rs in right hemisphere IC was 2.17±0.26 in NMD rats (n=4) and 1.00±0.13 in age-matched control rats (n=4). The relative value of P2X3Rs in left hemisphere IC was 1.68±0.28 in NMD rats (n=4) and 1.00±0.49 in age-matched control rats (n=4).
Figure 1.
Enhanced expression of P2X3Rs in right IC of NMD rats. (a) and (b) P2X3Rs expression was significantly increased at both protein and mRNA level in right IC of NMD rats compared to CON rats (n=4 for each group, *p<0.05, two sample t test). (c) and (d) P2X3Rs were expressed in in left IC but their amount was not altered at protein nor mRNA level of NMD rats (n=4 rats for each group, two sample t test). CON: control; NMD: neonatal maternal deprivation; IC: insular cortex.
NMD upregulated P2X3Rs expression in neurons and presynaptic terminals of IC
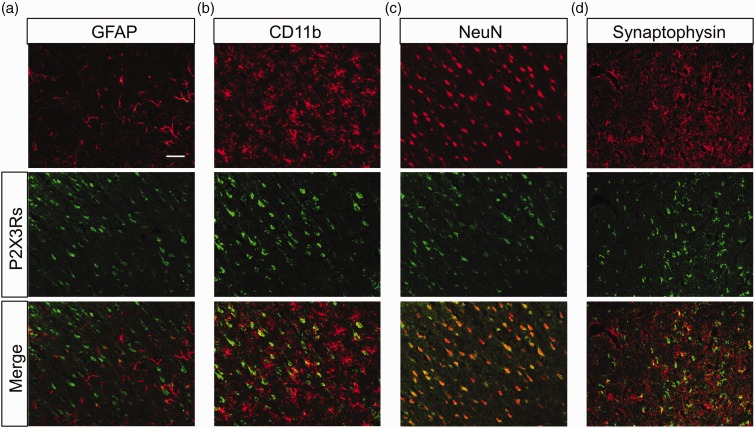
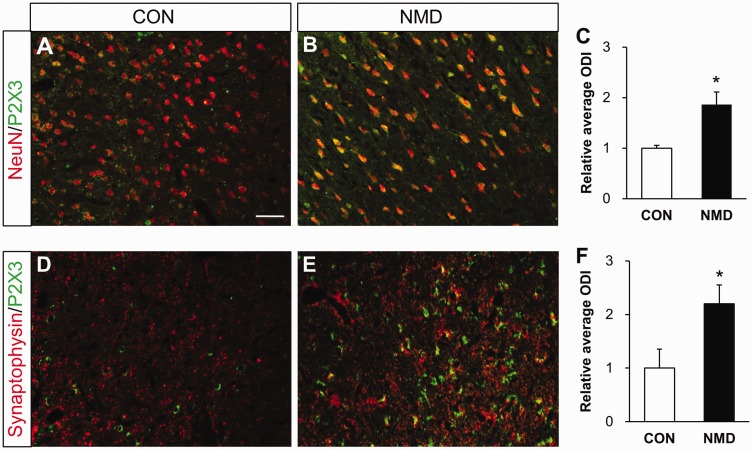
To determine the location of the P2X3Rs, we next performed immunohistochemistry in NMD rats. As shown in Figure 2, most P2X3Rs were co-localized with neurons labeled by NeuN and synaptophysin, which is a marker for presynaptic vesicles.23,24 However, P2X3Rs were not expressed in astrocytes or microglia of NMD rats. More importantly, the P2X3Rs co-expression with NeuN was markedly increased in right IC of NMD rats when compared with control rats (Figure 3(a) to (c)). The relative value was 1.88±0.36 in NMD rats (n=4) and 1.00±0.19 in age-matched control rats (n=4). Besides, the co-localization of P2X3Rs with synaptophysin was also significantly increased (Figure 3(d) to (f)). The relative value was 2.19±0.43 in NMD rats (n=4) and 1.00±0.41 in age-matched control rats (n=4). These results suggest that expression of P2X3Rs was upregulated in right IC in NMD rats. Since P2X3Rs were only upregulated in right IC, the following experiments were performed on the right IC unless mentioned otherwise.
