Abstract
Background
Dietary fish is a rich source of Omega-3 poly-unsaturated fatty acids (PUFAs). These compounds may have protective effect against cardiovascular events possibly by modifying lipid profiles. Consequently, fish oil supplements are produced commercially to complement low fish intake. It is not clear if both interventions have similar effects. The aim of this trial was to compare the anti-hyperlipidemic effect of omega3 fatty acid supplements with fresh fish.
Method
A total of 106 patients with hyperlipidemia were randomized. One group received 2 g/day of omega-3 capsules for a period of 8 weeks and the other group received a mean of 250 g trout fish twice weekly (for dinner and lunch) for the same time period. The effects of these diets on the lipid profile after the intervention were compared between the two groups.
Results
Data from 48 patients in fish oil group and 47 patients from fish group was used for final analysis. In both groups, total cholesterol, non-HDL cholesterol, triglyceride (TG) levels, and Castelli I index (total cholesterol/HDL ratio) were reduced significantly following the treatment; however, dietary-fish intake had a more pronounced effect (−85.08 ± 74.82 vs. −30.75 ± 89.00, P < 0.001; 75.06 ± 35.43 vs. −16.93 ± 40.21, P < 0.001; −66.55 ± 30.79 vs. −12.7 ± 35.48, P = 0.003; and −0.77 ± 1.39 vs. −3.02 ± 1.85, P < 0.001; respectively). HDL level was increased in both groups with a higher effect in dietary fish group (4.47 ± 7.83 vs. 8.51 ± 8.79, P = 0.022). Atherogenic (Log [TG/HDL ratio]) and Castelli II (LDL/HDL ratio) indices did not change in fish oil group while were reduced significantly by fresh fish consumption (−0.04 ± 0.27 vs. −0.26 ± 0.17, P < 0.001; and 0.15 ± 0.7 vs. -1.32 ± 1.15, P < 0.001, respectively). LDL level was increased in the supplementation group, while it was significantly reduced in the dietary-fish group (+18.7 ± 24.97 vs. −22.75 ± 27.28, P < 0.001).
Conclusion
Consumption of fresh fish seems to be superior in positively modifying the lipid profiles which may have important translations in the occurrence of cardiovascular events.
Introduction
Cardiovascular disease (CVD) is the major cause of death worldwide with an increasing trend in developing countries. The general risk factors associated with the diseases are known to be hypertension, smoking, and hyperlipidemia1, 2. Worldwide, high cholesterol levels cause some 56% of ischemic heart disease and 18% of strokes, amounting to 4.4 million deaths annually. It has been shown that 88 mg/dl increase in fasting circulating triacylglycerol (TG) levels elevates the risk of developing CVD by 14 and 37%, in males and females, respectively3–5. A 1-mg/dl increase in the low-density lipoprotein (LDL) level associates with a 2–3% increase in risk for CVDs, and elevations earlier in life may be associated with higher increases in risk. High-density lipoprotein (HDL) cholesterol independently predicts CVDs as well. Every 1-mg/dl decrease in HDL-cholesterol causes a 3–4% increase in the risk3, 4. Furthermore, other indices such as cholesterol ratio or Castelli I index (ratio of total cholesterol to HDL-cholesterol), Castelli II index (LDL/HDL), and atherogenic index (log of Triglyceride to HDL-cholesterol ratio) may predict CVD risk better than LDL alone6. So, modifying the lipid profile has become one of the most important goals in preventive cardiology. This can be achieved via medical therapy or adding beneficial dietary sources to daily regimen. Among the promising dietary sources those which are enriched in omega-3 poly-unsaturated fatty acids (PUFA) are shown to have considerable importance.
Many epidemiological studies have suggested the beneficial effects of omega-3 PUFAs on cardiovascular health7–10. Dietary fish is a rich source of Omega-3 PUFAs. Omega-3 PUFAs come in several forms but eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been most widely investigated with regard to their cardiovascular benefits. In this regard, the American Heart Association (AHA) has endorsed their nutritional value for the secondary prevention of cardiovascular events in patients with documented coronary heart disease (CHD)11. They recommend that patients with CHD should consume a total of 1 g per day of DHA and EPA, preferably from oily fish. However, the fish oil omega-3 supplements in the form of capsules or liquid have also been considered as an acceptable alternative1. In addition, supplementation of 4 g per day omega-3 fatty acids has been considered as an approved treatment for patients with very high triglyceride levels by the Food and Drug Administration (FDA). FDA has also approved an omega-3 supplement called Lovaze which contains 456 mg EPA and 375 mg DHA for hyperlipidemia patients. Based on Omega-3 PUFAs favorable effects on lipid profiles, AHA has recommended 2–4 g daily intake of DHA and EPA for patients with high triglyceride levels11.
