Abstract
Introduction
This study evaluates the concordance of treatment summaries (TSs) and survivorship care plans (SCPs) delivered to breast cancer survivors within the LIVESTRONG™ Network of Survivorship Centers of Excellence with Institute of Medicine (IOM) recommendations and describes additional structure/process variables.
Method
Seven NCI-designated comprehensive cancer centers and six community-based centers participated. TS/SCPs for 65 patients were rated against IOM recommendations using a study-derived checklist, and surveys were administered to better understand the structure and process of delivering TSs/SCPs.
Results
On average, fewer than half of IOM content recommendations were met for TSs (M=46%) and less than two thirds for SCPs (M=59%). No sites achieved ≥75% overall concordance with IOM recommendations for TSs and only two of 13 met this criterion for SCPs. Content domain scores across sites varied widely, as did the number of sites addressing domain content with ≥75% concordance. Nonetheless, resources required for document preparation and delivery were substantial.
Discussion
Gaps in concordance with IOM recommendations exist even in dedicated survivorship centers. A substantial time burden was also noted. Further research is needed to determine which informational elements are essential, to develop and test strategies for improving efficiency and reach, and to determine if outcomes of survivorship care planning warrant the resources required in their preparation and delivery.
Implications for survivors
TSs and SCPs have been recommended for all cancer survivors. Essential elements must be determined, approaches made more efficient, outcome improvements demonstrated, and cost-benefit analyses determined before survivors should expect widespread implementation of this recommendation for survivorship care.
Keywords: Treatment summaries, Survivorship care plans/planning, Breast cancer survivorship
Background
The 2006 Institute of Medicine (IOM) report, From Cancer Patient to Cancer Survivor: Lost in Transition, has been regarded by cancer survivors, families, advocacy groups, and providers as a key reference for the implementation of cancer survivorship recommendations and plans [1], stimulating a focus on cancer follow-up care and issues of long-term survivorship. The IOM report identified four components of survivorship care as “essential” and outlined ten recommendations that describe a wide array of activities and practice improvements. The assumption was that these recommendations would be implemented, improving cancer follow-up care and patient outcomes. However, after 5 years, cancer centers and survivorship programs continue to struggle with barriers to quality survivorship care, such as reimbursement issues, limited institutional resources including personnel, and requisite time commitment, as well as information and communications systems that fail to optimize communication and coordination of care. Systematic evaluations have been few.
Of the ten recommendations made by the IOM panel, the second focused on the development of comprehensive summaries and follow-up care plans for patients completing primary treatment, generally called treatment summaries (TSs) and survivorship care plans (SCPs). The treatment summary provides both disease and treatment history information, such as stage and tumor characteristics and cancer treatments received. The survivorship care plan acts as a guide for outlining and coordinating follow-up care, including surveillance tests, recommended health behaviors and resources, and education about and monitoring of potential long-term effects of cancer treatment. Implementation of these care plans has become a major concern for cancer care providers, in part because of the resources needed to complete and deliver them [2–4]. However, despite few evidence-based surveillance guidelines for adult cancer survivors [5], scant outcome data [6–9], and inadequate resources, the provision of TSs and SCPs is becoming a standard of care across the country with mandates to comply planned [10].
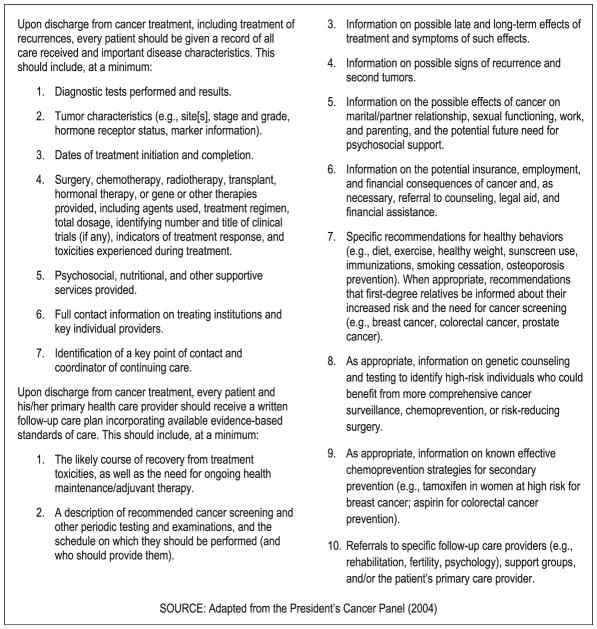
A committee of experts convened by the IOM stated that the TS and SCP should include information needed for the survivor’s long-term care, and outlined specific disease and treatment variables and follow-up care recommendations (see Fig. 1) that “at a minimum” should be covered in TSs and SCPs ([1] p. 151). In crafting these content recommendations, the committee noted that there was minimal literature on which to rely, as care plan implementation had not been examined empirically. Instead, the committee concluded that “some elements of care simply make sense—that is, they have strong face validity and can reasonably be assumed to improve care unless and until evidence accumulates to the contrary” ([1] p. 5). These recommendations then underwent independent external review before finalization. A number of templates have been developed by professional organizations and institutions to provide this information to patients. The IOM recommendations, however, are broadly defined and non-specific, providing a frame of reference for the widest possible range of cancer sites, treatments performed, follow-up plans, and long-term and late effects.
Fig. 1.
IOM recommendations for content of the survivorship care plan. Reprinted with permission from [1] by the National Academy of Sciences, Courtesy of the National Academies Press, Washington, D.C.
A growing number of centers now provide various TSs/SCPs to their patients. For example, LIVESTRONG™ has a Web-based patient-oriented tool that allows the generation of several versions of a care plan in order to meet the differing needs of patients and providers (http://live-strongcareplan.org). The American Society of Clinical Oncology (ASCO) developed SCP templates that allow the integration of ASCO recommendations to guide care (http://www.asco.org). Journey Forward generated a TS and SCP for providers and patients that integrates ASCO guidelines for follow-up care and allows tailoring of educational materials and resources to the individual survivor (http://journeyforward.org). Institutionally designed TSs and SCPs abound, as do supplementary documents. However, there has been little effort to determine the degree to which these heterogeneous tools are reflective of the IOM recommendations, describe the resources or efforts required to implement these plans, or examine the degree to which implementation improves clinical outcomes [6–9, 11–15].