Figure 2.
Co-localization of P2X3Rs with NeuN and synaptophysin. (a) and (b) P2X3Rs (green) did not express in GFAP labeled astrocytes (red) nor CD11b labeled microglia (red) in right IC of NMD rats. (c) P2X3Rs (green) were co-localized with NeuN labeled neurons (red) in right IC of NMD rats. (d) P2X3Rs (green) were partly co-expressed with synaptophysin (red). Bar=100 μm for all photos. GFAP: glial fibrillary acidic protein.
Figure 3.
Enhanced P2X3Rs expression in neurons and presynaptic terminals of right IC of NMD rats. (a) Merged immunofluorescence images showed minor co-localization of NeuN (red) and P2X3Rs (green) in right IC of CON rats. (b) Merged images showed many co-localizations of NeuN (red) and P2X3Rs (green) in right IC of NMD rats. (c) Statistic chart indicated that P2X3Rs positive neurons were significantly increased in right IC of NMD rats compared with CONs (n=4 for each group, *p<0.05 vs. CON, two sample t test). (d) Immunofluorescence images showed minor co-localization of synaptophysin (red) and P2X3Rs (green) in right IC of CON rats. (e) Images showed many co-localizations of synaptophysin (red) and P2X3Rs (green) in right IC of NMD rats. (f) Statistic chart showed that P2X3Rs positive synaptophysin was markedly increased in right IC of NMD rats compared with CONs (n=4 for each group, *p<0.05 vs. CON, two sample t test). Bar=100 μm for all photos. CON: control; NMD: neonatal maternal deprivation.
NMD enhanced mEPSC in IC
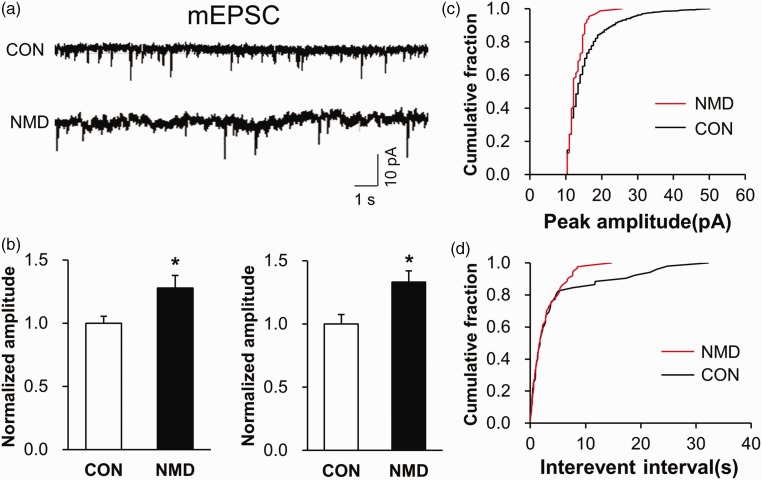
We have reported in previous paper that spontaneous excitatory postsynaptic current (sEPSC) was significantly strengthened in IC of NMD rats.12 Here, we showed that both the frequency and amplitude of mEPSC were markedly increased in the right IC of NMD rats (Figure 4). The normalized amplitude was 1.27±0.10 in NMD rats (n=8) and 1.00±0.06 in age-matched control rats (n=6). The normalized frequency was 1.33±0.09 in NMD rats (n=8) and 1.00±0.07 in age-matched control rats (n=6). These data proved again that NMD enhanced the neural synaptic transmission in right IC of NMD rats.
Figure 4.
Potentiation of mEPSC in right IC of NMD rats. (a) Recordings illustrating mEPSC of typical neurons in right IC of CON (top) and NMD (bottom) rats. (b) The frequency and amplitude of mEPSC were both greatly increased in right IC of NMD rats compared with CON ones (n=6 cells for CON group, n=8 cells for NMD group, *p<0.05 vs. CON, two sample t test). (c) Cumulative fraction of peak amplitude of mEPSC in an IC pyramidal neuron of NMD and CON rat, respectively. (d) Cumulative fraction of interevent interval of mEPSC in an IC pyramidal neuron of NMD and CON rat, respectively. mEPSC: miniature excitatory postsynaptic current; CON: control; NMD: neonatal maternal deprivation.