Although there are many reports on the positive effects of EPA and DHA on cardiovascular events, there are very few studies addressing which of omega-3 sources can serve this purpose the best. Dietary oily fish is a rich source of Omega-3 PUFAs. Consequently, fish oil supplements are produced commercially to complement low fish intake regimens. However, it is not clear if both sources have similar effects. In this study, we aimed to address this question by comparing the effect of dietary fish with omega-3 supplements on the lipid profile of patients suffering from hyperlipidemia.
Methods and patients
Trial design
This open-labeled, randomized trial was performed on 106 hyperlipidemic patients referring to Shiraz Healthy Heart House during a period of 6 months from 6 April 2015 to 12 October 2015. Medical Ethic Committee of Shiraz University of Medical Sciences approved the protocol of the study, and a signed written informed consent was obtained from each participant. The trial was registered at Iranian registry of clinical trials (www.IRCT.IR) under the registration number of IRCT201411141525N5
Study population
Patients with hyperlipidemia who referred to Shiraz Heart House were recruited. All participants were enrolled in the trial after a complete physical examination and medical history investigation in the hospital. Adults with LDL levels = 150–190 mg/dl or cholesterol levels >250 mg/dl or TG levels = 200–500 mg/dl were included in the study. The following patients were excluded from the study: 1—pregnant patients; 2—patients with systemic diseases; 3—patients who couldn’t tolerate the supplement or the fish intake; 4—patients who needed to be treated with statins for primary or secondary prevention; and 5—all patients already taking lipid lowering drugs.
Randomization
Randomization was done using a computer-based random digit generator based on the registration number of the patients (on the order of referral). The allocation was done in a 1:1 ratio. Apart from the project coordinator and the patients, the staff involved in clinical center, and members collecting and analyzing data were blinded to the intervention allocation.
Interventions and outcomes
106 eligible people were randomly assigned to the dietary-fish or omega-3 supplement groups, each consisting of 53 patients. Patients of the dietary-fish group were asked to consume 250 g farmed trout fish (which contained 1.4 g omega-3 (280 mg EPA and 160 mg DHA) per 100 g) two times a week for dinner and lunch for 2 months (Equal to consumption of 14 g of omega-3 [2.8 g EPA and 1.6 g DHA] per week) while they were advised not to consume fish oil supplement during the intervention period. The patients of the supplement group received 2 g of omega-3 supplement (21st century, USA; containing 180 mg EPA and 120 mg DHA in each soft-gel) every day (Equal to consumption of 14 g of omega-3 [2.5 g EPA and 1.7 g DHA] per week) for 2 months. All participants were instructed to maintain their habitual dietary style and physical activities during the trial. All the patients were regularly checked by phone calls to see if they are still adherent to the intervention or not?
The primary outcomes were lipid profiles changes including total cholesterol, triglyceride, HDL, LDL, and total cholesterol/HDL ratio at the beginning and 2 months after starting the intervention (Just after intervention was finished). Collection and analyses of all clinical and laboratory data were performed by the study personnel blinded for group assignment.
Measurements
The serum triglyceride was measured by GPO-PAP method providing a normal upper limit of 200 mg/dl (2.3 mmol/l). The total cholesterol was also checked by CHOD/PAP technique, which provided an upper limit normal value or 220 mg/dl (5.6 mmol/l). The HDL cholesterol was measured by dextran magnesium sulfate. LDL was measured directly using Solubilization LDL-C assay (SOL; Kyowa Medex)12. Some lipid profile indices were measured by following formulas:
Statistical analysis
In order to have 90% power to detect significant differences between changes of lipid profiles, 40 patients were needed to be required in each study group (P < 0.05, two-sided). To compensate for possible noncompliant patients and those who would possibly leave the study, we set the total sample size at 100. All principal analyses were performed with the intention to treat population, which consisted of all the patients who underwent randomization, regardless of the treatment received. The patients who were lost to follow-up and in whom no known event had occurred were not included in the denominator for calculation of binary end points. All statistical analyses were performed using the statistical Package for Social Sciences version 17.0 (SPSS Inc., Chicago, IL, USA). Baseline characteristics were analyzed by independent t-test or Chi-square tests. The 95% confidence intervals for the means of data were calculated, and the significance of the differences between results within groups was assessed using paired t-tests. A two-sided P value < 0.05 was considered statistically significantly. We also used a covariance analysis in order to minimize the effect of the baseline differences whenever needed.