The purpose of this study was to describe the concordance between IOM recommendations for content and TS/SCPs provided within a network of dedicated survivorship clinics serving breast cancer survivors. Sites within this network provide a variety of TSs and SCPs (e.g., Journey Forward, LIVESTRONG, and institutional templates), making it necessary to abstract and identify informational elements common across documents prior to examining outcomes. Although the IOM recommendations provide a framework for content, they are broad and provide no metric for assessing concordance or comparing or combining different materials. Our goal was to determine the degree to which TSs and SCPs provided map onto IOM-recommended content areas as a first step toward more evidence-based guidelines for essential elements in the care of cancer survivors. The study also examined basic structure and process variables related to TS/SCP delivery.
Method
Participating sites
This project took place within the LIVESTRONG™ Centers of Excellence in Cancer Survi-vorship Network [16]—a network of eight Centers of Excellence (COEs) each affiliated with one or more community-based centers (CBCs). The network has been described in detail elsewhere [16, 17]. We invited all eight COEs to participate by providing TSs and SCPs from both their site and one CBC affiliate, provided that each had delivered at least five TSs/SCPs to breast cancer survivors within the 18 preceding months.
Overview of methods
Following approval by their individual Institutional Review Boards, as well as that of the coordinating center, University of Pennsylvania, sites participated by providing de-identified TS/SCP materials for the final five breast cancer survivors seen for survivorship planning visits during 2009. Five TSs and five SCPs per site were specified a priori based on pragmatics of funding and time. The time period was chosen because sites were using the same clinical tools within their clinics during that period as during the study period, but the study had not yet been announced to the Network. Thus, these represented less biased samples of the TSs and SCPs provided to patients. All materials were taken into account in scoring TSs and SCPs, provided that the materials were distributed to breast cancer survivors themselves. In addition, sites identified and nominated one individual staff member to complete a brief questionnaire inquiring about structure and process elements related to TS/SCP preparation and delivery.
Concordance with IOM recommendations
This study required creation of two standardized checklists operationalizing the IOM recommendations for TSs and SCPs. The checklists were based upon the IOM-recommended elements of the TS and SCP (Fig. 1). This resulted in a 60-item TS Evaluation Tool and a 32-item SCP Evaluation Tool. The TS Evaluation Tool covered 13 content domains (Table 1). The SCP Evaluation Tool covered ten content domains (Table 1). Checklist items were scored as present or absent by two independent raters for each of the 65 TSs and SCPs. Differences in rating were resolved by consensus. Adequate inter-rater reliability has been established and reported at both the individual item and overall score levels. Further information on development and psychometric properties of the scoring tools, including a description of the individual items comprising each tool, were presented elsewhere [18]. Concordance scores reflect the proportion of materials that were rated as compliant for a given item or within a given domain. We also examined the degree to which sites met a minimum of ≥75% concordance with IOM recommendations within TS and SCP domains.
Table 1.
Domains of the treatment summary (TS) and survivorship care plan (SCP) evaluation tool
| No. of items | |
|---|---|
| Treatment summary domains scored | |
| Category | |
| Diagnosis | 2 |
| Staging and tumor characteristics | 8 |
| Surgery | 8 |
| Treating physician contact information | 8 |
| Clinical trials | 2 |
| Chemotherapy | 8 |
| Radiotherapy | 7 |
| Hormonal therapy | 3 |
| Targeted therapy | 3 |
| Toxicity | 1 |
| Genetic testing | 3 |
| Supportive therapy | 3 |
| Follow-up care contacts | 4 |
| Survivorship care plan domains scored | |
| Category | |
| Toxicities and late effects | 4 |
| Breast cancer surveillance | 3 |
| Cancer surveillance | 3 |
| Non-cancer surveillance | 3 |
| Signs of cancer (recurrent and second) | 2 |
| Psychosocial effects | 7 |
| Referrals and resources | 5 |
| Prevention/health promotion | 2 |
| Genetic testing recommendations | 1 |
| Relatives’ cancer risk | 2 |
Structure–process data
Self-report surveys were distributed to all sites to be completed by “the individual most responsible for coordinating the delivery of TSs and SCPs to breast cancer survivors.” Data collected included characteristics of the survivorship programs and staffing, such as numbers of patients served and staff/providers involved with survivorship care, as well as issues of time and reimbursement related to preparation and delivery of TSs/SCPs. The questionnaire used a mix of multiple choice questions with responses that included both pre-defined and open-ended response options.
Results
Description of participating sites
Out of a total of 16 possible centers invited (eight COEs and eight affiliated CBCs), 13 (seven COEs and six CBCs) participated in the study. The questionnaire response rate was 100% (i.e., 13/13). All participating COE institutions were NCI-designated Comprehensive Cancer Centers (Table 2). Among CBCs, community and public hospitals were the most common type of site, with diversity among the remaining sites. Sites were generally well staffed with oncology specialists, with eight sites (62%) having at least 11 oncologists, and oncology nurse practitioners being present at ten sites (77%). Only one site, a community-based health center, had neither oncologists nor oncology nurse practitioners on site. Participating facilities were actively seeing breast cancer survivors, with nine sites (69%) seeing more than 100 breast cancer survivors annually.
Table 2.
Characteristics of participating institutions and treatment summary (TS)/survivorship care plan (SCP) delivery
| Percent of sites (n=13) | ||
|---|---|---|
|
| ||
| N | % | |
| Type of institutions participating | ||
| NCI-designated comprehensive cancer center | 7 | 53.8 |
| Community hospital/public hospital | 3 | 23.1 |
| Community health center | 1 | 7.7 |
| University-based cancer treatment clinic | 1 | 7.7 |
| Multi-specialty group practice | 1 | 7.7 |
| Type of medical professionals on staff | ||
| Number of medical oncologists on staff per institution | ||
| 0 | 1 | 7.7 |
| 1–10 | 4 | 30.8 |
| 11–50 | 3 | 23.1 |
| >50 | 5 | 38.4 |
| Number of oncology nurse practitioners on staff per institution | ||
| 0 | 3 | 23.1 |
| 1–5 | 4 | 30.8 |
| 21–50 | 5 | 38.4 |
| >50 | 1 | 7.7 |
| Total breast cancer (BC) patients seen per year | ||
| Approximate number of BC patients seen each year | ||
| <50 | 0 | 0.0 |
| 51–100 | 4 | 30.8 |
| 101–500 | 3 | 23.1 |
| 501–1,000 | 1 | 7.7 |
| >1,000 | 5 | 38.4 |
| BC survivors receiving a TS and/or SCP in an average month | ||
| Number receiving a TS and/or SCP | ||
| ≤5 | 7 | 53.9 |
| 6–20 | 5 | 38.4 |
| >20 | 1 | 7.7 |
| Percentage receiving a TS and/or SCP | ||
| <10% | 8 | 61.5 |
| 10–49% | 1 | 7.7 |
| 51–75% | 1 | 7.7 |
| 76–99% | 2 | 15.4 |
| 100% | 1 | 7.7 |
TSs and SCPs delivered to survivors
All sites employed a practice of providing TSs and SCPs together rather than either document in isolation; thus, each site provided five TSs and five SCPs. As shown in Table 3, eight sites (62%) provided institutionally developed TSs to survivors. Three sites (23%) utilized the LIVESTRONG™ Care Plan [19] and two (15%) used Journey Forward [20] to create the TS document. A different approach characterized the creation of SCPs. Only four sites (31%) used institutionally developed SCPs. Use of publicly available documents predominated, with six sites (46%) using the LIVESTRONG™ Care Plan alone or in conjunction with additional materials. The Journey Forward [20] care plan was used by two (15%) sites and the ASCO [21] care plan was used by one site (8%), both also in conjunction with supplementary materials.