A317491 suppressed synaptic activities in IC of NMD rats
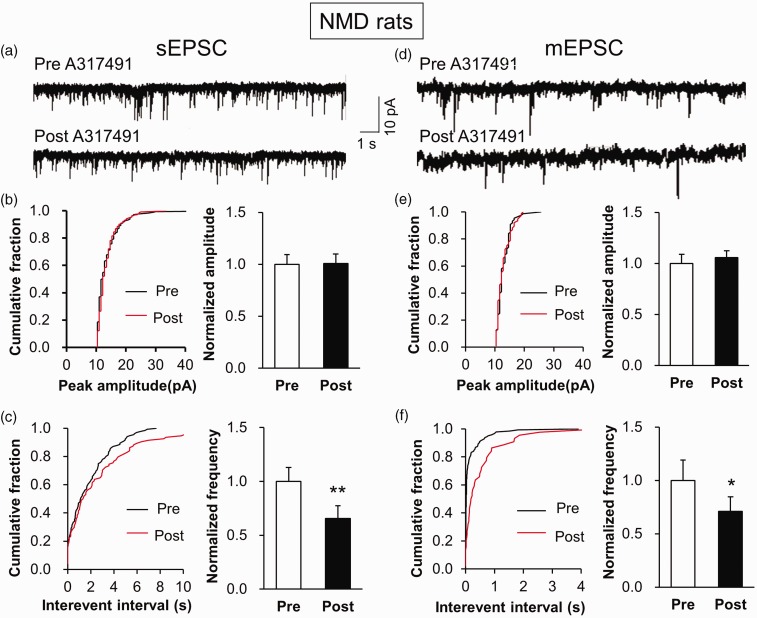
Since P2X3Rs expression was increased in IC neurons, we hypothesized that the upregulation of P2X3Rs expression contributed to the enhanced neural synaptic activity. Therefore, we selected a potent P2X3Rs antagonist A317491 to block P2X3Rs. The typical current traces showed a significant decrease in frequency of sEPSC in right IC slices when incubated with A317491. However, the amplitude of sEPSC was not altered after A317491 incubation (Figure 5(a)). The statistical data of cumulative fraction and charts were shown in Figure 5(b) and (c). The normalized frequency was 1.00±0.39 before incubation (Pre) and 0.66±0.35 after incubation (Post) (n=9). In addition, A317491 incubation markedly reduced the frequency but not amplitude of mEPSC in right IC (Figure 5(d) to (f)). The normalized frequency was 1.00±0.3 before incubation (Pre) and 0.70±0.27 after incubation (Post) (n=6). These data were consistent with the above result that P2X3Rs was co-localization with synaptophysin, thus indicating a presynaptic effect of P2X3Rs in the IC.
Figure 5.
Decreased frequency of sEPSC and mEPSC by A317491 incubation. (a) Representative traces illustrating sEPSC of a right IC neuron of control rats before and after an addition of A317491. (b) Cumulative fraction of peak amplitude of sEPSC in an IC pyramidal neuron before (Pre) and after (Post) drug application (left). Bar plots showing no significant change in sEPSC amplitude by A317491 treatment (right). (c) Cumulative fraction of interevent interval of sEPSC in an IC pyramidal neuron before (Pre) and after (Post) drug application (left). Bar plots showing that sEPSC frequency was significantly decreased by A317491 treatment (right, n=9 cells, **p<0.01 vs. Pre, paired sample t test). (d) Representative traces illustrating mEPSC of a right IC neuron of a control rat before and after an addition of A317491. (e) Cumulative fraction of peak amplitude of mEPSC in an IC pyramidal neuron before (Pre) and after (Post) drug application (left). Bar plots showing no significant change in mEPSC amplitude by A317491 treatment (right). (f) Cumulative fraction of interevent interval of mEPSC in an IC pyramidal neuron before (Pre) and after (Post) drug application (left). Bar plots showing that mEPSC frequency was markedly decreased by A317491 treatment (right, n=6 cells, *p<0.05 vs. Pre, paired sample t test). sEPSC: spontaneous excitatory postsynaptic current; mEPSC: miniature excitatory postsynaptic current; NMD: neonatal maternal deprivation.
