Boat noise is becoming increasingly common and is now recognized as an environmental pollutant (Slabbekoorn et al., 2010). We show, for the first time that boat noise elicits a stress response in fish embryos, and that their stress response is greater upon exposure to 2-stroke engines than 4-stroke engines.
Keywords: Anthropogenic noise, boats, embryo, fishes, pollution, stress
Abstract
Human generated noise is changing the natural underwater soundscapes worldwide. The most pervasive sources of underwater anthropogenic noise are motorboats, which have been found to negatively affect several aspects of fish biology. However, few studies have examined the effects of noise on early life stages, especially the embryonic stage, despite embryo health being critical to larval survival and recruitment. Here, we used a novel setup to monitor heart rates of embryos from the staghorn damselfish (Amblyglyphidodon curacao) in shallow reef conditions, allowing us to examine the effects of in situ boat noise in context with real-world exposure. We found that the heart rate of embryos increased in the presence of boat noise, which can be associated with the stress response. Additionally, we found 2-stroke outboard-powered boats had more than twice the effect on embryo heart rates than did 4-stroke powered boats, showing an increase in mean individual heart rate of 1.9% and 4.6%, respectively. To our knowledge this is the first evidence suggesting boat noise elicits a stress response in fish embryo and highlights the need to explore the ecological ramifications of boat noise stress during the embryo stage. Also, knowing the response of marine organisms caused by the sound emissions of particular engine types provides an important tool for reef managers to mitigate noise pollution.
Introduction
Human generated noise is changing natural soundscapes worldwide. Boat noise is the most prevalent source of underwater anthropogenic noise and is becoming recognized in international legislation as a prevalent anthropogenic pollutant that is increasing (Slabbekoorn et al., 2010; International Maritime Organization, 2011; Badino et al., 2012; Borsani et al., 2015). While boat noise has been found to have a variety of biological impacts on a broad range of taxa (Rolland et al., 2012; Nedelec et al., 2014; Simpson et al., 2016), data are insufficient to provide the evidence needed to inform policy geared toward mitigating biological and environmental impacts. Current boat noise regulations are developed based on assessments of airborne emissions affecting comfort of onboard living conditions or that of inhabitants near ports, but not the impacts of noise on aquatic life (Badino et al., 2012). Successful mitigation likely depends on altering boat noise production rather than decreasing boat prevalence, because boat numbers continue to increase. Yet, to our knowledge, no studies have examined the responses of aquatic organisms to noise from different types of boat engines.
The early life stages of marine organisms can be particularly susceptible to environmental perturbations, especially at key development stages when sensitivities are high (Mager et al., 2017). While most research that documents the importance of the early life history to population dynamics focuses on the larval phase (Peck et al., 2012), it is of course preceded in most species by an egg phase whose sole purpose is development driven and fuelled by maternally provisioned endogenous yolk reserves. Because of the small size and rapid development, embryos are particularly sensitive to disruption by environmental perturbations (e.g. temperature shock, pollutants) with carryover effects for neural, sensory, muscular and morphological development (Roussel, 2007; McCormick and Gagliano, 2010), that may flow on to effect growth and survival (Gagliano et al., 2007).
Here we investigate a coral reef fish species during the vulnerable embryonic life stage; a life stage identified as a research priority in relation to anthropogenic noise effects by the European Commission in the Marine Strategy Framework Directive (Borsani et al., 2015). Boat noise has been shown to affect many biological processes in fish including parental care (Nedelec et al., 2017), navigation (Holles et al., 2013), foraging (Voellmy et al., 2014) and survival under a predator threat (Simpson et al., 2016). However, to our knowledge only a single study has examined effects of noise on fish at the embryonic life stage (Bruintjes and Radford, 2014), despite evidence suggesting that fishes begin to respond to sound during embryonic development (Simpson et al., 2005) and that embryo health is important to larval growth and cohort survival (Bailey and Houde, 1989; McCormick and Nechaev, 2002; Simpson et al., 2005). The present study represents a significant advancement on Bruintjes and Radford’s study by manipulating the embryos’ acoustic environment in the field using real boats and by considering both the pressure and particle motion conditions during experimental exposures. Additionally, we compare effects of 2-stroke outboard engines to quieter 4-stroke engines.
We use heart rate as an indicator of the stress response in fish embryos. Heart rate is a reliable indicator of stress and has been frequently employed as an indicator in other studies (Nimon et al., 1996; Bunt et al., 2004; von Borell et al., 2007; Graham and Cooke, 2008; Atherton and McCormick, 2015). Heart rate increases (β-adrenoreceptor-mediated) directly in response to stressors caused by the stimulation of the hypothalmic-sympathetic-chromaffin-cell axis and the production of catecholamines (Barton, 2002; Bagatto, 2005). Therefore, heart rate provides a logistically feasible indicator of stress response, suggesting increased energy mobilization and use in fish embryo.