Table 3.
Types of treatment summaries and survivorship care plans
| Percent of sites (n=13) | ||
|---|---|---|
| Type of treatment summary | ||
| Institutionally created templates | 8 | 61.5 |
| LIVESTRONG™ care plan | 3 | 23.1 |
| Journey forward | 2 | 15.4 |
| Type of survivorship care plan | ||
| Institutionally created care plans and additional materials | 4 | 30.8 |
| LIVESTRONG™ care plan and additional materials | 4 | 30.8 |
| LIVESTRONG™ care plan alone | 2 | 15.4 |
| Journey forward care plan and additional materials | 2 | 15.4 |
| ASCO survivorship care plan and additional materials | 1 | 7.6 |
Concordance with IOM recommendations
Treatment summaries
Table 4 shows the average concordance within each of the domain areas outlined in the TS checklist. Overall, mean concordance with IOM recommendations for TSs content was 46.33% (SD=19.22%), though this ranged from a low of 14% to a high of 70%. As can be seen, details of hormonal therapy (79%), surgical treatment (73%), and staging and tumor characteristics (65%) were most commonly recorded. Least commonly addressed were details of supportive therapy (3%), follow-up care contact information (8%), and information regarding clinical trial participation (18%).
Table 4.
Treatment summary (TS) concordance with IOM recommendations
| Concordance with IOM recommendations | N=65 TSs/13 sites (for each domain) | No. of sites achieving adequate (≥75%) concordance | ||
|---|---|---|---|---|
|
| ||||
| Descriptive statistics | Mean | SD | Range | N (%) |
| Diagnosis | 0.46 | 0.31 | 0.00–1.00 | 2 (15.4) |
| Staging and tumor characteristics | 0.65 | 0.35 | 0.00–1.00 | 9 (69.2) |
| Surgery | 0.73 | 0.22 | 0.38–0.98 | 7 (53.8) |
| Treating physician contact information | 0.33 | 0.30 | 0.00–0.75 | 1 (7.7) |
| Clinical trials | 0.18 | 0.25 | 0.00–0.60 | 0 (0.0) |
| Chemotherapy | 0.52 | 0.24 | 0.10–0.83 | 3 (23.1) |
| Radiotherapy | 0.55 | 0.19 | 0.26–0.80 | 2 (15.4) |
| Hormonal therapy | 0.79 | 0.20 | 0.40–1.00 | 7 (53.8) |
| Targeted therapy | 0.34 | 0.38 | 0.00–1.00 | 3 (23.1) |
| Toxicity | 0.52 | 0.48 | 0.00–1.00 | 7 (53.8) |
| Genetic testing | 0.26 | 0.24 | 0.00–0.60 | 0 (0.0) |
| Supportive therapy | 0.03 | 0.08 | 0.00–0.27 | 0 (0.0) |
| Follow-up care contacts | 0.08 | 0.18 | 0.00–0.50 | 0 (0.0) |
| TS total | 0.46 | 0.19 | 0.14–0.70 | 0 (0.0) |
We also examined the degree to which sites demonstrated ≥75% concordance with IOM recommendations. Table 4 presents those data. More than half of the sites reached a level of 75% concordance for staging and tumor characteristics, surgery details, hormonal therapy details, and treatment toxicities. However, fewer than half of sites reached 75% concordance for diagnostic information, treating physician contact information, or details of chemotherapy, radiation therapy, or targeted therapy. No site reached such a level for information about clinical trials or genetic testing, or details of supportive therapy. Similarly, no site reached a level of 75% concordance for the TS content overall. Closer examination of individual items within domains revealed that granularity of data on cancer therapies was lacking; e.g., doses and dates of cycles of chemotherapy, although other items such as whether or not each type of therapy was received, and the names/site of therapy (if applicable) were included more consistently (Appendix).
Survivorship care plans
Sites were significantly more concordant with IOM recommendations for SCPs than for TSs (M=58.99%, SD=15.90%; t(12)=2.80, p =0.016), with concordance ranging from 37% to 83%. Concordance appeared to be partially a function of site, and concordance between SCPs and TSs was significantly associated (r= 0.58, p=0.036). Sites were most concordant with provision of information about treatment toxicities and potential late effects (98%), breast cancer-related surveillance recommendations (82%), and non-cancer-related surveillance recommendations (77%), as shown in Table 5. Concordance was less commonly achieved for information concerning familial cancer risk (1%) and signs of recurrent or secondary cancers (32%). Several individual items within domains were also infrequently addressed (see Appendix), such as possible effects of cancer on insurance, and documentation of the provider responsible for ordering routine cancer surveillance tests.
Table 5.