α,β-mATP enhanced synaptic transmission in IC of control rats
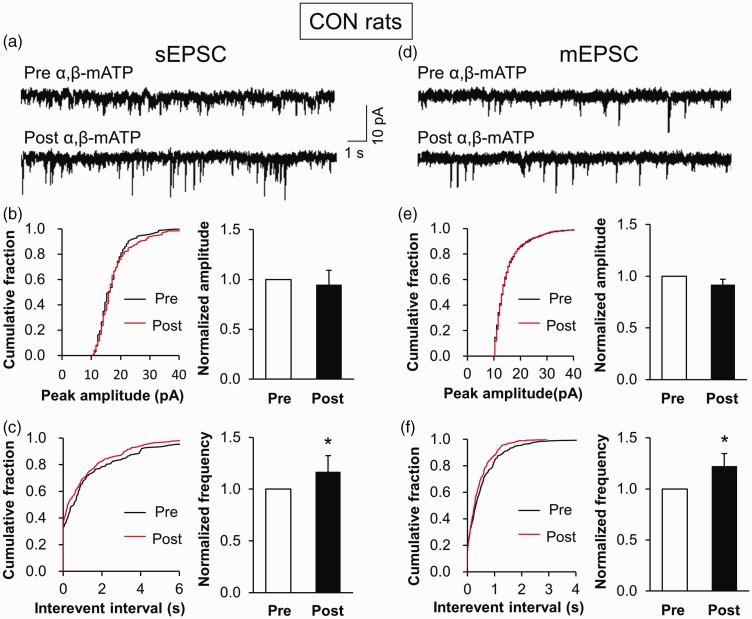
To further confirm the role of P2X3Rs on synaptic activity, we incubated IC slices of control rats with P2X3Rs agonist α,β-mATP. We showed that the frequency of sEPSC was remarkably increased in the presence of α,β-ATP, but the amplitude was not altered (Figure 6(a) to (c)). The normalized frequency was 1.00±0.24 before incubation (Pre) and 1.16±0.31 after incubation (Post) (n=4). Furthermore, α,β-mATP incubation obviously increased the frequency but not amplitude of mEPSC (Figure 6(d) to (f)). The normalized frequency was 1.00±0.22 before incubation (Pre) and 1.22±0.31 after incubation (Post) (n=6). These results demonstrate that the activation of P2X3Rs was an important element involved in synaptic transmission.
Figure 6.
Increased frequency of sEPSC and mEPSC by α,β-mATP incubation. (a) Representative traces illustrating sEPSC of a right IC neuron of a control rat before and after an addition of α,β-mATP. (b) Cumulative fraction of peak amplitude of sEPSC in an IC pyramidal neuron before (Pre) and after (Post) drug application (left). Bar plots showing no significant change in sEPSC amplitude by α,β-mATP treatment (right). (c) Cumulative fraction of interevent interval of sEPSC in an IC pyramidal neuron before (Pre) and after (Post) drug application (left). Bar plots showing that sEPSC frequency was obviously increased by α,β-mATP treatment (right, n=4 cells, *p<0.05 vs. Pre, paired sample t test). (d) Representative traces illustrating mEPSC of an right IC neuron of a control rat before and after an addition of α,β-mATP. (e) Cumulative fraction of peak amplitude of mEPSC in an IC pyramidal neuron before (Pre) and after (Post) drug application (left). Bar plots showing no significant change in mEPSC amplitude by α,β-mATP treatment (right). (f) Cumulative fraction of interevent interval of mEPSC in an IC pyramidal neuron before (Pre) and after (Post) drug application (left). Bar plots showing that mEPSC frequency was remarkably increased by α,β-mATP treatment (right, n=6 cells, *p<0.05 vs. Pre, paired sample t test). sEPSC: spontaneous excitatory postsynaptic current; mEPSC: miniature excitatory postsynaptic current; CON: control.