Results
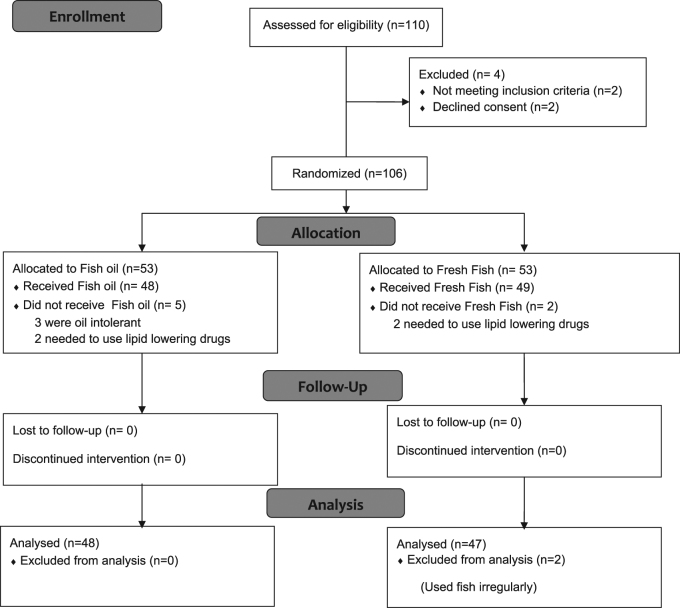
Of the 53 patients enrolled in the supplement group, 5 were excluded due to gastrointestinal oil intolerance, and a new need to use lipid-lowering drugs. Of 53 patients in the dietary-fish group, 4 were excluded from the study due to noncompliance with fish intake or a new need to use lipid-lowering drugs and two others were excluded from analysis because they consumed fish irregularly. Consort 2010 flow diagram is shown in Fig. 1.
Fig. 1.
CONSORT 2010 flow diagram for this randomized, double-blinded, clinical trial of effects of fish oil in comparison of fresh fish on lipid profiles in hyperlipidemic patients
There were no significant differences in terms of the patients’ gender between the two groups (P = 0.5). As shown in Table 1, the patients’ systolic and diastolic blood pressures, body mass index (BMI), and age was not different between the two groups at the beginning of the study and before our interventions. Prior to our interventions, the levels of cholesterol, LDL, HDL, and total cholesterol/HDL ratio were significantly different between the groups (Table 1). Therefore, we used the covariance analysis in order to minimize the effect of these differences.
Table 1.
Comparison of the mean age, BMI, SBP and DBP between the two groups at baselin
| Variables | Omega-3 group (n = 48) | Dietary-fish group (n = 47) | P-value |
|---|---|---|---|
| Age (years) | 48.7 | 50.73 | 0.257 |
| Sex | 0.5 | ||
| Male (%) | 25 (52.1%) | 23 (48.9%) | |
| Female (%) | 23 (47.9%) | 24 (51.1%) | |
| Body mass index (Kg/m2) | 26.24 | 26.57 | 0.901 |
| Systolic blood pressure (mmHg) | 173 ± 12 | 164 ± 12 | 0.47 |
| Diastolic blood pressure (mmHg) | 70 ± 8 | 71 ± 13 | 0.54 |
| Lipid profiles | |||
| Total cholesterol (mg/dl) | 230.20 ± 43.11 | 251.65 ± 47.87 | 0.023 |
| TG (mg/dl) | 270.02 ± 64.81 | 294.71 ± 109.56 | 0.181 |
| LDL (mg/dl) | 114.72 ± 25.15 | 133.61 ± 34.57 | 0.003 |
| HDL (mg/dl) | 42.34 ± 7.23 | 36.14 ± 9.27 | 0.001 |
HDL high density lipoprotein, LDL low density lipoprotein, TG triglyceride
Our results indicated that treatment by both omega-3 supplements and fresh fish caused a significant decrease in total cholesterol, non-HDL cholesterol, TG levels, and total cholesterol/HDL ratio but this reduction was more prominent in the fresh fish group. Blood LDL was another factor which was measured between the two groups. The fish oil supplement not only had no beneficial effect on the LDL level but also worsened the situation by significantly increasing the LDL level (18.95 ± 30.46%). However, in the dietary-fish group the LDL level was significantly decreased (−15.25 ± 17.97%, P < 0.001). HDL level was increased in both groups; again, dietary-fish was more effective in raising the HDL level (11.51 ± 17.93% vs. 27.39 ± 26.76%; P = 0.001). Both Atherogenic and Castelli II indices showed no significant change with fish oil supplementation but depicted significant decreases with fresh fish consumption. All the comparisons are shown in Table 2.