Survivorship care plan (SCP) concordance with IOM recommendations
| Concordance with IOM recommendations | N=65 SCPs/13 sites (for each domain) | No. of sites achieving adequate (≥75%) concordance | ||
|---|---|---|---|---|
|
| ||||
| Descriptive statistics | Mean | SD | Range | N (%) |
| Toxicities and late effects | 0.98 | 0.07 | 0.75–1.00 | 13 (100) |
| Breast cancer surveillance | 0.82 | 0.11 | 0.67–1.00 | 8 (61.5) |
| Cancer surveillance | 0.43 | 0.35 | 0.00–1.00 | 3 (23.1) |
| Non-cancer surveillance | 0.77 | 0.21 | 0.33–1.00 | 6 (46.2) |
| Signs of cancer (recurrent and second) | 0.32 | 0.27 | 0.00–1.00 | 1 (7.7) |
| Psychosocial effects | 0.52 | 0.35 | 0.00–0.86 | 6 (46.2) |
| Referrals and resources | 0.62 | 0.35 | 0.00–1.00 | 8 (61.5) |
| Prevention/health promotion | 0.46 | 0.14 | 0.00–0.50 | 0 (0.0) |
| Genetic testing recommendations | 0.62 | 0.51 | 0.00–1.00 | 8 (61.5) |
| Relatives’ cancer risk | 0.01 | 0.03 | 0.00–0.10 | 0 (0.0) |
| SCP total | 0.59 | 0.16 | 0.37–0.83 | 2 (15.4) |
We next examined the degree to which site SCP documents provided ≥75% concordance (see Table 5). All sites reached a level of 75% concordance when addressing potential toxicities and late effects and more than half of sites reached a similar level of concordance for recommendations about breast cancer surveillance, genetic testing recommendations, and information about referrals and resources. However, fewer than half of sites were ≥75% concordant for other cancer surveillance, signs of secondary or recurrent cancer, non-cancer surveillance recommendations, psychosocial effects, and no sites reached this degree of compliance for prevention/health promotion recommendations and information on relatives’ cancer risk. Only two sites (15%) achieved 75% concordance for overall SCP content.
Structure and process
Method of delivery and reach of survivorship care plans
The majority of centers (69%) provided TSs and SCPs at a dedicated survivorship visit, with fewer (15%) providing documents in the context of a routine oncology follow-up visit. Sites, however, reported limited reach of TS and SCP delivery, with more than half of centers serving five or fewer breast cancer survivors per month, and only one serving more than 20 per month (see Table 2). Most institutions (62%) reported providing SCPs to less than 10% of eligible breast cancer survivors, and only one reported providing these documents to 100% of breast cancer survivors (see Table 2).
Time utilization
Data representing time required for dissemination of TSs and SCPs to patients and providers are presented in Table 6. Median time for chart abstraction activities essential to the completion of TSs was 30–60 min, with over a third of sites estimating that it took more than one hour per patient. Median time for review of TS was 15–30 min, with 31% of sites reporting spending more than 60 min in reviewing TSs with each individual. Median time to review the SCP with the individual patient was also 15–30 min, with 46% of sites reporting that this activity usually took over 30 min to complete, and two sites reporting more than 1 h spent per patient in this activity.
Table 6.
Time spent/time burden
| Time it took to do the medical chart abstraction | Time it took to verify the medical chart abstraction | Time spent reviewing the TS with patient | Time spent reviewing the SCP with patient | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | % of sites (n=13) | N | % of sites (n=13) | N | % of sites (n=13) | N | % of sites (n=13) | |
| ≤30 min | 1 | 7.7 | 10 | 76.9 | 8 | 61.5 | 6 | 46.1 |
| 31–60 min | 7 | 53.8 | 3 | 23.1 | 4 | 30.8 | 4 | 30.8 |
| >60 min | 5 | 38.5 | 0 | 0.0 | 0 | 0.0 | 2 | 15.4 |
| Missing | 0 | 0.0 | 0 | 0.0 | 1 | 7.7 | 1 | 7.7 |
Dissemination of TSs and SCPs
Nine sites (69%) reported routinely sharing the SCP with the patient’s primary care provider (PCP). Three others (23%) reported that a copy was sent to all providers either noted on the patients chart or requested by the patient, so the PCP may have received a copy of the SCP through one of these mechanisms in particular cases. Our data indicated that all sites surveyed provided a printed copy of SCPs to patients, although one site did not provide a copy of the TS component directly to the patient. However, few sites provided electronic access to care plans through electronic medical records (EMRs) or non-institutional portals or Websites. Interestingly, the majority of sites were supplementing SCPs with tailored educational materials and community resources.
Discussion
The IOM report [1] initiated a number of clinical and policy initiatives to improve the care of adult cancer survivors, including those aimed at the provision of a “comprehensive care summary and follow-up plan… to cancer survivors following primary treatment.” [1, 4, 22–25]. National and international organizations (e.g., ASCO, Oncology Nursing Society, National Comprehensive Cancer Network, American Cancer Society, and Center for Disease Control and Prevention, National Cancer Institute) advocacy groups (e.g., National Coalition for Cancer Survivorship; LIVE-STRONG™), and other enterprises (e.g., Wellpoint/Journey Forward) have undertaken efforts to create tools and resources to support the implementation of such plans [19–21, 26–32], and clinical initiatives have been undertaken to deliver care plans [7, 12, 33, 34].
In spite of this activity, there has been little evaluation of the degree to which these efforts have reflected the content recommended by the IOM or if all domains and items are essential for improving outcomes. Furthermore, there has been little description of the structure and process of delivering TSs and SCPs, nor the quantification of their reach. The LIVE-STRONG™ COE Network provides a diverse forum for examining the state of TS and SCP content and delivery. Our study revealed marked variability and major gaps in concordance with IOM-recommended content for these documents. Whether these gaps influence outcomes or reflect a latent consensus as to essential components is not clear.
The challenges to implementing survivorship related care that is congruent with IOM recommendations are likely to be more onerous than it currently appears, as reflected in other attempts to adopt clinical practice guidelines. For example, in non-specialty mental health depression care, guideline congruent practice is the exception, rather than the rule [35], even though practice guidelines have been in existence since the early 1990s (AHCPR in Ref. [35]), adherence to guidelines has been shown to affect outcomes [36], and the focus has been on a single disease in a single treatment locale rather than an amalgamation of historical disease and treatment events, necessary personal health behaviors, and planned physician behaviors that often require patients to transition from specialty oncology settings to primary care. Survivorship care represents a much more complex set of patients, providers and system variables, and we can likely expect even greater difficulty coordinating these to improve patient outcomes, although perhaps lessons can be learned from other attempts at improving adherence to clinical practice guidelines [35].
What is currently done well? The majority of sites addressed staging and tumor characteristics, details of surgery and hormonal therapy, and treatment toxicities within TSs with at least 75% concordance. Similarly, most sites covered toxicities and late effects, recommendations for breast cancer surveillance, genetic testing recommendations, and information on referrals and resources within the SCP at the same level. Even within domains that were addressed less consistently, certain items were well addressed (see Appendix). For example, whether or not chemotherapy was administered and the individual names of chemotherapy drugs were recorded more than 90% of the time. Such domains and elements may be the “low-hanging fruit” within TS/SCPs that can be most easily obtained with the least need for additional resources and, thus, may be initial targets for clinical interventions and research demonstrating the effect of these informational domains on outcomes. It is unknown whether other therapy details (e.g., dose and dates) were less consistently included due to inadequate access to the information or whether sites deemed these of lesser importance to clinical outcomes.