Roles of insular P2X3Rs in visceral pain
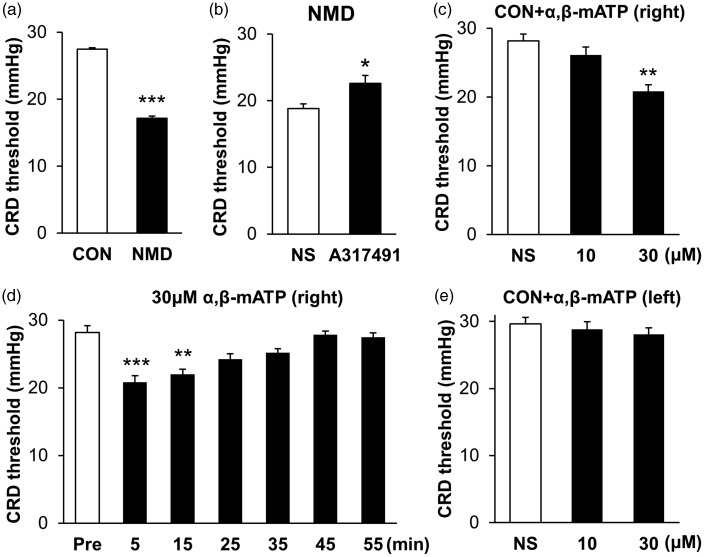
In agreement with our previous work,21 NMD significantly reduced the CRD threshold of adult rats (Figure 7(a)). The CRD threshold was 27.49±0.82 mmHg in NMD rats (n=7) and 17.14±0.54 mmHg in age-matched control rats (n=7). Importantly, the micro-injection of A317491, a potent antagonist of P2X3Rs, into right hemisphere IC significantly enhanced the CRD threshold (Figure 7(b)). The CRD threshold was 22.6±0.83 mmHg in A317491-treated NMD rats (n=5) and 18.80±1.41 mmHg in NS-treated NMD rats (n=5). We next determined whether activation of P2X3Rs in IC in vivo caused visceral pain in control rats. The CRD threshold was 26.13±1.15 mmHg in 10 μM α,β-mATP (n=8), 20.83±0.95 mmHg in 30 μM α,β-mATP (n=8), and 28.19±0.99 mmHg in NS-treated rats (n=8), respectively. After statistical analyses, injection α,β-mATP at 30 μM into right IC led to a significant reduction in CRD threshold while 10 μM α,β-mATP did not (Figure 7(c)). The time course of α,β-mATP effect was observed. The CRD threshold was 28.17±1.03, 20.83±0.95, 22±0.75, 24.25±0.76, 25.17±.63, 27.83±1.57, and 27.5±0.64 mmHg at Pre, 5, 15, 25, 35, 45, and 55 min after the injection, respectively (n=8 rats for each time point). Data showed that this effect maintained for at least 15 min (Figure 7(d)). However, the CRD threshold was not altered when the same dose of α,β-mATP was injected into left IC of control rats (Figure 7(e)). The CRD threshold was 29.62±0.50 mmHg in NS (n=7) and was 28.81±0.72 mmHg and 28.10±0.42 mmHg in 10 μM and 30 μM α,β-mATP-treated rats, respectively (n=7 for each group). These results further support our hypothesis that P2X3Rs in IC of right hemispheres might play an important role in visceral hypersensitivity of NMD rats.
Figure 7.