Table 2.
Mean values (Mean ± SD) for lipids levels in intervention and control groups before and after the study
| Lipid Parameters | Omega-3 group (n = 48) | Dietary-fish group (n = 47) | Treatment difference | 95% confidence interval | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | unit Change | P value | Before | After | unit change | P value | ||||
| Triglyceride (mg/dl) | 270.02 ± 64.81 | 239.27 ± 91.46 | −30.75 | 0.021 | 294.71 ± 109.56 | 209.63 ± 104.73 | −85.08 | 0.001 | −54.33 | −87.45 to −21.20 | <0.003 |
| Total cholesterol (mg/dl) | 230.20 ± 43.11 | 217.5 ± 42.92 | −12.7 | 0.017 | 251.65 ± 47.87 | 185.1 ± 38.63 | −66.55 | 0.001 | −53.84 | −67.23 to −40.45 | <0.001 |
| Low density lipoproteins (LDL) (mg/dl) | 114.72 ± 25.15 | 133.43 ± 34.42 | 18.7 | 0.001 | 133.61 ± 34.57 | 110.85 ± 28.73 | −22.75 | 0.001 | 141.46 | −52.01 to −30.91 | <0.001 |
| High density lipoproteins (HDL) (mg/dl) | 42.34 ± 7.23 | 46.33 ± 8.94 | 4.47 | <0.001 | 36.14 ± 9.27 | 44.65 ± 8.82 | 8.51 | <0.001 | 4.03 | 0.58 to 7.48 | 0.022 |
| Non-HDL cholesterol (mg/dl) | 186.09 ± 43.16 | 169.15 ± 41.74 | −16.93 | 0.008 | 215.51 ± 41.26 | 140.45 ± 35.53 | −75.06 | <0.001 | 58.12 | 42.54 to 73.71 | <0.001 |
| Total cholesterol to HDL ratio (cholesterol ratio, Castelli I Index) | 5.42 ± 1.28 | 4.78 ± 1.09 | −0.77 | <0.001 | 7.26 ± 1.81 | 4.24 ± 0.91 | 13.02 | <0.001 | −2.24 | −2.93 to −1.56 | <0.001 |
| LDL to HDL ratio (Castelli II index) | 2.70 ± 0.63 | 2.86 ± 0.78 | 0.15 | 0.162 | 3.84 ± 1.15 | 2.52 ± 0.62 | −1.32 | <0.001 | −1.47 | −1.87 to −1.07 | <0.001 |
| Log (triglyceride to HDL ratio) (Atherogenic index) | 0.71 ± 0.27 | 0.67 ± 0.20 | −0.04 | 0.27 | 0.90 ± 0.17 | 0.63 ± 0.22 | −0.26 | <0.001 | −0.22 | −0.31 to −0.13 | <0.001 |
Discussion
Here, by comparing the effect of omega-3 supplements and dietary fish on the blood lipid profile and indices of patients with hyperlipidemia, we have shown that dietary-fish intake had a significantly more pronounced effect on lowering the total cholesterol, non-HDL cholesterol, LDL, and TG levels; and indices of Castelli I (total cholesterol/HDL ratio), Castelli II (LDL/HDL ratio), and Atherogenic index (log [TG/HDL ratio]). In the case of HDL, fresh fish more effectively raised its level.