Materials and methods
Study species and collection
The staghorn damselfish (Amblyglyphidodon curacao) is an omnivorous damselfish that forms pairs during the breeding season when males make nests on vertical projections of dead substrate (Goulet, 1995). Eggs are laid in a monolayer and are defended from predators, principally by the male. At Lizard Island on the northern Great Barrier Reef, Australia (14°41′S, 145°27′E), during summer sea temperatures of approximately 28°C, embryos hatch 5 days post fertilization. The sagittal otoliths that form the basis of the acousticolateralis system form during embryogenesis, and it is likely that these embryos have a functioning acoustic system prior to hatching (Simpson et al., 2005). As embryo do not have a gas filled swim bladder, sound detection is likely driven by particle motion auditorily and via neuromast cells (Sarrazin et al., 2010). For the purposes of this paper, ‘hearing’ is used to describe the general detection of sound, via either mechanism.
Four-day-old A. curacao embryos were collected from the reefs around Lizard Island from 12 clutches and 9 different nesting sites/fathers between 21 and 29 October 2016. In order to collect and age the embryos, sheets of clear plastic were wrapped around dead coral branches at breeding sites and monitored daily for egg deposition. Plastic sheets were collected 4 days after egg deposition and placed into a seawater filled 9 L plastic bag, which was then placed into a seawater filled polystyrene box (to reduce noise disruption and temperature change) and driven slowly by boat (with a quiet 4-stroke engine) to a nearby beach (see Supplementary material Fig. S1 for an analysis of acoustic exposure during transport). Eggs were then kept in the plastic bags within the polystyrene box in the shade on the beach, isolated from any further boat noise, until their experimental treatment (less than 4 h later). Seawater was replaced in the plastic bags and box every 30 min, and water temperature was kept within 1°C of local sea temperature.
Acoustic stimuli
Three different acoustic stimuli were used in experimental treatments: ambient conditions (with background biophonic noise produced by fishes and invertebrates resident on patch reefs within the bay, but without any boats operating in the area), 2-stroke powered boats, and 4-stroke powered boats. Boat stimuli consisted of boats driven at 0–35 km/h at 10–200 m from the experimental setup. Seven boats were used in total; four aluminium-hulled 5m long boats with 30 hp Suzuki 2-stroke outboard engines (model DT30) and three boats of the same design but with 30 hp 4-stroke outboard engines (model DF30A).
In order to characterize the differences in acoustic conditions in the experiment, three recordings of acoustic pressure and particle motion conditions were made for each of the treatments, where a different boat was used in each of the boat noise recordings. Recordings were made at the location of the experimental trials, 1 m above the ocean bottom, from a kayak in 2–5 m water. Acoustic-pressure recordings were taken using an omnidirectional hydrophone (HiTech HTI-96-MIN with inbuilt preamplifier, manufacturer-calibrated sensitivity −164.3 dB re 1 V/μPa; frequency range 0.02–30 kHz; calibrated by manufacturers; High Tech Inc., Gulfport MS). Particle motion recordings were taken simultaneously using a triaxial accelerometer (M20L; sensitivity following a curve over the frequency range 0–2 kHz; calibrated by manufacturers; Geospectrum Technologies, Dartmouth, Canada). Both the accelerometer and hydrophone were connected to a digital 8-track recorder (F8 field recorder, sampling rate 48 kHz, Zoom Corporation, Tokyo, Japan). Using the same recording equipment, a recording was made in the polystyrene container of seawater on a boat to quantify the acoustic conditions to which embryos were exposed during transport (Fig. S2). Calibration parameters for the recording levels used were determined by recording a pure sine wave signal from a function generator, with the voltage measured using an in-line oscilloscope. Sound files were cropped in Audacity 2.1.2 (http://www.audacityteam.org), and acoustic analyses were calculated using PaPAM 0.872 (Nedelec et al., 2016) in Matlab Compiler Runtime 8.3 (https://au.mathworks.com).