There was considerable variability in the degree to which sites met IOM recommendations in the remaining domains. Some were rarely addressed, such as recording of supportive care, genetic testing, and clinical trial details in the TS. Some specific items (Appendix), such as specifying the name and contact information of a care coordinator, as well as defining familial cancer risk and need for surveillance, were covered by no sites or documents. Moreover, no sites achieved 75% concordance for the overall TS, and only two of 13 sites met this threshold for the overall SCP.
These findings are particularly thought-provoking considering the time reported in preparation and delivery of these documents. Over a third of sites reported spending more than 1 hour per patient on chart abstraction to create the TS/SCPs, and nearly half spent at least 30 min reviewing the SCP with each survivor. An additional 15 to 60 min was reported by the majority of sites to review the TS with each survivor. This time burden, even when compensated, is likely one factor limiting the reach of TS and SCP delivery, with the majority of sites delivering documents to less than 10% of breast cancer survivors.
These data raise a number of questions. If a network of centers with dedicated funding and documented institutional support for survivorship care [16, 17] does not achieve high concordance with IOM recommendations, can widespread concordance with these recommendations be expected? The American College of Surgeon’s Commission on Cancer intends to mandate SCPs among accredited institutions by 2015, and currently proposes using the IOM-recommended elements as the standard for evaluation [10]. It may be unrealistic to expect institutions to comply with these recommendations, especially given the dearth of evidence supporting an improvement in outcomes [6, 8, 9, 15]. Notably, several recommended elements were addressed by a few or no sites. It is not known if these elements are especially burdensome to collect or if they were not included due to lack of perceived value. Future investigations are needed to assess these and other possible explanatory factors, as well as to determine the importance of these elements to survivors, providers, and outcomes.
It is possible that the IOM recommendations are overreaching, and that less comprehensive TSs and SCPs would still be of value, especially given that most survivors currently receive no care plan at all. Perhaps mandates requiring implementation should be deferred or relaxed until we have a clearer understanding of which components are essential to patient outcomes. Certainly the results of this study suggest that many elements were not typically covered in our sample and it would appear that the current content recommendations exceed what can be met by most survivorship programs. It is also important to recognize that the provision of TS/SCPs is only one step in providing high quality cancer survivorship care. TS/SCPs alone are unlikely to overcome documented gaps and fragmentation in survivorship care unless linked to more comprehensive programs as well as high quality and accessible services recommended within SCPs [37]. In particular, the identification of care coordinators or survivorship navigators may be of use in ensuring that survivors receive adequate care and consistent follow-up [7], although this remains under-examined in the current literature, as well [38].
SCPs have been endorsed as having strong face validity [1, 3, 39], and cancer survivors desire these documents and endorse their potential utility in overcoming deficits in survivorship care [2, 34, 40–43]. While we await data on essential elements and outcomes, perhaps implementation efforts should be guided by the perspectives of key stakeholders, including not only survivors, but also PCPs who will increasingly assume survivors’ follow-up care [23, 44–47]. A growing number of studies are examining stakeholder perspectives [2, 34, 40–43, 48, 49]. For example, cancer survivors report preferences for plans that address resources and strategies for dealing with psychosocial effects and that clearly outline both follow-up care and health promotion including whom to contact with various concerns [2, 39, 40, 42, 43], areas not consistently addressed in the current study.
Another approach may be to assume that items most consistently addressed in care plans are not only implicitly endorsed by stakeholders but also the most feasible to address. A significant limitation of this approach, however, is that so few content domains (eight out of a possible 23) were consistently addressed by the majority of centers. This leaves a large number of areas unaddressed, including domains endorsed by patients as being important.
The substantial time required for preparation and delivery of SCPs will limit sustainability and reach if not addressed, and adequate reimbursement is a likely to be a prerequisite to systematic implementation. Creative solutions and novel approaches will be needed to improve efficiency. Some attention has already been paid to the need to leverage EMR systems and possibly tumor registries in helping to populate summaries [11, 34, 50–52] and decrease the inefficiencies of medical record abstraction. Our data indicate that very few sites included in the study are utilizing electronic routes to populate or disseminate SCP information. Other approaches to improve efficiency while overcoming identified gaps might include the utilization of standardized educational materials and/or the expansion of content in survivorship care planning programs such as the LIVESTRONG™ Care Plan or Journey Forward. Creative information technology solutions should also be explored for improving the efficiency of creating and tailoring SCPs, such as linking disease and treatment exposures with individualized follow-up care guidelines, although further research is needed if such guidelines are to be evidence based.
Limitations
There are a several limitations that must be taken into consideration in interpreting the results. Most importantly, our study focused on documents, not on the content of the visits during which these documents were provided. It is quite possible that issues under-addressed in the documents were addressed during the visit. In addition, we examined only those SCPs given to breast cancer survivors. The content and process of SCP delivery with other cancer survivors may be quite different, even at participating institutions. Moreover, concordance with IOM recommendations at the item level was scored as a dichotomous outcome; accuracy or breadth of content was not examined. Structure and processes of SCP delivery were assessed by self-report. Although we took care to target the person most engaged in SCP delivery at each site, it is possible that this individual was more familiar with some aspects of the process than others, resulting in variable accuracy of data. Furthermore, the content, structure, and process of SCP delivery may have further evolved since the time of study participation, thus limiting the contemporary accuracy of these data. Finally, data from this study have limited generalizability to other settings, as centers that are part of the LIVESTRONG™ Network differ from non-Network institutions in that they each receive LIVE-STRONG™ funding that helps to partially underwrite the cost of TS/SCP delivery. This, however, suggests that our findings may overestimate the concordance that may be expected in less well-resourced and focused settings.
Conclusions
The IOM has suggested that SCPs may address a number of deficits in survivorship care. However, it has been unclear how concordant various care plans are with IOM recommendations or whether many of the items embedded in these recommendations are necessary for providing optimal survivorship care, beyond the fact that there is at present limited evidence for their clinical effectiveness. Our data suggest that even institutions with dedicated funding and support for survivorship care deliver TSs and SCPs that are not fully concordant with IOM recommendations. In addition, content areas for which patients have expressed preferences and that may be essential to correcting deficits in survivorship care may not be currently well addressed. A variety of approaches should be explored to improve not only the efficiency and reach of SCP delivery, but also the acceptability and effectiveness of care plans in various populations of cancer survivors. The essential elements necessary for survivorship care planning should also be further investigated, especially given that the current scope of recommendations appears difficult to achieve. A number of studies directed at developing, refining, and evaluating the outcomes of models and methods of SCP delivery are underway, and results are eagerly awaited. In the interim, implementation of TSs and SCPs will continue, and we hope that lessons learned from our evaluation of their delivery within the LIVESTRONG™ Survivorship COE Network will provide useful insights that will enhance the quality and reach of SCP delivery.