Populations whose diets are based on regular fish intake (like Greenland Eskimos) have been noted to have a lower incidence of CHD13. This effect had been contributed to the presence of high amounts of omega-3 PUFAs in the fish14–16. Omega-3 PUFAs come in several forms but EPA and DHA have been most widely investigated with regard to their cardiovascular benefits. DHA and EPA are suggested to enrich the biological membrane phospholipids17; hence, they improve the arterial and endothelial function18. They are also reported to reduce the platelet aggregation19 and blood pressure20, 21. Omega-3 PUFAs may also reduce insulin resistance and suppress the pro-inflammatory responses triggered by cytokines22.
Several studies have addressed the question that how omega-3 supplementation would affect lipid profile. However, findings about this issue are highly controversial. In a study by Wei and colleagues, it was shown that LDL levels were elevated by 5% after DHA consumption, while those consuming EPA had a non-significant reduction in serum LDL of 1%23. In a systematic review, Eslick and colleagues showed that 3.25 g/day consumption of fish oil (1.9 g of EPA and 1.35 g of DHA) reduced the serum TG by 14%, while the LDL-c was not significantly increased24. Similar results were obtained in systematic reviews by Balk and Mori, both of which addressed the studies in which individuals consumed either fish, algal EPA or algal DHA oils25, 26. Leslie and coworkers reviewed the beneficial effect of omega-3 on circulating TG levels in normolipidemic to borderline hyperlipidemic healthy individuals. Their results indicated 9-26% reduction in circulating TG level where more than 4 g/day of omega-3 was consumed from either marine or EPA/DHA-enriched food sources, while a 4–51% reduction was found where 1–5 g/day of EPA and/or DHA was consumed through supplements27. In a randomized clinical trial, Yu Qin and colleagues found that 4 g of fish oil supplement consumption for 3 months effectively decreased the serum total cholesterol, triglyceride, apolipoprotein B and glucose concentrations in nonalcoholic fatty liver disease patients with hyperlipidemia28. In general, it is believed that fish oil consumption may decrease the TG level by 25–30%19. These findings are in agreement with ours. We saw nine percent TG reduction with fish oil consumption. The observed variable effects may arise from different dosages and sources used as the intervention in those studies. We know that different dosages of omega-3 supplements have different effects. For example, for patients with diagnosed ischemic heart disease, the AHA recommends about 1 g of EPA + DHA per day. For patients with elevated serum triglyceride levels, a higher dose of EPA + DHA, 2–4 g/day, is recommended. However, some experts believe that this recommendation should be revised to 3–4 g/day11, 29. Here we used a dosage of 2 g per day as our intervention. However, dietary fresh fish resulted in 29% TG reduction which is similar to effects of high dose fish oil supplements in previous studies. It should also be noticed that Omega-3 supplements also do contain DPA and increasing DPA may also be related to TG lowering effect of these supplements12.
Since many of food and supplement-based studies have utilized different sources and doses of omega-3 provided as fish oil supplements or dietary fish, it is not clear which form is bioactive in affecting the serum lipid levels27. Only few studies have taken into consideration the point that using an omega-3 supplement may not have the same effects as using fresh fish. In a randomized controlled trial study, it was shown that in patients who received omega-3 PUFAs after a myocardial infarction, the mortality rate was reduced by 29% with either using the oily fish (about 300 g per week) and fish-oil capsules (about 900 mg of DHA + EPA per day)30; although, no direct comparison was done between dietary fish and oil supplements. Here, we have noticed that fresh fish is far better than omega-3 supplements in modifying lipid profiles. This may be due to the fact that several important nutrients like selenium, vitamin D, and naturally occurring antioxidants are only found in oily fish and fish-oil supplements lack them. The dietary-fish can have another cardiovascular beneficial effect by consistently lowering the C-reactive protein levels, the effect which is absent from fish-oil supplements25, 31, 32. On the other hand, selenium has antithrombotic properties, reduces lipid peroxidation, myocardial infarct size, and ischemia-induced ventricular arrhythmias, and improves recovery from ischemia or reperfusion injury33. Moreover, the powerful antioxidant properties of selenium as well as its ability to attenuate the adverse effects of methyl mercury (which is present in fish in varying concentrations) can provide additional cardiovascular benefits33, 34. Finally, using fish in the regular diet means replacing a previous dietary component (such as red meat) with fish. Consequently, the probable undesirable effects of previous dietary contents are reduced, an effect that cannot be achieved by using fish oil supplement.