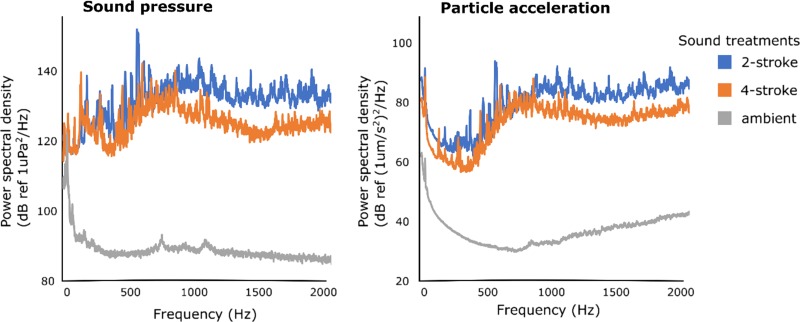
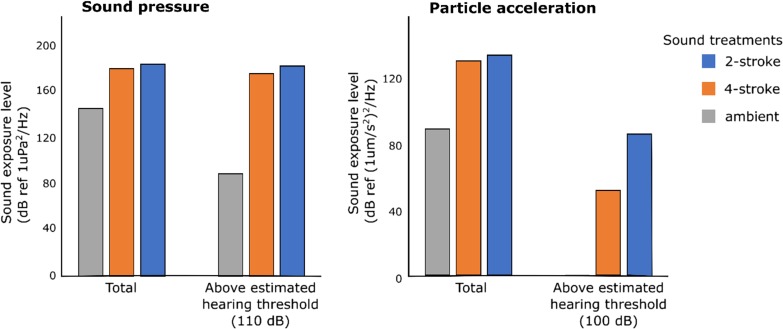
The root mean square of the power spectral density (PSD) was used to characterize the acoustic treatments. The PSD describes the acoustic power across frequencies, while the root mean square (RMS) of the PSD provides an average across frequencies (Merchant et al., 2015). The RMS PSD of each treatment (ambient, 2-stroke and 4-stroke) was calculated for 1 min tracks, where three passes of different boats of the same treatment were spliced together (to get an average between soundscape replicates), or three ambient tracks were spliced together in the case of the ambient treatment. The sound exposure level (SEL) within the estimated hearing range of the embryos (400–1200 Hz; Table 1), which describes the cumulative sound energy over time (Merchant et al., 2015), was calculated for 4 min tracks of each of the three soundscape replicates of each of the treatments, and then an average was taken for each treatment. For boat treatments, every sample consisted of a different boat passing the recording equipment 11 times. Consistency analysis, which indicates the percentage of time that the amplitude of sound is greater than a given threshold (Nedelec et al., 2016), was also calculated for these 4 min tracks at a threshold of 100 dB and 110 dB (at 400–1200 Hz) for particle motion and pressure, respectively. These thresholds are the best estimate of the embryos hearing thresholds based on previous studies with pomacentrid fishes (Table 1). Consistency was then multiplied by SELs to give an estimate of the cumulative sound energy that embryos were exposed to for each treatment (Fig. 1).
Table 1:
Approximate hearing thresholds found in other studies on pomacentrid fishes
| Reference | Species | Life stage | Hearing range (Hz) | Pressure threshold range (dB re 1 μPa) | Acceleration threshold range (dB re 1 μm/s2) |
|---|---|---|---|---|---|
| Wysocki et al. (2009) | Chromis chromis | Adult | 100–500 | 100–110 | 65–75 |
| Wright et al. (2011) | Pomacentrus nagasakiensis, Pomacentrus amboinensis | Settlement stage larvae | 100–2000 | 120–140 | 95–105 |
| Kenyon (1996) | Pomacentrus variablis | Post-settlement juvenile | 300–1200 |
|
|
| Egner (2004) | Abudefduf saxatilis | Post-settlement juveniles | 100–1200 | 110–150 | |
| Simpson et al. (2005) | Amphiprion ephippium | Embryo |
|
|
Figure 1:
Power spectral density of the sound treatments to which Amblyglyphidodon curacao embryos were exposed: 2-stroke powered boat noise, 4-stroke powered boat noise and natural ambient conditions. Spectral content is shown in sound pressure (left) and particle acceleration (right). Analyses were conducted in paPAM using one minute tracks that combined three separate recordings of each treatment to give the average sound profile of the three recordings. For boat tracks, each of the three recordings used in a track were from a different boat to account for variability between boats with the same engine type.
Experimental design
Heart rate was measured using a recording apparatus located on a shallow (2–5 m) sandy bottom site, adjacent to a reef (25 metres), in front of Lizard Island Research Station (14° 40′S, 145° 28′E), Great Barrier Reef, Australia. The apparatus consisted of an Olympus Stylus T-4 camera with an i-Das UCL-02 lens (125 mm/+8 macro lens), a Perspex stage in front of the lens, and a dive torch. To film the embryos, a strip of plastic sheet onto which embryos had been laid was attached to the stage and illuminated from behind by the torch (see Supplementary material Fig. S2 for photograph). Before each trial, a strip of the plastic sheet containing the egg clutch was cut off and taken by a snorkeler to the video apparatus. The camera was then focused on one to four individual embryos with visible heartbeats. Following 15 min of habituation in ambient conditions (natural ambient sound in the absence of boats), heartbeats were recorded using the camera for a further 2 min under pre-treatment, ambient conditions, followed by 4 min of one of three randomly selected acoustic stimuli treatments (ambient, 2-stroke, 4-stroke, Fig. 2).
Figure 2:
Sound exposure levels (SELs) are the cumulative sound energy at 400–1200 Hz (the estimated hearing range of Amblyglyphidodon curacao embryo) over 4 min. The SELs are shown in sound pressure (left) and particle acceleration (right) for each of three sound treatments: a 2-stroke powered boat, a 4-stroke powered boat noise, and natural ambient conditions. Total SELs as well as the SELs above the estimated hearing thresholds of A. curacao embryos (110 dB sound pressure; 100 dB particle acceleration) were calculated. Analyses were conducted using paPAM on three 4 min tracks for each treatment. The mean SEL of each treatment is represented in the graph. For boat tracks, each of the three recordings used in computing the average SEL were from different boats to account for variability between boats with the same engine type.