Acknowledgments
The authors would like to acknowledge contributions of both Donna Pucci and Alison Taggi, research coordinators at the University of Pennsylvania who were instrumental in conducting this study. We are also grateful to all of the clinical and research staff at each of the LIVESTRONG™ Survivorship Centers of Excellence and community-based affiliates who participated in and helped collect data for this study. This research was supported by funding from the LIVESTRONG™ Foundation.
Appendix
Table 7.
Individual treatment summary item concordance with IOM recommendations
| Individual treatment summary (TS) items |
n=65 TSs/13 sites Mean |
|---|---|
| Diagnosis | |
| Diagnostic tests performed | 0.20 |
| Diagnosis date | 0.48 |
| Staging and tumor characteristics | |
| Diagnosis (i.e., histology) | 0.75 |
| Laterality | 0.8 |
| Stage | 0.77 |
| Tumor grade | 0.28 |
| Estrogen receptor (ER) status | 0.69 |
| Progesterone receptor (PR) status | 0.66 |
| HER2/neu status | 0.58 |
| Lymph nodes positive (Y/N) | 0.69 |
| Surgery | |
| Surgery performed (Y/N) | 0.97 |
| Surgery type | 0.97 |
| Lymph node dissection performed (Y/N) | 0.89 |
| Type of lymph node dissection | 0.74 |
| Number of nodes dissected | 0.58 |
| Surgery date | 0.77 |
| Reconstruction performed (Y/N) | 0.51 |
| Reconstruction date | 0.40 |
| Treating physician contact information | |
| Surgeon(s) names | 0.35 |
| Complete contact information for the surgeon (s) | 0.29 |
| Radiation oncologist name | 0.52 |
| Complete contact information for the radiation oncologist | 0.23 |
| Supportive therapist(s) name(s) | 0.17 |
| Complete contact information for the supportive therapist | 0.02 |
| Primary oncology treatment provider noted (Y/N) | 0.58 |
| Complete contact information for primary oncology treatment provider | 0.46 |
| Clinical trials | |
| Received treatment on a clinical trial (Y/N) | 0.28 |
| Title and number of the clinical trial | 0.09 |
| Chemotherapy | |
| Chemotherapy received (Y/N) | 0.94 |
| Adjuvant vs. neoadjuvant | 0.11 |
| Chemotherapy start and stop dates | 0.58 |
| Full name(s) of chemotherapy agent(s) | 0.91 |
| Number of chemotherapy cycles received | 0.71 |
| Route of chemotherapy received | 0.20 |
| Dose-reduction required (Y/N) | 0.32 |
| Total dosage | 0.35 |
| Radiotherapy | |
| Radiotherapy received (Y/N) | 0.98 |
| Type of radiotherapy received | 0.31 |
| Radiotherapy subtype | 0.25 |
| Site (e.g., chest wall and breast) where radiotherapy received | 0.58 |
| Side where radiotherapy received | 0.51 |
| Radiotherapy start and stop dates | 0.58 |
| Radiotherapy dose | 0.62 |
| Hormonal therapy | |
| Hormonal therapy received (Y/N) | 0.91 |
| Type of hormonal therapy received | 0.86 |
| Start-stop (or continuing) dates for hormonal therapy | 0.62 |
| Targeted therapy | |
| Targeted therapy received (Y/N) | 0.45 |
| Type of targeted therapy received | 0.32 |
| Start-stop (or continuing) dates provided | 0.25 |
| Toxicity | |
| Toxicities or complications | 0.52 |
| Genetic testing | |
| Genetic testing performed (Y/N) | 0.43 |
| Date genetic testing performed | 0.08 |
| Results of genetic testing | 0.26 |
| Supportive therapy | |
| Supportive therapy received (Y/N) | 0.05 |
| Type of supportive therapy received | 0.05 |
| Start-stop (or continuing) dates provided | 0.00 |
| Follow-up care contacts | |
| Key contact for oncology treatment identified (Y/N) | 0.15 |
| Complete contact information for the key contact | 0.17 |
| Care coordinator identified, Y/N | 0.00 |
| Complete contact information for the care coordinator | 0.00 |
Table 8.
Individual survivorship care plan item concordance with IOM recommendations
| Domains and individual survivorship care plan (SCP) items |
n=65 SCPs/13 sites Mean |
|---|---|
| Toxicities and late effects | |
| Possible treatment toxicities | 1.00 |
| Course of recovery from treatment toxicities | 0.92 |
| Possible late effects identified | 1.00 |
| Symptoms of possible late effects | 1.00 |
| Breast cancer surveillance | |
| Breast cancer (BC) surveillance testing recommendations | 0.98 |
| Frequency of BC surveillance tests | 0.98 |
| Provider responsible for BC specific surveillance tests | 0.48 |
| Cancer surveillance (other than BC) | |
| Cancer surveillance testing recommendations | 0.66 |
| Frequency of other cancer surveillance tests | 0.46 |
| Provider responsible for other cancer surveillance tests | 0.17 |
| Non-cancer surveillance | |
| Non-cancer surveillance testing recommendations | 0.89 |
| Frequency of non-cancer surveillance tests | 0.89 |
| Provider responsible for non-cancer surveillance tests | 0.54 |
| Signs of cancer (recurrent and second) | |
| Signs of recurrence | 0.23 |
| Signs of second cancers | 0.40 |
| Psychosocial effects | |
| Effects on marital/partner relationship | 0.46 |
| Effects on sexual functioning | 0.86 |
| Effects on work/employment | 0.52 |
| Effects on parenting | 0.46 |
| Effects on insurance | 0.00 |
| Effects on finances | 0.54 |
| Potential need for future psychosocial support | 0.78 |
| Referrals and resources | |
| Referral sources for counseling | 0.68 |
| Referral sources for legal aid | 0.69 |
| Referral sources for financial assistance | 0.69 |
| Referrals to specific follow-up care providers | 0.17 |
| General cancer-related resources | 0.85 |
| Prevention/health promotion | |
| Specific healthy behavior recommendations | 0.92 |
| Chemoprevention strategies for secondary prevention | 0.00 |
| Genetic testing recommendations | |
| Information on who should consider genetic counseling/testing | 0.62 |
| Relatives’ cancer risk | |
| Relatives’ cancer risk | 0.02 |
| Recommendations for cancer screening tests for relatives | 0.00 |
Contributor Information
Carrie Tompkins Stricker, Abramson Cancer Center at the University of Pennsylvania, 3400 Civic Center Blvd. 3rd Floor West, Philadelphia, PA 19104, USA.