Our study had some limitations. In this study, we used the farmed trout fish which belongs to salmon family, as the source of dietary fish. This breed had a 1.4 g omega-3 per 100 g fish35. However, the routine dietary fish in our region (south of Iran) and many other areas of world do not contain this amount of omega-3 in their tissue36. Another important limitation of our study was that in spite of the fact that we performed a classic randomization, we found a significant difference in the baseline lipid profile of patients in the two groups. Consequently, we performed a multivariate analysis to minimize its effect. In addition, although we tried our best to supply equal amounts of omega-3 (14 g per week) to the both study groups, it was not possible to supply an exactly equal amount of EPA and DHA to both groups (2.8 g EPA and 1.6 g DHA; 2.5 g EPA and 1.7 g DHA per week for fresh fish and fish oil supplement groups respectively). That happened because EPA and DHA constituted different percentage of omega-3 acids in the fish oil and fresh fish. However, those amounts were very close and not statistically different. Another limitation was that, like other dietary studies, economic and social differences might have produced differences in lifestyle, activity level and baseline diet which made it difficult to distinguish their effects we couldn’t exclude. So in order to have more conclusive results, the enrollment of a larger population and with more diverse ethnicity background is demanded. We should also take another important note into consideration. Increasing the fish servings in a week may mean omitting or replacing other unhealthy diets such as fast foods or even diets with lower nutritional benefits such as red meats. This does not happen with adding fish oil supplement to daily diet. This effect could not be neutralized in this study as the study question demanded the current design. However, it should be noticed that the results are still highly viable. In fact, fish oil supplement cannot replace dietary fish whether the beneficial effect is driven by other fresh fish contents or by modifying the weekly pattern of nutrition. Finally, it should be noticed that we did not measure EPA, DHA or DPA levels before and after the intervention.
It can be concluded that fresh fish consumption can improve lipid profiles better than omega-3 supplementation, and using oil fish is not a substitute for fresh fish consumption. Based on our observations, consumption of fresh fish seems to be superior in lowering the total cholesterol, LDL, and TG levels as well as increasing HDL level. These effects may translate into a reduction in the risk of CVD. Future comparative large scale trials are needed to see whether these biochemical differences can be translated into clinical outcomes.
Acknowledgements
The authors would like to thank Shiraz University of Medical Sciences, Shiraz, Iran and also Center for Development of Clinical Research of Nemazee Hospital and Dr. Nasrin Shokrpour for editorial assistance. This study is extracted from a thesis supported by grant number 7391 from vice-chancellery of research in Shiraz University of Medical Sciences.
Competing interests
The authors declare that they have no competing financial interests
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Howard B. V., Wylie-Rosett J. Sugar and cardiovascular disease: a statement for healthcare professionals from the Committee on Nutrition of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation106, 523–527 (2002). [DOI] [PubMed]
- 2.Finks SW, et al. Key articles of dietary interventions that influence cardiovascular mortality. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012;32:e54–e87. doi: 10.1002/j.1875-9114.2011.01087.x. [DOI] [PubMed] [Google Scholar]
- 3.Assmann G, Schulte H, Cullen P. New and classical risk factors--the Munster heart study (PROCAM) Eur. J. Med. Res. 1997;2:237–242. [PubMed] [Google Scholar]
- 4.Tirosh A, et al. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann. Intern. Med. 2007;147:377–385. doi: 10.7326/0003-4819-147-6-200709180-00007. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, et al. Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis. 2013;12:159. doi: 10.1186/1476-511X-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolovou GD, et al. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr. Vasc. Pharmacol. 2011;9:258–270. doi: 10.2174/157016111795495549. [DOI] [PubMed] [Google Scholar]