Effect of IC injection of A317491 or α,β-mATP on CRD threshold. (a) NMD rats showed a lower CRD threshold than CON rats (n=7 for each group, ***p<0.001 vs. CON, Mann–Whiney test). (b) The injection of A317491 (10 nM in 1 μl) into right IC greatly increased CRD threshold of NMD rats (n=5 for each group, *p<0.05 vs. NS, Mann–Whiney test). (c) Injection of α,β-mATP (30 μM in 1 μl) into right IC significantly decreased CRD threshold of CON rats while α,β-mATP at 10 μM did not produce any effect (n=8 for each group, **p<0.01 vs. NS, Kruskal–Wallis test). (d) The CRD threshold was significantly lowered 5 and 15 min after 30 μM α,β-mATP injection into right IC of CON rats (n=8 for each group, **p<0.01 and ***p<0.001 vs. Pre, Kruskal–Wallis test). (e) The injection of α,β-mATP into left IC did not affect the CRD threshold of CON rats (n=7 for each group, Kruskal–Wallis test). CON: control; NMD: neonatal maternal deprivation; NS: normal saline.
Discussion
In the present study, we demonstrated for the first time that P2X3Rs in the right IC were involved in visceral pain in a rat model of IBS. This conclusion was based on the following observations. Inhibition of P2X3 receptors by A317491 significantly reduced the frequency without alteration in the amplitude of both sEPSC and mEPSC of right insular of NMD rats. Importantly, inhibition of P2X3Rs also markedly enhanced the CRD threshold of NMD rats. In contrast, application of right IC brain slice with P2X3Rs agonist enhanced the frequency without alteration in the amplitude of both sEPSC and mEPSC of healthy control rats. Injection of P2X3Rs agonist into the IC region of right hemisphere significantly lowered the CRD threshold of healthy control rats, while injection of P2X3Rs agonist into the left IC did not produce any effect. Together with our previous studies that P2X7Rs in right IC are involved in visceral pain,12 these findings provided additional evidence to support the idea that purinergic signaling in IC of right hemisphere play pivotal roles in the processes of chronic visceral pain of functional gastrointestinal disorders.
Research in the field of purinergic signaling has advanced our knowledge on their roles in the development of acute and chronic pain. This knowledge is gained mainly from the studies of purinergic receptors at the peripheral never system.25,26 Relatively little work has been done on the purinergic signaling in the central nerve system. In addition to expression in peripheral nerve system,27 P2X3Rs are reported to be expressed in some areas of the central nerve system such as hypothalamus,28 the anterior cingulate cortex, and the prefrontal cortex.29 By Western blot analysis, we have recently reported that P2X3Rs are expressed in the IC.12 In the present study, we provided new evidence by real-time quantitative polymerase chain reaction and immunofluorescence methods to confirm that P2X3Rs are expressed in the neural cells and terminals in the IC region. In addition, we showed that NMD significantly enhanced P2X3Rs expression both at mRNA and protein levels in right IC. Although it remains to be further investigated why the upregulation of P2X3Rs only observed in the right IC, our data indicate that P2X3Rs are not only physically expressed in the IC but also sensitized in an adult rat model of visceral pain with NMD.
More recently, our group has reported that P2X7Rs in the IC was involved in the development of visceral hypersensitivity of adult rats with NMD.21 Although the distinct effects of P2X7Rs and P2X3Rs need to be further investigated, we discussed their similarities and differences as follows. First of all, the P2X7Rs were not only expressed in neurons but also expressed in astrocytes and microglia although they are low under normal conditions.12 However, P2X3Rs were only expressed in neurons including cell bodies and terminals in IC. Second, the P2X3Rs are more sensitive to ATP than the P2X7Rs,30 and P2X3Rs can influx greater amounts of Ca2+ than most other types of channels.31 Therefore, we assume that the physiological pain process is most likely mediated by P2X3Rs since ATP release is low under normal conditions. Third, under injury or inflammatory conditions, a small increase in ATP release from neural and non-neural cells likely activates P2X3Rs first, thus causing mild pain; then P2X7Rs are likely activated only after a large amount of ATP is released from the damaged cells, thus causing severe pain or bursts of pain. Since activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses25 and ATP receptors are partially involved in glutamate deregulation and neuroinflammation in the brain after neonatal hypoxia,32 these data suggest a presynaptic mechanism in potentiation of synaptic transmission. Therefore, we assume that P2X3Rs and P2X7Rs are integrated presynaptically in IC to regulate chronic pain hypersensitivity, which needs to be further investigated.