Linda A. Jacobs, Abramson Cancer Center at the University of Pennsylvania, 3400 Civic Center Blvd. 3rd Floor West, Philadelphia, PA 19104, USA
Betsy Risendal, University of Colorado Cancer Center, 1665 Aurora Ct., Mail Stop F-704, Aurora, CO 80045, USA.
Alison Jones, University of Colorado Cancer Center, 1665 Aurora Ct., Mail Stop F-704, Aurora, CO 80045, USA.
Sarahlena Panzer, Abramson Cancer Center at the University of Pennsylvania, 3400 Civic Center Blvd. 3rd Floor West, Philadelphia, PA 19104, USA.
Patricia A. Ganz, UCLA Schools of Medicine & Public Health, Jonsson Comprehensive Cancer Center, 650 Charles E. Young Dr., Rm A2-125, Los Angeles, CA 90095-6900, USA
Karen L. Syrjala, Clinical Research Division, Fred Hutchinson Cancer Research Center: Survivorship Program, 1100 Fairview Ave. N, Mailstop LF-268, Seattle, WA 98109, USA
Mary S. McCabe, Memorial Sloan-Kettering Cancer Center: Survivorship Program, 1275 York Avenue, Room 2001K, New York, NY 10065, USA
K. Scott Baker, Pediatric Blood and Marrow Transplantation and Survivorship Programs, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N, Mailstop LF-268, Seattle, WA 98109, USA.
Kenneth Miller, Dana-Farber Cancer Institute, 450 Brookline Avenue # LW514, Boston, MA 02215, USA.
Jacqueline Casillas, David Geffen School of Medicine at UCLA, Department of Pediatrics, Division of Hematology/Oncology, 10833 Le Conte Ave., Rm A2-312 MDCC, Los Angeles, CA 90095, USA.
Donald L. Rosenstein, Departments of Psychiatry and Medicine, Comprehensive Cancer Support Program, North Carolina Cancer Hospital and Lineberger Cancer Center, University of North Carolina at Chapel Hill, CB 7512, Chapel Hill, NC 27514, USA
Marci Campbell, North Carolina Cancer Hospital and Lineberger Cancer Center, University of North Carolina at Chapel Hill, CB 7512, Chapel Hill, NC 27514, USA.
Steven C. Palmer, Abramson Cancer Center at the University of Pennsylvania, 7 Penn Tower 3400 Spruce Street, Philadelphia, PA 19104, USA
References
- 1.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. Washington: The National Academies; 2006. [Google Scholar]
- 2.Hewitt ME, et al. Perspectives on post-treatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270–3. doi: 10.1200/JCO.2006.10.0826. [DOI] [PubMed] [Google Scholar]
- 3.Earle CC. Failing to plan is planning to fail: improving the quality of care with survivorship care plans. J Clin Oncol. 2006;24(32):5112–6. doi: 10.1200/JCO.2006.06.5284. [DOI] [PubMed] [Google Scholar]
- 4.Erikson C, et al. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs LA, et al. Adult cancer survivorship: evolution, research, and planning care. CA A Cancer J Clin. 2009;59:391–410. doi: 10.3322/caac.20040. [DOI] [PubMed] [Google Scholar]
- 6.Earle CC. Long term care planning for cancer survivors: a health services research agenda. J Cancer Surviv. 2007;1(1):64–74. doi: 10.1007/s11764-006-0003-9. [DOI] [PubMed] [Google Scholar]
- 7.Faul LA, et al. Improving survivorship care for patients with colorectal cancer. Cancer Control. 2010;17(1):35–43. doi: 10.1177/107327481001700105. [DOI] [PubMed] [Google Scholar]
- 8.Oeffinger KC, et al. Increasing rates of breast cancer and cardiac surveillance among high-risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatr Blood Cancer. 2010;56(5):818–24. doi: 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunfeld E, et al. Clinical science symposium, patient and survivor care and health services research session: survivorship care plans, quality of care, and barriers to care. Eur J Clin Med Oncol; Presented at the Annual ASCO meeting; Chicago, IL. June 2011.2011. (suppl; abstr 9005) [Google Scholar]
- 10.Cancer Program Standards 2012: Ensuring Patient-Centered Care. Available online at: http://www.facs.org/cancer/coc/cps2012draft.pdf.
- 11.Ganz PA, Hahn EE. Implementing a survivorship care plan for patients with breast cancer. J Clin Oncol. 2008;26(5):759–67. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]
- 12.Hahn EE, Ganz PA. Survivorship programs and care plans in practice: variations on a theme. J Oncol Pract. 2011;7(2):70–5. doi: 10.1200/JOP.2010.000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casillas J, Ayanian JZ. Disparities in care for cancer survivors. In: Feuerstein M, Ganz PA, editors. Health services for cancer survivors. New York: Springer; 2011. pp. 153–68. [Google Scholar]
- 14.Malin J, Sayers EJ, Jefford M. What is quality health care for cancer survivors? In: Feuerstein M, Ganz PA, editors. Quality health care for cancer survivors. New York: Springer; 2011. [Google Scholar]
- 15.Jefford M, et al. Development and pilot testing of a nurse-led posttreatment support package for bowel cancer survivors. Cancer Nurs. 2011;34(3):E1–E10. doi: 10.1097/NCC.0b013e3181f22f02. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro CL, et al. The LIVESTRONG Center of Excellence network. J Cancer Surviv. 2009;3(1):4–11. doi: 10.1007/s11764-008-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell M, et al. Adult cancer survivorship care: experiences from the LIVESTRONG Centers of Excellence network. J Cancer Surviv. 2011;5(3):271–82. doi: 10.1007/s11764-011-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stricker CT, et al. Survivorship Care Plan Assessment Checklist (SCPAC): a tool to evaluate breast cancer survivorship care plans. [Abstract 6117]. J Clin Oncol; Presented at the Annual ASCO meeting; Chicago, IL. June 2011.2011. (suppl; abstr 6117) [Google Scholar]
- 19.LIVESTRONG. Develop my LIVESTRONG Care Plan. Available from: http://www.livestrongcareplan.org/
- 20.Journey Forward. About survivorship care planning. Available from: http://journeyforward.org/about-survivorship-care-planning.