Analyses
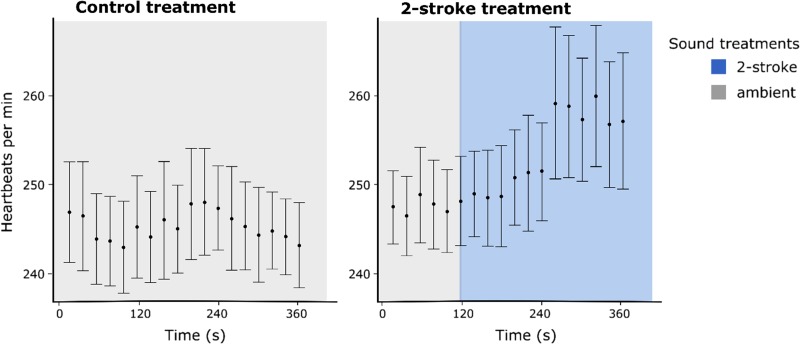
Heartbeats were counted in 20 s intervals, blind to treatment. To determine the time at which embryos were affected by boat noise, heart rate was initially plotted over time for embryos exposed to ambient conditions and those exposed to 2-stroke boat noise during treatment (see Supplementary material Fig. 3). These results suggested that it takes 140 s for embryos’ hearts to reach their full response to boat noise. Therefore, heart rate measurements taken in the 2 min following the first 140 s of boat noise were averaged within individuals to represent heart rate during treatments, and the heart rate measurement taken during the 2 min of pre-treatment, ambient conditions were averaged within individuals to represent heart rate during pre-treatment.
Figure 3:
Heart rate of 4 day old Amblyglyphidodon curacao embryos, following 15 min of habituation in the recording apparatus. Embryos were exposed to either ambient conditions for 360 s (left; sample size 13) or 120 s in ambient conditions followed by 240 s of 2-stroke powered boat noise driving at 0–35 km/h at 10–200 m from embryos (right; sample size 18). The full heart rate response to boat noise appears to occur 140 s after initiation of exposure to boat noise.
A linear mixed effects split-plot model was fitted to the data using maximum likelihood and implemented using the ‘lmer’ function in the ‘lme4’ R package (Bates et al., 2015). Treatment was included as a between individuals fixed effect, and time (pre-treatment/treatment) was included as a within individuals fixed effect, thereby incorporating the repeated measures element of the design into the analysis. A fixed treatment–time interaction was included to determine whether changes in heart rates within individuals differed with treatment. Individual and clutch were added as random factors without interactions. The assumption of normality was met, and the response variable (heart rate) was square root transformed to meet the assumption of homogeneity of variance. Within-group correlation structure did not improve the model and thus was not incorporated (Logan, 2010). The ‘lsmeans’ function in the ‘lsmeans’ package was used post-hoc to identify where differences among means occurred (Lenth, 2016).
Results
Acoustic analysis
In general, boats with 2-stroke engines generated more noise than boats 4-stroke engines. The RMS acoustic pressure (PSD) generated by boats within the estimated hearing range of A. curacao embryos (400–2000 Hz, Table 1) was 125 dB re μPa2Hz−1 for 4-stroke engines and 132 dB ref μPa2Hz−1 for 2-stroke engines (Fig. 2). Within this same frequency range the average particle acceleration was 77 dB re μms−2Hz−1 for 4-stroke engines and 84 dB re μms−2Hz−1 for 2-stroke engines. The cumulative sound energy over 4 min (SEL) above an estimated hearing threshold for A. curacao embryos of 110 dB re μPa2Hz−1 and within their estimated hearing range (400–2000 Hz) was 89 (ambient conditions), 175 (4-stroke engines) and 181 dB re μPa2s−2Hz−1 (2-stroke engines; Fig. 1). The SEL above an estimated hearing threshold of 100 dB re μms−2Hz−1 in terms of particle motion showed an even greater difference between 4-stroke and 2-stroke engines, averaging at 51 and 86 dB re μms−2Hz−1, respectively, while for ambient conditions gave an SEL of 0.006 dB re μms−2Hz−1.