In the previous study, we showed that NMD enhanced the synaptic transmission by an increase in the frequency of spontaneous EPSC in IC neurons.12 In the present study, we showed that the miniature EPSCs were significantly enhanced both at frequency and amplitude, further confirming an enhanced synaptic transmission both at presynaptic and postsynaptic levels. Of note is that either the inhibitor or the agonist of P2X3Rs only affected the frequency rather than amplitude of both sEPSC and mEPSC. This indicates that other mechanisms such as postsynaptic plasticity might be involved in the enhanced synaptic transmission.33 In addition to IC, the anterior cingulate cortex is also thought to be involved in complex visceral motor function and the pain response.34 Basal lateral amygdale is another brain area in the regulation of visceral pain.21 Further investigation into detailed neural circuitry among these brain areas in regulating visceral pain is definitely warranted.
In summary, the present study demonstrated that IC is a brain region that receives and integrates visceral sensations. Purinergic signaling in right IC might play an important role in the development of visceral pain. In this exploratory study, findings of atypical function of the right IC point to the importance of future work investigating the role of visceral afferent signaling in understanding chronic visceral pain in patients with functional gastrointestinal disorders.
Author Contributions
P-AZ, H-YZ, and Q-YX performed experiments, analyzed data, and prepared figures and the manuscript. W-JD and SH performed experiments. G-YX designed experiments, supervised the experiments, and finalized the manuscript. All the authors have read and approved the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval
Care and handling of the animals were approved by the Institutional Animal Care and Use Committee of Soochow University and were in accordance with the guidelines of the International Association for the Study of Pain.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from National Natural Science Foundation of China (31730040, 81471137 and 81500952) and from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
References
- 1.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 2006; 27: 166–176. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y Mackes J Chan S Haughey NJ Guo Z Ouyang X Furukawa K Ingram DK andMattson MP.. Impaired long-term depression in P2X3 deficient mice is not associated with a spatial learning deficit. J Neurochem 2006; 99: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 3.Cao S Xiao Z Sun M andLi Y.. D-serine in the midbrain periaqueductal gray contributes to morphine tolerance in rats. Mol Pain 2016; 12: 1–12. DOI: 10.1177/1744806916646786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakatsuka T Tsuzuki K Ling JX Sonobe H andGu JG.. Distinct roles of P2X receptors in modulating glutamate release at different primary sensory synapses in rat spinal cord. J Neurophysiol 2003; 89: 3243–3252. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y Li H Li TT Luo H Gu XY Lu N Ji RR andZhang YQ.. Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18. J Neurosci 2015; 35: 7950–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou LJ andLiu XG.. Glial activation. A common mechanism underlying spinal synaptic plasticity? Neurosci Bull 2017; 33: 121–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benison AM Chumachenko S Harrison JA Maier SF Falci SP Watkins LR andBarth DS.. Caudal granular insular cortex is sufficient and necessary for the long-term maintenance of allodynic behavior in the rat attributable to mononeuropathy. J Neurosci 2011; 31: 6317–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baier B zu Eulenburg P Geber C Rohde F Rolke R Maihofner C Birklein F andDieterich M.. Insula and sensory insular cortex and somatosensory control in patients with insular stroke. Eur J Pain 2014; 18: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 9.Gauriau C andBernard JF.. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci 2004; 24: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C Yang T Zhao H Zhang M Meng F Fu H Xie Y andXu H.. Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull 2016; 32: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson LA Gandevia SC andMacefield VG.. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain 2007; 128: 20–30. [DOI] [PubMed] [Google Scholar]