- 7.Parsanezhad M.E, Attar A., Namavar-Jahromi B., Khoshkhou S., Khosravi-Maharlooei M., Monabati A., Habibagahi M. Changes in endothelial progenitor cell subsets in normal pregnancy compared with preeclampsia. J. Chin. Med. Assoc. 2015;78:345–352. doi: 10.1016/j.jcma.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 9.Zibaeenezhad, M. J., Shahamat, M., Mosavat, S. H., Attar, A., Bahramali, E. Effect of Amygdalus scoparia kernel oil consumption on lipid profile of the patients with dyslipidemia: a randomized, openlabel controlled clinical trial. Oncotarget (2017) [In press]. 45, 79636–79641 (2017). [DOI] [PMC free article] [PubMed]
- 10.Zibaeenezhad MJ, et al. Effects of walnut oil on lipid profiles in hyperlipidemic type 2 diabetic patients: a randomized, double-blind, placebo-controlled trial. Nutr. Diabetes. 2017;7:e259. doi: 10.1038/nutd.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.CIR.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 12.Skulas-Ray AC, et al. Red blood cell docosapentaenoic acid (DPA n-3) is inversely associated with triglycerides and c-reactive protein (CRP) in healthy adults and dose-dependently increases following n-3 fatty acid supplementation. Nutrients. 2015;7:6390–6404. doi: 10.3390/nu7085291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang H, Dyerberg J, Nielsen An. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971;297:1143–1146. doi: 10.1016/S0140-6736(71)91658-8. [DOI] [PubMed] [Google Scholar]
- 14.Miller M, et al. Triglycerides and cardiovascular disease a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 15.Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br. J. Nutr. 2012;107(S2):S201–S213. doi: 10.1017/S0007114512001596. [DOI] [PubMed] [Google Scholar]
- 16.Skulas-Ray AC, West SG, Davidson MH, Kris-Etherton PM. Omega-3 fatty acid concentrates in the treatment of moderate hypertriglyceridemia. Expert. Opin. Pharmacother. 2008;9:1237–1248. doi: 10.1517/14656566.9.7.1237. [DOI] [PubMed] [Google Scholar]
- 17.Harris WS. Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol. Res. 2007;55:217–223. doi: 10.1016/j.phrs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thies F, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 19.Din JN, et al. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis. 2008;197:290–296. doi: 10.1016/j.atherosclerosis.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 20.O’Keefe JH, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am. J. Cardiol. 2006;97:1127–1130. doi: 10.1016/j.amjcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Ueshima H, et al. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure INTERMAP study. Hypertension. 2007;50:313–319. doi: 10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh M, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler. Thromb. Vasc. Biol. 2007;27:1918–1925. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 23.Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011;13:474–483. doi: 10.1007/s11883-011-0210-3. [DOI] [PubMed] [Google Scholar]
- 24.Eslick GD, Howe PR, Smith C, Priest R, Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int. J. Cardiol. 2009;136:4–16. doi: 10.1016/j.ijcard.2008.03.092. [DOI] [PubMed] [Google Scholar]
- 25.Balk EM, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 27.Leslie MA, Cohen D, Liddle DM, Robinson LE, Ma D. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 2015;14:14–53. doi: 10.1186/s12944-015-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Y, et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial. PLoS One. 2015;10:e0133496. doi: 10.1371/journal.pone.0133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris WS, Dayspring TD, Moran TJ. Omega-3 fatty acids and cardiovascular disease: new developments and applications. Postgrad. Med. 2013;125:100–113. doi: 10.3810/pgm.2013.11.2717. [DOI] [PubMed] [Google Scholar]
- 30.Burr ML, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;334:757–761. doi: 10.1016/S0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 31.Madsen T, Schmidt EB, Christensen JH. The effect of n-3 fatty acids on C-reactive protein levels in patients with chronic renal failure. J. Ren. Nutr. 2007;17:258–263. doi: 10.1053/j.jrn.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Ouellet V, et al. Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J. Nutr. 2008;138:2386–2391. doi: 10.3945/jn.108.092346. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int. J. Environ. Res. Public Health. 2009;6:1894–1916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown K, Arthur J. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4(2b):593–599. doi: 10.1079/PHN2001143. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, O’Keefe JH, Lavie CJ, Harris WS. Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat. Rev. Cardiol. 2009;6:753–758. doi: 10.1038/nrcardio.2009.188. [DOI] [PubMed] [Google Scholar]
- 36.Nezhad MZ, Khosravi M, Baniasadi N, Daneshvar Z. Omega-3 fatty acid content in various tissues of different Persian Gulf fish. Int. Cardiovasc. Res. J. 2008;2:24–31. [Google Scholar]