Effect of boat noise
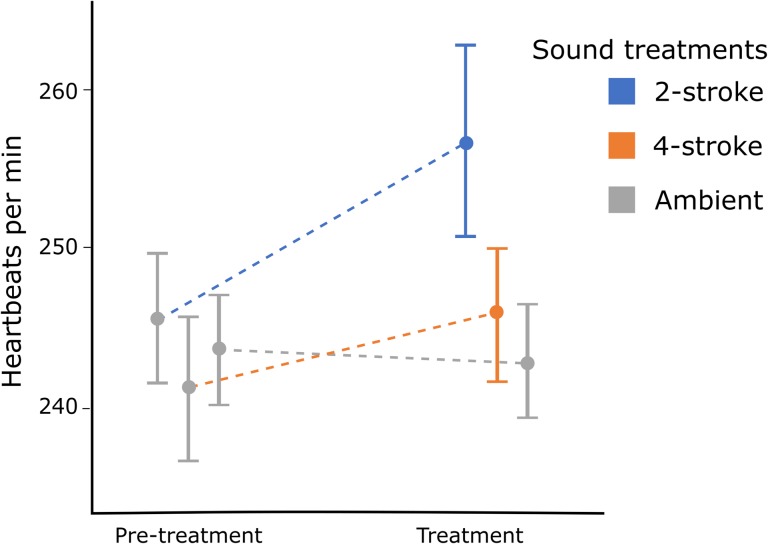
When compared to embryos under ambient conditions, heart rate of A. curacao embryos significantly increased during exposure to boat noise, as demonstrated by a significant treatment–time (time = pre-treatment or treatment) interaction (F2,20 = 21.0, P < 0.001; Fig. 4). Embryos that were exposed only to ambient conditions did not show a significant change in heart rate between pre-treatment and treatment periods (t66 = 0.5, P = 0.6). Embryos exposed to 2-stroke engine noise showed a statistically significant mean increase in heart rate of 4.6% ± 3.5 above that under ambient conditions (t66 = −8.6, P < 0.001). The effect of 4-stroke engine noise was less than half of the effect of 2-stroke engine noise, showing a mean increase in heart rate of 1.9 % ± 1.7 from ambient conditions (t66 = −3.7, P < 0.001).
Figure 4.
: Change in heart rate of Amblyglyphidodon curacao embryos from pre-treatment conditions (ambient) to treatment conditions (ambient, 2-stroke engine or 4-stroke engine), where heart rate was negated for the first 140 s of the treatment conditions to allow the response to be reached. Both 2-stroke and 4-stroke treatments involved a boat driving at 0–35 km/h at 10–200 m from embryos. The graph displays inter-individual means, and bars represent 95% confidence intervals.
Discussion
We found increased heart rate in A. curacao embryos in response to boat passage. Increased heart rate indicates the initiation of an adrenergic stress response, which is ultimately responsible for activating metabolic pathways and the mobilization of energy substrates to deal with perceived challenges (Armstrong, 1986; Lucas, 1994; Barton, 2002). In the case of boat noise, the perceived challenge is not a threat, so the energy expenditure associated with the stress could be detrimental to the embryos by depleting energy that could have otherwise been allocated to fitness promoting processes. However, from the data collected in this study, we cannot say whether the energetic cost of the stress induced by boat noise is sufficiently large to have impacts on body condition and fitness (Frid and Dill, 2002). If boat noise induced stress significantly depletes embryonic energy reserves, it may affect subsequent recruitment to coral reefs. Growth until feeding in the post-yolk sac larval stage is dependent on the available yolk sac energy reserves (McCormick and Nechaev, 2002). Thus, depletion of the endogenous embryonic energy reserves can reduce larval growth. Studies have found that larger and faster growing larvae have higher survival, which is related to larger larvae having increased ability to compete for food, resist starvation, and avoid predation (Bailey and Houde, 1989; Sogard, 1997; Jenkins and King, 2006; Peck et al., 2012). Furthermore, in many populations, a strong link has been found between larval abundance and recruitment (Cushing, 1990; Leggett and Deblois, 1994; Karjalainen et al., 2000). Thus, the depletion of energy reserves associated with boat noise induced stress may affect young-of-year recruitment by reducing growth. Further experiments are required to quantify the magnitude of these energetic costs associated with boat noise induced stress and determine whether these costs affect recruitment by affecting embryo survival or causing carryover effects to later life stages (McCormick and Gagliano, 2010).
The embryos heart rates increased by 1.9% and 4.9% on average with the passage of 2-stroke and 4-stroke powered boats, respectively. Any additional stress caused by the experimental procedure likely make these estimates more conservative, as they would decrease the ability of the embryo to respond to other stressors. An increase in heart rate of 4.9% with the passage of 2-stroke powered boats may indicate a considerably severe stress response when compared to increases in heart rate associated with conspecific alarm odours found in other fish species. A study on another pomacendrid (Amphiprion melanopus) found that embryos responded to conspecific alarm odours, arguably the most stressful cues that could be perceived, with an average increase in heart rate of 6.6% and 12.2% on Days 6 and 7 of development over an 8 day development period (Atherton and McCormick, 2015). On Day 4 of development, Melanotaenia duboulayi showed a 8.9% increase in heart rate in response to conspecific alarm odour (Oulton et al., 2013). In a detailed experimental study of the affect of cortisol on developmental rhythms during embryogenesis, McCormick & Nechaev (2002) found that the experimental elevation of cortisol resulted in a 4–14% increase in heart rate, and that magnitude of increase was dependent upon developmental stage. Overall these changes were enough to alter the size of larvae at hatching such that larvae with higher heart rates were smaller in size. These findings were further supported by a study that looked at the interrelationships between egg, embryo and larval characteristics at the individual level (Gagliano and McCormick, 2009), suggesting that perturbations within the embryonic stage can have strong carryover effects into future life stages. Therefore, it is possible that the increases in heart rate observed in our study in the presence of boat noise may indicate a stress response that could have carryover effects to future life stages.