- 21.American Society of Clinical Oncology. Chemotherapy treatment plan and summary. Available from: http://www.asco.org/ASCOv2/Practice+%26+Guidelines/Quality+Care/Quality+Measurement+%26+Improvement/Chemotherapy+Treatment+Plan+and+Summary.
- 22.Shulman LN, et al. Cancer care and cancer survivorship care in the United States: will we be able to care for these patients in the future? J Oncol Pract. 2009;5(3):119–23. doi: 10.1200/JOP.0932001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starfield B, Fryer GE., Jr The primary care physician workforce: ethical and policy implications. Ann Fam Med. 2007;5(6):486–91. doi: 10.1370/afm.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salsberg E, Grover A. Physician workforce shortages: implications and issues for academic health centers and policymakers. Acad Med. 2006;81(9):782–7. doi: 10.1097/00001888-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 25.President’s Cancer Panel 2005–2006 annual report. Department of Health and Human Services, National Institutes on Health; Bethesda, MD: 2006. NCI, Assessing progress, advancing change. [Google Scholar]
- 26.Haylock PJ, et al. The cancer survivor’s prescription for living. Am J Nurs. 2007;107(4):58–70. doi: 10.1097/01.NAJ.0000265276.92317.b2. [DOI] [PubMed] [Google Scholar]
- 27.Oncology Nursing Society. ONS joins Journey Forward collaborative to benefit cancer survivors. Available from: http://www.ons.org/news.aspx?id=108.
- 28.National Comprehensive Cancer Network. Moving forward after cancer. Available from: http://www.nccn.com/component/content/article/69-life-after-cancer-overview/50-moving-forward-after-cancer.html.
- 29.American Cancer Society. Survivorship care plans. Available from: http://www.cancer.org/Treatment/SurvivorshipDuringandAfterTreatment/SurvivorshipCarePlans/index.
- 30.Center for Disease Control. Basic information about cancer survivor-ship. Available from: http://www.cdc.gov/cancer/survivorship/basic_info/
- 31.National Cancer Institute. Follow up care after cancer treatment. Available from: http://www.cancer.gov/cancertopics/factsheet/Therapy/followup.
- 32.National Coalition for Cancer Survivorship. Cancer care planning. Available from: http://www.canceradvocacy.org/careplanning/
- 33.Miller R. Implementing a survivorship care plan for patients with breast cancer. Clin J Oncol Nurs. 2008;12(3):479–87. doi: 10.1188/08.CJON.479-487. [DOI] [PubMed] [Google Scholar]
- 34.Ganz PA, Casillas J, Hahn EE. Ensuring quality care for cancer survivors: implementing the survivorship care plan. Semin Oncol Nurs. 2008;24(3):208–17. doi: 10.1016/j.soncn.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Belnap B, et al. Challenges of implementing depression care management in the primary care setting. Adm Policy Ment Health Ment Health Serv Res. 2006;33(1):65–75. doi: 10.1007/s10488-005-4237-z. [DOI] [PubMed] [Google Scholar]
- 36.Hepner KA, et al. The effect of adherence to practice guidelines on depression outcomes. Ann Intern Med. 2007;147(5):320–9. doi: 10.7326/0003-4819-147-5-200709040-00007. [DOI] [PubMed] [Google Scholar]
- 37.Silver JK. Strategies to overcome cancer survivorship care barriers. Phys Med Rehabil: J Inj Funct Rehabil. 2011;3(6):503–6. doi: 10.1016/j.pmrj.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Wells KJ, et al. Patient navigation: state of the art or is it science? Cancer. 2008;113(8):1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adler N, Page A, editors. Institute of Medicine (IOM) Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: The National Academies; 2008. [PubMed] [Google Scholar]
- 40.Marbach TJ, Griffie J. Patient preferences concerning treatment plans, survivorship care plans, education, and support services. Oncol Nurs Forum. 2011;38(3):335–42. doi: 10.1188/11.ONF.335-342. [DOI] [PubMed] [Google Scholar]
- 41.Horning SJ. Follow-up of adult cancer survivors: new paradigms for survivorship care planning. Hematol Oncol Clin North Am. 2008;22(2):201–10. doi: 10.1016/j.hoc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Smith S, et al. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011:1–8. doi: 10.1007/s11764-011-0185-7. [DOI] [PubMed] [Google Scholar]
- 43.Baravelli C, et al. The views of bowel cancer survivors and health care professionals regarding survivorship care plans and post treatment follow up. J Cancer Surviv. 2009;3(2):99–108. doi: 10.1007/s11764-009-0086-1. [DOI] [PubMed] [Google Scholar]
- 44.Cheung WY, et al. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27:2489–95. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- 45.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;40:25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Giudice ME, et al. Primary care physicians’ views of routine follow-up care of cancer survivors. J Clin Oncol. 2009;27(20):3338–45. doi: 10.1200/JCO.2008.20.4883. [DOI] [PubMed] [Google Scholar]
- 47.Mao JJ, et al. Delivery of survivorship care by primary care physicians: the perspective of breast cancer patients. J Clin Oncol. 2009;27(6):933–8. doi: 10.1200/JCO.2008.18.0679. [DOI] [PubMed] [Google Scholar]
- 48.Grunfeld E. Looking beyond survival: how are we looking at survivorship? J Clin Oncol. 2006;24(32):5166–9. doi: 10.1200/JCO.2006.06.5953. [DOI] [PubMed] [Google Scholar]
- 49.Watson E, Sugden E, Rose P. Views of primary care physicians and oncologists on cancer follow-up initiatives in primary care: an online survey. J Cancer Surviv. 2010;4(2):159–66. doi: 10.1007/s11764-010-0117-y. [DOI] [PubMed] [Google Scholar]
- 50.Anonymous. Ensuring continuity of care through electronic health records. J Oncol Pract. 2007;3(3):137–42. doi: 10.1200/JOP.0733501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill-Kayser CE, et al. An internet tool for creation of cancer survivorship care plans for survivors and health care providers: design, implementation, use and user satisfaction. J Med Internet Res. 2009;11(3):e39. doi: 10.2196/jmir.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grant M, Economou D, Ferrell BR. Oncology nurse participation in survivorship care. Clin J Oncol Nurs. 2010;14(6):709–15. doi: 10.1188/10.CJON.709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]