- 12.Zhang PA Xu QY Xue L Zheng H Yan J Xiao Y andXu GY.. Neonatal maternal deprivation enhances presynaptic P2X7 receptor transmission in insular cortex in an adult rat model of visceral hypersensitivity. CNS Neurosci Ther 2017; 23: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson MB Tillisch K Craig AD Engstrom M Labus J Naliboff B Lundberg P Strom M Mayer EA andWalter SA.. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology 2012; 142: 463–472.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guleria A Karyampudi A Singh R Khetrapal CL Verma A Ghoshal UC andKumar D.. Mapping of brain activations to rectal balloon distension stimuli in male patients with irritable bowel syndrome using functional magnetic resonance imaging. J Neurogastroenterol Motil 2017; 23: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong JY Naliboff B Labus JS Gupta A Kilpatrick LA Ashe-McNalley C Stains J Heendeniya N Smith SR Tillisch K andMayer EA.. Altered brain responses in subjects with irritable bowel syndrome during cued and uncued pain expectation. Neurogastroenterol Motil 2016; 28: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S Xiao Y Zhu L Li L Hu CY Jiang X andXu GY.. Neonatal maternal deprivation sensitizes voltage-gated sodium channel currents in colon-specific dorsal root ganglion neurons in rats. Am J Physiol Gastrointest Liver Physiol 2013; 304: G311–G321. [DOI] [PubMed] [Google Scholar]
- 17.Ren J Bian X DeVries M Schnegelsberg B Cockayne DA Ford AP andGalligan JJ.. P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J Physiol 2003; 552: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HH Hu J Zhou YL Qin X Song ZY Yang PP Hu S Jiang X andXu GY.. Promoted interaction of nuclear factor-kappaB with demethylated purinergic P2X3 receptor gene contributes to neuropathic pain in rats with diabetes. Diabetes 2015; 64: 4272–4284. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YL Jiang GQ Wei J Zhang HH Chen W Zhu H Hu S Jiang X andXu GY.. Enhanced binding capability of nuclear factor-kappaB with demethylated P2X3 receptor gene contributes to cancer pain in rats. Pain 2015; 156: 1892–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting JT Daigle TL Chen Q andFeng G.. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 2014; 1183: 221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao Y Chen X Zhang PA Xu Q Zheng H andXu GY.. TRPV1-mediated presynaptic transmission in basolateral amygdala contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Sci Rep 2016; 6: 29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washburn MS andMoises HC.. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 1992; 12: 4066–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon HT Bolshakov VY Janz R Hammer RE Siegelbaum SA andSudhof TC.. Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc Natl Acad Sci U S A 1996; 93: 4760–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonova I Arancio O Trillat AC Wang HG Zablow L Udo H Kandel ER andHawkins RD.. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science 2001; 294: 1547–1550. [DOI] [PubMed] [Google Scholar]
- 25.Gu JG andMacDermott AB.. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 1997; 389: 749–753. [DOI] [PubMed] [Google Scholar]
- 26.Cockayne DA Hamilton SG Zhu QM Dunn PM Zhong Y Novakovic S Malmberg AB Cain G Berson A Kassotakis L Hedley L Lachnit WG Burnstock G McMahon SB andFord AP.. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000; 407: 1011–1015. [DOI] [PubMed] [Google Scholar]
- 27.Gu Y Wang C Li G andHuang LY.. EXPRESS: F-actin links Epac-PKC signaling to purinergic P2X3 receptors sensitization in dorsal root ganglia following inflammation. Mol Pain 2016; 12: 1–11. DOI: 10.1177/1744806916660557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitlock A Burnstock G andGibb AJ.. The single-channel properties of purinergic P2X ATP receptors in outside-out patches from rat hypothalamic paraventricular parvocells. Pflugers Arch 2001; 443: 115–122. [DOI] [PubMed] [Google Scholar]
- 29.Weng ZJ Wu LY Zhou CL Dou CZ Shi Y Liu HR andWu HG.. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal 2015; 11: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci 2001; 2: 165–174. [DOI] [PubMed] [Google Scholar]
- 31.Egan TM andKhakh BS.. Contribution of calcium ions to P2X channel responses. J Neurosci 2004; 24: 3413–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao J Huang Y Li X Li L Yang T Huang L Yang L Jiang H Li H andLi F.. TNP-ATP is beneficial for treatment of neonatal hypoxia-induced hypomyelination and cognitive decline. Neurosci Bull 2016; 32: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliet SH Piet R andPoulain DA.. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 2001; 292: 923–926. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs PN Peng YB Boyette-Davis JA andUhelski ML.. The anterior cingulate cortex and pain processing. Front Integr Neurosci 2014; 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]