Our finding that A. curacao embryos exhibit a stress response when exposed to motorboat noise contributes to the growing body of evidence that vessel noise can have detrimental effects on fishes. At juvenile and adult life stages, several other studies have found boat noise to instigate a physiological stress response (Spiga et al., 2012; Nichols, 2014; Simpson et al., 2016) and behavioural changes (Holles et al., 2013; Voellmy et al., 2014) in fishes. At the embryonic life stage, the few studies related to boat noise show variability in the sensitivity of embryos to boat noise. The playback of chronic boat noise was not found to effect growth and survival of embryonic cichlids (Neolamprologus pulcher) in the laboratory (Bruintjes and Radford, 2014). In the marine mollusc Stylocheilus striatus, chronic boat noise playback decreased embryonic survival by 21% and by a further 22% upon hatching (Nedelec et al., 2014). Differences among species in their tolerance and reaction to anthropogenic noise may be expected from differences in the development of hearing systems and their sensitivities (Wright et al., 2011); a topic that remains unexplored for most species of fishes and invertebrates.
Our study is the first to assess the effects of in situ boat noise on embryonic fish. Using real boat noise in a field setting is an important advancement because sound is altered through its replication by speakers and by resonance, reflection, and differential absorption within a tank environment (see Rogers et al., 2016 for a discussion of tank acoustics and drawbacks). Additionally, while particle motion and sound pressure components of sound have a direct relationship in the far field (fish would often experience sound in the far field in their natural environment), they do not when the sound source is in close proximity (such as a speaker in a tank). Many fishes hear both particle motion and sound pressure components of sound, and when a fish responds to sound, it is often uncertain to which component the fish is responding. Thus, it is difficult to adjust sound exposure levels in a tank experiment to the levels fish would experience in their natural environment, and therefore, it is more informative to conduct aquatic noise pollution studies in the field. It is an important advancement to find evidence that embryonic fish can display a stress response to boat noise, suggesting that it is important for future studies to examine the consequences of this stress response and whether effects carryover to later life stages. An examination of the capacity to habituate to chronic exposure would be another research direction and for future studies. However, there are potentially many confounding factors in a long term field experiment (e.g. effects on parental care, nest predators, etc.); so, our current finding that boat noise elicits an acute stress response in forms an important foundation for future work.
Another important advancement of our study is that we found the effect of boat noise on embryos differed with source of the acoustic disturbance (i.e. engine type). We found the effect of 2-stroke powered boats on embryo heart rates to be more than twice that of 4-stroke powered boats. When comparing the acoustic signatures of the two engine types, only a small difference was found in PSD, the most common metric used in noise pollution studies (Fig. 2). We suggest that measuring the total SEL above the sound pressure and particle motion hearing threshold and within the hearing range of the species and life stage may be a more appropriate metric for determining effects of noise pollution, as it is more indicative of what the organism may actually experience. There is a marked difference between 2-stroke and 4-stroke engines in the SEL produced above the estimated particle motion hearing threshold and within the hearing range of A. curacao embryos, which may account for the differences in heart rate responses found between the two engine types.
It is currently unclear whether the relatively small but significant changes in heart rate caused by boat noise are ecologically relevant and have repercussions for subsequent early life history dynamics. Our study lays a strong methodological foundation for further studies that will examine the potential for habituation to boat noise by embryos and the relative importance of carryover effects to later life stages. It is only by examining how noise perturbations affect all major life stages that the importance of windows of developmental sensitivity (sensuFawcett and Frankenhuis, 2015) and carryover effects can be integrated into our understanding of how environmental perturbations such as noise affect the dynamics of marine organisms. Knowing whether different types of engines produce different magnitudes of disturbance is important as it gives aquatic resource managers an effective tool with which to mitigate the impacts of noise through restrictions on maximum sound outputs.
Supplementary Material
Acknowledgements
We thank H. Harding, T. Gordon and Lizard Island Research Station staff for their assistance with fieldwork.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Ethics
Work complied with JCU Animal Ethics Committee regulations (permit: #A2089 and #A4208).
Funding
Research was funded by the ARC Center of Excellence for Coral Reef Studies (EI140100117), an International Postgraduate Research Scholarship awarded to S.J.S. from James Cook University and a UK Natural Environment Research Council grant to S.D.S. (NE/P001572/1).
Authors’s contributions
All authors contributed to study design and writing the manuscript and approved it prior to submission. S.J.S., S.D.S. and E.F. collected data. S.J.S. conducted analyses.
References
- Armstrong JD. (1986) Heart rate as an indicator of activity, metabolic rate, food intake and digestion in pike, Esox lucius. J Fish Biol 29: 207–221. [Google Scholar]
- Atherton JA, McCormick MI (2015) Active in the sac: damselfish embryos use innate recognition of odours to learn predation risk before hatching. Anim Behav 103: 1–6. [Google Scholar]
- Badino A, Borelli D, Gaggero T, Rizzuto E, Schenone C (2012) Normative framework for ship noise: present and situation and future trends. Noise Control Eng J 60: 740–762. [Google Scholar]
- Bagatto B. (2005) Ontogeny of cardiovascular control in zebrafish (Danio rerio): effects of developmental environment. Comp Biochem Physiol A Mol Integr Physiol 141: 391–400. [DOI] [PubMed] [Google Scholar]
- Bailey KM, Houde ED (1989) Predation on eggs and larvae of marine fishes and the recruitment problem. Adv Mar Biol 25: 1–83. [Google Scholar]
- Barton BA. (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42: 517–525. [DOI] [PubMed] [Google Scholar]
- Bates D, Machler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using “lme4”. J Stat Softw 67: 1–48. [Google Scholar]
- Borsani J, Faulk R, Merchant ND (2015) Impacts of noise and use of propagation models to predict. Cefas Contract Rep C6082 doi:10.13140/RG.2.1.1512.5841
- Bruintjes R, Radford AN (2014) Chronic playback of boat noise does not impact hatching success or post-hatching larval growth and survival in a cichlid fish. PeerJ 2: e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt CM, Cooke SJ, Schreer JF, Philipp DP (2004) Effects of incremental increases in silt load on the cardiovascular performance of riverine and lacustrine rock bass, Ambloplites rupestris. Environ Pollut 128: 437–444. [DOI] [PubMed] [Google Scholar]
- Cushing DH. (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv Mar Biol 26: 249–293. [Google Scholar]
- Egner SA. (2004) Auditory sensitivity of the sergeant majors (Abudefduf saxatilis) from post-settlement juvenile to adult.
- Fawcett TW, Frankenhuis WE (2015) Adaptive explanations for sensitive windows in development. Front Zool 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid A, Dill LM (2002) Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol 6: 11. [Google Scholar]
- Gagliano M, McCormick MI (2009) Hormonally mediated maternal effects shape offspring survival potential in stressful environments. Oecologia 160: 657–665. [DOI] [PubMed] [Google Scholar]
- Gagliano M, McCormick MI, Meekan MG (2007) Survival against the odds: ontogenetic changes in selective pressure mediate growth-mortality tradeoffs. Proc R Soc London Ser B Biol Sci 274: 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet D. (1995) Temporal patterns of reproduction in the Red Sea damselfish Amblyglyphidon leucogaster. Bull Mar Sci 57: 582–595. [Google Scholar]
- Graham AL, Cooke SJ (2008) The effects of noise disturbance from various recreational boating activities common to inland waters on the cardiac physiology of a freshwater fish, the largemouth bass (Micropterus salmoides). Aquat Conserv Freshw Ecosyst 18: 1315–1324. [Google Scholar]
- Holles S, Simpson SD, Radford AN, Berten L, Lecchini D (2013) Boat noise disrupts orientation behaviour in a coral reef fish. Mar Ecol: Prog Ser 485: 295–300. [Google Scholar]
- International Maritime Organization (2011) Noise from Commercial Shipping and Its Adverse Impacts on Marine Life. Germany.
- Jenkins GP, King D (2006) Variation in larval growth can predict the recruitment of a temperate, seagrass-associated fish. Oecologia 147: 641–649. [DOI] [PubMed] [Google Scholar]
- Karjalainen J, Auvinen H, Helminen H, Marjomaki TJ, Niva T, Sarvala J, Viljanen M (2000) Unpredictability of fish recruitment: interannual variation in young-of-the-year abundance. J Fish Biol 56: 837–857. [Google Scholar]
- Kenyon TN. (1996) Ontogenetic changes in the auditory sensitivity of damselfishes (pomacentridae). J Comp Physiol 179: 553–561. [Google Scholar]
- Leggett WC, Deblois E (1994) Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Netherlands J Sea Res 32: 119–134. [Google Scholar]
- Lenth RV. (2016) Least-Squares Means: the R Package lsmeans. J Stat Softw 69: 1–33. [Google Scholar]
- Logan M. (2010) Biostatistical Design and Analysis Using R: A Practical Guide. Willey, Blackwell. [Google Scholar]
- Lucas MC. (1994) Heart rate as an indicator of metabolic rate and activity in adult Atlantic salmon, Salmo salar. J Fish Biol 44: 889–903. [Google Scholar]
- McCormick MI, Gagliano M (2010) Carry-over effects: the importance of a good start. 11th Int Coral Reef Symp, 305–310.
- McCormick MI, Nechaev IV (2002) Influence of cortisol on developmental rhythms during embryogenesis in a tropical damselfish. J Exp Zool 293: 456–466. [DOI] [PubMed] [Google Scholar]
- Merchant ND, Fristrup KM, Johnson MP, Tyack PL, Matthew J, Blondel P, Parks SE (2015) Measuring acoustic habitats, 257–265. [DOI] [PMC free article] [PubMed]
- Mager EM, Pasparakis C, Schlenker LS, Yao Z, Bodinier C, Stieglitz JD, Hoenig R, Morris JM, Benetti DD, Grosell M (2017) Assessment of early life stage mahi-mahi windows of sensitivity during acute exposures to Deepwater Horizon crude oil. Environ Toxicol Chem 36: 1887–1895. [DOI] [PubMed] [Google Scholar]
- Nedelec SL, Campbell J, Radford AN, Simpson SD, Merchant ND (2016) paPAM User Manual for version 0.872, 0–34.
- Nedelec SL, Radford AN, Pearl L, Nedelec B, Mccormick MI, Meekan MG, Simpson SD (2017) Motorboat noise impacts parental behaviour and offspring survival in a reef fish. [DOI] [PMC free article] [PubMed]
- Nedelec SL, Radford AN, Simpson SD, Nedelec B, Lecchini D, Mills SC (2014) Anthropogenic noise playback impairs embryonic development and increases mortality in a marine invertebrate. Sci Rep 4: 5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TA. (2014) Boat Engine Noise Induces Physiological Stress and Reduces Predation Risk in Coastal Marine Fish.
- Nimon AJ, Schroter RC, Oxenham RC (1996) Artificial eggs : measuring heart rate and effects of disturbance in nesting penguins. Physiol Behav 60: 1019–1022. [DOI] [PubMed] [Google Scholar]
- Oulton LJ, Haviland V, Brown C (2013) Predator recognition in rainbowfish, Melanotaenia duboulayi, Embryos. PLoS One 8: 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck MA, Huebert KB, Llopiz JK (2012) Intrinsic and Extrinsic Factors Driving Match-mismatch Dynamics During the Early Life History of Marine Fishes, First Edition Advances in Ecological Research, 47, pp 177–302. Elsevier Ltd. [Google Scholar]
- Rogers PH, Hawkins AD, Popper AN, Fay RR, Gray MD (2016) Parvulescu revisited: small tank acoustics for bioacousticians In AN Popper, Hawkins A, eds, The Effects of Noise on Aquatic Life II. Springer, New York, pp 933–941. [DOI] [PubMed] [Google Scholar]
- Rolland RM, Parks SE, Hunt KE, Castellote M, Corkeron PJ, Nowacek DP, Wasser SK, Kraus SD (2012) Evidence that ship noise increases stress in right whales. Proc Biol Sci 279: 2363–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel J-M. (2007) Carry-over effects in brown trout (Salmo trutta): hypoxia on embryos impairs predator avoidance by alevins in experimental channels. Can J Fish Aquat Sci 64: 786–792. [Google Scholar]
- Sarrazin AF, Nunez VA, Sapede D, Tassin V, Dambly-Chaudiere C, Ghysen A (2010) Origin and early development of the posterior lateral line system of Zebrafish. J Neurosci 30: 8234–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SD, Radford AN, Nedelec SL, Ferrari MCO, Chivers DP, McCormick MI, Meekan MG (2016) Anthropogenic noise increases fish mortality by predation. Nat Commun 7: 10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SD, Yan HY, Wittenrich ML, Meekan MG (2005) Response of embryonic coral reef fishes (Pomacentridae: Amphiprion spp.) to noise. Mar Ecol: Prog Ser 287: 201–208. [Google Scholar]
- Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN (2010) A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol Evol 25: 419–427. [DOI] [PubMed] [Google Scholar]
- Sogard SM. (1997) Size selective mortality in the juvenile stages of teleost fishes: a review. Bull Mar Sci 60: 1129–1157. [Google Scholar]
- Spiga I, Fox J, Benson R (2012) Potential effects of long-term exposure to boat noise on the growth, survival and nutrient retention in juvenile fish In AN Popper, A Hawkins, eds, The Effects of Noise on Aquatic Life. Springer, New York, pp 255–257. [DOI] [PubMed] [Google Scholar]
- Voellmy IK, Purse J, Flynn D, Kennedy P, Simpson SD, Radford AN (2014) Acoustic noise reduces foraging success in two sympatric fish species via different mechanisms. Anim Behav 89: 191–198. [Google Scholar]
- von Borell E, Langbein J, Després G, Hansen S, Leterrier C, Marchant-forde J, Marchant-forde R, Minero M, Mohr E, Prunier A, et al. (2007) Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—a review. Physiol Behav 92: 293–316. [DOI] [PubMed] [Google Scholar]
- Wright KJ, Higgs DM, Leis JM (2011) Ontogenetic and interspecific variation in hearing ability in marine fish larvae. Mar Ecol: Prog Ser 424: 1–13. [Google Scholar]
- Wysocki L, Codarin A, Ladich F, Picciulin M (2009) Sound pressure and particle acceleration audiograms in three marine fish species from the Adriatic Sea. J Acoust Soc Am 126: 4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.